Chitosan-Based Composite Membranes with Different Biocompatible Metal Oxide Nanoparticles: Physicochemical Properties and Drug-Release Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Membrane Preparation
2.3. Membrane Characterization
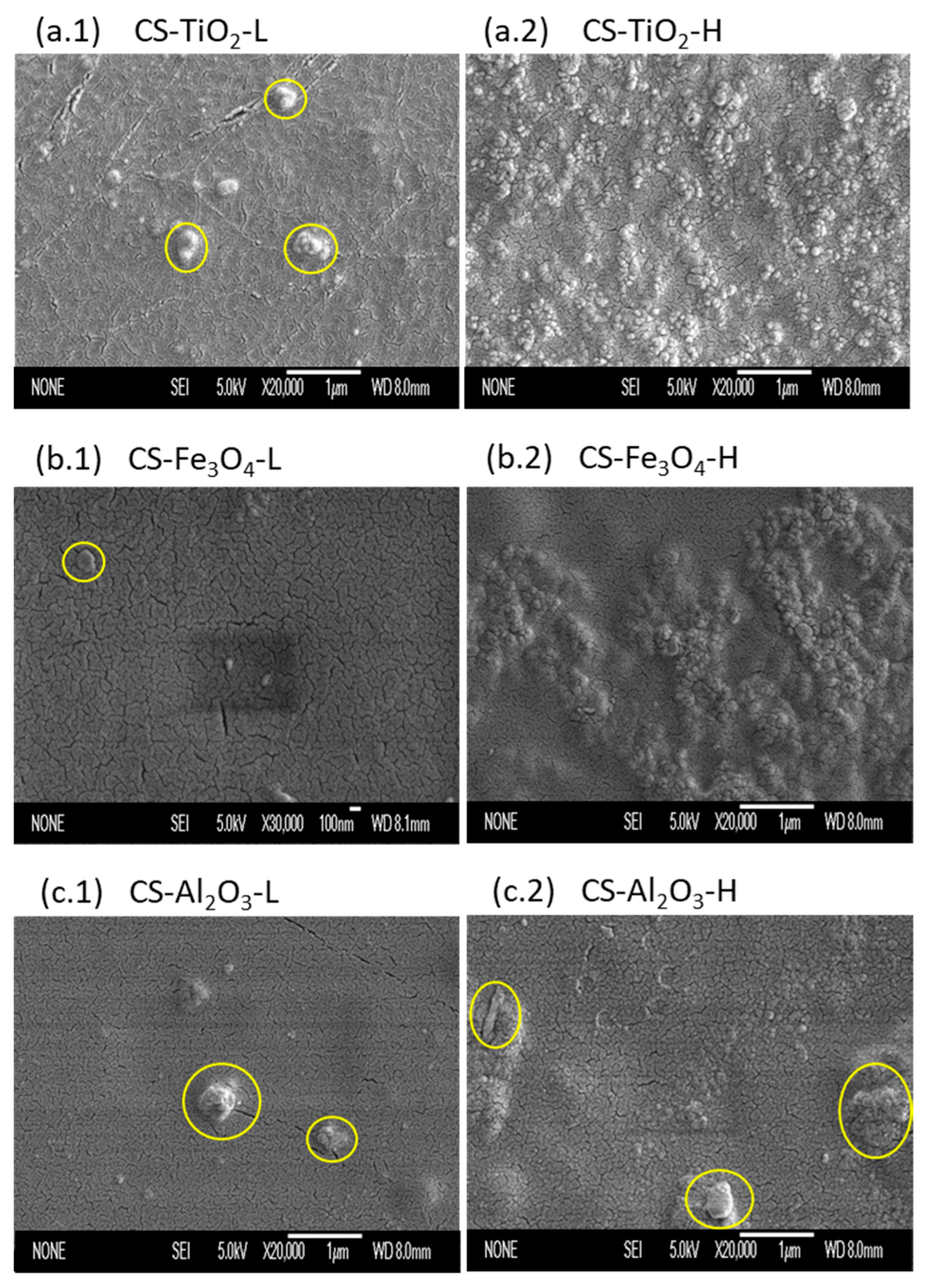
2.3.1. Scanning Electron Microscopy (SEM)
2.3.2. X-ray Diffraction (XRD)
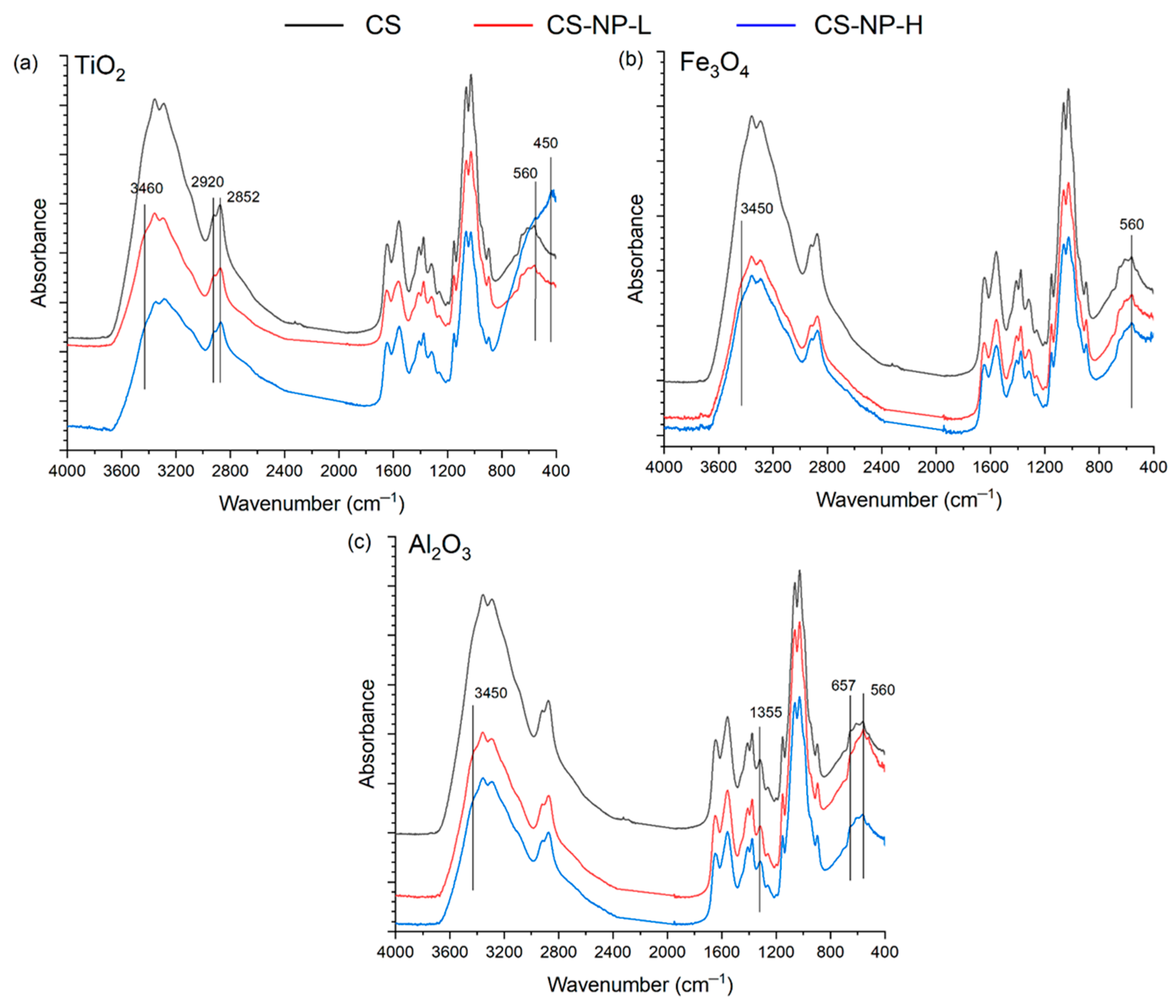
2.3.3. Attenuated Total Reflectance/Fourier Transforms Infrared Spectroscopy (ATR-FTIR)
2.3.4. Mechanical Properties
2.3.5. Swelling
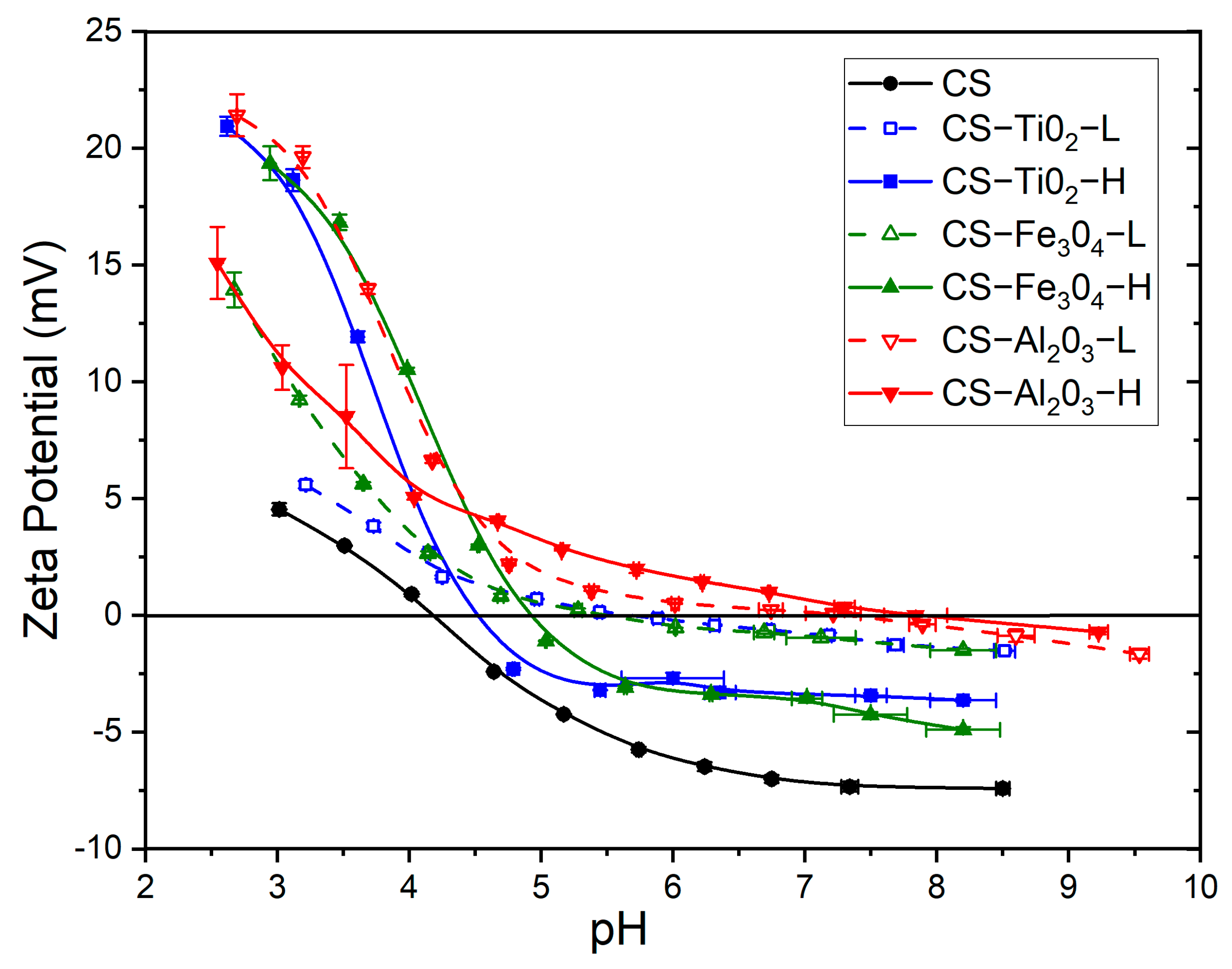
2.3.6. Zeta Potential
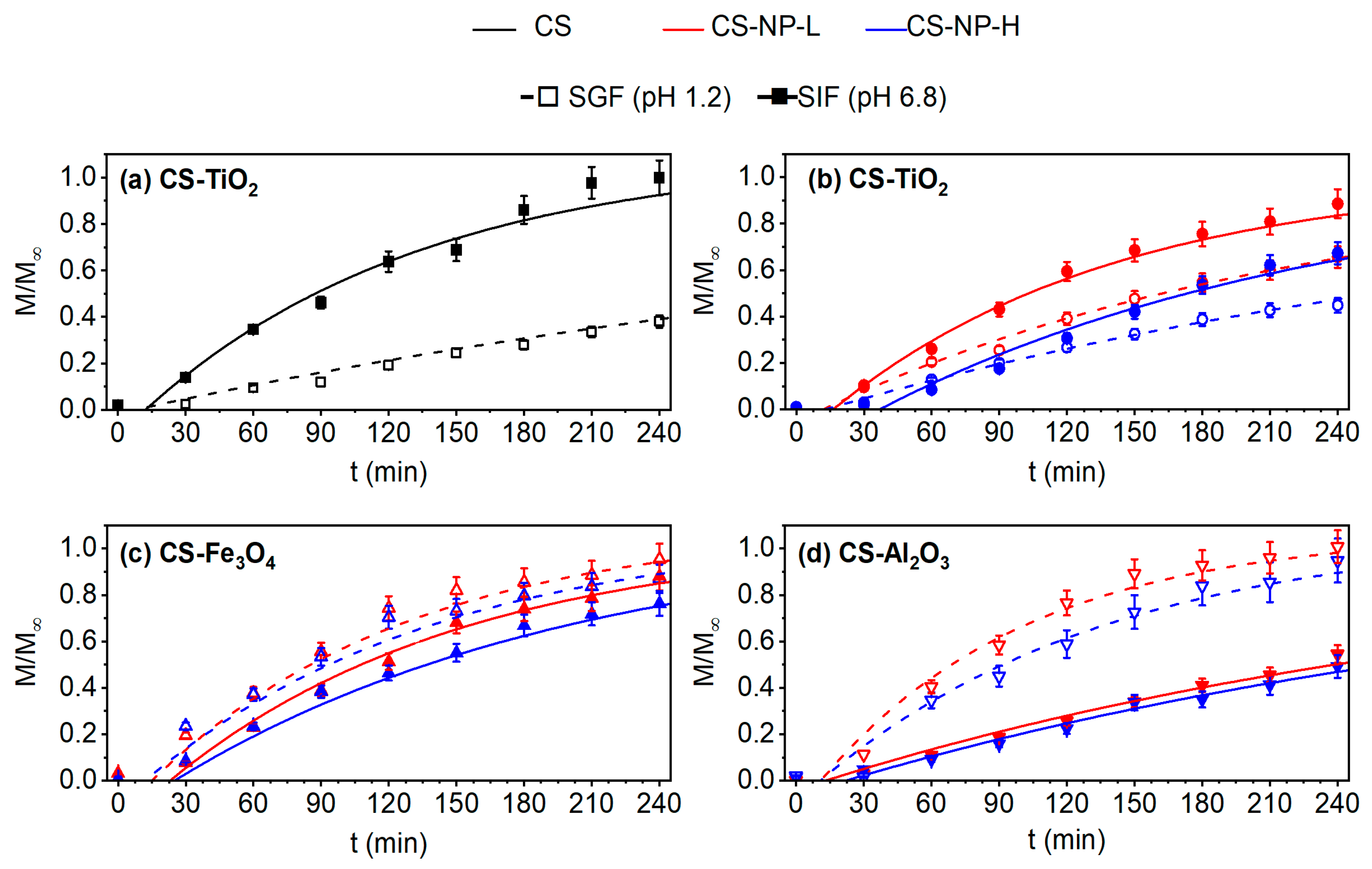
2.4. Drug Permeability Experiments
3. Results
3.1. SEM
3.2. X-ray Diffraction
3.3. ATR-FTIR
3.4. Mechanical Properties
3.5. Swelling
3.6. Zeta Potential
3.7. Drug Permeability Experiments
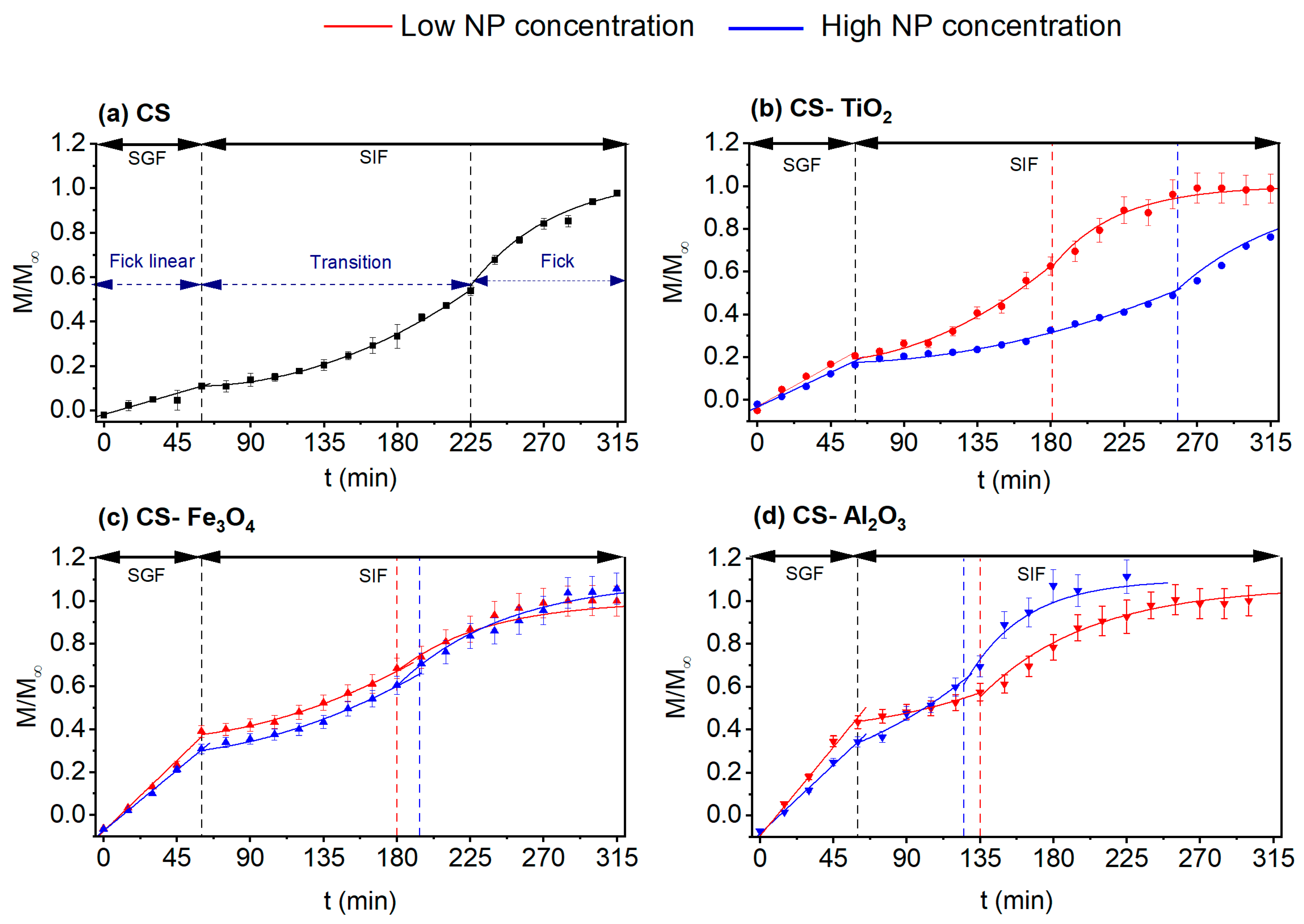
3.7.1. Transport according to pH
3.7.2. Simulation Experiments of Gastrointestinal Conditions (SGIT)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Baroudi, A.; García-Payo, C.; Khayet, M. Structural, Mechanical, and Transport Properties of Electron Beam-Irradiated Chitosan Membranes at Different Doses. Polymers 2018, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Zainal, Z.; Hui, L.K.; Hussein, M.Z.; Abdullah, A.H.; Hamadneh, I.M. Characterization of TiO2-chitosan/glass photocatalyst for the removal of a monoazo dye via photodegradation-adsorption process. J. Hazard. Mater. 2009, 164, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Ramachandran, R.; Divyarani, V.V.; Chennazhi, K.P.; Tamura, H.; Nair, S.V. Fabrication of chitin-chitosan/nano TiO2-composite scaffolds for tissue engineering applications. Int. J. Biol. Macromol. 2011, 48, 336–344. [Google Scholar] [CrossRef] [PubMed]
- Ražem, D.; Katušin-Ražem, B. The effects of irradiation on controlled drug delivery/controlled drug release systems. Radiat. Phys. Chem. 2008, 77, 288–344. [Google Scholar] [CrossRef]
- Movahedi, M.; Bishop, D.T.; Macrae, F.; Mecklin, J.-P.; Moeslein, G.; Olschwang, S.; Eccles, D.; Evans, D.G.; Maher, E.R.; Bertario, L. Obesity, Aspirin, and Risk of Colorectal Cancer in Carriers of Hereditary Colorectal Cancer: A Prospective Investigation in the CAPP2 Study. J. Clin. Oncol. 2015, 33, 3591–3597. [Google Scholar] [CrossRef]
- Herova, M.; Schmid, M.; Gemperle, C.; Loretz, C.; Hersberger, M. Low dose aspirin is associated with plasma chemerin levels and may reduce adipose tissue inflammation. Atherosclerosis 2014, 235, 256–262. [Google Scholar] [CrossRef]
- Robless, P.M.D.; Stansby, G. Systematic review of antiplatelet therapy for the prevention of myocardial infarction, stroke or vascular death in patients with peripheral vascular disease. Br. J. Surg. 2001, 88, 787–800. [Google Scholar] [CrossRef]
- Islam, A.; Yasin, T.; Bano, I.; Riaz, M. Controlled release of aspirin from pH-sensitive chitosan/poly(vinyl alcohol) hydrogel. J. Appl. Polym. Sci. 2012, 124, 4184–4192. [Google Scholar] [CrossRef]
- Kamari, Y.; Ghiaci, M. Preparation and characterization of ibuprofen/modified chitosan/TiO2 hybrid composite as a controlled drug-delivery system. Microporous Mesoporous Mater. 2016, 234, 361–369. [Google Scholar] [CrossRef]
- Mi, F.L.; Shyu, S.S.; Lee, S.T.; Wong, T.B. Kinetic study of chitosan-tripolyphosphate complex reaction and acid-resistive properties of the chitosan-tripolyphosphate gel beads prepared by in-liquid curing method. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1551–1564. [Google Scholar] [CrossRef]
- Kono, H.; Teshirogi, T. Cyclodextrin-grafted chitosan hydrogels for controlled drug delivery. Int. J. Biol. Macromol. 2015, 72, 299–308. [Google Scholar] [CrossRef]
- Gierszewska, M.; Ostrowska-Czubenko, J. Chitosan-based membranes with different ionic crosslinking density for pharmaceutical and industrial applications. Carbohydr. Polym. 2016, 153, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Bhumkar, D.R.; Pokharkar, V.B. Studies on effect of pH on crossinking of chitosan with sodium tripolyphosphate: A technical note. AAPS PharmSciTech 2006, 7, E138–E143. [Google Scholar] [CrossRef] [PubMed]
- Velickova, E.; Winkelhausen, E.; Kuzmanova, S.; Alves, V.D.; Moldão-Martins, M. Impact of chitosan-beeswax edible coatings on the quality of fresh strawberries (Fragaria ananassa cv Camarosa) under commercial storage conditions. Food Sci. Technol. Int. 2013, 52, 80–92. [Google Scholar] [CrossRef]
- Müller, K.; Bugnicourt, E.; Latorre, M.; Jorda, M.; Echegoyen Sanz, Y.; Lagaron, J.M.; Miesbauer, O.; Bianchin, A.; Hankin, S.; Bölz, U.; et al. Review on the processing and properties of polymer nanocomposites and nanocoatings and their applications in the packaging, automotive and solar energy fields. Nanomaterials 2017, 7, 74. [Google Scholar] [CrossRef]
- Kloster, G.A.; Marcovich, N.E.; Mosiewicki, M.A. Composite films based on chitosan and nanomagnetite. Eur. Polym. J. 2015, 66, 386–396. [Google Scholar] [CrossRef]
- Madhumathi, K.; Sudheesh Kumar, P.T.; Kavya, K.C.; Furuike, T.; Tamura, H.; Nair, S.V.; Jayakumar, R. Novel chitin/nanosilica composite scaffolds for bone tissue engineering applications. Int. J. Biol. Macromol. 2009, 45, 289–292. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Park, D.; Lee, Y.C. Chitosan Combined with ZnO, TiO2 and Ag Nanoparticles for Antimicrobial Wound Healing Applications: A Mini Review of the Research Trends. Polymers 2017, 9, 21. [Google Scholar] [CrossRef]
- Amin, K.A.M.; Panhuis, M.I.H. Reinforced Materials Based on Chitosan, TiO2 and Ag Composites. Polymers 2012, 4, 590–599. [Google Scholar] [CrossRef]
- Díaz-Visurraga, J.; Meléndrez, M.F.; García, A.; Paulraj, M.; Cárdenas, G. Semitransparent chitosan-TiO2 nanotubes composite film for food package applications. J. Appl. Polym. Sci. 2010, 116, 3503–3515. [Google Scholar]
- Revia, R.A.; Zhang, M. Magnetite nanoparticles for cancer diagnosis, treatment, and treatment monitoring: Recent advances. Mater. Today 2016, 19, 157–168. [Google Scholar] [CrossRef]
- Ding, Y.; Shen, S.Z.; Sun, H.; Sun, K.; Liu, F.; Qi, Y.; Yan, J. Design and construction of polymerized-chitosan coated Fe3O4 magnetic nanoparticles and its application for hydrophobic drug delivery. Mater. Sci. Eng. C 2015, 48, 487–498. [Google Scholar] [CrossRef]
- Viswanathan, N.; Meenakshi, S. Enriched fluoride sorption using alumina/chitosan composite. J. Hazard. Mater. 2010, 178, 226–232. [Google Scholar] [CrossRef]
- Gong, D.; Yadavalli, V.; Paulose, M.; Pishko, M.; Grimes, C.A. Controlled molecular release using nanoporous alumina capsules. Biomed. Microdevices 2003, 5, 75–80. [Google Scholar] [CrossRef]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.M.; Argüelles-Monal, W.; Desbrières, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Krevelen, D.W.V.; Nijenhuis, K.T. Properties of Polymers; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar]
- Khayet, M.; García-Payo, M.C. X-Ray diffraction study of polyethersulfone polymer, flat-sheet and hollow fibers prepared from the same under different gas-gaps. Desalination 2009, 245, 494–500. [Google Scholar] [CrossRef]
- Park, S.B.; You, J.O.; Park, H.Y.; Haam, S.J.; Kim, W.S. A novel pH-sensitive membrane from chitosan-TEOS IPN; preparation and its drug permeation characteristics. Biomaterials 2001, 22, 323–330. [Google Scholar] [CrossRef]
- Schmiedel, D.A.-V.D.R. European Pharmacopoeia, 9th Edition 2016, English: Subscription to Main Volume + Supplement 1 + Supplement 2; Deutscher Apotheker Verlag: Stuttgart, Germany, 2016. [Google Scholar]
- Singco, B.; Liu, L.-H.; Chen, Y.-T.; Shih, Y.-H.; Huang, H.-Y.; Lin, C.-H. Approaches to drug delivery: Confinement of aspirin in MIL-100(Fe) and aspirin in the de novo synthesis of metal–organic frameworks. Microporous Mesoporous Mater. 2016, 223, 254–260. [Google Scholar] [CrossRef]
- Ruiz-Medina, A.; Fernández de Córdova, M.L.; Ortega-Barrales, P.; Molina Díaz, A. Flow-through UV spectrophotometric sensor for determination of (acetyl)salicylic acid in pharmaceutical preparations. Int. J. Pharm. 2001, 216, 95–104. [Google Scholar] [CrossRef]
- Bruschi, M. Strategies to Modify the Drug Release from Pharmaceutical Systems; 5. Mathematical models of drug release; Woodhead Publishing: Cambridge, UK, 2015; pp. 63–89. [Google Scholar]
- Siepmann, J.; Siepmann, F. Modeling of diffusion controlled drug delivery. J. Control. Release 2012, 161, 351–362. [Google Scholar] [CrossRef]
- Peppas, N.A.; Sahlin, J.J. A simple equation for the description of solute release. III. Coupling of diffusion and relaxation. Int. J. Pharm. 1989, 57, 169–172. [Google Scholar] [CrossRef]
- Ritger, P.L.; Peppas, N.A. A simple equation for description of solute release II. Fickian and anomalous release from swellable devices. J. Control. Release 1987, 5, 37–42. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Nikfar, Z. Synthesis and antibacterial activities of novel nanocomposite films of chitosan/phosphoramide/Fe3O4 NPs. Int. J. Biol. Macromol. 2013, 60, 226–234. [Google Scholar] [CrossRef]
- Liqiang, J.; Xiaojun, S.; Baifu, X.; Baiqi, W.; Weimin, C.; Honggang, F. The preparation and characterization of La doped TiO2 nanoparticles and their photocatalytic activity. J. Solid State Chem. 2004, 177, 3375–3382. [Google Scholar] [CrossRef]
- Karunakaran, C.; Anilkumar, P.; Gomathisankar, P. Photoproduction of iodine with nanoparticulate semiconductors and insulators. Chem. Cent. J. 2011, 5, 31. [Google Scholar] [CrossRef]
- Castelló, J.; Gallardo, M.; Busquets, M.A.; Estelrich, J. Chitosan (or alginate)-coated iron oxide nanoparticles: A comparative study. Colloids Surf. A 2015, 468, 151–158. [Google Scholar] [CrossRef]
- Ghamsari, M.S.; Mahzar, Z.A.S.; Radiman, S.; Hamid, A.M.A.; Khalilabad, S.R. Facile route for preparation of highly crystalline γ-Al2O3 nanopowder. Mater. Lett. 2012, 72, 32–35. [Google Scholar] [CrossRef]
- de Barros, A.; Ferreira, M.; Constantino, C.J.L.; Ferreira, M. Nanocomposites based on LbL films of polyaniline and sodium montmorillonite clay. Synth. Met. 2014, 197, 119–125. [Google Scholar] [CrossRef]
- Essawy, H.A.; Youssef, A.M.; Abd El-Hakim, A.A.; Rabie, A.M. Exfoliation of Kaolinite Nanolayers in Poly(methylmethacrylate) Using Redox Initiator System Involving Intercalating Component. Polym. Plast. Technol. Eng. 2009, 48, 177–184. [Google Scholar] [CrossRef]
- Osman, Z.; Arof, A.K. FTIR studies of chitosan acetate based polymer electrolytes. Electrochim. Acta 2003, 48, 993–999. [Google Scholar] [CrossRef]
- Shen, K.; Hu, Q.; Wang, Z.; Qu, J. Effect of 60Co irradiation on the properties of chitosan rod. Mater. Sci. Eng. C 2011, 31, 866–872. [Google Scholar] [CrossRef]
- Antony, R.; Theodore David, S.; Karuppasamy, K.; Sanjeev, G.; Balakumar, S. Influence of electron beam irradiation on spectral, thermal, morphological and catalytic properties of Co(II) complex immobilized on chitosan’s Schiff base. Spectrochim. Acta Part A 2014, 124, 178–186. [Google Scholar] [CrossRef]
- Kavitha, K.; Sutha, S.; Prabhu, M.; Rajendran, V.; Jayakumar, T. In situ synthesized novel biocompatible titania-chitosan nanocomposites with high surface area and antibacterial activity. Carbohydr. Polym. 2013, 93, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Taşkın Çakıcı, G. Nano TiO2-doped sodium alginate/hydroxypropyl methylcellulose synthesis of bionanocomposite membrane and its use in controlled release of anti-cancer drug 5-fluorouracil. Polym. Bull. 2023. [Google Scholar] [CrossRef]
- Dutta, B.; Shetake, N.G.; Barick, B.K.; Barick, K.C.; Pandey, B.N.; Priyadarsini, K.I.; Hassan, P.A. pH sensitive surfactant-stabilized Fe3O4 magnetic nanocarriers for dual drug delivery. Colloids Surf. B Biointerfaces 2018, 162, 163–171. [Google Scholar] [CrossRef]
- Alamouti, A.F.; Nadafan, M.; Dehghani, Z.; Ara, M.H.M.; Noghreiyan, A.V. Structural and Optical Coefficients Investigation of γ-Al2O3 Nanoparticles using Kramers-Kronig Relations and Z–scan Technique. J. Asian Ceram. Soc. 2021, 9, 366–373. [Google Scholar] [CrossRef]
- Chai, L.; Wang, Y.; Zhao, N.; Yang, W.; You, X. Sulfate-doped Fe3O4/Al2O3 nanoparticles as a novel adsorbent for fluoride removal from drinking water. Water Res. 2013, 47, 4040–4049. [Google Scholar] [CrossRef]
- Jastrzębska, A.M.; Karwowska, E.; Olszyna, A.R.; Kunicki, A. Influence of bacteria adsorption on zeta potential of Al2O3 and Al2O3/Ag nanoparticles in electrolyte and drinking water environment studied by means of zeta potential. Surf. Coat. Technol. 2015, 271, 225–233. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Guo, Z.; Pereira, T.; Choi, O.; Wang, Y.; Hahn, H.T. Surface functionalized alumina nanoparticle filled polymeric nanocomposites with enhanced mechanical properties. J. Mater. Chem. 2006, 16, 2800–2808. [Google Scholar] [CrossRef]
- Franks, G.V.; Gan, Y. Charging behavior at the alumina–water interface and implications for ceramic processing. J. Am. Ceram. Soc. 2007, 90, 3373–3388. [Google Scholar] [CrossRef]
- Tsykhanovska, I.; Evlash, V.; Alexandrov, A.; Gontar, T. Dissolution Kinetics of Fe3O4 Nanoparticles in the Acid Media. Chem. Chem. Technol. 2019, 2, 170–184. [Google Scholar] [CrossRef]
- Ziemniak, S.; Jones, M.; Combs, K. Magnetite solubility and phase stability in alkaline media at elevated temperatures. J. Solution Chem. 1995, 24, 837–877. [Google Scholar] [CrossRef]
- Wu, Y. 15—Preparation of ultrafin powders by reaction-precipitation in impinging streams III: Nanotitania. In Impinging Streams; Wu, Y., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 301–315. [Google Scholar] [CrossRef]
- Yang, D.; Li, J.; Jiang, Z.; Lu, L.; Chen, X. Chitosan/TiO2 nanocomposite pervaporation membranes for ethanol dehydration. Chem. Eng. Sci. 2009, 64, 3130–3137. [Google Scholar] [CrossRef]
- Kosmulski, M. The pH-dependent surface charging and the points of zero charge. J. Colloid Interface Sci. 2002, 253, 77–87. [Google Scholar] [CrossRef]
- Bouhaik, I.S.; Leroy, P.; Ollivier, P.; Azaroual, M.; Mercury, L. Influence of surface conductivity on the apparent zeta potential of TiO2 nanoparticles: Application to the modeling of their aggregation kinetics. J. Colloid Interface Sci. 2013, 406, 75–85. [Google Scholar] [CrossRef]
- Marsalek, R.; Kotyrba, M.; Volna, E.; Jarusek, R. Neural Network Modelling for Prediction of Zeta Potential. Mathematics 2021, 9, 3089. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, Y.-S.; Ho, J.-M. Adsorption of Reactive Red 2 from aqueous solutions using Fe3O4 nanoparticles prepared by co-precipitation in a rotating packed bed. J. Alloys Compd. 2016, 666, 153–158. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, L.; Yang, Y.; Li, M.; Li, Y.; Dong, Y. The efficient removal of Cu(II) from aqueous solutions by Fe3O4@hexadecyl trimethoxysilane@chitosan composites. J. Mol. Liq. 2016, 219, 341–349. [Google Scholar] [CrossRef]
- Cacua, K.; Ordoñez, F.; Zapata, C.; Herrera, B.; Pabón, E.; Buitrago-Sierra, R. Surfactant concentration and pH effects on the zeta potential values of alumina nanofluids to inspect stability. Colloids Surf. A Physicochem. Eng. Asp. 2019, 583, 123960. [Google Scholar] [CrossRef]
- Franks, G.V.; Meagher, L. The isoelectric points of sapphire crystals and alpha-alumina powder. Colloids Surf. A Physicochem. Eng. Asp. 2003, 214, 99–110. [Google Scholar] [CrossRef]
- Mattsson, T.; Sedin, M.; Theliander, H. Zeta-potential and local filtration properties: Constitutive relationships for TiO2 from experimental filtration measurements. Chem. Eng. Sci. 2011, 66, 4573–4581. [Google Scholar] [CrossRef]
- Riedel, S.; Heyart, B.; Apel, K.S.; Mayr, S.G. Programing stimuli-responsiveness of gelatin with electron beams: Basic effects and development of a hydration-controlled biocompatible demonstrator. Sci. Rep. 2017, 7, 17436. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, H.T.; Wu, G.J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, Y.; Zhao, L.; Huang, X. Preparation of acetylsalicylic acid-acylated chitosan as a novel polymeric drug for drug controlled release. Int. J. Biol. Macromol. 2015, 78, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Maia, G.D.; Giulietti, M. Solubility of Acetylsalicylic Acid in Ethanol, Acetone, Propylene Glycol, and 2-Propanol. J. Chem. Eng. Data 2008, 53, 256–258. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, J.; Peng, X.; Jin, Y. Preparation and release behavior of carboxymethylated chitosan/alginate microspheres encapsulating bovine serum albumin. J. Appl. Polym. Sci. 2004, 92, 878–882. [Google Scholar] [CrossRef]
- Boonsongrit, Y.; Mitrevej, A.; Mueller, B.W. Chitosan drug binding by ionic interaction. Eur. J. Pharm. Biopharm. 2006, 62, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, S.; Goyal, N.; Gupta, S. A recipe for optimizing TiO2 nanoparticles for drug delivery applications. OpenNano 2022, 8, 100096. [Google Scholar] [CrossRef]
- Ajun, W.; Yan, S.; Li, G.; Huili, L. Preparation of aspirin and probucol in combination loaded chitosan nanoparticles and in vitro release study. Carbohydr. Polym. 2009, 75, 566–574. [Google Scholar] [CrossRef]
- Luo, S.; Man, H.; Jia, X.; Li, Y.; Pan, A.; Zhang, X.; Song, Y. Preparation and characterization of acetylsalicylic acid/chitosan nanoparticles and its antithrombotic effects. Des. Monomers Polym. 2018, 21, 172–181. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, X.; Kaneti, Y.V.; Yu, A. Design and construction of polymerized-glucose coated Fe3O4 magnetic nanoparticles for delivery of aspirin. Powder Technol. 2013, 236, 157–163. [Google Scholar] [CrossRef]
- Argin, S.; Kofinas, P.; Lo, Y.M. The cell release kinetics and the swelling behavior of physically crosslinked xanthan–chitosan hydrogels in simulated gastrointestinal conditions. Food Hydrocoll. 2014, 40, 138–144. [Google Scholar] [CrossRef]
- Ferrero, C.; Muñoz-Ruiz, A.; Jiménez-Castellanos, M.R. Fronts movement as a useful tool for hydrophilic matrix release mechanism elucidation. Int. J. Pharm. 2000, 202, 21–28. [Google Scholar] [CrossRef]
- Paños, I.; Acosta, N.; Heras, A. New drug delivery system based on chitosan. Curr. Drug Discov. Technol. 2008, 5, 333–341. [Google Scholar] [CrossRef] [PubMed]



| Diffusion Exponent (m) | Mechanism | ||
|---|---|---|---|
| Film | Cylinder | Sphere | |
| 0.5 | 0.45 | 0.43 | Fickian diffusion |
| 0.5 < m < 1.00 | 0.45 < m < 0.89 | 0.43 < m < 0.85 | Anomalous transport |
| 1.00 | 0.89 | 0.85 | Case-II transport |
| Nanoparticle | Position (2θ, °) | FWHM (2θ, °) | (nm) | (nm) |
|---|---|---|---|---|
| TiO2 | 25.3 | 0.3454 | 23.6 ± 0.3 | 21 |
| Fe3O4 | 36.6 | 0.3070 | 27.2 ± 0.3 | <50 |
| Al2O3 | 18.8 | 0.2303 | 35.0 ± 0.3 | <50 |
| Membrane | E (GPa) | τs (MPa) | εb (%) | IEP (-) |
|---|---|---|---|---|
| CS | 4.8 ± 0.6 | 114 ± 6 | 7.9 ± 0.4 | 4.19 ± 0.06 |
| CS-TiO2-L | 6.7 ± 0.4 | 122 ± 6 | 3.5 ± 0.3 | 5.69 ± 0.05 |
| CS-TiO2-H | 7.1 ± 0.5 | 174 ± 24 | 2.6 ± 0.4 | 4.50 ± 0.19 |
| CS-Fe3O4-L | 5.1 ± 0.6 | 117 ± 16 | 7.2 ± 0.9 | 5.50 ± 0.14 |
| CS-Fe3O4-H | 6.5± 0.3 | 133 ± 8 | 4.1 ± 0.5 | 4.90 ± 0.14 |
| CS-Al2O3-L | 5.4 ± 0.5 | 121 ± 10 | 4.0 ± 0.9 | 7.30 ± 0.12 |
| CS-Al2O3-H | 6.2 ± 0.2 | 144 ± 17 | 3.0 ± 0.7 | 7.86 ± 0.08 |
| Membrane | pH | R2 | (10−10 m2/s) | Tδ (min) |
|---|---|---|---|---|
| CS | 1.2 | 0.976 | 1.01 ± 0.06 | 7 ± 1 |
| 6.8 | 0.973 | 3.8 ± 0.3 | 9 ± 2 | |
| CS-TiO2-L | 1.2 | 0.989 | 2.2 ± 0.1 | 12 ± 2 |
| 6.8 | 0.992 | 3.8 ± 0.2 | 16 ± 4 | |
| CS-TiO2-H | 1.2 | 0.991 | 1.3 ± 0.5 | 13 ± 2 |
| 6.8 | 0.969 | 2.4 ± 0.2 | 37 ± 6 | |
| CS-Fe3O4-L | 1.2 | 0.991 | 4.1 ± 0.2 | 15 ± 2 |
| 6.8 | 0.989 | 3.6 ± 0.2 | 24 ± 3 | |
| CS-Fe3O4-H | 1.2 | 0.981 | 3.6 ± 0.2 | 12 ± 2 |
| 6.8 | 0.993 | 2.6 ± 0.1 | 25 ± 3 | |
| CS-Al2O3-L | 1.2 | 0.982 | 5.2 ± 0.3 | 11 ± 2 |
| 6.8 | 0.978 | 1.5 ± 0.1 | 15 ± 2 | |
| CS-Al2O3-H | 1.2 | 0.960 | 3.6 ± 0.3 | 11 ± 2 |
| 6.8 | 0.978 | 1.4 ± 0.1 | 22 ± 3 |
| Membrane | pH | Model | R2 | (10−10 m2/s) |
|---|---|---|---|---|
| CS | 1.2 | Lineal | 0.986 | 1.0 ± 0.1 |
| 6.8 | Exponential | 0.985 | 8.5 ± 0.5 | |
| CS-TiO2-L | 1.2 | Lineal | 0.960 | 2.3 ± 0.2 |
| 6.8 | Exponential | 0.962 | 13 ± 1 | |
| CS-TiO2-H | 1.2 | Lineal | 0.998 | 1.59 ± 0.04 |
| 6.8 | Exponential | 0.985 | 6.8 ± 0.5 | |
| CS-Fe3O4-L | 1.2 | Lineal | 0.977 | 4.0 ± 0.4 |
| 6.8 | Exponential | 0.987 | 11.3 ± 6 | |
| CS-Fe3O4-H | 1.2 | Lineal | 0.995 | 3.2 ± 0.1 |
| 6.8 | Exponential | 0.959 | 8.1 ± 0.7 | |
| CS-Al2O3-L | 1.2 | Lineal | 0.989 | 4.3 ± 0.2 |
| 6.8 | Exponential | 0.964 | 13 ± 1 | |
| CS-Al2O3-H | 1.2 | Lineal | 0.995 | 3.4 ± 0.1 |
| 6.8 | Exponential | 0.965 | 20 ± 3 |
| Membrane | R2 | k1 (10−3 min−0.8) | k2 (10−4 min−1.6) | ttrans-exp (min) | ttrans-theorical (min) | Error (%) |
|---|---|---|---|---|---|---|
| CS | 0.996 | −1.4 ± 0.3 | 1.4 ± 0.1 | 216 | 207 | 4 |
| CS-TiO2-L | 0.989 | −1.1 ± 0.7 | 2.1 ± 0.2 | 178 | 191 | 7 |
| CS-TiO2-H | 0.991 | −1.8 ± 0.3 | 0.92 ± 0.04 | 270 | 261 | 3 |
| CS-Fe3O4-L | 0.998 | −1.1 ± 0.2 | 1.53 ± 0.05 | 198 | 183 | 8 |
| CS-Fe3O4-H | 0.994 | −1.6 ± 0.8 | 1.7 ± 0.1 | 207 | 214 | 3 |
| CS-Al2O3-L | 0.984 | −1.3 ±0.7 | 1.9 ± 0.2 | 175 | 179 | 2 |
| CS-Al2O3-H | 0.977 | −2 ± 1 | 4.2 ± 0.7 | 136 | 153 | 13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroudi, A.; García-Payo, C.; Khayet, M. Chitosan-Based Composite Membranes with Different Biocompatible Metal Oxide Nanoparticles: Physicochemical Properties and Drug-Release Study. Polymers 2023, 15, 2804. https://doi.org/10.3390/polym15132804
Baroudi A, García-Payo C, Khayet M. Chitosan-Based Composite Membranes with Different Biocompatible Metal Oxide Nanoparticles: Physicochemical Properties and Drug-Release Study. Polymers. 2023; 15(13):2804. https://doi.org/10.3390/polym15132804
Chicago/Turabian StyleBaroudi, Alia, Carmen García-Payo, and Mohamed Khayet. 2023. "Chitosan-Based Composite Membranes with Different Biocompatible Metal Oxide Nanoparticles: Physicochemical Properties and Drug-Release Study" Polymers 15, no. 13: 2804. https://doi.org/10.3390/polym15132804
APA StyleBaroudi, A., García-Payo, C., & Khayet, M. (2023). Chitosan-Based Composite Membranes with Different Biocompatible Metal Oxide Nanoparticles: Physicochemical Properties and Drug-Release Study. Polymers, 15(13), 2804. https://doi.org/10.3390/polym15132804








