Conjugated Polymer Modifying TiO2 Performance for Visible-Light Photodegradation of Organics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Mesoporous TiO2
2.3. Synthesis of Mesoporous TiO2/PEDOT Systems
2.4. Characterization of the TiO2/PEDOT Systems
2.5. Investigation of Photocatalytic Activities of TiO2/PEDOT Composites
3. Results and Discussion
3.1. Characterization of the Synthesized Titania Samples
3.1.1. XRD Measurements
3.1.2. N2 Sorption Measurements
3.1.3. Morphological Characterization
3.1.4. Investigation of the Surface Chemistry by FTIR Spectroscopy
3.1.5. X-ray Photoelectron Spectroscopy
3.1.6. Optical Properties
3.2. Photocatalytic Degradation of Congo Red Dye
3.2.1. Effect of Catalyst Dose
3.2.2. Determination of CR Dye Removal Capacity
3.2.3. Identification of Reactive Species Responsible for CR Photo-Oxidation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Becker, D.; Jungfer, C.; Track, T. Integrated Industrial Water Management—Challenges, Solutions, and Future Priorities. Chem. Ing. Tech. 2019, 91, 1367–1374. [Google Scholar] [CrossRef]
- Edward Kwaku, A.; Maggie, C.; Jeremiah Adebisi, A.; Donald Tyoker, K.; Boldwin, M.; Khaya Pearlman, S.; Babatunde Femi, B. Emerging Trends in Wastewater Treatment Technologies: The Current Perspective. In Promising Techniques for Wastewater Treatment and Water Quality Assessment; Iqbal Ahmed, M., Summers, J.K., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter. 4. [Google Scholar]
- Katančić, Z.; Chen, W.-T.; Waterhouse, G.I.N.; Kušić, H.; Lončarić Božić, A.; Hrnjak-Murgić, Z.; Travas-Sejdic, J. Solar-active photocatalysts based on TiO2 and conductive polymer PEDOT for the removal of bisphenol A. J. Photochem. Photobiol. A Chem. 2020, 396, 112546. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Ignat, M.; Samoila, P.; Coromelci, C.; Sacarescu, L.; Asaftei, I.; Harabagiu, V.; Miron, C. Plasma generation in liquid as a new efficient synthesis approach of titania–zinc ferrite nano(photo)catalyst. Comptes Rendus Chim. 2018, 21, 310–317. [Google Scholar] [CrossRef]
- Ignat, M.; Rotaru, R.; Samoila, P.; Sacarescu, L.; Timpu, D.; Harabagiu, V. Relationship between the component synthesis order of zinc ferrite–titania nanocomposites and their performances as visible light-driven photocatalysts for relevant organic pollutant degradation. Comptes Rendus Chim. 2018, 21, 263–269. [Google Scholar] [CrossRef]
- Coromelci-Pastravanu, C.; Ignat, M.; Popovici, E.; Harabagiu, V. TiO2-coated mesoporous carbon: Conventional vs. microwave-annealing process. J. Hazard. Mater. 2014, 278, 382–390. [Google Scholar] [CrossRef]
- Zhou, Q.; Shi, G. Conducting Polymer-Based Catalysts. J. Am. Chem. Soc. 2016, 138, 2868–2876. [Google Scholar] [CrossRef]
- Saianand, G.; Gopalan, A.-I.; Wang, L.; Venkatramanan, K.; Roy, V.A.L.; Sonar, P.; Lee, D.-E.; Naidu, R. Conducting polymer based visible light photocatalytic composites for pollutant removal: Progress and prospects. Environ. Technol. Innov. 2022, 28, 102698. [Google Scholar] [CrossRef]
- Sangareswari, M.; Meenakshi Sundaram, M. Development of efficiency improved polymer-modified TiO2 for the photocatalytic degradation of an organic dye from wastewater environment. Appl. Water Sci. 2017, 7, 1781–1790. [Google Scholar] [CrossRef] [Green Version]
- Dimitrijevic, N.M.; Tepavcevic, S.; Liu, Y.; Rajh, T.; Silver, S.C.; Tiede, D.M. Nanostructured TiO2/Polypyrrole for Visible Light Photocatalysis. J. Phys. Chem. C 2013, 117, 15540–15544. [Google Scholar] [CrossRef]
- Ghosh, S.; Kouame, N.A.; Remita, S.; Ramos, L.; Goubard, F.; Aubert, P.-H.; Dazzi, A.; Deniset-Besseau, A.; Remita, H. Visible-light active conducting polymer nanostructures with superior photocatalytic activity. Sci. Rep. 2015, 5, 18002. [Google Scholar] [CrossRef]
- Zhang, L.; Jamal, R.; Zhao, Q.; Wang, M.; Abdiryim, T. Preparation of PEDOT/GO, PEDOT/MnO2, and PEDOT/GO/MnO2 nanocomposites and their application in catalytic degradation of methylene blue. Nanoscale Res. Lett. 2015, 10, 148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dagar, A.; Narula, A.K. Photo-degradation of methyl orange under visible light by PEDOT/NiO/Fly ash cenosphere. Mater. Chem. Phys. 2016, 183, 561–570. [Google Scholar] [CrossRef]
- Abdiryim, T.; Ali, A.; Jamal, R.; Osman, Y.; Zhang, Y. A facile solid-state heating method for preparation of poly(3,4-ethelenedioxythiophene)/ZnO nanocomposite and photocatalytic activity. Nanoscale Res. Lett. 2014, 9, 89. [Google Scholar] [CrossRef] [Green Version]
- Vavilapalli, D.S.; Rosen, J.; Singh, S. Immobilization of a TiO2–PEDOT:PSS hybrid heterojunction photocatalyst for degradation of organic effluents. RSC Adv. 2023, 13, 3095–3101. [Google Scholar] [CrossRef]
- Liu, J.; McCarthy, D.L.; Tong, L.; Cowan, M.J.; Kinsley, J.M.; Sonnenberg, L.; Skorenko, K.H.; Boyer, S.M.; DeCoste, J.B.; Bernier, W.E.; et al. Poly(3,4-ethylenedioxythiophene) (PEDOT) infused TiO2 nanofibers: The role of hole transport layer in photocatalytic degradation of phenazopyridine as a pharmaceutical contaminant. RSC Adv. 2016, 6, 113884–113892. [Google Scholar] [CrossRef]
- Mahu, E.; Ignat, M.; Cojocaru, C.; Samoila, P.; Coromelci, C.; Asaftei, I.; Harabagiu, V. Development of porous titania structure with improved photocatalytic activity: Response surface modeling and multi-objective optimization. Nanomaterials 2020, 10, 998. [Google Scholar] [CrossRef]
- JCPDS-International Centre for Diffraction Data 2002, PDF Card 86-1155.
- JCPDS-International Centre for Diffraction Data 2002, PDF Card 76-1937.
- Kouhnavard, M.; Yifan, D.; D’ Arcy, J.M.; Mishra, R.; Biswas, P. Highly conductive PEDOT films with enhanced catalytic activity for dye-sensitized solar cells. Sol. Energy 2020, 211, 258–264. [Google Scholar] [CrossRef]
- Yang, B.; Chen, G.; Tian, H.; Wen, L. Improvement of the Photoelectrochemical Performance of TiO2 Nanorod Array by PEDOT and Oxygen Vacancy Co-Modification. Catalysts 2019, 9, 407. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Liu, Y.; Zhu, H.; Wang, X. Hierarchical bead chain ZnFe2O4-PEDOT composites with enhanced Li-ion storage properties as anode materials for lithium-ion batteries. Appl. Surf. Sci. 2020, 529, 147078. [Google Scholar] [CrossRef]
- Yu, Z.; Jamal, R.; Zhang, R.; Zhang, W.; Yan, Y.; Liu, Y.; Ge, Y.; Abdiryim, T. PEDOT-Type Conducting Polymers/Black TiO2 Composites for Electrochemical Determination of Cd2+ and Pb2+. J. Electrochem. Soc. 2020, 167, 067514. [Google Scholar] [CrossRef]
- Ramesh, G.; Palaniappan, S.; Basavaiah, K. One-step synthesis of PEDOT-PSS•TiO2 by peroxotitanium acid: A highly stable electrode for a supercapacitor. Ionics 2018, 24, 1475–1485. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Eren, E.; Celik, G.; Uygun, A.; Tabačiarová, J.; Omastová, M. Synthesis of poly(3,4-ethylenedioxythiophene)/titanium dioxide nanocomposites in the presence of surfactants and their properties. Synth. Met. 2012, 162, 1451–1458. [Google Scholar] [CrossRef]
- Kadac, K.; Nowaczyk, A.; Nowaczyk, J. Synthesis and characterization of new copolymer of pyrrole and 3,4-ethylenedioxythiophene synthesized by electrochemical route. Synth. Met. 2015, 206, 145–153. [Google Scholar] [CrossRef]
- Isari, A.A.; Hayati, F.; Kakavandi, B.; Rostami, M.; Motevassel, M.; Dehghanifard, E. N, Cu co-doped TiO2@functionalized SWCNT photocatalyst coupled with ultrasound and visible-light: An effective sono-photocatalysis process for pharmaceutical wastewaters treatment. Chem. Eng. J. 2020, 392, 123685. [Google Scholar] [CrossRef]
- Djellabi, R.; Yang, B.; Xiao, K.; Gong, Y.; Cao, D.; Sharif, H.M.A.; Zhao, X.; Zhu, C.; Zhang, J. Unravelling the mechanistic role of TiOC bonding bridge at titania/lignocellulosic biomass interface for Cr(VI) photoreduction under visible light. J. Colloid Interface Sci. 2019, 553, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Ajimsha, R.; Joshi, M.; Mohan, R.; Das, A.; Misra, P.; Kukreja, L.; Phase, D. Band offset at TiO2/MDMO PPV and TiO2/PEDOT PSS interfaces studied using photoelectron spectroscopy. RSC Adv. 2015, 5, 97891–97897. [Google Scholar] [CrossRef]
- Coromelci, C.; Neamtu, M.; Ignat, M.; Samoila, P.; Zaltariov, M.F.; Palamaru, M. Ultrasound assisted synthesis of heterostructured TiO2/ZnFe2O4 and TiO2/ZnFe1.98La0.02O4 systems as tunable photocatalysts for efficient organic pollutants removal. Ceram. Int. 2022, 48, 4829–4840. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.-M.; Yu, L.-m. A Cu2O/PEDOT/graphene-modified electrode for the enzyme-free detection and quantification of glucose. J. Electroanal. Chem. 2021, 897, 115558. [Google Scholar] [CrossRef]
- Schultheiss, A.; Gueye, M.; Carella, A.; Benayad, A.; Pouget, S.; Faure-Vincent, J.; Demadrille, R.; Revaux, A.; Simonato, J.-P. Insight into the Degradation Mechanisms of Highly Conductive Poly(3,4-ethylenedioxythiophene) Thin Films. ACS Appl. Polym. Mater. 2020, 2, 2686–2695. [Google Scholar] [CrossRef]
- Peng, F.; Lai, Q.; Chang, W.; Cui, Y. Preparation, Characterization and Visible Light Photocatalytic Activities of Samarium-Doped Mixed Crystalline Phase TiO2 Nanoparticles. IOP Conf. Ser. Mater. Sci. Eng. 2019, 562, 012031. [Google Scholar] [CrossRef]
- Matouk, Z.; Islam, M.; Gutiérrez, M.; Pireaux, J.J.; Achour, A. X-ray Photoelectron Spectroscopy (XPS) Analysis of Ultrafine Au Nanoparticles Supported over Reactively Sputtered TiO2 Films. Nanomaterials 2022, 12, 3692. [Google Scholar] [CrossRef]
- Ghafourisaleh, S.; Popov, G.; Leskelä, M.; Putkonen, M.; Ritala, M. Oxidative MLD of Conductive PEDOT Thin Films with EDOT and ReCl5 as Precursors. ACS Omega 2021, 6, 17545–17554. [Google Scholar] [CrossRef]
- Rahman, K.H.; Kar, A.K. Effect of band gap variation and sensitization process of polyaniline (PANI)-TiO2 p-n heterojunction photocatalysts on the enhancement of photocatalytic degradation of toxic methylene blue with UV irradiation. J. Environ. Chem. Eng. 2020, 8, 104181. [Google Scholar] [CrossRef]
- Aprilita, N.H.; Amalia, D.; Wahyuni, E.T. Removal of the Hazardous Congo Red Dye through Degradation under Visible Light Photocatalyzed by C,N Co-Doped TiO2 Prepared from Chicken Egg White. Sci. World J. 2022, 2022, 2613841. [Google Scholar] [CrossRef]
- Abd Elkodous, M.; El-Khawaga, A.M.; Abouelela, M.M.; Abdel Maksoud, M.I.A. Cocatalyst loaded Al-SrTiO3 cubes for Congo red dye photo-degradation under wide range of light. Sci. Rep. 2023, 13, 6331. [Google Scholar] [CrossRef]
- de Moraes, N.P.; Torezin, F.A.; Jucá Dantas, G.V.; de Sousa, J.G.M.; Valim, R.B.; da Silva Rocha, R.; Landers, R.; da Silva, M.L.C.P.; Rodrigues, L.A. TiO2/Nb2O5/carbon xerogel ternary photocatalyst for efficient degradation of 4-chlorophenol under solar light irradiation. Ceram. Int. 2020, 46, 14505–14515. [Google Scholar] [CrossRef]
- Fónagy, O.; Szabó-Bárdos, E.; Horváth, O. 1,4-Benzoquinone and 1,4-hydroquinone based determination of electron and superoxide radical formed in heterogeneous photocatalytic systems. J. Photochem. Photobiol. A Chem. 2021, 407, 113057. [Google Scholar] [CrossRef]
- Mancuso, A.; Sacco, O.; Vaiano, V.; Sannino, D.; Pragliola, S.; Venditto, V.; Morante, N. Visible light active Fe-Pr co-doped TiO2 for water pollutants degradation. Catal. Today 2021, 380, 93–104. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Mills, A.; Lee, S.-K. A web-based overview of semiconductor photochemistry-based current commercial applications. J. Photochem. Photobiol. A Chem. 2002, 152, 233–247. [Google Scholar] [CrossRef]
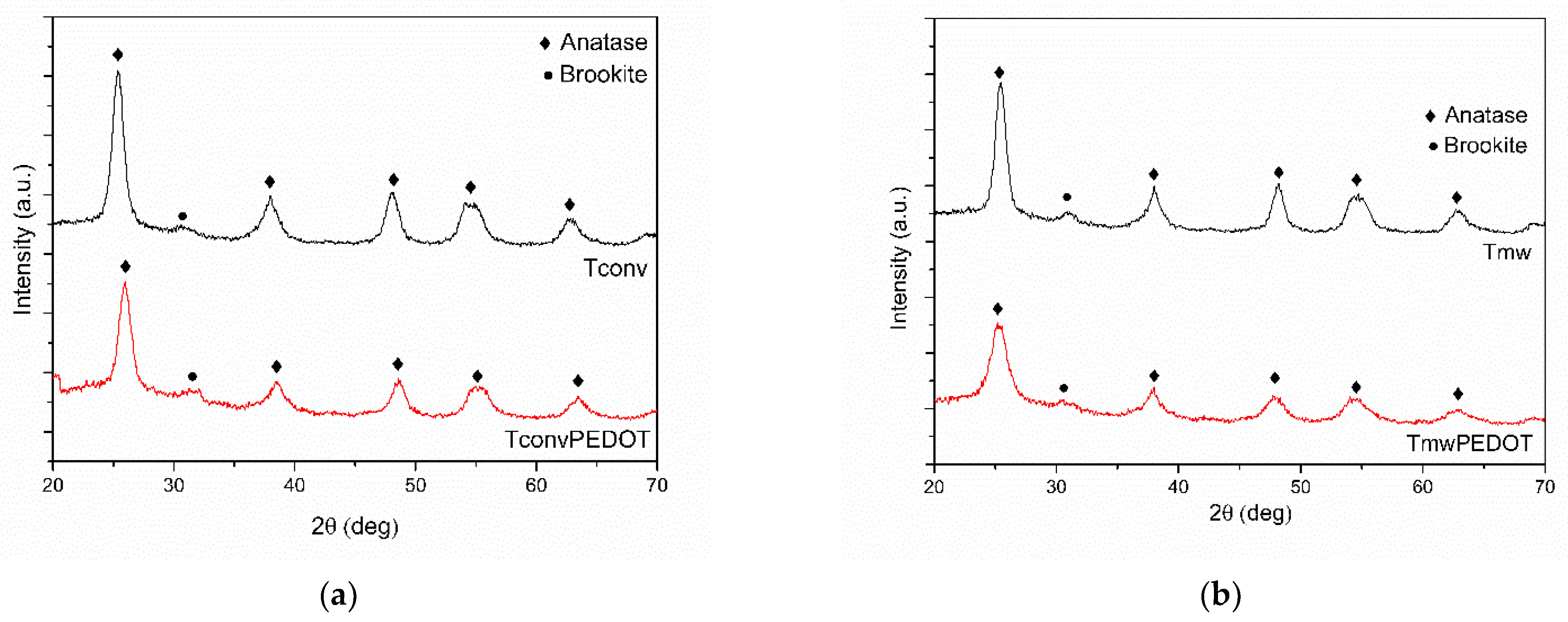

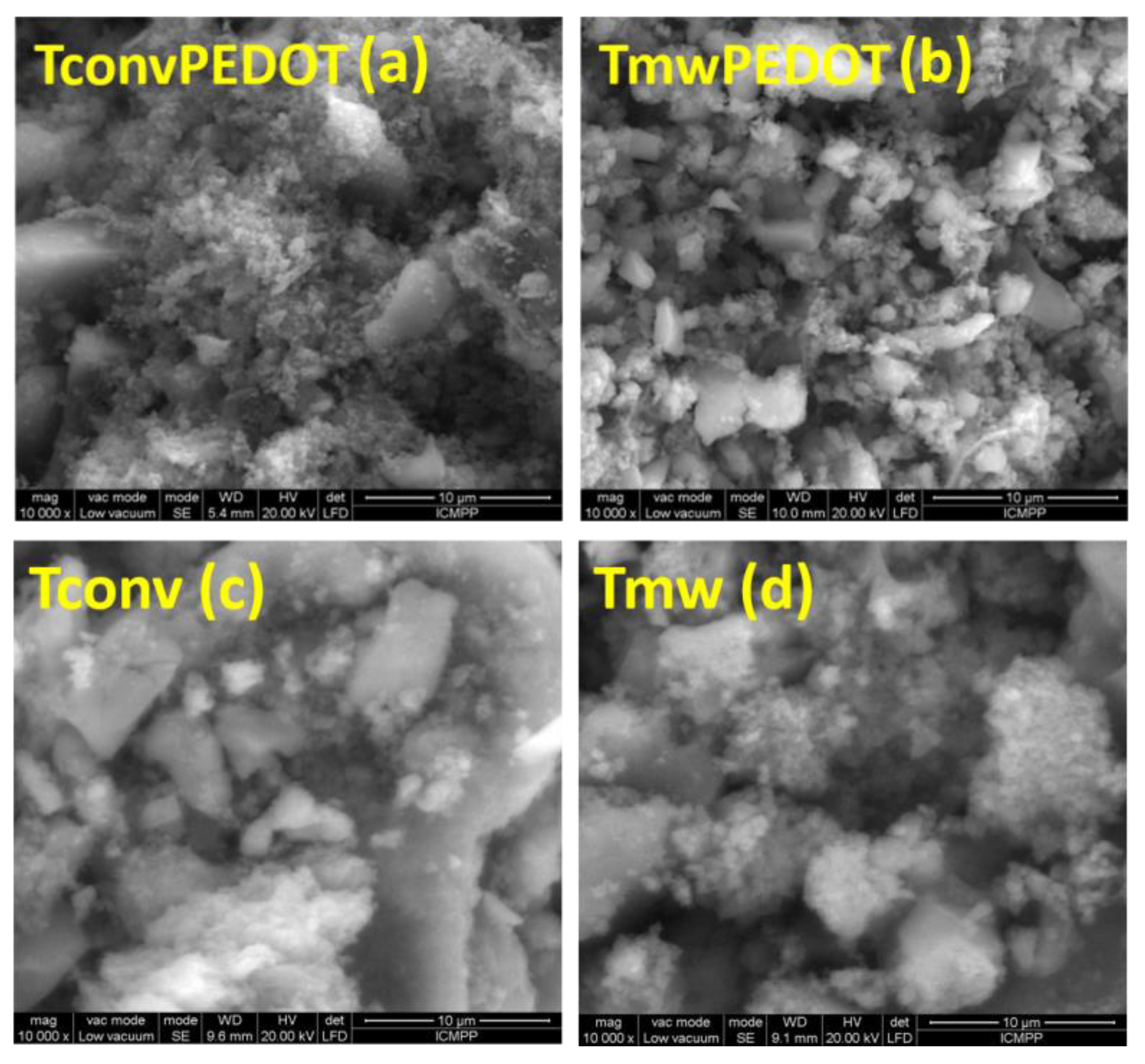

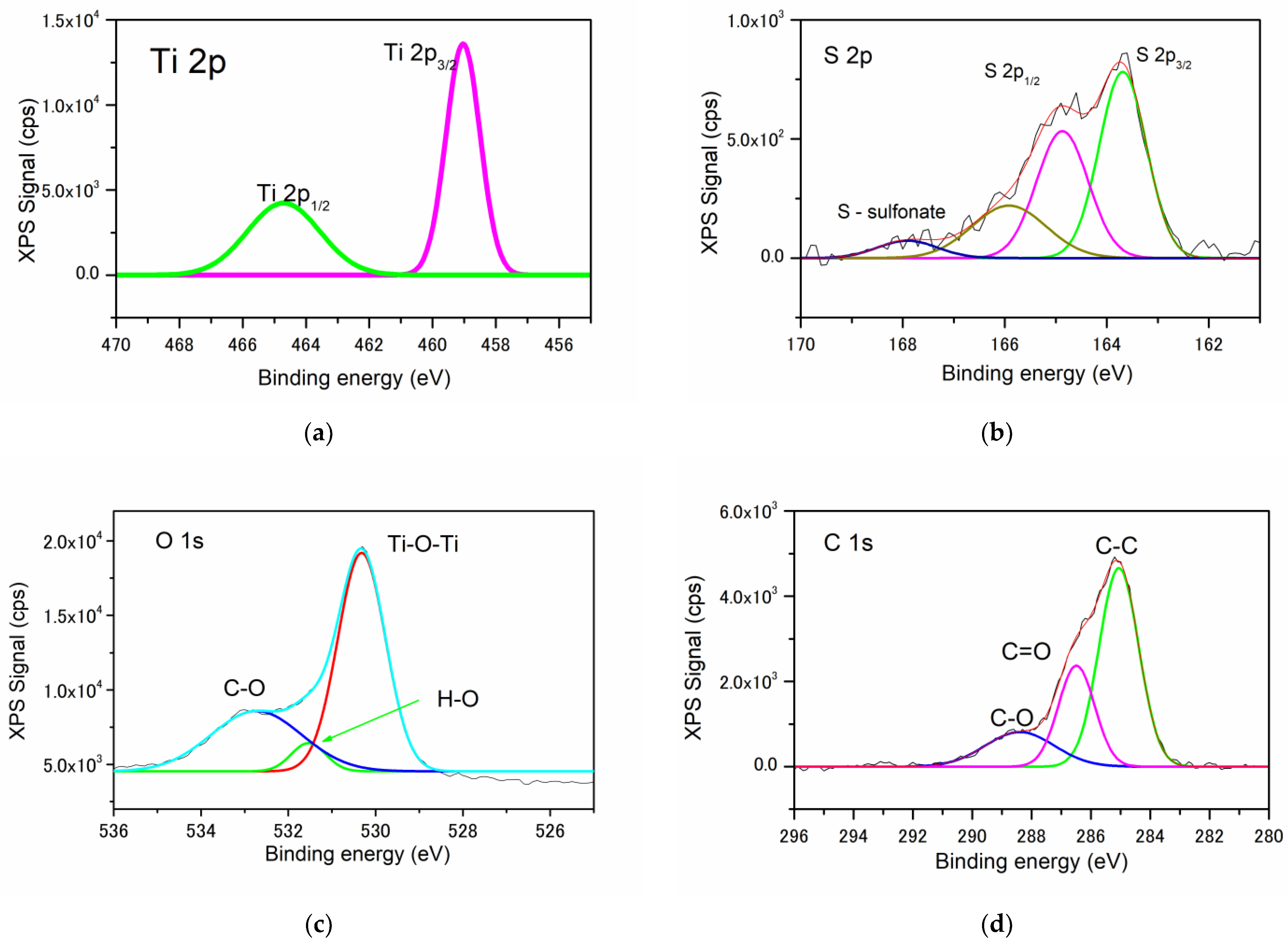
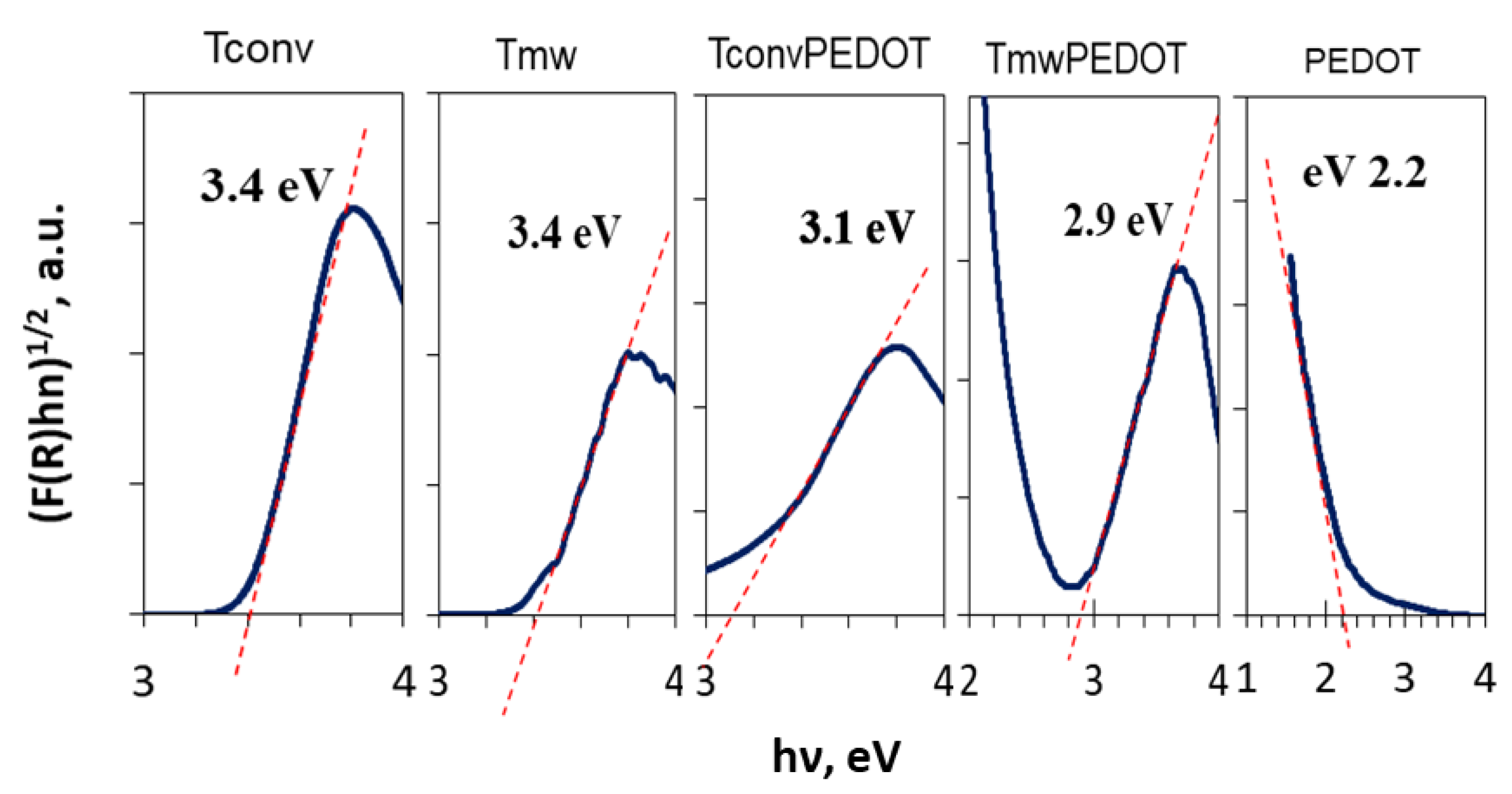
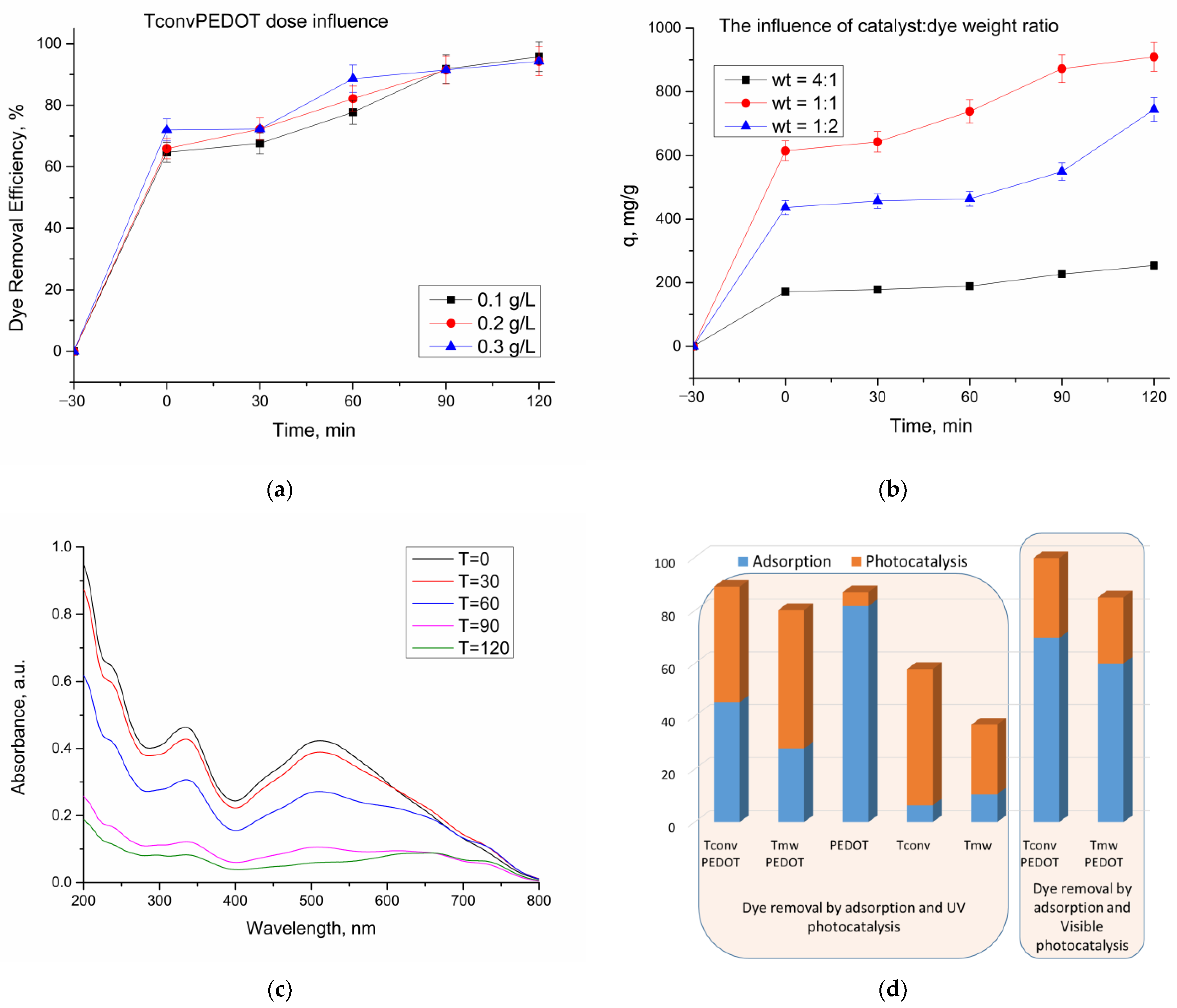
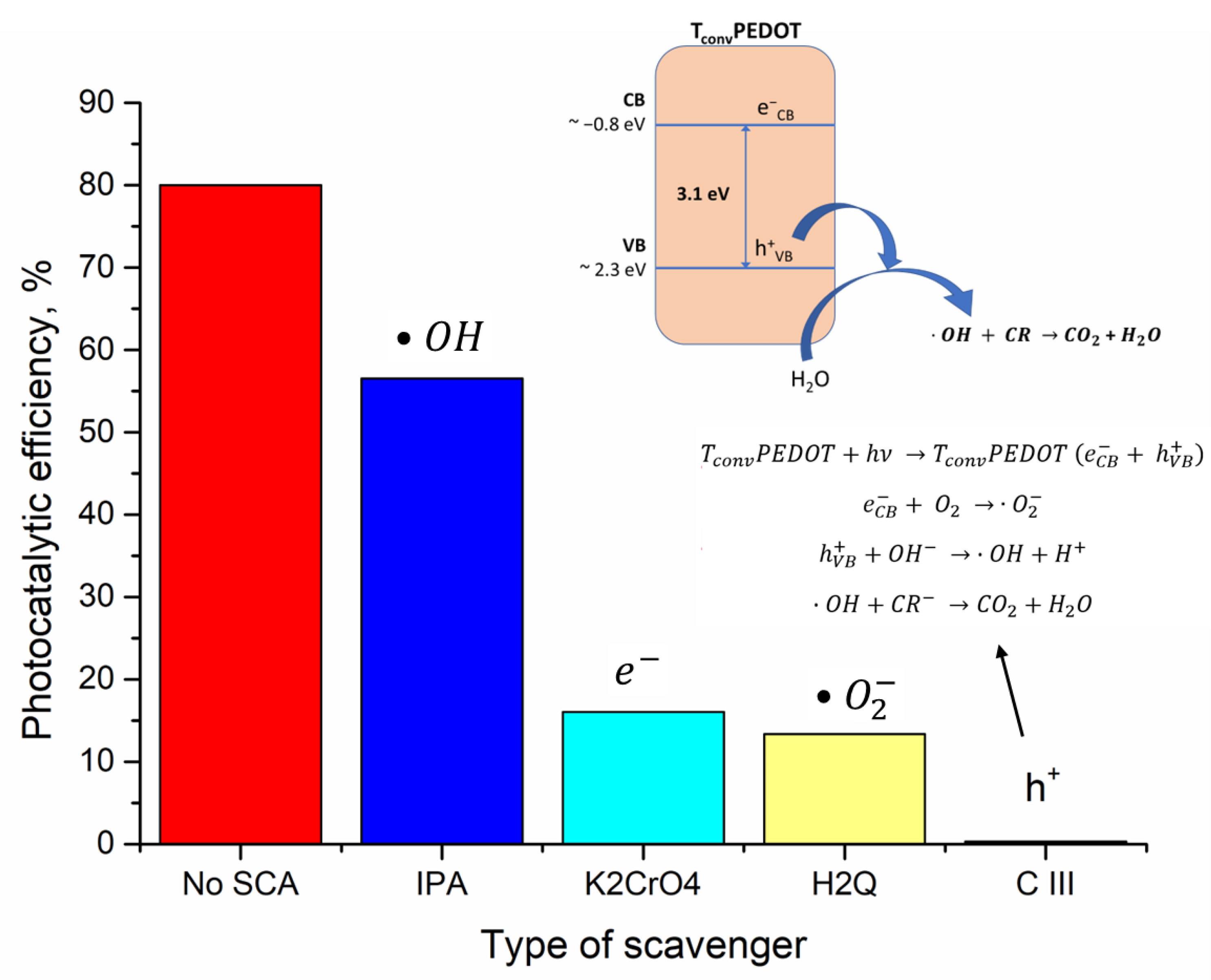
| Sample | FWHM (deg) | D101 (nm) | d101 (Å) | a0 (Å) | DWH (nm) | Ε (*10−2) | Eg, (eV) | SBET, (m2/g) | Vtot, (cm3/g) | dpore (nm) | E (%) UV Photocatalysis | E (%) Vis Photocatalysis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tconv | 1.469 | 5.48 | 3.516 | 4.06 | 10.71 | 1.03 | 3.4 | 129 | 0.24 | 5.6 3.2 | 37 | - |
| Tmw | 0.920 | 8.75 | 3.500 | 4.04 | 11.92 | 0.96 | 3.4 | 215 | 0.27 | 4.7 2.9 | 58 | - |
| PEDOT | 5.122 | 1.57 | 3.481 | 4.02 | - | - | 12 | 0.05 | - | 86 | - | |
| TconvPEDOT | 1.588 | 5.07 | 3.514 | 4.06 | 9.33 | 0.78 | 3.1 | 87 | 0.13 | 4.5 3.1 | 90 | 99 |
| TmwPEDOT | 2.44 | 3.3 | 3.537 | 4.084 | 5.19 | 0.0036 | 2.5 | 137 | 0.18 | 4.6 2.9 | 80 | 85 |
| Sample | C 1s | O 1s | S 2p | Ti 2p3 |
|---|---|---|---|---|
| PEDOT | 71.3 | 21.8 | 6.9 | - |
| TconvPEDOT | 39.0 | 41.6 | 2.4 | 17.1 |
| TmwPEDOT | 38.9 | 41.3 | 2.1 | 17.7 |
| Sample | Mineralization (%) | |
|---|---|---|
| UV Light | Visible Light | |
| TconvPEDOT | 59.32 | 52.32 |
| TmwPEDOT | 43.74 | 39.34 |
| Sample | Light Source | CR Solution | Sample, mg | Time of Exposure (min) | Photo-Decolorization (%) | Reference |
|---|---|---|---|---|---|---|
| TiO2-C,N | Visible | 100 mL 10 mg/L | 50 mg | 45 | 91 | [39] |
| UV | 100 mL 10 mg/L | 50 mg | 45 | 98 | ||
| Al-SrTiO3 | Visible | 100 mL 10 mg/L | 20 mg | 90 | 68 | [40] |
| UV | 100 mL 10 mg/L | 20 mg | 90 | 81 | ||
| TconvPEDOT | Visible | 50 mL 100 mg/L | 5 mg | 120 | 99 | This work |
| UV | 50 mL 100 mg/L | 5 mg | 120 | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coromelci, C.G.; Turcu, E.; Doroftei, F.; Palamaru, M.N.; Ignat, M. Conjugated Polymer Modifying TiO2 Performance for Visible-Light Photodegradation of Organics. Polymers 2023, 15, 2805. https://doi.org/10.3390/polym15132805
Coromelci CG, Turcu E, Doroftei F, Palamaru MN, Ignat M. Conjugated Polymer Modifying TiO2 Performance for Visible-Light Photodegradation of Organics. Polymers. 2023; 15(13):2805. https://doi.org/10.3390/polym15132805
Chicago/Turabian StyleCoromelci, Cristina Giorgiana, Elvira Turcu, Florica Doroftei, Mircea Nicolae Palamaru, and Maria Ignat. 2023. "Conjugated Polymer Modifying TiO2 Performance for Visible-Light Photodegradation of Organics" Polymers 15, no. 13: 2805. https://doi.org/10.3390/polym15132805
APA StyleCoromelci, C. G., Turcu, E., Doroftei, F., Palamaru, M. N., & Ignat, M. (2023). Conjugated Polymer Modifying TiO2 Performance for Visible-Light Photodegradation of Organics. Polymers, 15(13), 2805. https://doi.org/10.3390/polym15132805






