A Review of Chitosan and Chitosan Nanofiber: Preparation, Characterization, and Its Potential Applications
Abstract
1. Introduction
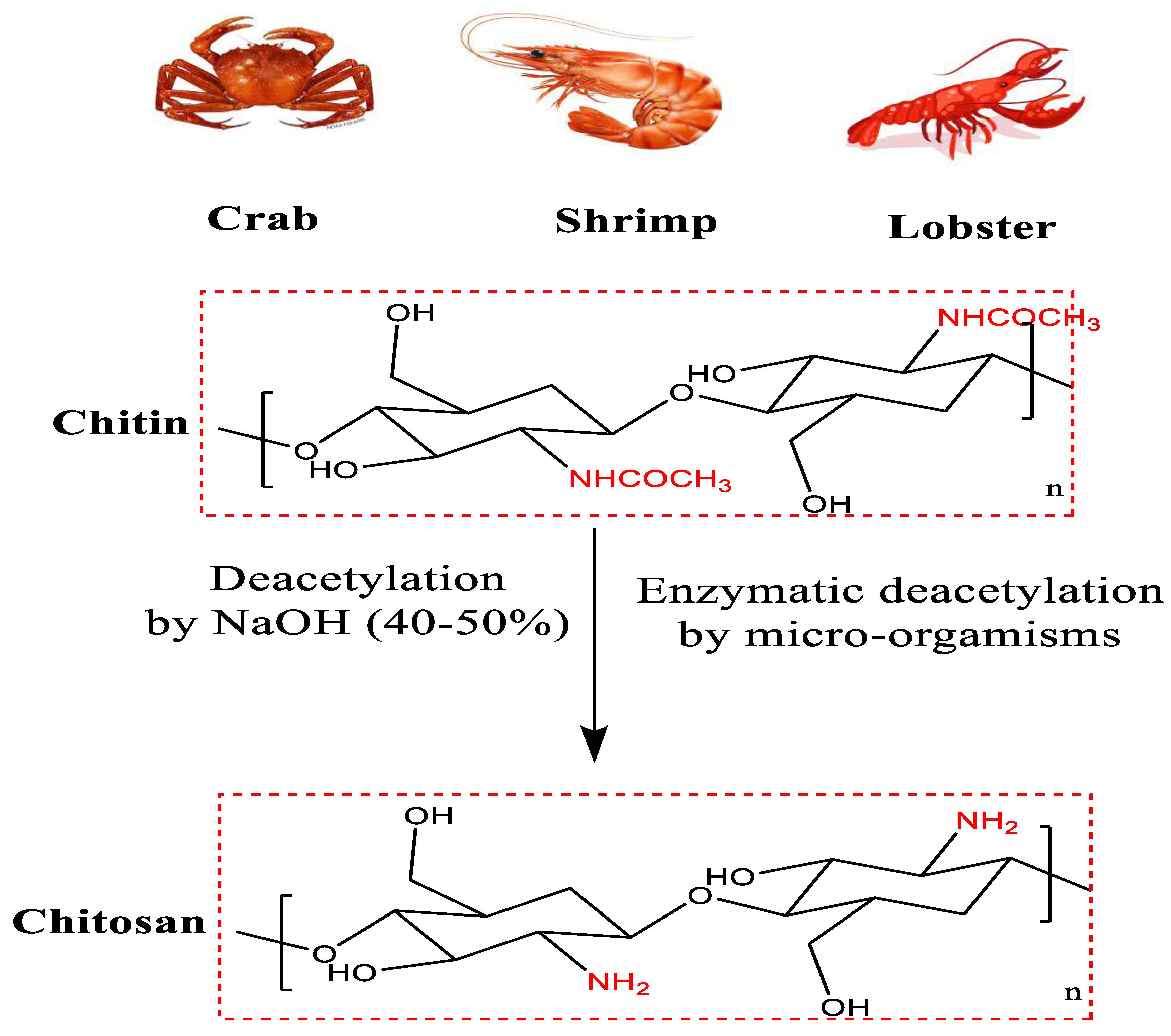
2. Structure of Chitin and Chitosan
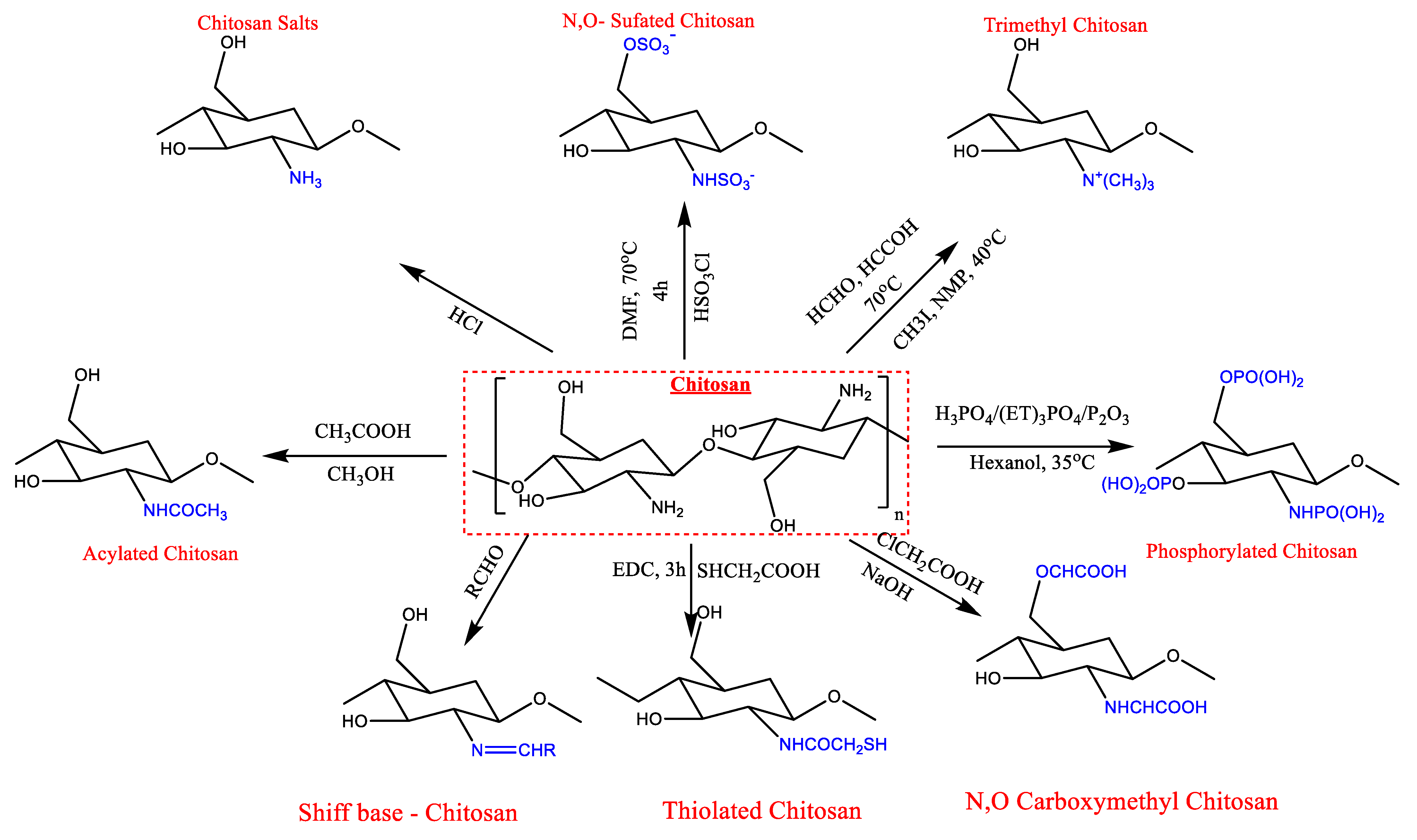
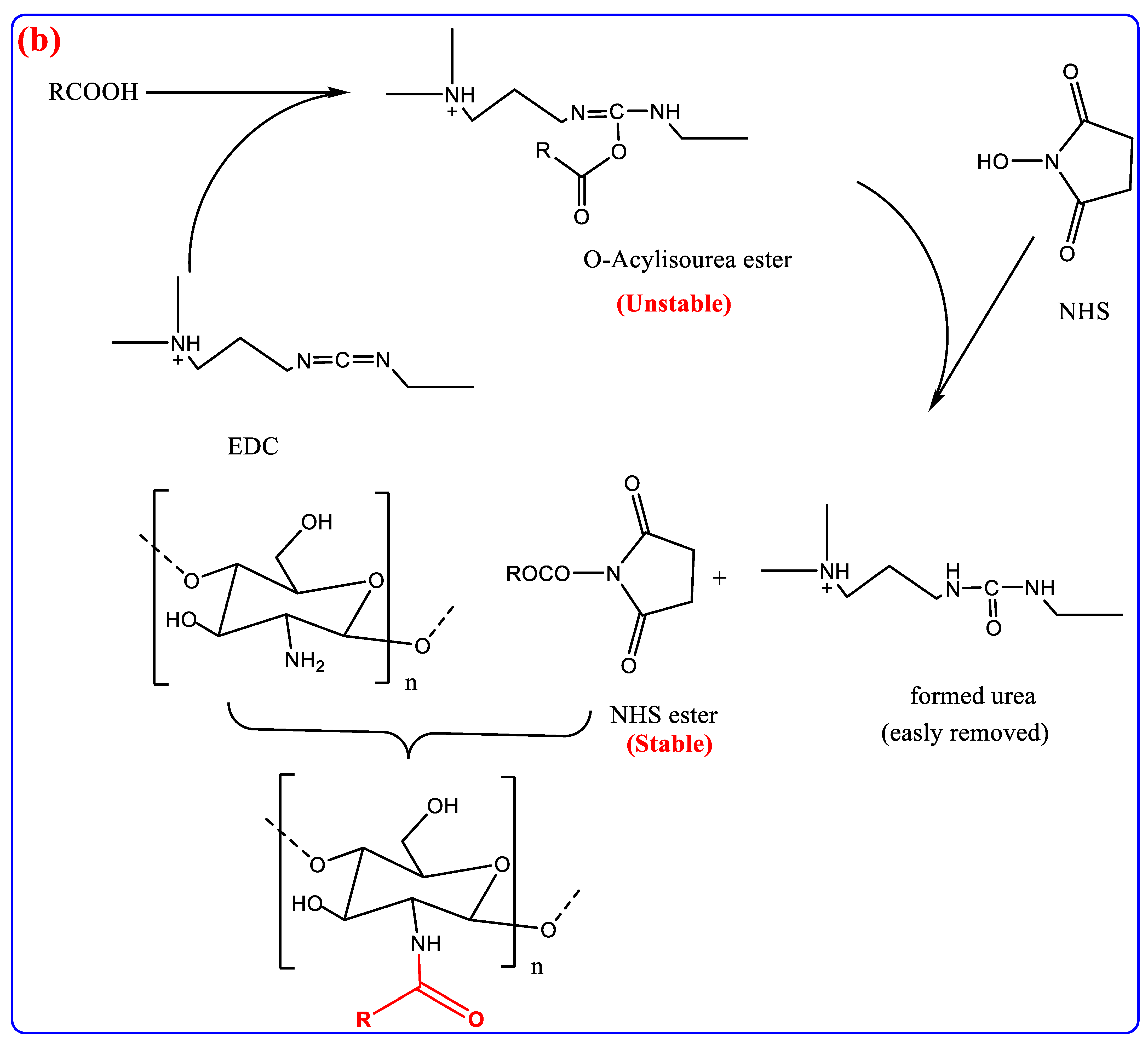
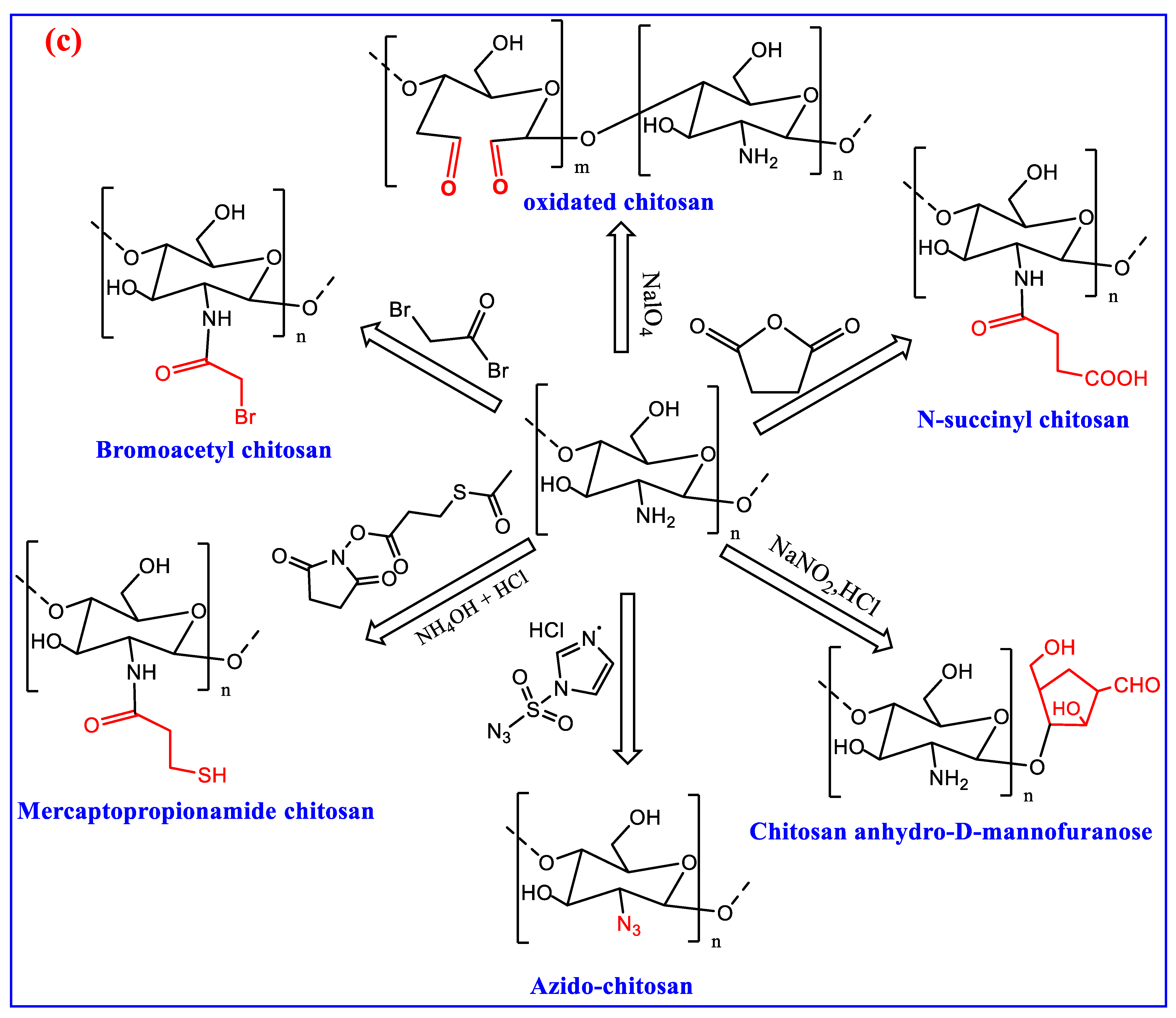
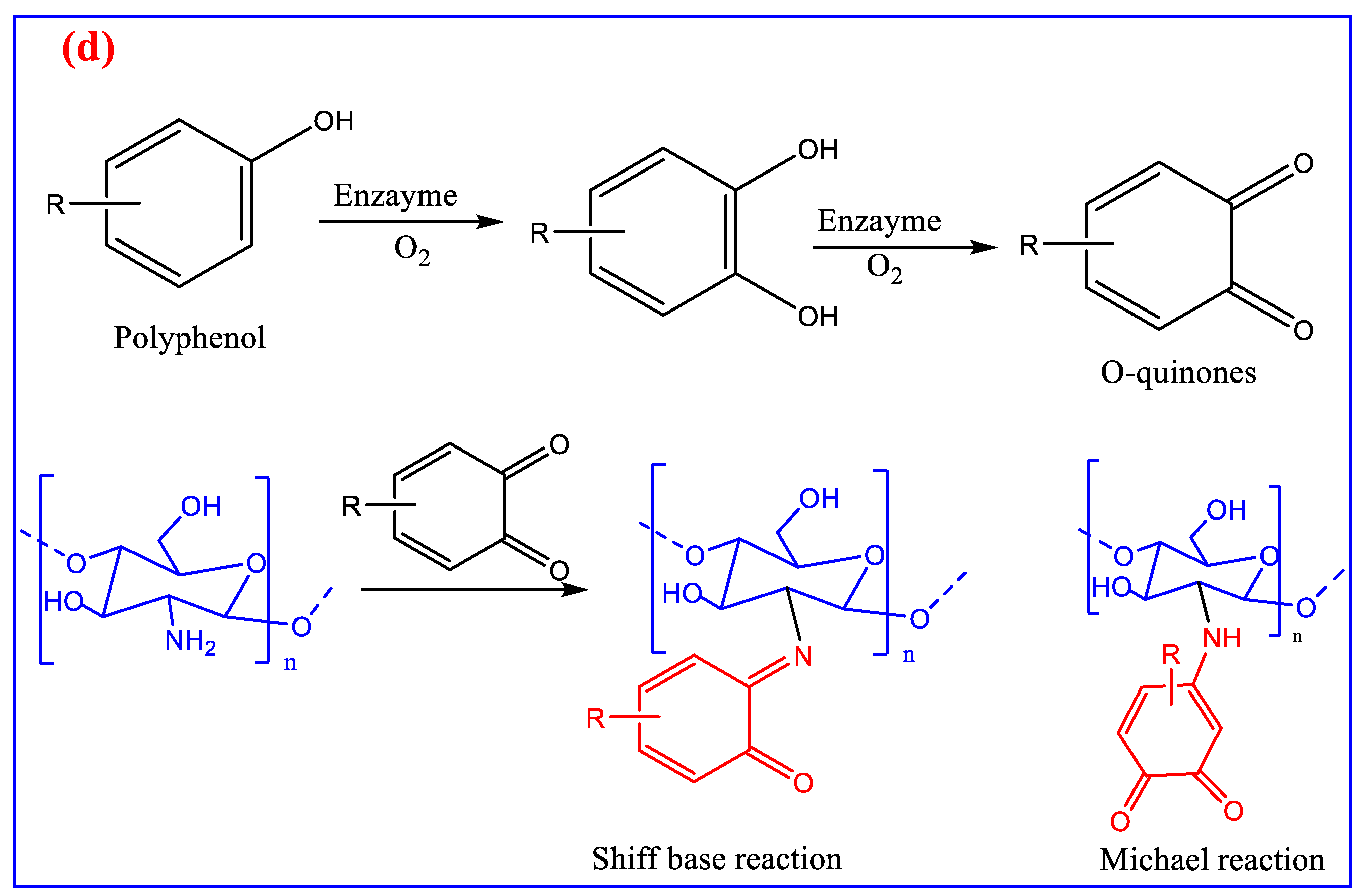
3. Modification of Chitin and Chitosan
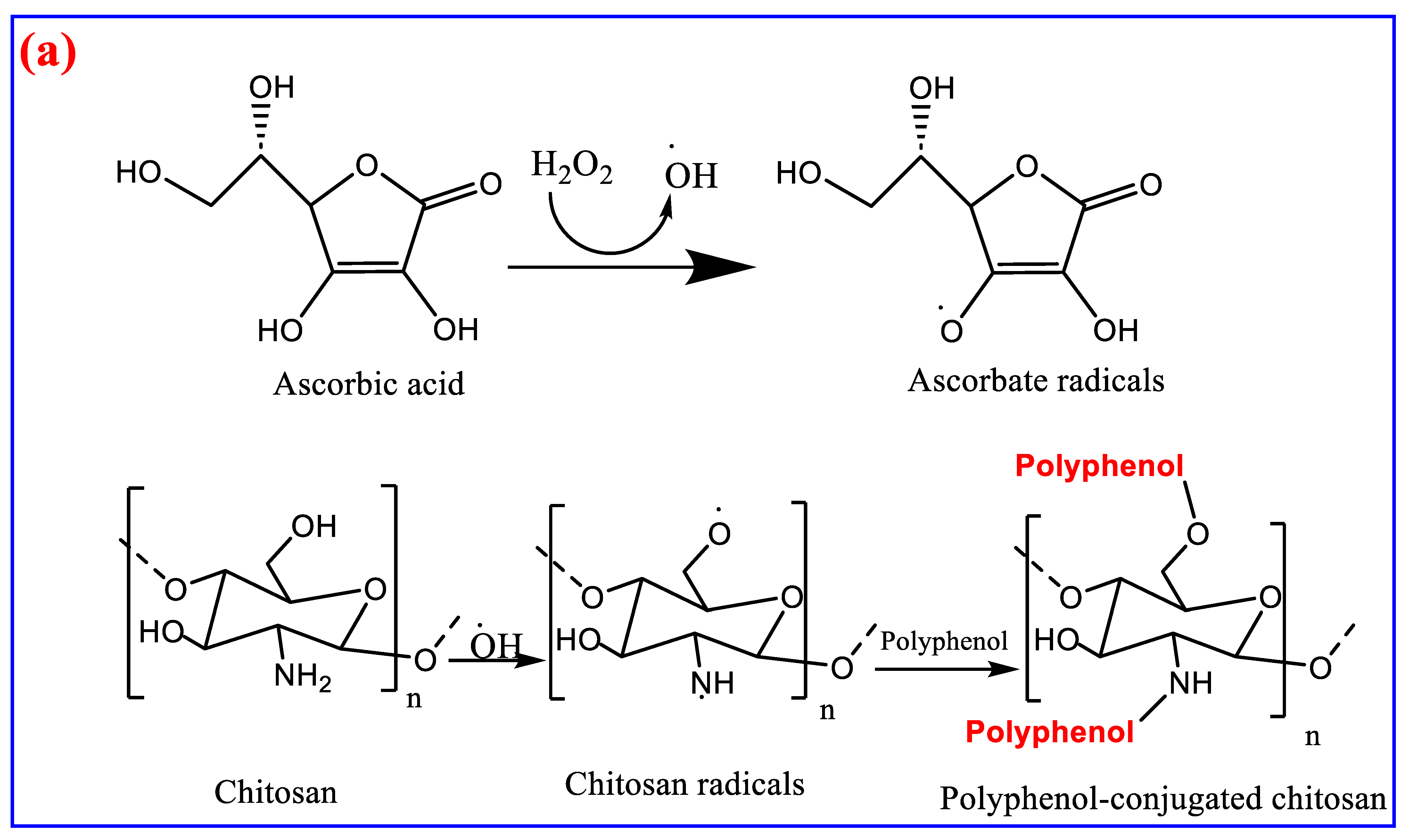
4. Chitosan as Biomaterial
5. Applications of Chitosan and Chitosan Derivatives
5.1. Agricultural Application
5.2. Wastewater Treatment Applications
5.3. Food Industry Applications
5.4. Medical Applications
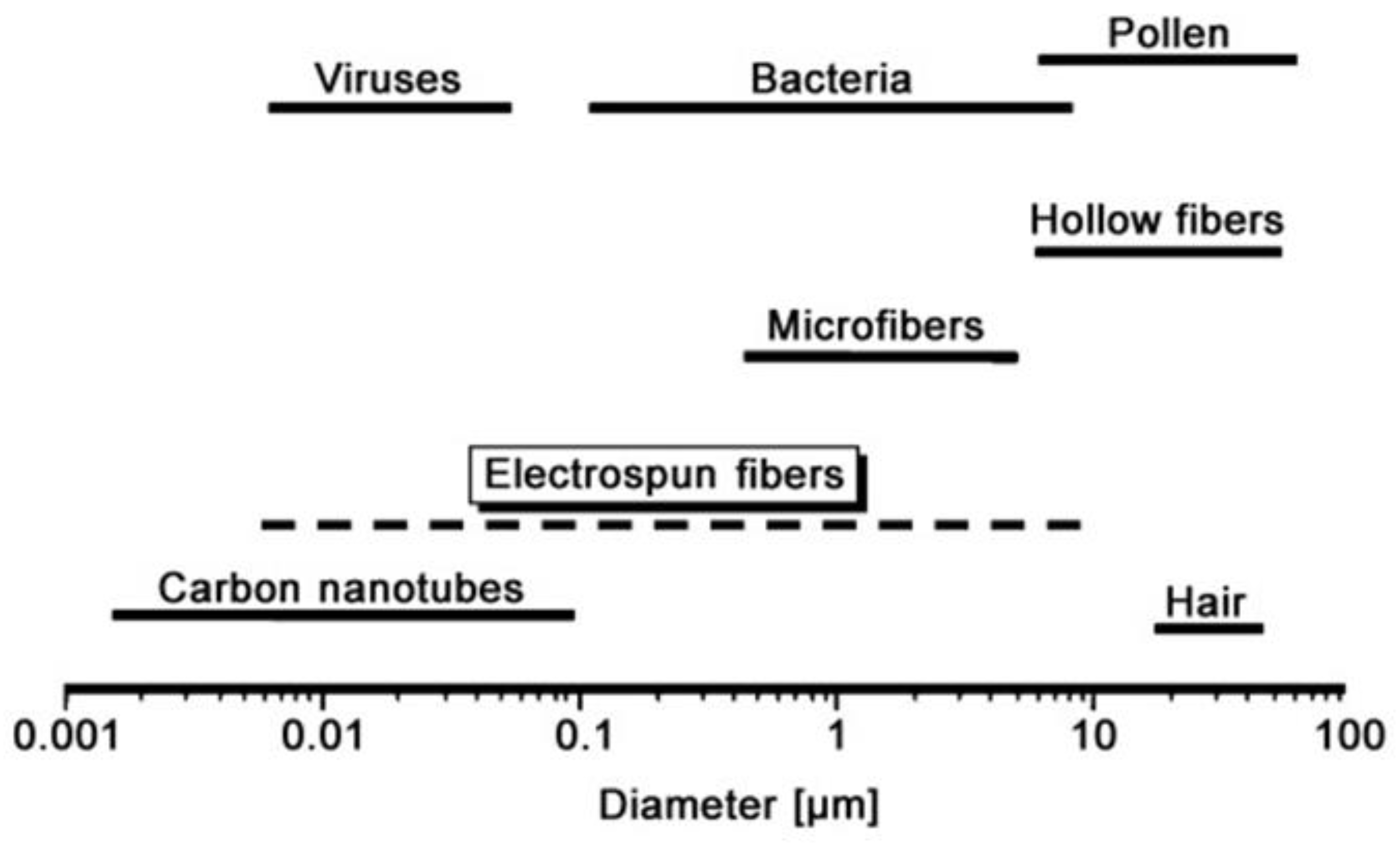
6. Electro-Spun Nanofibers: Process and Application
6.1. History of Electrospinning
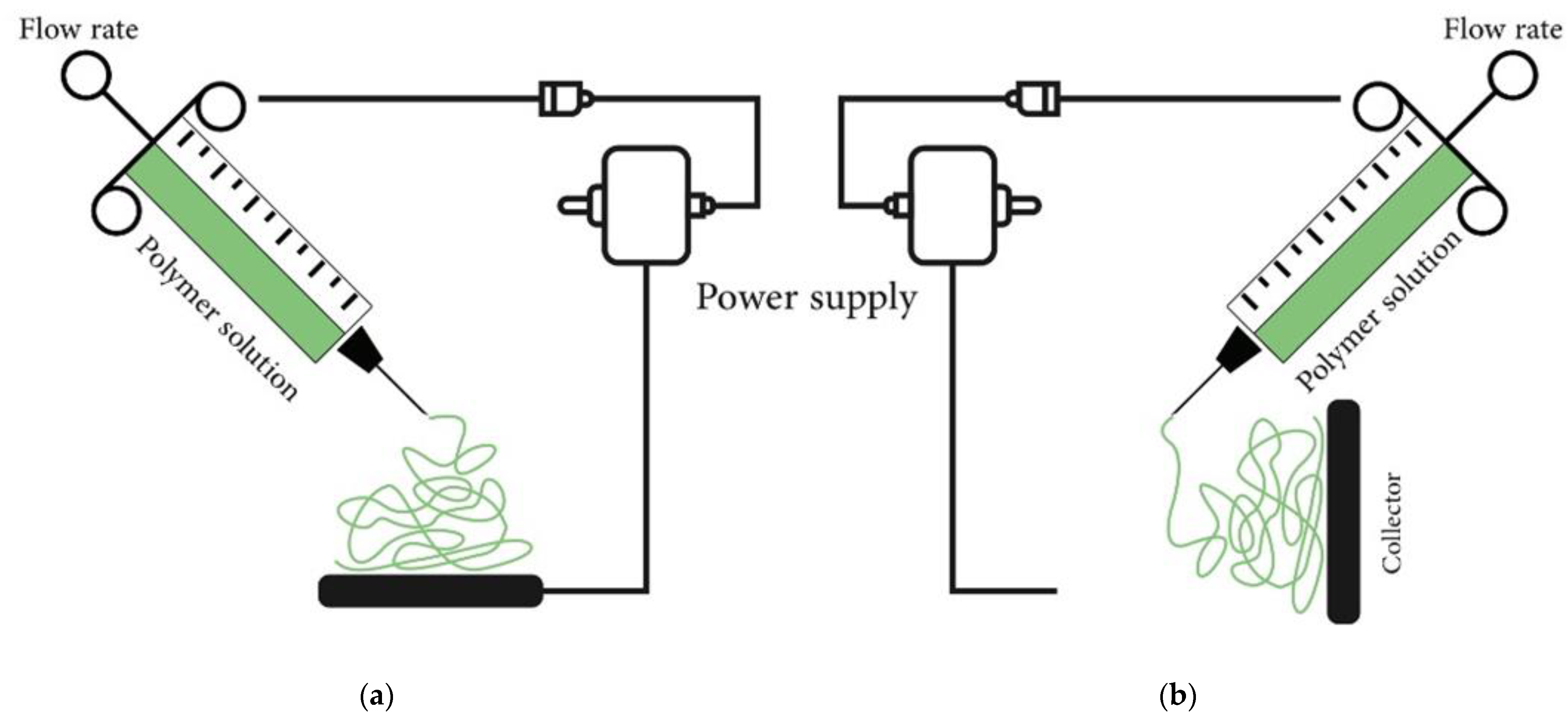
6.2. Electrospinning Process
6.3. Application of Electro-Spun Nanofibers
6.3.1. Tissue Engineering Applications
6.3.2. Drug Delivery Applications
6.3.3. Enzymes Immobilization Applications
6.3.4. Wound Dressing Applications
6.3.5. Antibacterial Applications
6.4. Electrospinning of Chitosan
6.5. Applications of Chitosan Nanofibers
6.5.1. Chitosan Nanofibers in Tissue Engineering
| Composition | Nanofiber Diameter | Cells | Application | Reference |
|---|---|---|---|---|
| Chitosan/montmorillonite/PVA nanofiber composite mesh | 60–140 nm | Human dental pulp stem cells | Nerve tissue engineering | [208] |
| PVA/chitosan nanofiber composite scaffolds | 94–410 nm | PC12 nerve cells | Nerve tissue engineering and repair | [209] |
| Polycaprolactone/chitosan nanofibers composite | 110–240 nm | Schwann cell (SC) | Nerve tissue engineering | [210] |
| Chitosan/PVA/graphene oxide nanofibers composite | 123–160 nm | ATDC5 cells | Cartilage tissue engineering | [211] |
| Chitosan/polyethylene oxide nanofiber composite | 140 ± 41 nm | C2C12 myoblast cells | Tendon tissue engineering | [212] |
| Chitosan nanofiber scaffolds | 50–450 nm | Primary ventricular cardiomyocytes | Cardiac tissue engineering | [213] |
| Polycaprolactone/chitosan nanofibers | 150 ± 2 nm | Mouse model and sheep | Vascular tissue engineering | [214] |
| Chitosan/poly (vinyl alcohol) nanofibrous composite scaffold | 137 nm | rMSC | Skeletal muscle regeneration | [215] |
| Chitosan nano-/micro fibrous double-layered composite | 20–300 nm | Bovine chondrocytes | Cartilage tissue engineering | [216] |
6.5.2. Chitosan Nanofibers in Enzyme Immobilization
6.5.3. Chitosan Nanofibers in Cancer Treatment
6.5.4. Chitosan Nanofibers in Food Technology
7. Limitations and Future Perspectives for Chitosan Application
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brück, W.M.; Slater, J.W.; Carney, B.F. Chitin and chitosan from marine organisms. In Chitin, Chitosan, Oligosaccharides and Their Derivatives: Biological Activities and Applications; Taylor & Francis: Boca Raton, FL, USA, 2010; pp. 11–19. [Google Scholar]
- Zhou, A.; Wei, Y.; Wu, B.; Chen, Q.; Xing, D. Pyropheophorbide A and c (RGDyK) comodified chitosan-wrapped upconversion nanoparticle for targeted near-infrared photodynamic therapy. Mol. Pharm. 2012, 9, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Vinsova, J.; Vavrikova, E. Chitosan derivatives with antimicrobial, antitumour and antioxidant activities—A review. Curr. Pharm. Des. 2011, 17, 3596–3607. [Google Scholar] [CrossRef] [PubMed]
- Philippova, O.; Korchagina, E. Chitosan and its hydrophobic derivatives: Preparation and aggregation in dilute aqueous solutions. Polym. Sci. Ser. A 2012, 54, 552–572. [Google Scholar] [CrossRef]
- Dutta, P.K.; Ravikumar, M.; Dutta, J. Chitin and chitosan for versatile applications. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 307–354. [Google Scholar] [CrossRef]
- Salama, R.; Osman, H.; Ibrahim, H.M. Preparation of biocompatible chitosan nanoparticles loaded with Aloe vera extract for use as a novel drug delivery mechanism to improve the antibacterial characteristics of cellulose-based fabrics. Egypt. J. Chem. 2022, 65, 581–595. [Google Scholar] [CrossRef]
- Mosaad, R.M.; Alhalafi, M.H.; Emam, E.A.M.; Ibrahim, M.A.; Ibrahim, H. Enhancement of Antimicrobial and Dyeing Properties of Cellulosic Fabrics via Chitosan Nanoparticles. Polymers 2022, 14, 4211. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Sheier, M.B.; Reda, M.M.; Ibrahim, H.M. Synthesis, Application, and Antibacterial Activity of New Direct Dyes based on Chromene Derivatives. Curr. Org. Synth. 2022, 19, 757–766. [Google Scholar] [CrossRef]
- Ali, M.A.; Bydoon, E.A.; Ibrahim, H.M. Bioactive Composite Nonwoven Surgical Dressing based on Cellulose Coated with Nanofiber Membrane using the layer-by-layer technique. Egypt. J. Chem. 2022, 65, 525–542. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Wang, J.; Chen, C. Chitosan-based biosorbents: Modification and application for biosorption of heavy metals and radionuclides. Bioresour. Technol. 2014, 160, 129–141. [Google Scholar] [CrossRef]
- Zappino, M.; Cacciotti, I.; Benucci, I.; Nanni, F.; Liburdi, K.; Valentini, F.; Esti, M. Bromelain immobilization on microbial and animal source chitosan films, plasticized with glycerol, for application in wine-like medium: Microstructural, mechanical and catalytic characterisations. Food Hydrocoll. 2015, 45, 41–47. [Google Scholar] [CrossRef]
- Salmon, S.; Hudson, S.M. Crystal morphology, biosynthesis, and physical assembly of cellulose, chitin, and chitosan. J. Macromol. Sci. Part C Polym. Rev. 1997, 37, 199–276. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Gupta, K.; Kumar, M.N.R. pH dependent hydrolysis and drug release behavior of chitosan/poly (ethylene glycol) polymer network microspheres. J. Mater. Sci. Mater. Med. 2001, 12, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Menon, D.; Manzoor, K.; Nair, S.; Tamura, H. Biomedical applications of chitin and chitosan based nanomaterials—A short review. Carbohydr. Polym. 2010, 82, 227–232. [Google Scholar] [CrossRef]
- Wang, J.; Zhuang, S. Chitosan-based materials: Preparation, modification and application. J. Clean. Prod. 2022, 355, 131825. [Google Scholar] [CrossRef]
- Mourya, V.; Inamdar, N.N.; Tiwari, A. Carboxymethyl chitosan and its applications. Adv. Mat. Lett. 2010, 1, 11–33. [Google Scholar] [CrossRef]
- Peniche, C.; Argüelles-Monal, W.; Goycoolea, F. Chitin and chitosan: Major sources, properties and applications. Monomers Polym. Compos. Renew. Resour. 2008, 1, 517–542. [Google Scholar]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan amphiphilic derivatives. Chemistry and applications. Curr. Org. Chem. 2010, 14, 308. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural modification, biological activity and application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Ahmed, F.; Soliman, F.M.; Adly, M.A.; Soliman, H.A.M.; El-Matbouli, M.; Saleh, M. Recent progress in biomedical applications of chitosan and its nanocomposites in aquaculture: A review. Res. Vet. Sci. 2019, 126, 68–82. [Google Scholar] [CrossRef]
- Zhao, D.; Yu, S.; Sun, B.; Gao, S.; Guo, S.; Zhao, K. Biomedical Applications of Chitosan and Its Derivative Nanoparticles. Polymers 2018, 10, 462. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Klingner, A. A review on electrospun polymeric nanofibers: Production parameters and potential applications. Polym. Test. 2020, 90, 106647. [Google Scholar] [CrossRef]
- Qin, Y.; Li, P. Antimicrobial Chitosan Conjugates: Current Synthetic Strategies and Potential Applications. Int. J. Mol. Sci. 2020, 21, 499. [Google Scholar] [CrossRef]
- Crini, G. Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci. 2005, 30, 38–70. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.; Abdel-Hay, F.; Mohy Eldin, M.; Tamer, T.; Ibrahim, E. Novel Aminated Chitosan-Aromatic Aldehydes Schiff Bases: Synthesis, Characterization and Bio-evaluation. Int. J. Adv. Res 2015, 3, 563–572. [Google Scholar]
- Sashiwa, H.; Shigemasa, Y. Chemical modification of chitin and chitosan 2: Preparation and water soluble property of N-acylated or N-alkylated partially deacetylated chitins. Carbohydr. Polym. 1999, 39, 127–138. [Google Scholar] [CrossRef]
- Martău, G.A.; Mihai, M.; Vodnar, D.C. The use of chitosan, alginate, and pectin in the biomedical and food sector—biocompatibility, bioadhesiveness, and biodegradability. Polymers 2019, 11, 1837. [Google Scholar] [CrossRef]
- Di Martino, A.; Sedlarik, V. Amphiphilic chitosan-grafted-functionalized polylactic acid based nanoparticles as a delivery system for doxorubicin and temozolomide co-therapy. Int. J. Pharm. 2014, 474, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Bosquez-Molina, E.; Zavaleta-Avejar, L. New Bioactive Biomaterials Based on Chitosan. In Chitosan in the Preservation of Agricultural Commodities; Academic Press: Cambridge, MA, USA, 2016; pp. 33–64. [Google Scholar]
- Furuike, T.; Komoto, D.; Hashimoto, H.; Tamura, H. Preparation of chitosan hydrogel and its solubility in organic acids. Int. J. Biol. Macromol. 2017, 104, 1620–1625. [Google Scholar] [CrossRef] [PubMed]
- Schügerl, K. Integrated processing of biotechnology products. Biotechnol. Adv. 2000, 18, 581–599. [Google Scholar] [CrossRef]
- Calvo, P.; Remunan-Lopez, C.; Vila-Jato, J.L.; Alonso, M.J. Novel hydrophilic chitosan-polyethylene oxide nanoparticles as protein carriers. J. Appl. Polym. Sci. 1997, 63, 125–132. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016, 151, 321–325. [Google Scholar] [CrossRef]
- Ramezani, Z.; Zarei, M.; Raminnejad, N. Comparing the effectiveness of chitosan and nanochitosan coatings on the quality of refrigerated silver carp fillets. Food Control 2015, 51, 43–48. [Google Scholar] [CrossRef]
- Janes, K.A.; Fresneau, M.P.; Marazuela, A.; Fabra, A.; Alonso, M.A.J. Chitosan nanoparticles as delivery systems for doxorubicin. J. Control. Release 2001, 73, 255–267. [Google Scholar] [CrossRef]
- De Campos, A.M.; Sánchez, A.; Alonso, M.a.J. Chitosan nanoparticles: A new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int. J. Pharm. 2001, 224, 159–168. [Google Scholar] [CrossRef]
- Pan, Y.; Li, Y.-J.; Zhao, H.-Y.; Zheng, J.-M.; Xu, H.; Wei, G.; Hao, J.-S. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002, 249, 139–147. [Google Scholar] [CrossRef]
- Tian, X.X.; Groves, M.J. Formulation and biological activity of antineoplastic proteoglycans derived from Mycobacterium vaccae in chitosan nanoparticles. J. Pharm. Pharmacol. 1999, 51, 151–157. [Google Scholar] [CrossRef]
- Hassan, E.A.; Hassan, M.L.; Abou-Zeid, R.E.; El-Wakil, N.A. Novel nanofibrillated cellulose/chitosan nanoparticles nanocomposites films and their use for paper coating. Ind. Crops Prod. 2016, 93, 219–226. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Park, Y.-J.; Lee, S.-J.; Ku, Y.; Han, S.-B.; Klokkevold, P.R.; Choi, S.-M.; Chung, C.-P. Tissue engineered bone formation using chitosan/tricalcium phosphate sponges. J. Periodontol. 2000, 71, 410–417. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Gihm, S.H.; Park, C.R.; Lee, K.Y.; Kim, T.W.; Kwon, I.C.; Chung, H.; Jeong, S.Y. Structural characteristics of size-controlled self-aggregates of deoxycholic acid-modified chitosan and their application as a DNA delivery carrier. Bioconjugate Chem. 2001, 12, 932–938. [Google Scholar] [CrossRef]
- Duan, B.; Dong, C.; Yuan, X.; Yao, K. Electrospinning of chitosan solutions in acetic acid with poly(ethylene oxide). J. Biomater. Sci. Polym. Ed. 2004, 15, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Abou-Okeil, A.; Refaei, R.; Khalil, E.M.; Refaei, R.; Moustafa, S.E.; Ibrahim, H.M. Fabrication of novel green magnetic electrospun nanofibers based on Fe3O4 nanoparticles/PVA/chitosan/collagen. Egypt. J. Chem. 2022, 65, 283–299. [Google Scholar] [CrossRef]
- Bakr, M.M.; Taha, M.A.; Osman, H.; Ibrahim, H.M. Novel green printing of cotton, wool and polyester fabrics with natural safflower dye nanoparticles. Egypt. J. Chem. 2021, 64, 6221–6230. [Google Scholar] [CrossRef]
- Abou-Okeil, A.; Fahmy, H.M.; Fouda, M.M.G.; Aly, A.A.; Ibrahim, H.M. Hyaluronic Acid/Oxidized K-Carrageenan Electrospun Nanofibers Synthesis and Antibacterial Properties. BioNanoScience 2021, 11, 687–695. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Mostafa, M.; Kandile, N.G. Potential use of N-carboxyethylchitosan in biomedical applications: Preparation, characterization, biological properties. Int. J. Biol. Macromol. 2020, 149, 664–671. [Google Scholar] [CrossRef]
- Ibrahim, H.; El- Zairy, E.M.R.; Emam, E.A.M.; Adel, E. Combined antimicrobial finishing dyeing properties of cotton, polyester fabrics and their blends with acid and disperse dyes. Egypt. J. Chem. 2019, 62, 965–976. [Google Scholar] [CrossRef]
- Aranaz, I.; Gutiérrez, M.C.; Ferrer, M.L.; Del Monte, F. Preparation of chitosan nanocompositeswith a macroporous structure by unidirectional freezing and subsequent freeze-drying. Mar. Drugs 2014, 12, 5619–5642. [Google Scholar] [CrossRef]
- de Oliveira, J.L.; Campos, E.V.R.; Bakshi, M.; Abhilash, P.; Fraceto, L.F. Application of nanotechnology for the encapsulation of botanical insecticides for sustainable agriculture: Prospects and promises. Biotechnol. Adv. 2014, 32, 1550–1561. [Google Scholar] [CrossRef] [PubMed]
- Pichyangkura, R.; Chadchawan, S. Biostimulant activity of chitosan in horticulture. Sci. Hortic. 2015, 196, 49–65. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Tzortzakis, N.; Petropoulos, S.A. Sustainable Agriculture Systems in Vegetable Production Using Chitin and Chitosan as Plant Biostimulants. Biomolecules 2021, 11, 819. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi Pirbalouti, A.; Malekpoor, F.; Salimi, A.; Golparvar, A. Exogenous application of chitosan on biochemical and physiological characteristics, phenolic content and antioxidant activity of two species of basil (Ocimum ciliatum and Ocimum basilicum) under reduced irrigation. Sci. Hortic. 2017, 217, 114–122. [Google Scholar] [CrossRef]
- Saharan, V.; Kumaraswamy, R.V.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-Chitosan Nanoparticle Mediated Sustainable Approach To Enhance Seedling Growth in Maize by Mobilizing Reserved Food. J. Agric. Food Chem. 2016, 64, 6148–6155. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.; Li, C.; An, H.; Wan, T.; Zhang, P. Application of Chitosan and Its Derivative Polymers in Clinical Medicine and Agriculture. Polymers 2022, 14, 958. [Google Scholar] [CrossRef]
- Vakili, M.; Rafatullah, M.; Salamatinia, B.; Abdullah, A.Z.; Ibrahim, M.H.; Tan, K.B.; Gholami, Z.; Amouzgar, P. Application of chitosan and its derivatives as adsorbents for dye removal from water and wastewater: A review. Carbohydr. Polym. 2014, 113, 115–130. [Google Scholar] [CrossRef]
- Lodhi, G.; Kim, Y.-S.; Hwang, J.-W.; Kim, S.-K.; Jeon, Y.-J.; Je, J.-Y.; Ahn, C.-B.; Moon, S.-H.; Jeon, B.-T.; Park, P.-J. Chitooligosaccharide and its derivatives: Preparation and biological applications. BioMed Res. Int. 2014, 2014, 654913. [Google Scholar] [CrossRef]
- Dwivedi, C.; Gupta, A.; Chaudhary, A.; Nandi, C.K. Gold nanoparticle chitosan composite hydrogel beads show efficient removal of methyl parathion from waste water. RSC Adv. 2014, 4, 39830–39838. [Google Scholar] [CrossRef]
- Yadla, S.V.; Sridevi, V.; Lakshmi, M.V.V.C. A review on adsorption of heavy metals from aqueous solution. J. Chem. Biol. Phys. Sci. 2012, 2, 1585. [Google Scholar]
- Chen, A.-H.; Yang, C.-Y.; Chen, C.-Y.; Chen, C.-Y.; Chen, C.-W. The chemically crosslinked metal-complexed chitosans for comparative adsorptions of Cu (II), Zn (II), Ni (II) and Pb (II) ions in aqueous medium. J. Hazard. Mater. 2009, 163, 1068–1075. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef]
- Kyzas, G.Z.; Bikiaris, D.N. Recent modifications of chitosan for adsorption applications: A critical and systematic review. Mar. Drugs 2015, 13, 312–337. [Google Scholar] [CrossRef] [PubMed]
- Cazón, P.; Vázquez, M. Applications of chitosan as food packaging materials. In Sustainable Agriculture Reviews 36; Springer: Berlin/Heidelberg, Germany, 2019; pp. 81–123. [Google Scholar]
- Falguera, V.; Ceron, J.P.Q.; Jiménez, A.; Muñoz, A. Películas y recubrimientos comestibles: Estructuras, funciones activas y tendencias en su uso. Tend. Cienc. Tecnol. Los Aliment. 2011, 22, 292–303. [Google Scholar]
- Aider, M. Chitosan application for active bio-based films production and potential in the food industry. LWT-Food Sci. Technol. 2010, 43, 837–842. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; De La Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.-J.; Sheu, F.; Lin, H.-R. Coating citrus (Murcott tangor) fruit with low molecular weight chitosan increases postharvest quality and shelf life. Food Chem. 2007, 100, 1160–1164. [Google Scholar] [CrossRef]
- Chien, P.-J.; Sheu, F.; Yang, F.-H. Effects of edible chitosan coating on quality and shelf life of sliced mango fruit. J. Food Eng. 2007, 78, 225–229. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Y. Effects of chitosan coating on postharvest life and quality of longan fruit. Food Chem. 2001, 73, 139–143. [Google Scholar] [CrossRef]
- Ferreira, L.F.; de Abreu, G.F.; Lago, A.M.T.; Figueiredo, L.P.; Borém, F.M.; Martins, M.A.; Borges, S.V.; Dias, M.V. Development and application of biopolymer coatings to specialty green coffee beans: Influence on water content, color and sensory quality. LWT 2018, 96, 274–280. [Google Scholar] [CrossRef]
- Soares, N.M.; Mendes, T.S.; Vicente, A.A. Effect of chitosan-based solutions applied as edible coatings and water glazing on frozen salmon preservation—A pilot-scale study. J. Food Eng. 2013, 119, 316–323. [Google Scholar] [CrossRef]
- Chien, P.J.; Chou, C.C. Antifungal activity of chitosan and its application to control post-harvest quality and fungal rotting of Tankan citrus fruit (Citrus tankan Hayata). J. Sci. Food Agric. 2006, 86, 1964–1969. [Google Scholar] [CrossRef]
- Choi, W.Y.; Park, H.J.; Ahn, D.J.; Lee, J.; Lee, C.Y. Wettability of chitosan coating solution on ‘Fuji’apple skin. J. Food Sci. 2002, 67, 2668–2672. [Google Scholar] [CrossRef]
- Shao, X.F.; Tu, K.; Tu, S.; Tu, J. A combination of heat treatment and chitosan coating delays ripening and reduces decay in “Gala” apple fruit. J. Food Qual. 2012, 35, 83–92. [Google Scholar] [CrossRef]
- Eissa, H.A.A. Effect of chitosan coating on shelf life and quality of fresh-cut mushroom. J. Food Qual. 2007, 30, 623–645. [Google Scholar] [CrossRef]
- Djioua, T.; Charles, F.; Freire, M., Jr.; Filgueiras, H.; Ducamp-Collin, M.N.; Sallanon, H. Combined effects of postharvest heat treatment and chitosan coating on quality of fresh-cut mangoes (Mangifera indica L.). Int. J. Food Sci. Technol. 2010, 45, 849–855. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Jiang, W. Effects of chitosan coating on shelf life of cold-stored litchi fruit at ambient temperature. LWT-Food Sci. Technol. 2005, 38, 757–761. [Google Scholar] [CrossRef]
- Dong, C.; Chen, W.; Liu, C. Flocculation of algal cells by amphoteric chitosan-based flocculant. Bioresour. Technol. 2014, 170, 239–247. [Google Scholar] [CrossRef]
- Souza, C.P.; Almeida, B.C.; Colwell, R.R.; Rivera, I.N.G. The Importance of Chitin in the Marine Environment. Mar. Biotechnol. 2011, 13, 823–830. [Google Scholar] [CrossRef]
- Ahmed, K.B.M.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production—A review. Carbohydr. Polym. 2020, 227, 115331. [Google Scholar] [CrossRef]
- Pen, L.T.; Jiang, Y.M. Effects of chitosan coating on shelf life and quality of fresh-cut Chinese water chestnut. LWT-Food Sci. Technol. 2003, 36, 359–364. [Google Scholar] [CrossRef]
- Fan, M.; Hu, Q. Chitosan-LiOH-urea aqueous solution—A novel water-based system for chitosan processing. Carbohydr. Res. 2009, 344, 944–947. [Google Scholar] [CrossRef]
- Patrulea, V.; Ostafe, V.; Borchard, G.; Jordan, O. Chitosan as a starting material for wound healing applications. Eur. J. Pharm. Biopharm. 2015, 97, 417–426. [Google Scholar] [CrossRef]
- Farag, S.; Ibrahim, H.M.; Amr, A.; Asker, M.S.; El-Shafai, A. Preparation and characterization of ion exchanger based on bacterial cellulose for heavy metal cation removal. Egypt. J. Chem. 2019, 62, 457–466. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Ibrahim, H.M.; Aly, A.A.; El-Alfy, E.A. Improvement of dyeability and antibacterial properties of gelatin treated cotton fabrics with synthesized reactive dye. Biosci. Res. 2018, 15, 4403–4408. [Google Scholar]
- Mohamed, F.A.; Abd El-Megied, S.A.; Bashandy, M.S.; Ibrahim, H.M. Synthesis, application and antibacterial activity of new reactive dyes based on thiazole moiety. Pigment Resin Technol. 2018, 47, 246–254. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Farid, O.A.; Samir, A.; Mosaad, R.M. Preparation of chitosan antioxidant nanoparticles as drug delivery system for enhancing of anti-cancer drug. In Key Engineering Materials; Trans Tech Publications Ltd.: Wollerau, Switzerland, 2018; Volume 759, pp. 92–97. [Google Scholar]
- Mosaad, R.M.; Samir, A.; Ibrahim, H.M. Median lethal dose (LD50) and cytotoxicity of Adriamycin in female albino mice. J. Appl. Pharm. Sci. 2017, 7, 77–80. [Google Scholar] [CrossRef]
- Liu, L.; Xin, Y.; Liu, J.; Zhang, E.; Li, W. Inhibitory effect of chitosan oligosaccharide on human hepatoma cells in vitro. Afr. J. Tradit. Complement. Altern. Med. 2017, 14, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Ngo, D.N.; Kim, S.-K.; Vo, T.S. Antiproliferative effect of aminoethyl-chitooligosaccharide on human lung A549 cancer cells. Biomolecules 2019, 9, 195. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, S.; Hou, J.; Chi, J.; Wang, S.; Shao, K.; Liu, W.; Sun, R.; Han, B. Effects of carboxymethyl chitosan oligosaccharide on regulating immunologic function and inhibiting tumor growth. Carbohydr. Polym. 2020, 250, 116994. [Google Scholar] [CrossRef]
- Zhai, X.; Li, C.; Ren, D.; Wang, J.; Ma, C.; Abd El-Aty, A.M. The impact of chitooligosaccharides and their derivatives on the in vitro and in vivo antitumor activity: A comprehensive review. Carbohydr. Polym. 2021, 266, 118132. [Google Scholar] [CrossRef]
- Muanprasat, C.; Chatsudthipong, V. Chitosan oligosaccharide: Biological activities and potential therapeutic applications. Pharmacol. Ther. 2017, 170, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Cherng, J.-H.; Liu, C.-C.; Fang, T.-J.; Hong, Z.-J.; Chang, S.-J.; Fan, G.-Y.; Hsu, S.-D. Procoagulant and antimicrobial effects of chitosan in wound healing. Int. J. Mol. Sci. 2021, 22, 7067. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.; Han, W.; Jiang, T.; Liu, F.; Zhao, X. Alginate-chitosan oligosaccharide-ZnO composite hydrogel for accelerating wound healing. Carbohydr. Polym. 2021, 266, 118100. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Dai, K.; Yu, Y.; Wang, J.; Liu, C. Sulfated chitosan rescues dysfunctional macrophages and accelerates wound healing in diabetic mice. Acta Biomater. 2020, 117, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef]
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef]
- Wu, L.; Liu, M. Preparation and properties of chitosan-coated NPK compound fertilizer with controlled-release and water-retention. Carbohydr. Polym. 2008, 72, 240–247. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitosan Effects on Plant Systems. Int. J. Mol. Sci. 2016, 17, 996. [Google Scholar] [CrossRef]
- Abd El-Naby, F.S.; Naiel, M.A.E.; Al-Sagheer, A.A.; Negm, S.S. Dietary chitosan nanoparticles enhance the growth, production performance, and immunity in Oreochromis niloticus. Aquaculture 2019, 501, 82–89. [Google Scholar] [CrossRef]
- Abd El-Naby, A.S.; Al-Sagheer, A.A.; Negm, S.S.; Naiel, M.A.E. Dietary combination of chitosan nanoparticle and thymol affects feed utilization, digestive enzymes, antioxidant status, and intestinal morphology of Oreochromis niloticus. Aquaculture 2020, 515, 734577. [Google Scholar] [CrossRef]
- Sinha, V.R.; Singla, A.K.; Wadhawan, S.; Kaushik, R.; Kumria, R.; Bansal, K.; Dhawan, S. Chitosan microspheres as a potential carrier for drugs. Int. J. Pharm. 2004, 274, 1–33. [Google Scholar] [CrossRef]
- Azad, A.K.; Sermsintham, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan Membrane as a Wound-Healing Dressing: Characterization and Clinical Application. J. Biomed. Mater. Res. Part B Appl. Biomater. 2004, 69, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-B.; Yu, S.-H.; Mi, F.-L.; Wu, C.-W.; Shyu, S.-S.; Peng, C.-K.; Chao, A.-C. Preparation and characterization on mechanical and antibacterial properties of chitsoan/cellulose blends. Carbohydr. Polym. 2004, 57, 435–440. [Google Scholar] [CrossRef]
- Fan, L.; Du, Y.; Zhang, B.; Yang, J.; Zhou, J.; Kennedy, J.F. Preparation and properties of alginate/carboxymethyl chitosan blend fibers. Carbohydr. Polym. 2006, 65, 447–452. [Google Scholar] [CrossRef]
- Chen, J.-P.; Chang, G.-Y.; Chen, J.-K. Electrospun collagen/chitosan nanofibrous membrane as wound dressing. Colloids Surf. A Physicochem. Eng. Asp. 2008, 313–314, 183–188. [Google Scholar] [CrossRef]
- Qasim, S.B.; Najeeb, S.; Delaine-Smith, R.M.; Rawlinson, A.; Ur Rehman, I. Potential of electrospun chitosan fibers as a surface layer in functionally graded GTR membrane for periodontal regeneration. Dent. Mater. 2017, 33, 71–83. [Google Scholar] [CrossRef]
- Ma, Y.; Xin, L.; Tan, H.; Fan, M.; Li, J.; Jia, Y.; Ling, Z.; Chen, Y.; Hu, X. Chitosan membrane dressings toughened by glycerol to load antibacterial drugs for wound healing. Mater. Sci. Eng. C 2017, 81, 522–531. [Google Scholar] [CrossRef]
- Behera, S.S.; Das, U.; Kumar, A.; Bissoyi, A.; Singh, A.K. Chitosan/TiO2 composite membrane improves proliferation and survival of L929 fibroblast cells: Application in wound dressing and skin regeneration. Int. J. Biol. Macromol. 2017, 98, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Ghannam, S.F.; Korayem, H.E.; Farghaly, L.M.; Hosny, S. The effect of chitosan nanosilver dressing versus mesenchymal stem cells on wound healing. J. Afr. Assoc. Physiol. Sci. 2018, 6, 23–31. [Google Scholar]
- Denkbaş, E.B.; Öztürk, E.; Özdemir, N.; Keçeci, K.; Agalar, C. Norfloxacin-loaded chitosan sponges as wound dressing material. J. Biomater. Appl. 2004, 18, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.-M.; He, L.-Z.; Zhao, M.; Yang, D.; Liu, Y. Biological properties of the chitosan-gelatin sponge wound dressing. Carbohydr. Polym. 2007, 69, 583–589. [Google Scholar] [CrossRef]
- Obara, K.; Ishihara, M.; Ishizuka, T.; Fujita, M.; Ozeki, Y.; Maehara, T.; Saito, Y.; Yura, H.; Matsui, T.; Hattori, H.; et al. Photocrosslinkable chitosan hydrogel containing fibroblast growth factor-2 stimulates wound healing in healing-impaired db/db mice. Biomaterials 2003, 24, 3437–3444. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xing, X.; Tan, H.; Jia, Y.; Zhou, T.; Chen, Y.; Ling, Z.; Hu, X. Covalently antibacterial alginate-chitosan hydrogel dressing integrated gelatin microspheres containing tetracycline hydrochloride for wound healing. Mater. Sci. Eng. C 2017, 70, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89. [Google Scholar] [CrossRef]
- Celebioglu, A.; Uyar, T. Electrospinning of nanofibers from non-polymeric systems: Electrospun nanofibers from native cyclodextrins. J. Colloid Interface Sci. 2013, 404, 1–7. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.; Tock, R.; Parameswaran, S.; Ramkumar, S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Ghorani, B.; Tucker, N. Fundamentals of electrospinning as a novel delivery vehicle for bioactive compounds in food nanotechnology. Food Hydrocoll. 2015, 51, 227–240. [Google Scholar] [CrossRef]
- Pillay, V.; Dott, C.; Choonara, Y.E.; Tyagi, C.; Tomar, L.; Kumar, P.; du Toit, L.C.; Ndesendo, V.M. A review of the effect of processing variables on the fabrication of electrospun nanofibers for drug delivery applications. J. Nanomater. 2013, 2013, 789289. [Google Scholar] [CrossRef]
- Dalton, P.D.; Klinkhammer, K.; Salber, J.; Klee, D.; Möller, M. Direct in vitro electrospinning with polymer melts. Biomacromolecules 2006, 7, 686–690. [Google Scholar] [CrossRef]
- Greiner, A.; Wendorff, J.H. Electrospinning: A fascinating method for the preparation of ultrathin fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Agarwal, S.; Greiner, A.; Wendorff, J.H. Functional materials by electrospinning of polymers. Prog. Polym. Sci. 2013, 38, 963–991. [Google Scholar] [CrossRef]
- Haider, A.; Haider, S.; Kang, I.-K. A comprehensive review summarizing the effect of electrospinning parameters and potential applications of nanofibers in biomedical and biotechnology. Arab. J. Chem. 2018, 11, 1165–1188. [Google Scholar] [CrossRef]
- Liang, D.; Hsiao, B.S.; Chu, B. Functional electrospun nanofibrous scaffolds for biomedical applications. Adv. Drug Deliv. Rev. 2007, 59, 1392–1412. [Google Scholar] [CrossRef] [PubMed]
- Adomavičiūtė, E.; Milašius, R. The influence of applied voltage on poly (vinyl alcohol)(PVA) nanofibre diameter. Fibres Text. East. Eur. 2007, 15, 63. [Google Scholar]
- Reneker, D.; Yarin, A.; Zussman, E.; Xu, H. Electrospinning of nanofibers from polymer solutions and melts. Adv. Appl. Mech. 2007, 41, 43–346. [Google Scholar]
- Shin, Y.; Hohman, M.; Brenner, M.; Rutledge, G. Electrospinning: A whipping fluid jet generates submicron polymer fibers. Appl. Phys. Lett. 2001, 78, 1149–1151. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Fujihara, K.; Teo, W.-E.; Lim, T.-C.; Ma, Z. An Introduction to Electrospinning and Nanofibers; World Scientific: Singapore, 2005; Volume 90. [Google Scholar]
- Kidoaki, S.; Kwon, I.K.; Matsuda, T. Mesoscopic spatial designs of nano-and microfiber meshes for tissue-engineering matrix and scaffold based on newly devised multilayering and mixing electrospinning techniques. Biomaterials 2005, 26, 37–46. [Google Scholar] [CrossRef]
- Stankus, J.J.; Freytes, D.O.; Badylak, S.F.; Wagner, W.R. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J. Biomater. Sci. Polym. Ed. 2008, 19, 635–652. [Google Scholar] [CrossRef]
- El-Alfy, E.A.; El-Bisi, M.K.; Taha, G.M.; Ibrahim, H.M. Preparation of biocompatible chitosan nanoparticles loaded by tetracycline, gentamycin and ciprofloxacin as novel drug delivery system for improvement the antibacterial properties of cellulose based fabrics. Int. J. Biol. Macromol. 2020, 161, 1247–1260. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Liao, Y.; Loh, C.-H.; Tian, M.; Wang, R.; Fane, A.G. Progress in electrospun polymeric nanofibrous membranes for water treatment: Fabrication, modification and applications. Prog. Polym. Sci. 2018, 77, 69–94. [Google Scholar] [CrossRef]
- Senthil, T.; George, G.; Srinivasan, A. Electrospinning: From Fundamentals to Applications. In Advances in Polymer Materials and Technology; CRC Press: Boca Raton, FL, USA, 2016; pp. 171–240. [Google Scholar]
- Zagho, M.M.; Elzatahry, A. Recent trends in electrospinning of polymer nanofibers and their applications as templates for metal oxide nanofibers preparation. In Electrospinning: Material, Techniques, and Biomedical Applications; IntechOpen: London, UK, 2016. [Google Scholar]
- Bode-Aluko, C.A.; Laatikainen, K.; Pereao, O.; Nechaev, A.; Kochnev, I.; Rossouw, A.; Dobretsov, S.; Branger, C.; Sarbu, A.; Petrik, L. Fabrication and characterisation of novel nanofiltration polymeric membrane. Mater. Today Commun. 2019, 20, 100580. [Google Scholar] [CrossRef]
- Huang, Z.-M.; Zhang, Y.-Z.; Kotaki, M.; Ramakrishna, S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Compos. Sci. Technol. 2003, 63, 2223–2253. [Google Scholar] [CrossRef]
- Mohamed, F.A.; Reda, M.M.; Ibrahim, H. Enhancement of Dyeing and Antimicrobial Properties of Chitosan and Chitosan Nanoparticles-Treated Cotton and Viscose Fabrics with Acid Dyes. Egypt. J. Chem. 2022, 65, 339–344. [Google Scholar]
- Teo, W.-E.; Inai, R.; Ramakrishna, S. Technological advances in electrospinning of nanofibers. Sci. Technol. Adv. Mater. 2016, 12, 013002. [Google Scholar] [CrossRef]
- Metreveli, G.; Wågberg, L.; Emmoth, E.; Belák, S.; Strømme, M.; Mihranyan, A. A Size-Exclusion Nanocellulose Filter Paper for Virus Removal. Adv. Healthc. Mater. 2014, 3, 1546–1550. [Google Scholar] [CrossRef]
- Haider, A.; Gupta, K.C.; Kang, I.-K. Morphological effects of HA on the cell compatibility of electrospun HA/PLGA composite nanofiber scaffolds. BioMed Res. Int. 2014, 2014, 308306. [Google Scholar] [CrossRef]
- Haider, A.; Gupta, K.C.; Kang, I.-K. PLGA/nHA hybrid nanofiber scaffold as a nanocargo carrier of insulin for accelerating bone tissue regeneration. Nanoscale Res. Lett. 2014, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Tysseling-Mattiace, V.M.; Sahni, V.; Niece, K.L.; Birch, D.; Czeisler, C.; Fehlings, M.G.; Stupp, S.I.; Kessler, J.A. Self-assembling nanofibers inhibit glial scar formation and promote axon elongation after spinal cord injury. J. Neurosci. 2008, 28, 3814–3823. [Google Scholar] [CrossRef]
- Woo, K.M.; Chen, V.J.; Ma, P.X. Nano-fibrous scaffolding architecture selectively enhances protein adsorption contributing to cell attachment. J. Biomed. Mater. Res. Part A 2003, 67, 531–537. [Google Scholar] [CrossRef]
- Gupta, K.C.; Haider, A.; Choi, Y.-R.; Kang, I.-K. Nanofibrous scaffolds in biomedical applications. Biomater. Res. 2014, 18, 5. [Google Scholar] [CrossRef]
- Haider, S.; Binagag, F.F.; Haider, A.; Mahmood, A.; Shah, N.; Al-Masry, W.A.; Khan, S.U.-D.; Ramay, S.M. Adsorption kinetic and isotherm of methylene blue, safranin T and rhodamine B onto electrospun ethylenediamine-grafted-polyacrylonitrile nanofibers membrane. Desalination Water Treat. 2015, 55, 1609–1619. [Google Scholar] [CrossRef]
- Haider, S.; Binagag, F.F.; Haider, A.; Mahmood, A.; Al Masry, W.A.; Alhoshan, M.; Khan, S.U.-D. Fabrication of the Diethylenetriamine Grafted Polyacrylonitrile Electrospun Nanofibers Membrane for the Aqueous Removal of Cationic Dyes. Sci. Adv. Mater. 2015, 7, 309–318. [Google Scholar] [CrossRef]
- Haider, S.; Haider, A.; Ahmad, A.; Khan, S.U.-D.; Almasry, W.A.; Sarfarz, M. Electrospun nanofibers affinity membranes for water hazards remediation. Nanotechnol. Res. J. 2015, 8, 511. [Google Scholar]
- Haider, S.; Al-Zeghayer, Y.; Ali, F.A.A.; Haider, A.; Mahmood, A.; Al-Masry, W.A.; Imran, M.; Aijaz, M.O. Highly aligned narrow diameter chitosan electrospun nanofibers. J. Polym. Res. 2013, 20, 105. [Google Scholar] [CrossRef]
- Vasita, R.; Katti, D.S. Nanofibers and their applications in tissue engineering. Int. J. Nanomed. 2006, 1, 15. [Google Scholar] [CrossRef]
- Sun, B.; Long, Y.; Zhang, H.; Li, M.; Duvail, J.; Jiang, X.; Yin, H. Advances in three-dimensional nanofibrous macrostructures via electrospinning. Prog. Polym. Sci. 2014, 39, 862–890. [Google Scholar] [CrossRef]
- Li, W.J.; Laurencin, C.T.; Caterson, E.J.; Tuan, R.S.; Ko, F.K. Electrospun nanofibrous structure: A novel scaffold for tissue engineering. J. Biomed. Mater. Res. 2002, 60, 613–621. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Bowlin, G.L.; Mansfield, K.; Layman, J.; Simpson, D.G.; Sanders, E.H.; Wnek, G.E. Release of tetracycline hydrochloride from electrospun poly (ethylene-co-vinylacetate), poly (lactic acid), and a blend. J. Control. Release 2002, 81, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Thomopoulos, S.; Xia, Y. Electrospun nanofibers for regenerative medicine. Adv. Healthc. Mater. 2012, 1, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, S.; Zhou, G.; Huang, Y.; Xie, Z.; Jing, X. Electrospinning of polymeric nanofibers for drug delivery applications. J. Control. Release 2014, 185, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Kanani, A.G.; Bahrami, S.H. Effect of changing solvents on poly (ε-caprolactone) nanofibrous webs morphology. J. Nanomater. 2011, 2011, 31. [Google Scholar]
- Yang, D.; Li, Y.; Nie, J. Preparation of gelatin/PVA nanofibers and their potential application in controlled release of drugs. Carbohydr. Polym. 2007, 69, 538–543. [Google Scholar] [CrossRef]
- Xie, J.; Hsieh, Y.-L. Ultra-high surface fibrous membranes from electrospinning of natural proteins: Casein and lipase enzyme. J. Mater. Sci. 2003, 38, 2125–2133. [Google Scholar] [CrossRef]
- Ye, P.; Xu, Z.-K.; Che, A.-F.; Wu, J.; Seta, P. Chitosan-tethered poly (acrylonitrile-co-maleic acid) hollow fiber membrane for lipase immobilization. Biomaterials 2005, 26, 6394–6403. [Google Scholar] [CrossRef]
- Huang, X.J.; Xu, Z.K.; Wan, L.S.; Innocent, C.; Seta, P. Electrospun nanofibers modified with phospholipid moieties for enzyme immobilization. Macromol. Rapid Commun. 2006, 27, 1341–1345. [Google Scholar] [CrossRef]
- Kim, H.S.; Yoo, H.S. MMPs-responsive release of DNA from electrospun nanofibrous matrix for local gene therapy: In vitro and in vivo evaluation. J. Control. Release 2010, 145, 264–271. [Google Scholar] [CrossRef]
- Im, J.S.; Yun, J.; Lim, Y.-M.; Kim, H.-I.; Lee, Y.-S. Fluorination of electrospun hydrogel fibers for a controlled release drug delivery system. Acta Biomater. 2010, 6, 102–109. [Google Scholar] [CrossRef]
- Theron, S.; Yarin, A.; Zussman, E.; Kroll, E. Multiple jets in electrospinning: Experiment and modeling. Polymer 2005, 46, 2889–2899. [Google Scholar] [CrossRef]
- Gallant-Behm, C.L.; Yin, H.Q.; Liu, S.; Heggers, J.P.; Langford, R.E.; Olson, M.E.; Hart, D.A.; Burrell, R.E. Comparison of in vitro disc diffusion and time kill-kinetic assays for the evaluation of antimicrobial wound dressing efficacy. Wound Repair Regen. 2005, 13, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Bowler, P.G.; Walker, M.; Parsons, D. Controlling wound bioburden with a novel silver-containing Hydrofiber® dressing. Wound Repair Regen. 2004, 12, 288–294. [Google Scholar] [CrossRef]
- Rim, N.G.; Shin, C.S.; Shin, H. Current approaches to electrospun nanofibers for tissue engineering. Biomed. Mater. 2013, 8, 014102. [Google Scholar] [CrossRef]
- Gao, Y.; Bach Truong, Y.; Zhu, Y.; Louis Kyratzis, I. Electrospun antibacterial nanofibers: Production, activity, and in vivo applications. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef]
- Smith, D.J.; Reneker, D.H.; McManus, A.T.; Schreuder-Gibson, H.L.; Mello, C.; Sennett, M.S. Electrospun Fibers and an Apparatus Therefor. U.S. Patent 6,753,454, 22 June 2004. [Google Scholar]
- Si, Y.; Tang, X.; Yu, J.; Ding, B. Electrospun Nanofibers: Solving Global Issues. In Electrospun Nanofibers for Energy and Environmental Applications; Springer: Berlin/Heidelberg, Germany, 2014; pp. 3–38. [Google Scholar]
- Nakielski, P.; Pierini, F. Blood interactions with nano- and microfibers: Recent advances, challenges and applications in nano- and microfibrous hemostatic agents. Acta Biomater. 2019, 84, 63–76. [Google Scholar] [CrossRef]
- Huang, J.; Cheng, Y.; Wu, Y.; Shi, X.; Du, Y.; Deng, H. Chitosan/tannic acid bilayers layer-by-layer deposited cellulose nanofibrous mats for antibacterial application. Int. J. Biol. Macromol. 2019, 139, 191–198. [Google Scholar] [CrossRef]
- Ren, X.; Xu, Z.; Wang, L.; Meng, K.; Wang, H.; Zhao, H. Silk fibroin/chitosan/halloysite composite medical dressing with antibacterial and rapid haemostatic properties. Mater. Res. Express 2019, 6, 125409. [Google Scholar] [CrossRef]
- Sasmal, P.; Datta, P. Tranexamic acid-loaded chitosan electrospun nanofibers as drug delivery system for hemorrhage control applications. J. Drug Deliv. Sci. Technol. 2019, 52, 559–567. [Google Scholar] [CrossRef]
- Leonhardt, E.E.; Kang, N.; Hamad, M.A.; Wooley, K.L.; Elsabahy, M. Absorbable hemostatic hydrogels comprising composites of sacrificial templates and honeycomb-like nanofibrous mats of chitosan. Nat. Commun. 2019, 10, 2307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Luo, J.; Menkhaus, T.J.; Varadaraju, H.; Sun, Y.; Fong, H. Antimicrobial nano-fibrous membranes developed from electrospun polyacrylonitrile nanofibers. J. Membr. Sci. 2011, 369, 499–505. [Google Scholar] [CrossRef]
- Subramanian, A.; Krishnan, U.M.; Sethuraman, S. Fabrication of uniaxially aligned 3D electrospun scaffolds for neural regeneration. Biomed. Mater. 2011, 6, 025004. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Reneker, D.H.; Yarin, A.L. Electrospinning jets and polymer nanofibers. Polymer 2008, 49, 2387–2425. [Google Scholar] [CrossRef]
- Augustine, R.; Rehman, S.R.U.; Ahmed, R.; Zahid, A.A.; Sharifi, M.; Falahati, M.; Hasan, A. Electrospun chitosan membranes containing bioactive and therapeutic agents for enhanced wound healing. Int. J. Biol. Macromol. 2020, 156, 153–170. [Google Scholar] [CrossRef]
- Ranjith, R.; Balraj, S.; Ganesh, J.; Milton, M.C.J. Therapeutic agents loaded chitosan-based nanofibrous mats as potential wound dressings: A review. Mater. Today Chem. 2019, 12, 386–395. [Google Scholar] [CrossRef]
- Yang, X.; Wang, J.; Guo, H.; Liu, L.; Xu, W.; Duan, G. Structural design toward functional materials by electrospinning: A review. e-Polymers 2020, 20, 682–712. [Google Scholar] [CrossRef]
- Guo, H.; Chen, Y.; Li, Y.; Zhou, W.; Xu, W.; Pang, L.; Fan, X.; Jiang, S. Electrospun fibrous materials and their applications for electromagnetic interference shielding: A review. Compos. Part A Appl. Sci. Manuf. 2021, 143, 106309. [Google Scholar] [CrossRef]
- Zhao, L.; Duan, G.; Zhang, G.; Yang, H.; He, S.; Jiang, S. Electrospun Functional Materials toward Food Packaging Applications: A Review. Nanomaterials 2020, 10, 150. [Google Scholar] [CrossRef]
- Tao, F.; Cheng, Y.; Shi, X.; Zheng, H.; Du, Y.; Xiang, W.; Deng, H. Applications of chitin and chitosan nanofibers in bone regenerative engineering. Carbohydr. Polym. 2020, 230, 115658. [Google Scholar] [CrossRef] [PubMed]
- Homayoni, H.; Ravandi, S.A.H.; Valizadeh, M. Electrospinning of chitosan nanofibers: Processing optimization. Carbohydr. Polym. 2009, 77, 656–661. [Google Scholar] [CrossRef]
- Liu, L.; Xu, W.; Ding, Y.; Agarwal, S.; Greiner, A.; Duan, G. A review of smart electrospun fibers toward textiles. Compos. Commun. 2020, 22, 100506. [Google Scholar] [CrossRef]
- Neamnark, A.; Rujiravanit, R.; Supaphol, P. Electrospinning of hexanoyl chitosan. Carbohydr. Polym. 2006, 66, 298–305. [Google Scholar] [CrossRef]
- Li, S.; Guo, H.; He, S.; Yang, H.; Liu, K.; Duan, G.; Jiang, S. Advanced electrospun nanofibers as bifunctional electrocatalysts for flexible metal-air (O2) batteries: Opportunities and challenges. Mater. Des. 2022, 214, 110406. [Google Scholar] [CrossRef]
- Geng, X.; Kwon, O.-H.; Jang, J. Electrospinning of chitosan dissolved in concentrated acetic acid solution. Biomaterials 2005, 26, 5427–5432. [Google Scholar] [CrossRef]
- Okutan, N.; Terzi, P.; Altay, F. Affecting parameters on electrospinning process and characterization of electrospun gelatin nanofibers. Food Hydrocoll. 2014, 39, 19–26. [Google Scholar] [CrossRef]
- Frenot, A.; Chronakis, I.S. Polymer nanofibers assembled by electrospinning. Curr. Opin. Colloid Interface Sci. 2003, 8, 64–75. [Google Scholar] [CrossRef]
- Haghi, A.K.; Akbari, M. Trends in electrospinning of natural nanofibers. Phys. Status Solidi (A) 2007, 204, 1830–1834. [Google Scholar] [CrossRef]
- Burger, C.; Hsiao, B.S.; Chu, B. NANOFIBROUS MATERIALS AND THEIR APPLICATIONS. Annu. Rev. Mater. Res. 2006, 36, 333–368. [Google Scholar] [CrossRef]
- Su, H.; Liu, K.-Y.; Karydis, A.; Abebe, D.G.; Wu, C.; Anderson, K.M.; Ghadri, N.; Adatrow, P.; Fujiwara, T.; Bumgardner, J.D. In vitro and in vivo evaluations of a novel post-electrospinning treatment to improve the fibrous structure of chitosan membranes for guided bone regeneration. Biomed. Mater. 2016, 12, 015003. [Google Scholar] [CrossRef]
- Rasouli, R.; Barhoum, A.; Bechelany, M.; Dufresne, A. Nanofibers for biomedical and healthcare applications. Macromol. Biosci. 2019, 19, 1800256. [Google Scholar] [CrossRef]
- Singh, B.N.; Pramanik, K. Development of novel silk fibroin/polyvinyl alcohol/sol–gel bioactive glass composite matrix by modified layer by layer electrospinning method for bone tissue construct generation. Biofabrication 2017, 9, 015028. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Pangon, A.; Saesoo, S.; Saengkrit, N.; Ruktanonchai, U.; Intasanta, V. Hydroxyapatite-hybridized chitosan/chitin whisker bionanocomposite fibers for bone tissue engineering applications. Carbohydr. Polym. 2016, 144, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Deng, H.; Du, Y.; Shi, X.; Wang, Q. Emerging chitin and chitosan nanofibrous materials for biomedical applications. Nanoscale 2014, 6, 9477–9493. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Afifi, A.M.; Jahangirian, H.; Webster, T.J. Biomedical applications of chitosan electrospun nanofibers as a green polymer—Review. Carbohydr. Polym. 2019, 207, 588–600. [Google Scholar] [CrossRef]
- Hokmabad, V.R.; Davaran, S.; Aghazadeh, M.; Alizadeh, E.; Salehi, R.; Ramazani, A. A Comparison of the Effects of Silica and Hydroxyapatite Nanoparticles on Poly(ε-caprolactone)-Poly(ethylene glycol)-Poly(ε-caprolactone)/Chitosan Nanofibrous Scaffolds for Bone Tissue Engineering. Tissue Eng. Regen. Med. 2018, 15, 735–750. [Google Scholar] [CrossRef]
- Lastra, M.L.; Molinuevo, M.S.; Blaszczyk-Lezak, I.; Mijangos, C.; Cortizo, M.S. Nanostructured fumarate copolymer-chitosan crosslinked scaffold: An in vitro osteochondrogenesis regeneration study. J. Biomed. Mater. Res. Part A 2018, 106, 570–579. [Google Scholar] [CrossRef]
- Ghasemi Hamidabadi, H.; Rezvani, Z.; Nazm Bojnordi, M.; Shirinzadeh, H.; Seifalian, A.M.; Joghataei, M.T.; Razaghpour, M.; Alibakhshi, A.; Yazdanpanah, A.; Salimi, M.; et al. Chitosan-Intercalated Montmorillonite/Poly(vinyl alcohol) Nanofibers as a Platform to Guide Neuronlike Differentiation of Human Dental Pulp Stem Cells. ACS Appl. Mater. Interfaces 2017, 9, 11392–11404. [Google Scholar] [CrossRef]
- Alhosseini, S.N.; Moztarzadeh, F.; Mozafari, M.; Asgari, S.; Dodel, M.; Samadikuchaksaraei, A.; Kargozar, S.; Jalali, N. Synthesis and characterization of electrospun polyvinyl alcohol nanofibrous scaffolds modified by blending with chitosan for neural tissue engineering. Int. J. Nanomed. 2012, 25–34. [Google Scholar]
- Bolaina-Lorenzo, E.; Martínez-Ramos, C.; Monleón-Pradas, M.; Herrera-Kao, W.; Cauich-Rodríguez, J.V.; Cervantes-Uc, J.M. Electrospun polycaprolactone/chitosan scaffolds for nerve tissue engineering: Physicochemical characterization and Schwann cell biocompatibility. Biomed. Mater. 2016, 12, 015008. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Zhang, F.; Wang, Q.; Wu, X. Fabrication of chitosan/graphene oxide polymer nanofiber and its biocompatibility for cartilage tissue engineering. Mater. Sci. Eng. C 2017, 79, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Tonda-Turo, C.; Ruini, F.; Ramella, M.; Boccafoschi, F.; Gentile, P.; Gioffredi, E.; Falvo D’Urso Labate, G.; Ciardelli, G. Non-covalently crosslinked chitosan nanofibrous mats prepared by electrospinning as substrates for soft tissue regeneration. Carbohydr. Polym. 2017, 162, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Collins, G.; Yip, D.; Cho, C.H. Functional 3-D cardiac co-culture model using bioactive chitosan nanofiber scaffolds. Biotechnol. Bioeng. 2013, 110, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Fukunishi, T.; Best, C.A.; Sugiura, T.; Shoji, T.; Yi, T.; Udelsman, B.; Ohst, D.; Ong, C.S.; Zhang, H.; Shinoka, T. Tissue-engineered small diameter arterial vascular grafts from cell-free nanofiber PCL/chitosan scaffolds in a sheep model. PLoS ONE 2016, 11, e0158555. [Google Scholar] [CrossRef] [PubMed]
- Kheradmandi, M.; Vasheghani-Farahani, E.; Ghiaseddin, A.; Ganji, F. Skeletal muscle regeneration via engineered tissue culture over electrospun nanofibrous chitosan/PVA scaffold. J. Biomed. Mater. Res. Part A 2016, 104, 1720–1727. [Google Scholar] [CrossRef]
- Shim, I.K.; Suh, W.H.; Lee, S.Y.; Lee, S.H.; Heo, S.J.; Lee, M.C.; Lee, S.J. Chitosan nano-/microfibrous double-layered membrane with rolled-up three-dimensional structures for chondrocyte cultivation. J. Biomed. Mater. Res. Part A 2009, 90A, 595–602. [Google Scholar] [CrossRef]
- De Farias, B.S.; Sant’Anna Cadaval, T.R., Jr.; de Almeida Pinto, L.A. Chitosan-functionalized nanofibers: A comprehensive review on challenges and prospects for food applications. Int. J. Biol. Macromol. 2019, 123, 210–220. [Google Scholar] [CrossRef]
- Sharifi, M.; Sohrabi, M.J.; Hosseinali, S.H.; Hasan, A.; Kani, P.H.; Talaei, A.J.; Karim, A.Y.; Nanakali, N.M.Q.; Salihi, A.; Aziz, F.M.; et al. Enzyme immobilization onto the nanomaterials: Application in enzyme stability and prodrug-activated cancer therapy. Int. J. Biol. Macromol. 2020, 143, 665–676. [Google Scholar] [CrossRef]
- Bösiger, P.; Tegl, G.; Richard, I.M.T.; Le Gat, L.; Huber, L.; Stagl, V.; Mensah, A.; Guebitz, G.M.; Rossi, R.M.; Fortunato, G. Enzyme functionalized electrospun chitosan mats for antimicrobial treatment. Carbohydr. Polym. 2018, 181, 551–559. [Google Scholar] [CrossRef]
- Ismail, A.R.; Baek, K.-H. Lipase immobilization with support materials, preparation techniques, and applications: Present and future aspects. Int. J. Biol. Macromol. 2020, 163, 1624–1639. [Google Scholar] [CrossRef]
- Srivastava, B.; Singh, H.; Khatri, M.; Singh, G.; Arya, S.K. Immobilization of keratinase on chitosan grafted-β-cyclodextrin for the improvement of the enzyme properties and application of free keratinase in the textile industry. Int. J. Biol. Macromol. 2020, 165, 1099–1110. [Google Scholar] [CrossRef]
- de Oliveira, R.L.; da Silva, M.F.; da Silva, S.P.; de Araújo, A.C.V.; Cavalcanti, J.V.F.L.; Converti, A.; Porto, T.S. Fructo-oligosaccharides production by an Aspergillus aculeatus commercial enzyme preparation with fructosyltransferase activity covalently immobilized on Fe3O4–chitosan-magnetic nanoparticles. Int. J. Biol. Macromol. 2020, 150, 922–929. [Google Scholar] [CrossRef] [PubMed]
- Soares, A.M.B.F.; Gonçalves, L.M.O.; Ferreira, R.D.S.; de Souza, J.M.; Fangueiro, R.; Alves, M.M.M.; Carvalho, F.A.A.; Mendes, A.N.; Cantanhêde, W. Immobilization of papain enzyme on a hybrid support containing zinc oxide nanoparticles and chitosan for clinical applications. Carbohydr. Polym. 2020, 243, 116498. [Google Scholar] [CrossRef] [PubMed]
- Tavernini, L.; Ottone, C.; Illanes, A.; Wilson, L. Entrapment of enzyme aggregates in chitosan beads for aroma release in white wines. Int. J. Biol. Macromol. 2020, 154, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Duru Kamaci, U.; Peksel, A. Fabrication of PVA-chitosan-based nanofibers for phytase immobilization to enhance enzymatic activity. Int. J. Biol. Macromol. 2020, 164, 3315–3322. [Google Scholar] [CrossRef]
- Haghju, S.; Bari, M.R.; Khaled-Abad, M.A. Affecting parameters on fabrication of β-d-galactosidase immobilized chitosan/poly (vinyl alcohol) electrospun nanofibers. Carbohydr. Polym. 2018, 200, 137–143. [Google Scholar] [CrossRef]
- Kutlu, N.; İspirli Doğaç, Y.; Deveci, İ.; Teke, M. Urease immobilized electrospun PVA/chitosan nanofibers with improved stability and reusability characteristics: An application for removal of urea from artificial blood serum. Prep. Biochem. Biotechnol. 2020, 50, 425–437. [Google Scholar] [CrossRef]
- Park, J.-M.; Kim, M.; Park, H.-S.; Jang, A.; Min, J.; Kim, Y.-H. Immobilization of lysozyme-CLEA onto electrospun chitosan nanofiber for effective antibacterial applications. Int. J. Biol. Macromol. 2013, 54, 37–43. [Google Scholar] [CrossRef]
- Jhuang, J.-R.; Lou, S.-N.; Lin, S.-B.; Chen, S.H.; Chen, L.-C.; Chen, H.-H. Immobilizing laccase on electrospun chitosan fiber to prepare time-temperature indicator for food quality monitoring. Innov. Food Sci. Emerg. Technol. 2020, 63, 102370. [Google Scholar] [CrossRef]
- Srbová, J.; Slováková, M.; Křípalová, Z.; Žárská, M.; Špačková, M.; Stránská, D.; Bílková, Z. Covalent biofunctionalization of chitosan nanofibers with trypsin for high enzyme stability. React. Funct. Polym. 2016, 104, 38–44. [Google Scholar] [CrossRef]
- Maryšková, M.; Ardao, I.; García-González, C.A.; Martinová, L.; Rotková, J.; Ševců, A. Polyamide 6/chitosan nanofibers as support for the immobilization of Trametes versicolor laccase for the elimination of endocrine disrupting chemicals. Enzym. Microb. Technol. 2016, 89, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Demirkan, E.; Avci, T.; Aykut, Y. Protease immobilization on cellulose monoacetate/chitosan-blended nanofibers. J. Ind. Text. 2017, 47, 2092–2111. [Google Scholar] [CrossRef]
- Teepoo, S.; Dawan, P.; Barnthip, N. Electrospun Chitosan-Gelatin Biopolymer Composite Nanofibers for Horseradish Peroxidase Immobilization in a Hydrogen Peroxide Biosensor. Biosensors 2017, 7, 47. [Google Scholar] [CrossRef] [PubMed]
- Radmansouri, M.; Bahmani, E.; Sarikhani, E.; Rahmani, K.; Sharifianjazi, F.; Irani, M. Doxorubicin hydrochloride-Loaded electrospun chitosan/cobalt ferrite/titanium oxide nanofibers for hyperthermic tumor cell treatment and controlled drug release. Int. J. Biol. Macromol. 2018, 116, 378–384. [Google Scholar] [CrossRef]
- Lu, L.; Cao, X.; Shen, Z.; Li, L.; Huo, J.; Chen, W.; Liu, C.; Liu, H. Electrospun nitrogen-doped carbon nanofibers for electrocatalysis. Sustain. Mater. Technol. 2020, 26, e00221. [Google Scholar] [CrossRef]
- Jouybari, M.H.; Hosseini, S.; Mahboobnia, K.; Boloursaz, L.A.; Moradi, M.; Irani, M. Simultaneous controlled release of 5-FU, DOX and PTX from chitosan/PLA/5-FU/g-C3N4-DOX/g-C3N4-PTX triaxial nanofibers for breast cancer treatment in vitro. Colloids Surf. B Biointerfaces 2019, 179, 495–504. [Google Scholar] [CrossRef]
- Balan, P.; Indrakumar, J.; Murali, P.; Korrapati, P.S. Bi-faceted delivery of phytochemicals through chitosan nanoparticles impregnated nanofibers for cancer therapeutics. Int. J. Biol. Macromol. 2020, 142, 201–211. [Google Scholar] [CrossRef]
- Ardeshirzadeh, B.; Anaraki, N.A.; Irani, M.; Rad, L.R.; Shamshiri, S. Controlled release of doxorubicin from electrospun PEO/chitosan/graphene oxide nanocomposite nanofibrous scaffolds. Mater. Sci. Eng. C 2015, 48, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Rostami, M.; Ghorbani, M.; Aman Mohammadi, M.; Delavar, M.; Tabibiazar, M.; Ramezani, S. Development of resveratrol loaded chitosan-gellan nanofiber as a novel gastrointestinal delivery system. Int. J. Biol. Macromol. 2019, 135, 698–705. [Google Scholar] [CrossRef]
- Yan, E.; Cao, M.; Wang, Y.; Hao, X.; Pei, S.; Gao, J.; Wang, Y.; Zhang, Z.; Zhang, D. Gold nanorods contained polyvinyl alcohol/chitosan nanofiber matrix for cell imaging and drug delivery. Mater. Sci. Eng. C 2016, 58, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, U.; Goyal, A.K.; Rath, G. Development and characterization of the cisplatin loaded nanofibers for the treatment of cervical cancer. Mater. Sci. Eng. C 2017, 75, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Liu, Y.; Peng, C.; Fang, D.; He, B.; Nie, J. Paclitaxel loaded electrospun porous nanofibers as mat potential application for chemotherapy against prostate cancer. Carbohydr. Polym. 2011, 86, 505–512. [Google Scholar] [CrossRef]
- Samadi, S.; Moradkhani, M.; Beheshti, H.; Irani, M.; Aliabadi, M. Fabrication of chitosan/poly(lactic acid)/graphene oxide/TiO2 composite nanofibrous scaffolds for sustained delivery of doxorubicin and treatment of lung cancer. Int. J. Biol. Macromol. 2018, 110, 416–424. [Google Scholar] [CrossRef]
- Sedghi, R.; Shaabani, A.; Mohammadi, Z.; Samadi, F.Y.; Isaei, E. Biocompatible electrospinning chitosan nanofibers: A novel delivery system with superior local cancer therapy. Carbohydr. Polym. 2017, 159, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Irani, M.; Mir Mohamad Sadeghi, G.; Haririan, I. A novel biocompatible drug delivery system of chitosan/temozolomide nanoparticles loaded PCL-PU nanofibers for sustained delivery of temozolomide. Int. J. Biol. Macromol. 2017, 97, 744–751. [Google Scholar] [CrossRef]
- Zhou, X.; Chen, L.; Wang, W.; Jia, Y.; Chang, A.; Mo, X.; Wang, H.; He, C. Electrospun nanofibers incorporating self-decomposable silica nanoparticles as carriers for controlled delivery of anticancer drug. RSC Adv. 2015, 5, 65897–65904. [Google Scholar] [CrossRef]
- Zhao, J.; Zhu, Y.; Ye, C.; Chen, Y.; Wang, S.; Zou, D.; Li, Z. Photothermal transforming agent and chemotherapeutic co-loaded electrospun nanofibers for tumor treatment. Int. J. Nanomed. 2019, 3893–3909. [Google Scholar] [CrossRef] [PubMed]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Bio-inspired synthesis of chitosan/copper oxide nanocomposite using rutin and their anti-proliferative activity in human lung cancer cells. Int. J. Biol. Macromol. 2019, 141, 476–483. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Reda, M.M.; Klingner, A. Preparation and characterization of green carboxymethylchitosan (CMCS) – Polyvinyl alcohol (PVA) electrospun nanofibers containing gold nanoparticles (AuNPs) and its potential use as biomaterials. Int. J. Biol. Macromol. 2020, 151, 821–829. [Google Scholar] [CrossRef]
- Farhoodi, M. Nanocomposite Materials for Food Packaging Applications: Characterization and Safety Evaluation. Food Eng. Rev. 2016, 8, 35–51. [Google Scholar] [CrossRef]
- Mousavi Khaneghah, A.; Hashemi, S.M.B.; Limbo, S. Antimicrobial agents and packaging systems in antimicrobial active food packaging: An overview of approaches and interactions. Food Bioprod. Process. 2018, 111, 1–19. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Calderón, L.; Harris, R.; Cordoba-Diaz, M.; Elorza, M.; Elorza, B.; Lenoir, J.; Adriaens, E.; Remon, J.P.; Heras, A.; Cordoba-Diaz, D. Nano and microparticulate chitosan-based systems for antiviral topical delivery. Eur. J. Pharm. Sci. 2013, 48, 216–222. [Google Scholar] [CrossRef]
- Helander, I.M.; Nurmiaho-Lassila, E.L.; Ahvenainen, R.; Rhoades, J.; Roller, S. Chitosan disrupts the barrier properties of the outer membrane of Gram-negative bacteria. Int. J. Food Microbiol. 2001, 71, 235–244. [Google Scholar] [CrossRef]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal Activity of Chitosan Nanoparticles and Correlation with Their Physical Properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef]
- Jaime, M.D.L.A.; Lopez-Llorca, L.V.; Conesa, A.; Lee, A.Y.; Proctor, M.; Heisler, L.E.; Gebbia, M.; Giaever, G.; Westwood, J.T.; Nislow, C. Identification of yeast genes that confer resistance to chitosan oligosaccharide (COS) using chemogenomics. BMC Genom. 2012, 13, 267. [Google Scholar] [CrossRef] [PubMed]
- Abdul Khalil, H.P.S.; Banerjee, A.; Saurabh, C.K.; Tye, Y.Y.; Suriani, A.B.; Mohamed, A.; Karim, A.A.; Rizal, S.; Paridah, M.T. Biodegradable Films for Fruits and Vegetables Packaging Application: Preparation and Properties. Food Eng. Rev. 2018, 10, 139–153. [Google Scholar] [CrossRef]
- Arkoun, M.; Daigle, F.; Heuzey, M.-C.; Ajji, A. Mechanism of action of electrospun chitosan-based nanofibers against meat spoilage and pathogenic bacteria. Molecules 2017, 22, 585. [Google Scholar] [CrossRef]
- Cui, H.; Bai, M.; Li, C.; Liu, R.; Lin, L. Fabrication of chitosan nanofibers containing tea tree oil liposomes against Salmonella spp. in chicken. LWT 2018, 96, 671–678. [Google Scholar] [CrossRef]
- Ceylan, Z.; Unal Sengor, G.F.; Yilmaz, M.T. Nanoencapsulation of liquid smoke/thymol combination in chitosan nanofibers to delay microbiological spoilage of sea bass (Dicentrarchus labrax) fillets. J. Food Eng. 2018, 229, 43–49. [Google Scholar] [CrossRef]
- He, X.; Hwang, H.-M. Nanotechnology in food science: Functionality, applicability, and safety assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Cook, S.L.; Bull, S.P.; Methven, L.; Parker, J.K.; Khutoryanskiy, V.V. Mucoadhesion: A food perspective. Food Hydrocoll. 2017, 72, 281–296. [Google Scholar] [CrossRef]
- Martins, J.T.; Ramos, Ó.L.; Pinheiro, A.C.; Bourbon, A.I.; Silva, H.D.; Rivera, M.C.; Cerqueira, M.A.; Pastrana, L.; Malcata, F.X.; González-Fernández, Á.; et al. Edible Bio-Based Nanostructures: Delivery, Absorption and Potential Toxicity. Food Eng. Rev. 2015, 7, 491–513. [Google Scholar] [CrossRef]
- Ali, A.; Ahmed, S. A review on chitosan and its nanocomposites in drug delivery. Int. J. Biol. Macromol. 2018, 109, 273–286. [Google Scholar] [CrossRef]
- Darbasizadeh, B.; Motasadizadeh, H.; Foroughi-Nia, B.; Farhadnejad, H. Tripolyphosphate-crosslinked chitosan/poly (ethylene oxide) electrospun nanofibrous mats as a floating gastro-retentive delivery system for ranitidine hydrochloride. J. Pharm. Biomed. Anal. 2018, 153, 63–75. [Google Scholar] [CrossRef]
- Hasani, S.; Ojagh, S.M.; Ghorbani, M. Nanoencapsulation of lemon essential oil in Chitosan-Hicap system. Part 1: Study on its physical and structural characteristics. Int. J. Biol. Macromol. 2018, 115, 143–151. [Google Scholar] [CrossRef]
- Omwenga, E.O.; Hensel, A.; Shitandi, A.; Goycoolea, F.M. Chitosan nanoencapsulation of flavonoids enhances their quorum sensing and biofilm formation inhibitory activities against an E.coli Top 10 biosensor. Colloids Surf. B Biointerfaces 2018, 164, 125–133. [Google Scholar] [CrossRef]
- Shekarforoush, E.; Ajalloueian, F.; Zeng, G.; Mendes, A.C.; Chronakis, I.S. Electrospun xanthan gum-chitosan nanofibers as delivery carrier of hydrophobic bioactives. Mater. Lett. 2018, 228, 322–326. [Google Scholar] [CrossRef]
- Kuddus, M. Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Wang, Z.-G.; Wan, L.-S.; Liu, Z.-M.; Huang, X.-J.; Xu, Z.-K. Enzyme immobilization on electrospun polymer nanofibers: An overview. J. Mol. Catal. B Enzym. 2009, 56, 189–195. [Google Scholar] [CrossRef]
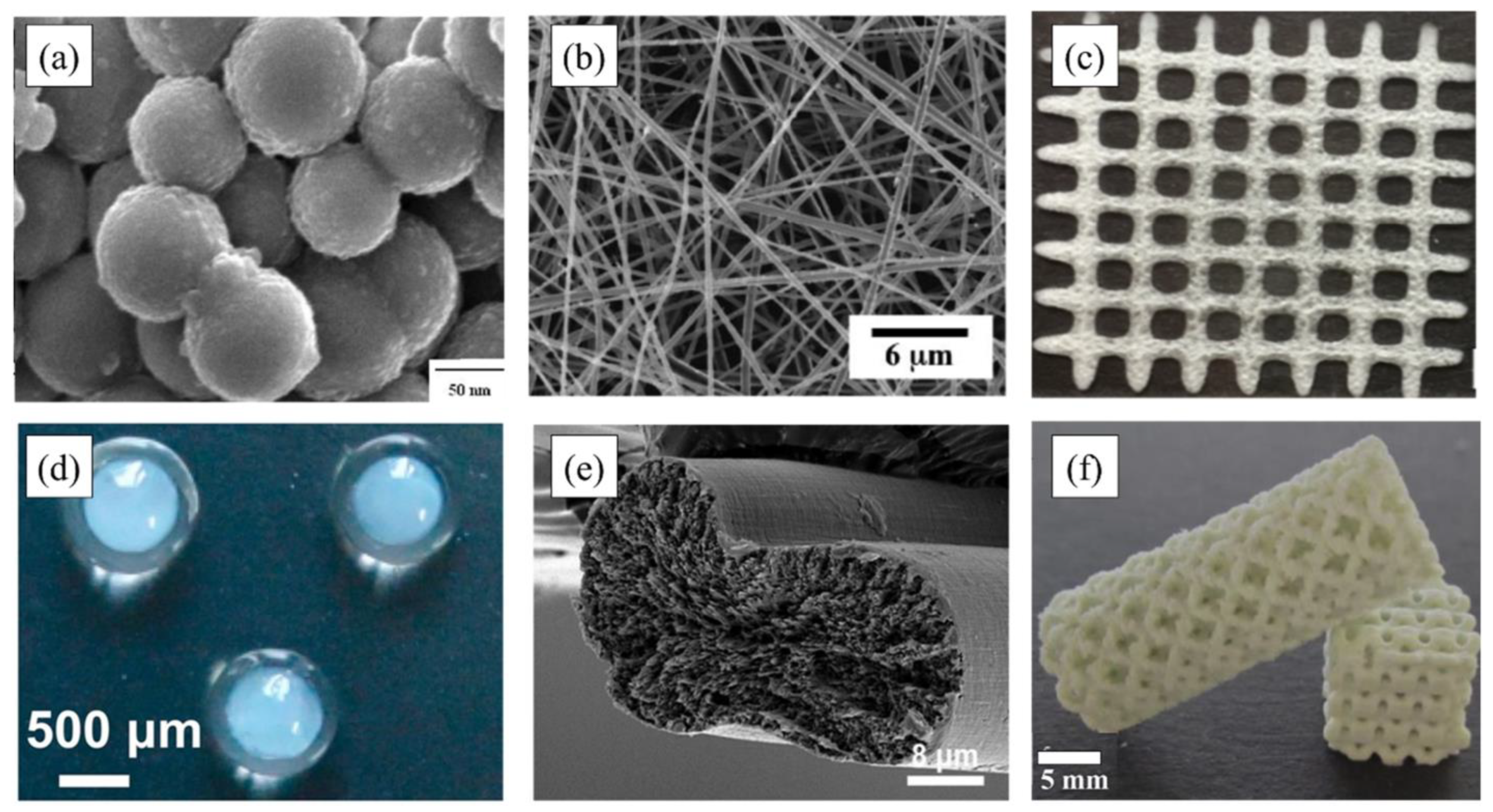
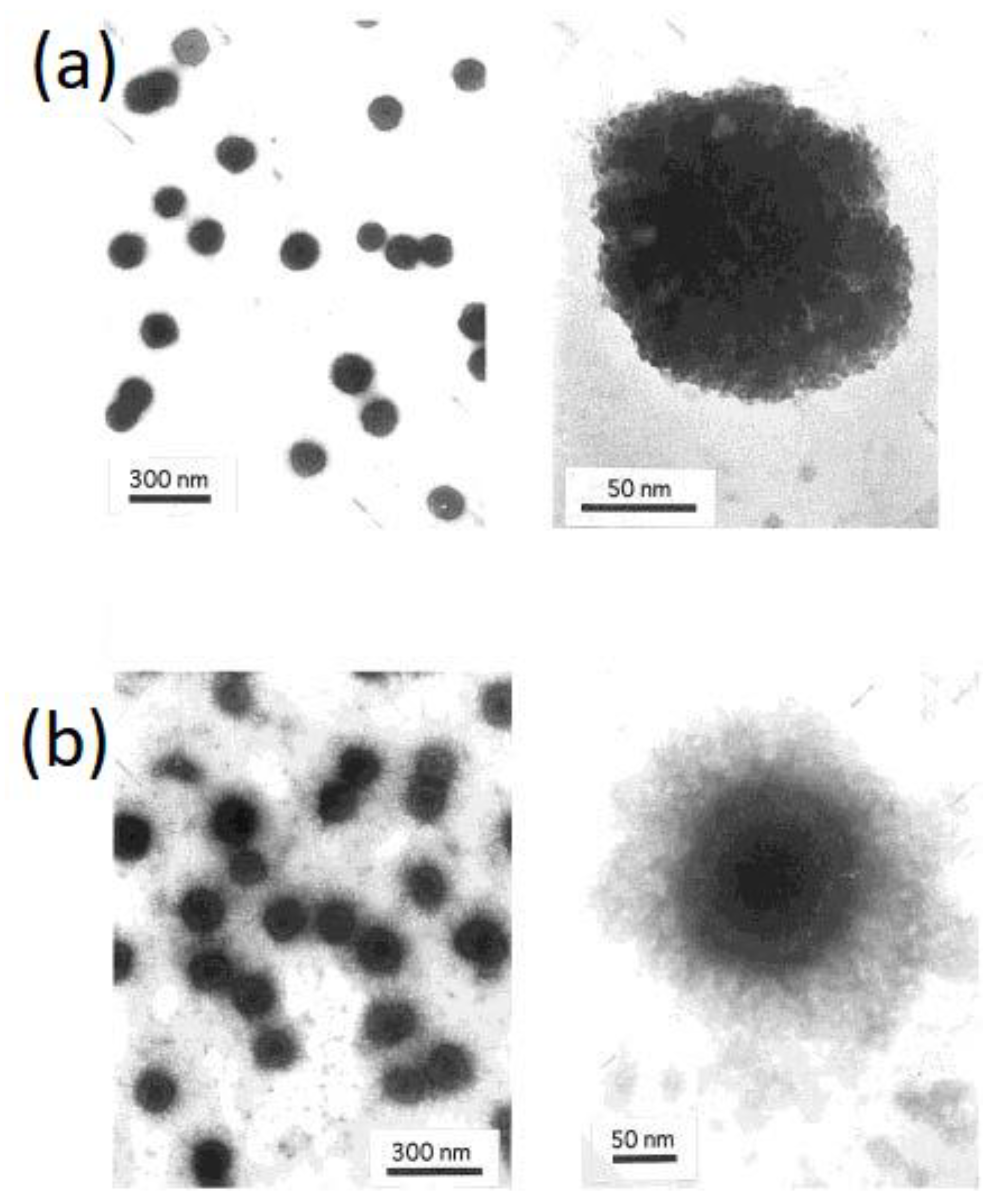
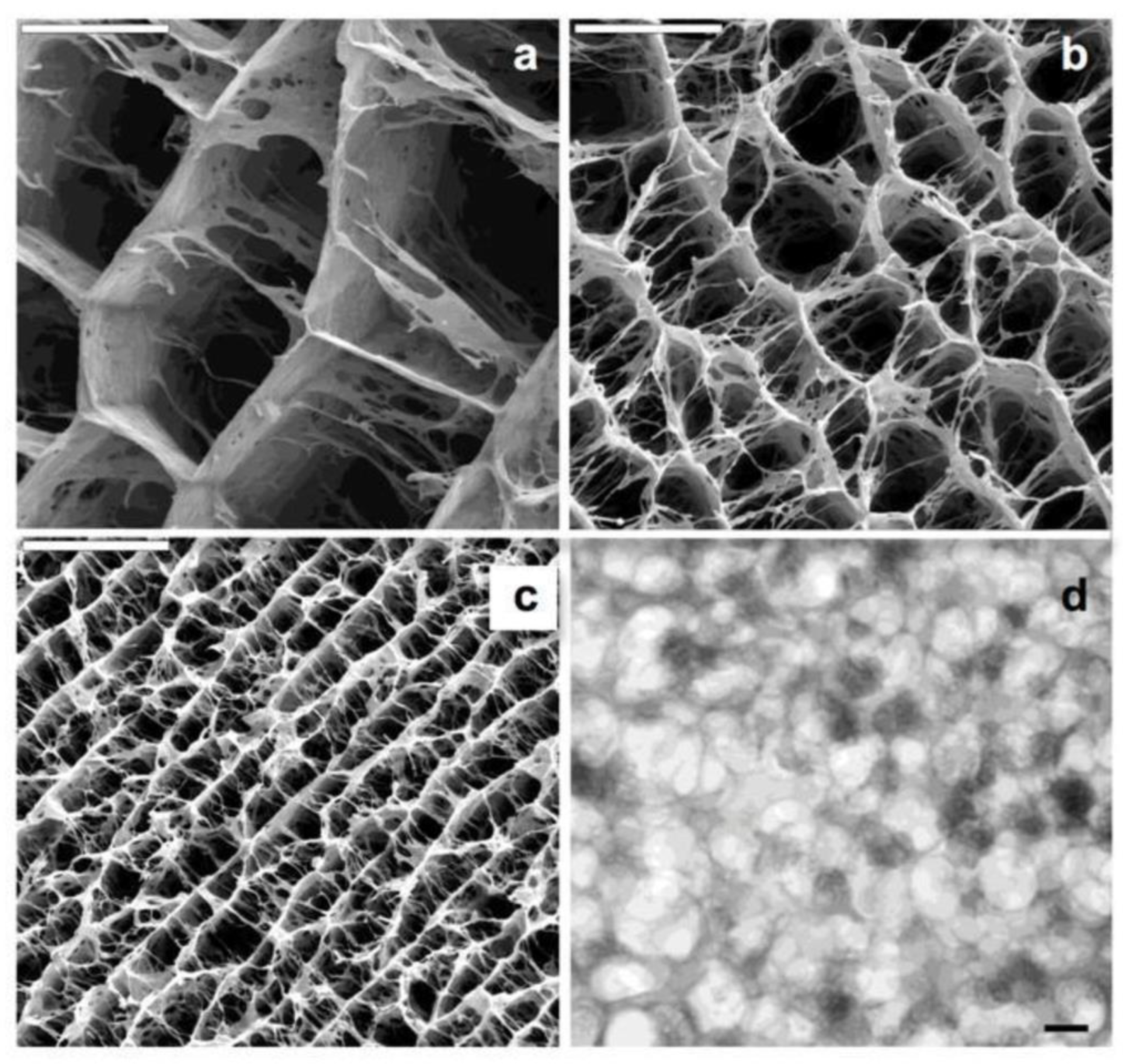
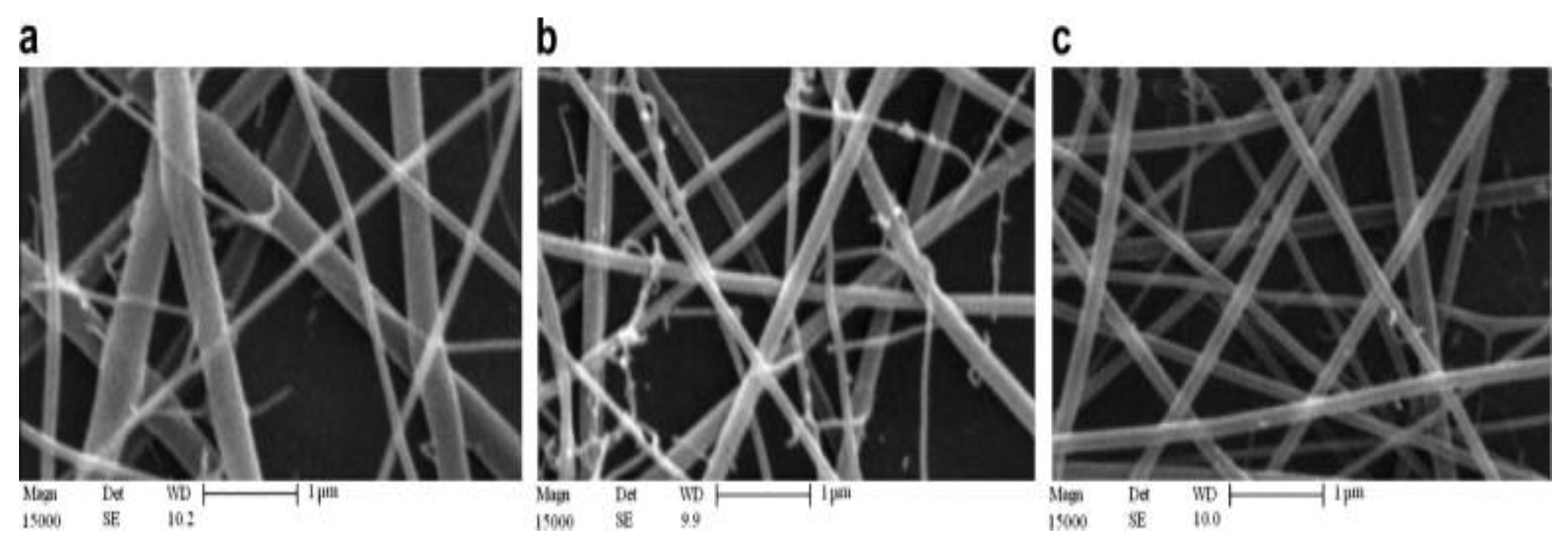
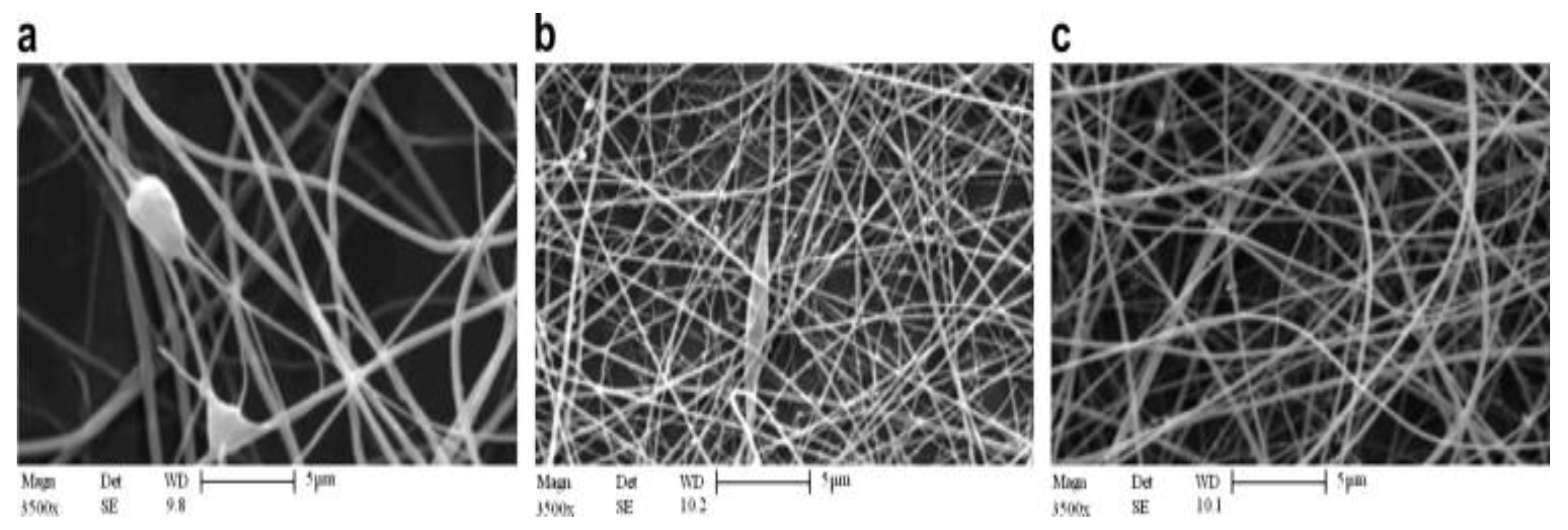
| Polymer | Protein Loaded | Size (nm) | Potential (mV) | Theoretical Lading (%) | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| PLA | Tetanus Toxoid | 192 ± 12 | −47.9 ± 1.5 | 1 | 36.7 ± 0.3 |
| PEG-PLA | Tetanus Toxoid | 196 ± 20 | −23.9 ± 1.2 | 1 | 31.1 ± 0.5 |
| CS-PLGA | Tetanus Toxoid | 500 ± 29 | −21.8 ± 1.1 | 1 | 90.0 ± 3.6 |
| CS | Tetanus Toxoid | 354 ± 27 | −37.1 ± 5.9 | 10 | 55.1 ± 3.4 |
| CS | Insulin | 337 ± 14 | −36.9 ± 0.3 | 40 | 94.7 ± 2.1 |
| Chitosan Film/Coated | Food | References |
|---|---|---|
| Chitosan | Citrus fruit | [70,71] |
| Chitosan | Logan fruit | [72] |
| Chitosan | Green coffee beans | [73] |
| Chitosan | Frozen salmon | [74] |
| Chitosan | Tankan citrus fruit | [75] |
| Chitosan | Apples | [76,77] |
| Chitosan | Mushroom | [78] |
| Chitosan | Mangoes | [70,71,79] |
| Chitosan | Litchi fruit | [80,81] |
| Chitosan | Salmon fillets | [82] |
| Chitosan | Tomatoes | [83] |
| Chitosan | Strawberries | [83] |
| Chitosan | Peach fruit | [72] |
| Chitosan | Fresh-cut Chinese water chestnut L.T. | [84] |
| Chitosan | Silver carp | [85] |
| Composite/Properties | Stimulatory Effects | References |
|---|---|---|
| Environmental purposes | ||
| Flocculation | Removes a variety of contaminants from wastewater in an efficient manner | [101] |
| Metals and organic compounds adsorption | Removes pathogens, radioactive materials, heavy metals, colors, organic chemicals, and inorganic nutrients (nitrates and phosphates) | [101] |
| Agricultural purposes | ||
| Biocontrol agent | Safe alternative to the use of pesticides and agrochemicals as a biocontrol material against many pathogenic microorganisms | [102] |
| Enhance crop production |
| [103] |
| Aquafeed additives | Positively impacts the growth, digestive enzymes, body composition, intestinal bacterial count, immunological response, and hematological and liver health of commercial freshwater fish | [104,105] |
| Biomedical purposes | ||
| Chitosan microspheres |
| [106] |
| Chitosan mesh membrane | Decreases wound healing time and increases the recovery of the granular layer | [107] |
| Chitosan collagen blend membrane | Increases the antibacterial activity against E. coli and S. aureus and decreases the excessive dehydration of the wound | [108] |
| Alginate/carboxymethyl chitosan blend fibers | Increased water-retention and increased antibacterial activity against S. aureus | [109] |
| Composite nanofibrous membranes (NFM) of collagen and chitosan | Increased wound-healing and increased tissue regeneration | [110] |
| Electro spun chitosan fiber with polyethylene oxide | Used effectively as surface layers on the wound site in periodontal disease | [111] |
| Chitosan membranes loaded with Tetracycline hydrochloride or silver sulfadiazine | Increased wettability, decreased swelling rate, water vapor permeability, and tensile strength, and increased antimicrobial activity against E. coli and S. aureus | [112] |
| Chitosan titanium dioxide composite membranes | Increased antimicrobial activity against S. aureus, decreased oxidative stress and apoptosis of fibroblast cell sand, increased proliferation in L929 fibroblast cells | [113] |
| Chitosan nano silver dressing | Increased wound-healing using the non-invasive dressing | [114] |
| Chitosan sponges loaded with norfloxacin | The dressing can conduct the role of normal skin and the antibiotic release is swelling-controlled | [115] |
| Chitosan-gelatin sponge | Increased antimicrobial activity against E. coli K88 over penicillin Increased antimicrobial activity against S. aureus over cefradine | [116] |
| Photo cross linkable chitosan hydrogel containing fibroblast growth factor-2 | Increased wound healing in diabetic and normal mice | [117] |
| Carboxymethyl chitosan alginate hydrogel | Increases Bactericidal properties toward S. aureus and E. coli, and antibiotic continues to be released from the hydrogel | [118] |
| Composition | Component’s Ratio | Enzyme | References |
|---|---|---|---|
| Chitosan/poly (vinyl alcohol) composite in 0.1 M Sodium acetate | 1:5,4 | Phytase by entrapment | [225] |
| Chitosan/poly (vinyl alcohol) composite in 1% acetic acid | 1:1 | B-d-galactosidase by entrapment | [226] |
| Chitosan/poly (vinyl alcohol) composite in water | 1:6 | Urease by Cross-linking | [227] |
| Chitosan/poly (vinyl alcohol) composite in 0.5% acetic acid | 1:10 | Lysozyme by Cross-linking | [228] |
| Chitosan/poly (vinyl alcohol) composite in 2% acetic acid | 1:4 | Laccase by Cross-linking | [229] |
| Chitosan/polyethylene oxide composite in 1% acetic acid | 22:1 | Trypsin by Adsorption/Covalent | [230] |
| Chitosan/poly (ethylene oxide) composite in 90% acetic acid | 95:5 | Glucose oxidase by Cross-linking | [219] |
| Chitosan/polyamide 6 composite in Formic acid and Acetic acid (2:1 v/v) | 1:9 | Laccase by Cross-linking | [231] |
| Chitosan/Cellulose monoacetate composite in acetone 99% | 1:5, 2:5, 1:1, 7:5 | Protease by Adsorption/Cross linking | [232] |
| Chitosan/gelatin composite in 60% acetic acid | 4:6 | Peroxidase by Cross-linking | [233] |
| Composition | Anticancer Drug | Cancer Type | References |
|---|---|---|---|
| Chitosan | Fe3O4/under magnetic field | HFL1 | [234] |
| Chitosan | Fe2+/under magnetic field | HFL1 | [234] |
| Chitosan | Glutaraldehyde/under magnetic field | HFL1 | [234] |
| Chitosan/poly(ε-caprolactone) composite | 5-Fluorouracil | B16F10 | [235] |
| Chitosan/polyvinyl alcohol-g-C3N4-g-C3N4 composite | 5-Fluorouracil, Doxorubicin, Paclitaxel | MCF-7 | [236] |
| Chitosan/polycaprolactone composite | Resveratrol, ferulic acid | HaCat, A431 | [237] |
| Chitosan/polyethylene oxide composite | Berberine | HeLa, BT474, MCF-7, MDA-MB-468 | [237] |
| Polyethylene oxide/chitosan/graphene oxide composite | Doxorubicin | A549 | [238] |
| Chitosan/gelatin composite | Resveratrol | HT29 | [239] |
| Polyvinyl alcohol/chitosan/Au composite | Doxorubicin | SKOV3 | [240] |
| Polycaprolactone/chitosan composite | Cisplatin | Erlich ascites carcinoma | [241] |
| Chitosan/polyethylene oxide/hyaluronic acid composite | Paclitaxel | DU145 | [242] |
| Chitosan/cobalt ferrite/TiO2 composite | Doxorubicin | B16F10 | [234] |
| Chitosan/poly (lactic acid)/TiO2/graphene oxide | Doxorubicin | A549 | [243] |
| Chitosan/PVA/graphene oxide/Si composite | Curcumin | MCF-7 | [244] |
| Poly (ε-caprolactone diol)/polyurethane/chitosan/Au TiO2 | Temozolomide | U-87 MG | [245] |
| Graphene oxide/chitosan composite | Curcumin | MCF-7, HepG2, L929 | [244] |
| Poly (lactic-co-glycolic acid)/chitosan/SiO2 composite | Doxorubicin | HeLa | [246] |
| Chitosan/polyvinyl alcohol/MoS2 composite | Doxorubicin | HT29, HT29 cell lines | [247] |
| Chitosan | Cupric oxide | A549 | [248] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahim, M.A.; Alhalafi, M.H.; Emam, E.-A.M.; Ibrahim, H.; Mosaad, R.M. A Review of Chitosan and Chitosan Nanofiber: Preparation, Characterization, and Its Potential Applications. Polymers 2023, 15, 2820. https://doi.org/10.3390/polym15132820
Ibrahim MA, Alhalafi MH, Emam E-AM, Ibrahim H, Mosaad RM. A Review of Chitosan and Chitosan Nanofiber: Preparation, Characterization, and Its Potential Applications. Polymers. 2023; 15(13):2820. https://doi.org/10.3390/polym15132820
Chicago/Turabian StyleIbrahim, Marwan A., Mona H. Alhalafi, El-Amir M. Emam, Hassan Ibrahim, and Rehab M. Mosaad. 2023. "A Review of Chitosan and Chitosan Nanofiber: Preparation, Characterization, and Its Potential Applications" Polymers 15, no. 13: 2820. https://doi.org/10.3390/polym15132820
APA StyleIbrahim, M. A., Alhalafi, M. H., Emam, E.-A. M., Ibrahim, H., & Mosaad, R. M. (2023). A Review of Chitosan and Chitosan Nanofiber: Preparation, Characterization, and Its Potential Applications. Polymers, 15(13), 2820. https://doi.org/10.3390/polym15132820







