Modification of Starches and Flours by Acetylation and Its Dual Modifications: A Review of Impact on Physicochemical Properties and Their Applications
Abstract
:1. Introduction
2. Applications of Acetylated Modified Starch/Flour
3. Acetylation Modification Process in Starch/Flour
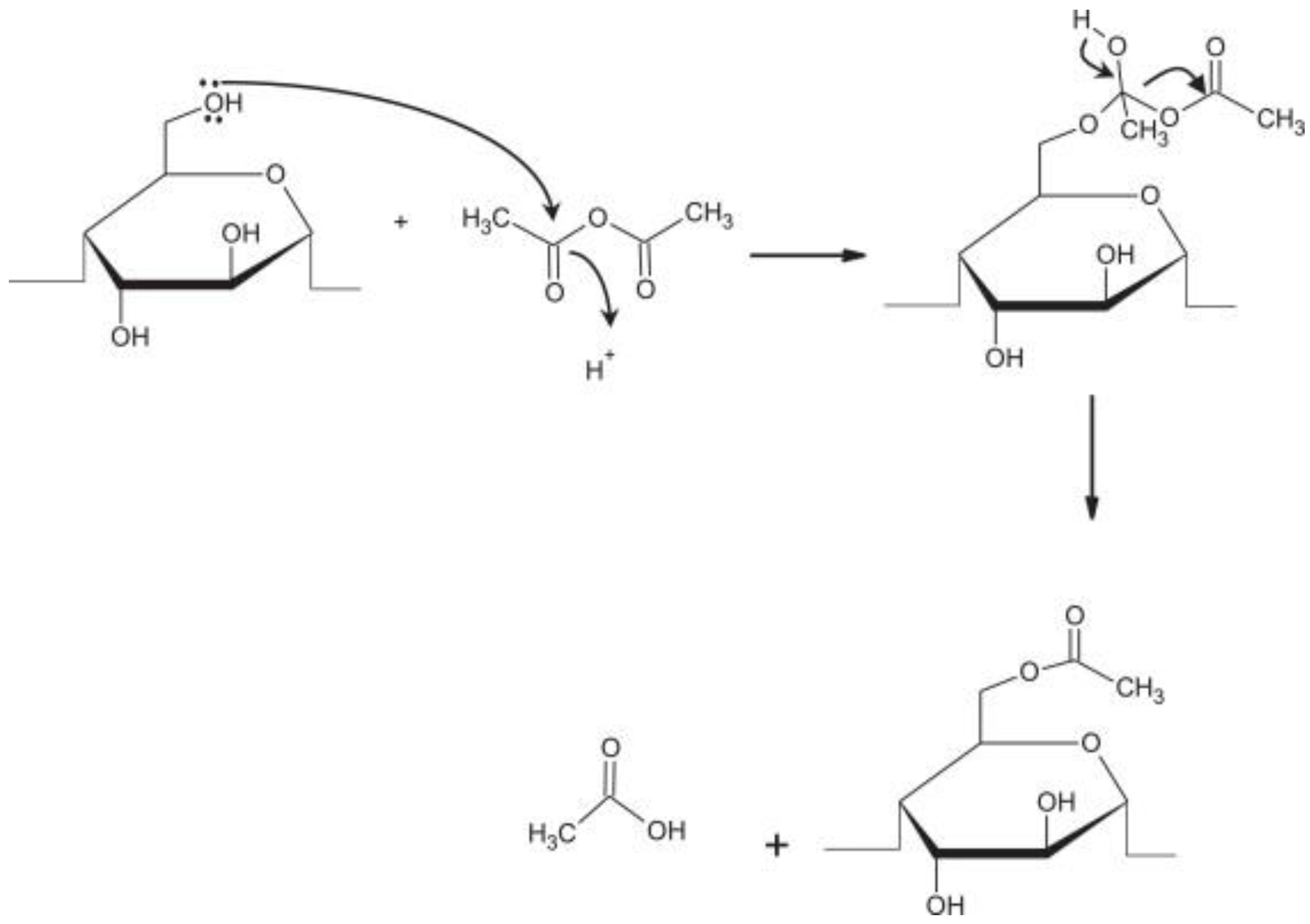
3.1. Mechanism of Acetylation
3.2. Effect of Acetylation Methods on Properties of Starch/Flour
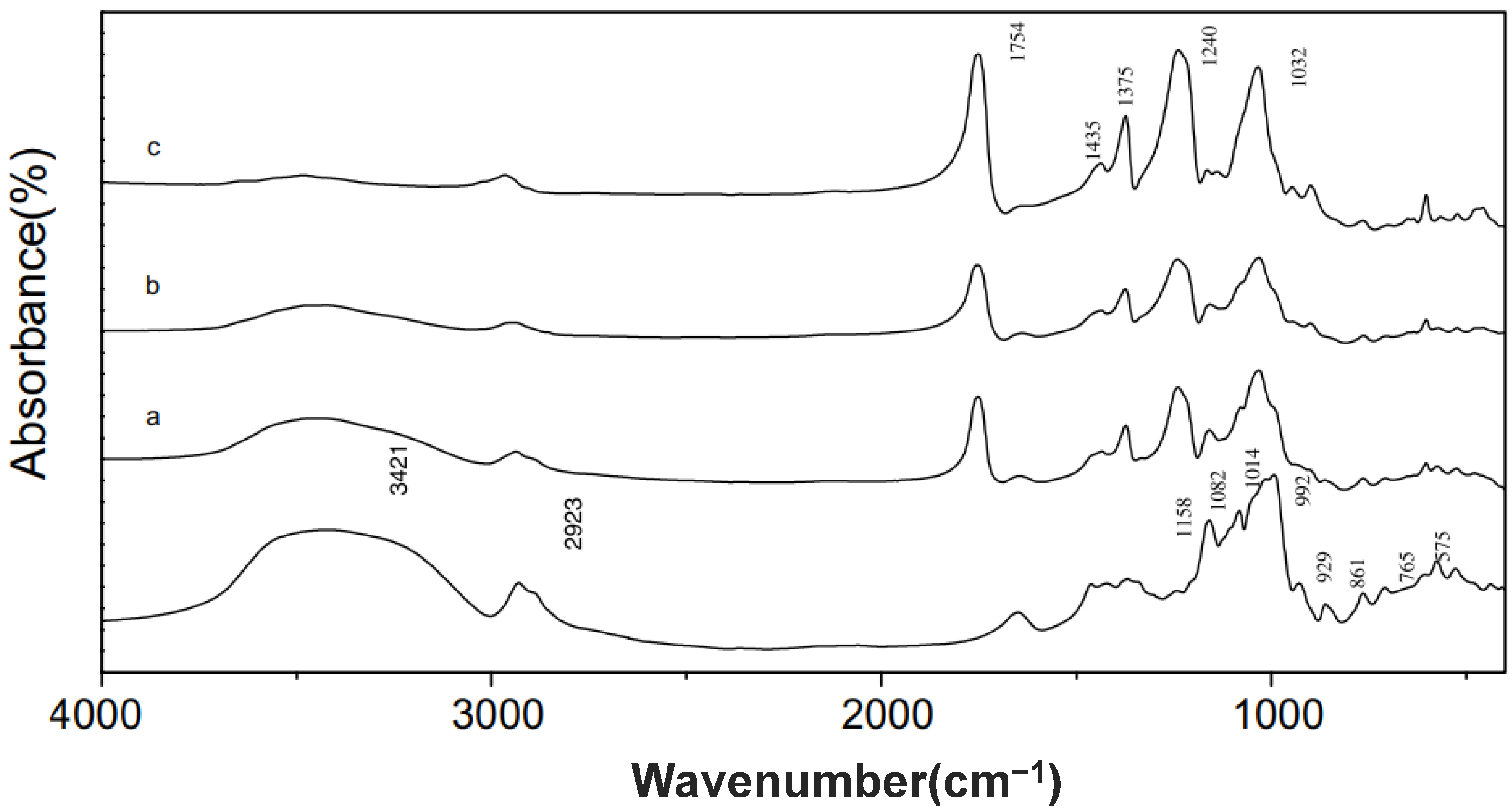
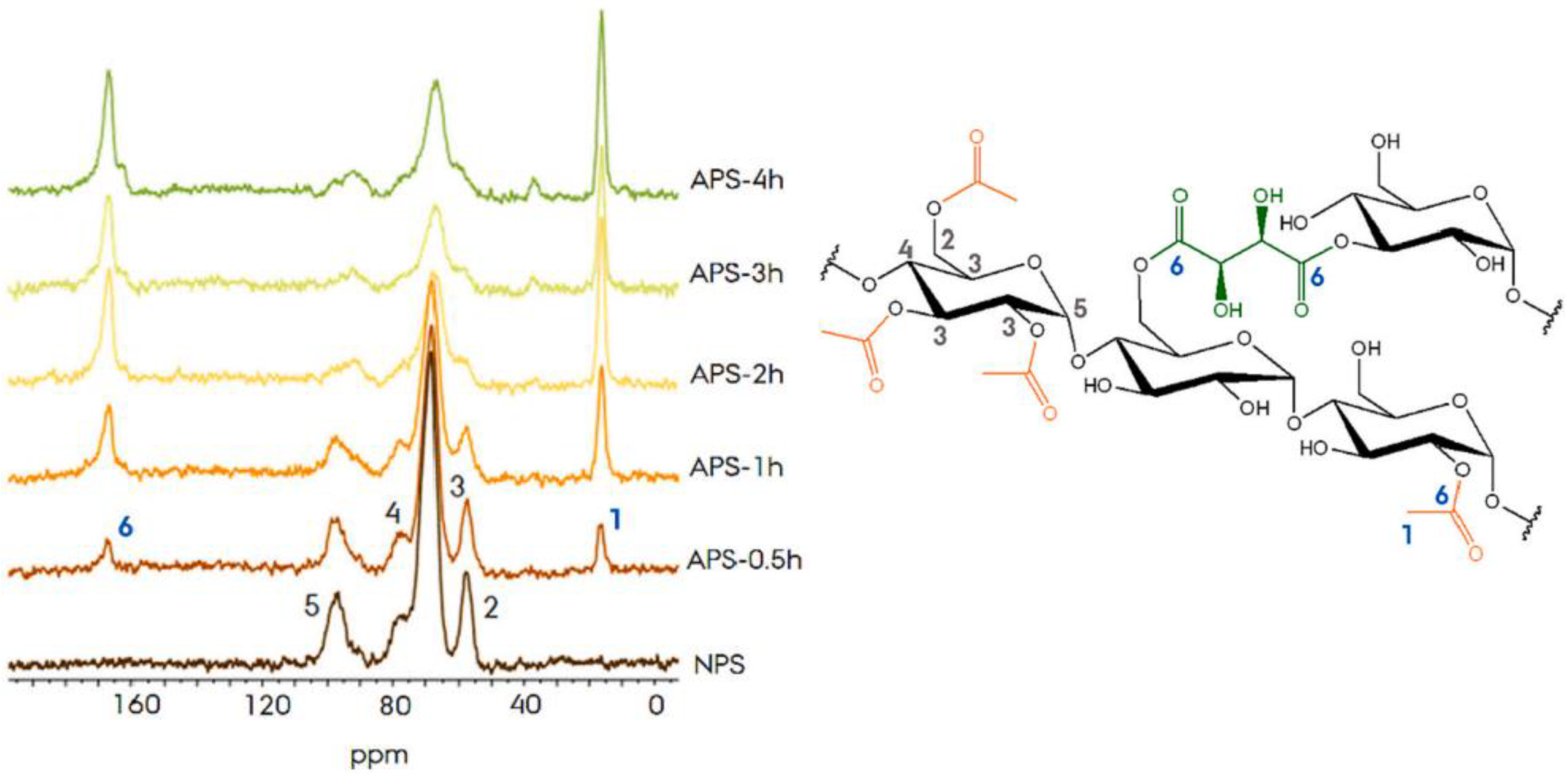
4. Characteristics of Acetylated Modified Starch/Flour
4.1. Functional Properties of Acetylated Modified Starch/Flour
4.2. Pasting Properties of Acetylated Modified Starch/Flour
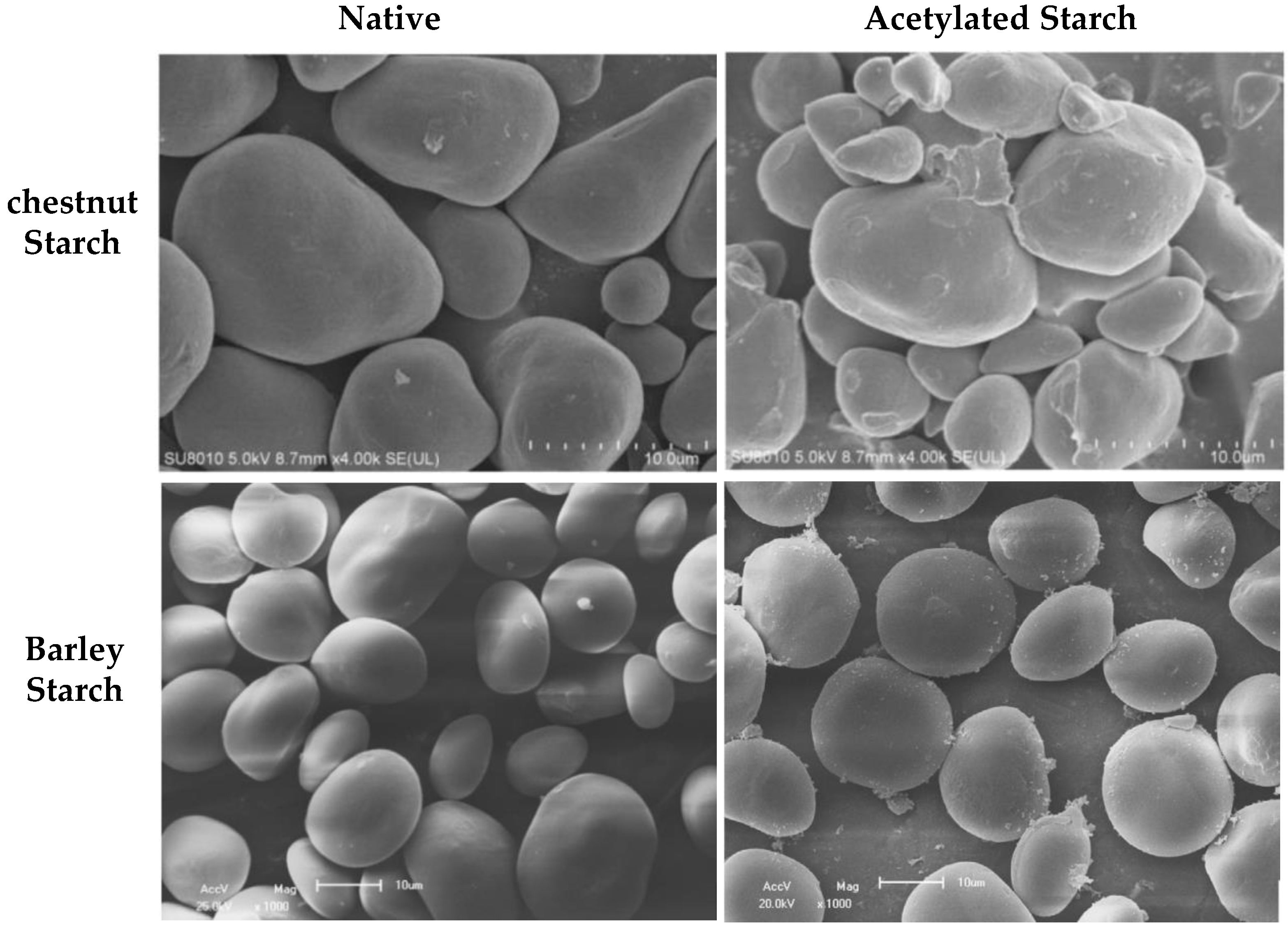
4.3. Starch Granule Morphology of Acetylated Modified Starch/Flour
4.4. Starch Crystallinity of Acetylated Modified Starch/Flour
4.5. Comparison of Acetylated Modified Starch/Flour with Other Modifications
5. Conclusions and Future Research
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fonseca, L.M.; El Halal, S.L.M.; Dias, A.R.G.; da Rosa Zavareze, E. Physical Modification of Starch by Heat-Moisture Treatment and Annealing and Their Applications: A Review. Carbohydr. Polym. 2021, 274, 118665. [Google Scholar] [CrossRef] [PubMed]
- Majzoobi, M.; Pesaran, Y.; Mesbahi, G.; Farahnaky, A. Evaluation of the Effects of Hydrothermal Treatment on Rice Flour and Its Related Starch. Int. J. Food Prop. 2016, 19, 2135–2145. [Google Scholar] [CrossRef]
- Majzoobi, M.; Farahnaky, A. Granular Cold-Water Swelling Starch; Properties, Preparation and Applications, a Review. Food Hydrocoll. 2021, 111, 106393. [Google Scholar] [CrossRef]
- Subroto, E.; Sitha, N.; Filianty, F.; Indiarto, R.; Sukri, N. Freeze Moisture Treatment and Ozonation of Adlay Starch (Coix Lacryma-Jobi): Effect on Functional, Pasting, and Physicochemical Properties. Polymers 2022, 14, 3854. [Google Scholar] [CrossRef]
- Chang, Q.; Zheng, B.; Zhang, Y.; Zeng, H. A Comprehensive Review of the Factors Influencing the Formation of Retrograded Starch. Int. J. Biol. Macromol. 2021, 186, 163–173. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Compr. Rev. Food Sci. Food Saf. 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Liu, X.; Chao, C.; Yu, J.; Copeland, L.; Wang, S. Mechanistic Studies of Starch Retrogradation and Its Effects on Starch Gel Properties. Food Hydrocoll. 2021, 120, 106914. [Google Scholar] [CrossRef]
- Chung, H.J.; Liu, Q.; Hoover, R. Effect of Single and Dual Hydrothermal Treatments on the Crystalline Structure, Thermal Properties, and Nutritional Fractions of Pea, Lentil, and Navy Bean Starches. Food Res. Int. 2010, 43, 501–508. [Google Scholar] [CrossRef]
- Colussi, R.; Pinto, V.Z.; El Halal, S.L.M.; Vanier, N.L.; Villanova, F.A.; Marques e Silva, R.; da Rosa Zavareze, E.; Dias, A.R.G. Structural, Morphological, and Physicochemical Properties of Acetylated High-, Medium-, and Low-Amylose Rice Starches. Carbohydr. Polym. 2014, 103, 405–413. [Google Scholar] [CrossRef] [Green Version]
- Egodage, R. The Impact of Annealing, Acetylation, and Dual Modification on the Structure and Physicochemical Properties of Waxy Starches; Memorial University of Newfoundland: Corner Brook, NL, Canada, 2019. [Google Scholar]
- Shaari, S.; Samsudin, H.; Uthumporn, U. Effect of Acetylation Treatment on Surface Modified Tapioca Starches. Food Res. 2021, 5, 340–347. [Google Scholar] [CrossRef]
- Gagneten, M.; Cáceres, S.G.; Rodríguez Osuna, I.A.; Olaiz, N.M.; Schebor, C.; Leiva, G.E. Modification of Cassava Starch by Acetylation and Pulsed Electric Field Technology: Analysis of Physical and Functional Properties. Innov. Food Sci. Emerg. Technol. 2023, 85, 103344. [Google Scholar] [CrossRef]
- Abba, H.; Ibrahim, A.; Shallangwa, G.A.; Uba, S.; Dallatu, Y.A. Effect of Acetylation on Stability to Retrogradation of Starch Extracted from Wild Polynesian Arrowroot (Tacca Leontopetaloides (L.) Kuntze) for Utilization as Adhesive on Paper. J. Polym. 2014, 2014, 732174. [Google Scholar] [CrossRef]
- Liu, C.; Yan, H.; Liu, S.; Chang, X. Influence of Phosphorylation and Acetylation on Structural, Physicochemical and Functional Properties of Chestnut Starch. Polymers 2022, 14, 172. [Google Scholar] [CrossRef] [PubMed]
- Colussi, R.; El Halal, S.L.M.; Pinto, V.Z.; Bartz, J.; Gutkoski, L.C.; da Rosa Zavareze, E.; Dias, A.R.G. Acetylation of Rice Starch in an Aqueous Medium for Use in Food. LWT Food Sci. Technol. 2015, 62, 1076–1082. [Google Scholar] [CrossRef] [Green Version]
- Eshag Osman, M.F.; Mohamed, A.A.; Mohamed Ahmed, I.A.; Alamri, M.S.; Al Juhaimi, F.Y.; Hussain, S.; Ibraheem, M.A.; Qasem, A.A. Acetylated Corn Starch as a Fat Replacer: Effect on Physiochemical, Textural, and Sensory Attributes of Beef Patties during Frozen Storage. Food Chem. 2022, 388, 132988. [Google Scholar] [CrossRef]
- Miyazaki, M.; Maeda, T.; Morita, N. Gelatinization Properties and Bread Quality of Flours Substituted with Hydroxypropylated, Acetylated and Phosphorylated Cross-Linked Tapioca Starches. J. Appl. Glycosci. 2005, 52, 345–350. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Shi, C.; Zhu, J.; Li, Y.; Huang, Q. Structure of Starch-Fatty Acid Complexes Produced via Hydrothermal Treatment. Food Hydrocoll. 2019, 88, 58–67. [Google Scholar] [CrossRef]
- Pukkahuta, C.; Suwannawat, B.; Shobsngob, S.; Varavinit, S. Comparative Study of Pasting and Thermal Transition Characteristics of Osmotic Pressure and Heat-Moisture Treated Corn Starch. Carbohydr. Polym. 2008, 72, 527–536. [Google Scholar] [CrossRef]
- Subroto, E.; Indiarto, R.; Marta, H.; Shalihah, S. Effect of Heat Moisture Treatment on Functional and Pasting Properties of Potato. Food Res. 2019, 3, 469–476. [Google Scholar] [CrossRef]
- Mirmoghtadaie, L.; Kadivar, M.; Shahedi, M. Effects of Cross-Linking and Acetylation on Oat Starch Properties. Food Chem. 2009, 116, 709–713. [Google Scholar] [CrossRef]
- Ashogbon, A.O. Dual Modification of Various Starches: Synthesis, Properties and Applications. Food Chem. 2021, 342, 128325. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, A.; Corke, H. Effect of Hydroxypropylation and Alkaline Treatment in Hydroxypropylation on Some Structural and Physicochemical Properties of Heat-Moisture Treated Wheat, Potato and Waxy Maize Starches. Carbohydr. Polym. 2007, 68, 305–313. [Google Scholar] [CrossRef]
- Ačkar, Đ.; Babić, J.; Jozinović, A.; Miličević, B.; Jokić, S.; Miličević, R.; Rajič, M.; Šubarić, D. Starch Modification by Organic Acids and Their Derivatives: A Review. Molecules 2015, 20, 19554–19570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawaz, M.A.; Singh, T.K.; Tan, M.; Prakash, S.; Fukai, S.; Bhandari, B. Acetylation of Intact White Rice Grains to Alter the Physicochemical Properties. J. Cereal Sci. 2020, 92, 102928. [Google Scholar] [CrossRef]
- Zdybel, E.; Wilczak, A.; Kapelko-Żeberska, M.; Tomaszewska-Ciosk, E.; Gryszkin, A.; Gawrońska, A.; Zięba, T. Physicochemical Properties and Digestion Resistance of Acetylated Starch Obtained from Annealed Starch. Polymers 2021, 13, 4141. [Google Scholar] [CrossRef]
- Subroto, E.; Mahani; Indiarto, R.; Yarlina, V.P.; Izzati, A.N. A Mini Review of Physicochemical Properties of Starch and Flour by Using Hydrothermal Treatment. Polymers 2022, 14, 5447. [Google Scholar] [CrossRef]
- Zavareze, E.D.R.; Dias, A.R.G. Impact of Heat-Moisture Treatment and Annealing in Starches: A Review. Carbohydr. Polym. 2011, 83, 317–328. [Google Scholar] [CrossRef]
- Raina, C.S.; Singh, S.; Bawa, A.S.; Saxena, D.C. Some Characteristics of Acetylated, Cross-Linked and Dual Modified Indian Rice Starches. Eur. Food Res. Technol. 2006, 223, 561–570. [Google Scholar] [CrossRef]
- de Oliveira, N.R.; Fornaciari, B.; Mali, S.; Carvalho, G.M. Acetylated Starch-Based Nanoparticles: Synthesis, Characterization, and Studies of Interaction With Antioxidants. Starch-Stärke 2018, 70, 1700170. [Google Scholar] [CrossRef]
- Xiao, H.; Yang, F.; Lin, Q.; Zhang, Q.; Tang, W.; Zhang, L.; Xu, D.; Liu, G.-Q. Preparation and Properties of Hydrophobic Films Based on Acetylated Broken-Rice Starch Nanocrystals for Slow Protein Delivery. Int. J. Biol. Macromol. 2019, 138, 556–564. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Ji, N.; Dai, L.; Xiong, L.; Sun, Q. Acetylated Debranched Starch Micelles as a Promising Nanocarrier for Curcumin. Food Hydrocoll. 2021, 111, 106253. [Google Scholar] [CrossRef]
- Gangopadhyay, A.; Bose, A.; Rout, S.S.; Mohapatra, R. Application of Dual Modified Corn Starch as a Polymer for the Colon Targeted Direct Compressible Budesonide Tablet. J. Drug Deliv. Sci. Technol. 2022, 74, 103556. [Google Scholar] [CrossRef]
- Promhuad, K.; Bumbudsanpharoke, N.; Wadaugsorn, K.; Sonchaeng, U.; Harnkarnsujarit, N. Maltol-Incorporated Acetylated Cassava Starch Films for Shelf-Life-Extension Packaging of Bakery Products. Polymers 2022, 14, 5342. [Google Scholar] [CrossRef] [PubMed]
- Fitch-Vargas, P.R.; Camacho-Hernández, I.L.; Rodríguez-González, F.J.; Martínez-Bustos, F.; Calderón-Castro, A.; Zazueta-Morales, J.d.J.; Aguilar-Palazuelos, E. Effect of Compounding and Plastic Processing Methods on the Development of Bioplastics Based on Acetylated Starch Reinforced with Sugarcane Bagasse Cellulose Fibers. Ind. Crops Prod. 2023, 192, 116084. [Google Scholar] [CrossRef]
- Nasseri, R.; Ngunjiri, R.; Moresoli, C.; Yu, A.; Yuan, Z.; Xu, C. (Charles) Poly(Lactic Acid)/Acetylated Starch Blends: Effect of Starch Acetylation on the Material Properties. Carbohydr. Polym. 2020, 229, 115453. [Google Scholar] [CrossRef]
- Yao, X.; Lin, R.; Liang, Y.; Jiao, S.; Zhong, L. Characterization of Acetylated Starch Nanoparticles for Potential Use as an Emulsion Stabilizer. Food Chem. 2023, 400, 133873. [Google Scholar] [CrossRef]
- Cui, B.; Lu, Y.; Tan, C.; Wang, G.; Li, G.-H. Effect of Cross-Linked Acetylated Starch Content on the Structure and Stability of Set Yoghurt. Food Hydrocoll. 2014, 35, 576–582. [Google Scholar] [CrossRef]
- Rahim, A.; Kadir, S.; Jusman, J.; Zulkipli, Z.; Hambali, T.N.A. Physical, Chemical and Sensory Characteristics of Bread with Different Concentrations of Acetylated Arenga Starches. Int. Food Res. J. 2019, 26, 841–848. [Google Scholar]
- Lin, D.; Zhou, W.; Yang, Z.; Zhong, Y.; Xing, B.; Wu, Z.; Chen, H.; Wu, D.; Zhang, Q.; Qin, W.; et al. Study on Physicochemical Properties, Digestive Properties and Application of Acetylated Starch in Noodles. Int. J. Biol. Macromol. 2019, 128, 948–956. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Liu, M.; Strappe, P.; Li, M.; Wang, A.; Zhuang, M.; Liu, J.; Blanchard, C.; Zhou, Z. Association of Starch Crystalline Pattern with Acetylation Property and Its Influence on Gut Microbota Fermentation Characteristics. Food Hydrocoll. 2022, 128, 107556. [Google Scholar] [CrossRef]
- Gu, Y.; Cheng, L.; Gu, Z.; Hong, Y.; Li, Z.; Li, C. Preparation, Characterization and Properties of Starch-Based Adhesive for Wood-Based Panels. Int. J. Biol. Macromol. 2019, 134, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Gu, Z.; Hong, Y.; Cheng, L. Preparation, Characterization and Properties of Starch-Based Wood Adhesive. Carbohydr. Polym. 2012, 88, 699–706. [Google Scholar] [CrossRef]
- Posada-Velez, M.C.; Pineda-Gomez, P.; Martinez-Hernandez, H.D. Acetylated Corn and Potato Starches as an Alternative to the Toxic Inorganic Coagulants/Flocculants for Wastewater Treatment. Environ. Nanotechnol. Monit. Manag. 2023, 20, 100786. [Google Scholar] [CrossRef]
- Pang, Z.; Xu, R.; Luo, T.; Che, X.; Bansal, N.; Liu, X. Physiochemical Properties of Modified Starch under Yogurt Manufacturing Conditions and Its Relation to the Properties of Yogurt. J. Food Eng. 2019, 245, 11–17. [Google Scholar] [CrossRef]
- Singh, H.; Sodhi, N.S.; Singh, N. Structure and Functional Properties of Acetylated Sorghum Starch. Int. J. Food Prop. 2012, 15, 312–325. [Google Scholar] [CrossRef]
- Li, H.; Wang, Y.; Zhao, P.; Guo, L.; Huang, L.; Li, X.; Gao, W. Naturally and Chemically Acetylated Polysaccharides: Structural Characteristics, Synthesis, Activities, and Applications in the Delivery System: A Review. Carbohydr. Polym. 2023, 313, 120746. [Google Scholar] [CrossRef] [PubMed]
- Collar, C.; Rosell, C.M. Bakery and Confectioneries. In Valorization of by Products from Plant Based Food Processing Industries; Chandrasekaran, M., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 554–582. [Google Scholar]
- Watcharakitti, J.; Win, E.E.; Nimnuan, J.; Smith, S.M. Modified Starch-Based Adhesives: A Review. Polymers 2022, 14, 2023. [Google Scholar] [CrossRef]
- Zia-ud-Din; Xiong, H.; Fei, P. Physical and Chemical Modification of Starches: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2691–2705. [Google Scholar] [CrossRef]
- Adiyanti, T.; Subroto, E. Modifications Of Banana Starch And Its Characteristics: A Review. Int. J. Sci. Technol. Res. 2020, 9, 3–6. [Google Scholar]
- Masina, N.; Choonara, Y.E.; Kumar, P.; du Toit, L.C.; Govender, M.; Indermun, S.; Pillay, V. A Review of the Chemical Modification Techniques of Starch. Carbohydr. Polym. 2017, 157, 1226–1236. [Google Scholar] [CrossRef]
- Hong, J.; Zeng, X.-A.; Brennan, C.S.; Brennan, M.; Han, Z. Recent Advances in Techniques for Starch Esters and the Applications: A Review. Foods 2016, 5, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojogbo, E.; Ogunsona, E.O.; Mekonnen, T.H. Chemical and Physical Modifications of Starch for Renewable Polymeric Materials. Mater. Today Sustain. 2020, 7–8, 100028. [Google Scholar] [CrossRef]
- Tharanathan, R.N. Starch-Value Addition by Modification. Crit. Rev. Food Sci. Nutr. 2005, 45, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mhaske, P.; Farahnaky, A.; Kasapis, S.; Majzoobi, M. Cassava Starch: Chemical Modification and Its Impact on Functional Properties and Digestibility, a Review. Food Hydrocoll. 2022, 129, 107542. [Google Scholar] [CrossRef]
- Hermansson, A.M.; Svegmark, K. Developments in the Understanding of Starch Functionality. Trends Food Sci. Technol. 1996, 7, 345–353. [Google Scholar] [CrossRef]
- Nurmilah, S.; Subroto, E. Chemical Modification of Starch for the Production of Resistant Starch Type-4 (Rs4): A Review. Int. J. Eng. Trends Technol. 2021, 69, 45–50. [Google Scholar] [CrossRef]
- Sarkar, S. Influence of Acetylation and Heat-Moisture Treatment on Physio-Chemical, Pasting and Morphological Properties of Buckwheat (Fagopyrum esculentum) Starch. Asian J. Dairy Food Res. 2016, 35, 298–303. [Google Scholar] [CrossRef]
- Xue, X.; Liang, Q.; Gao, Q.; Luo, Z. One-Step Synthesis of Cross-Linked Esterified Starch and Its Properties. Appl. Sci. 2022, 12, 4075. [Google Scholar] [CrossRef]
- Wang, Y.J.; Wang, L. Characterization of Acetylated Waxy Maize Starches Prepared under Catalysis by Different Alkali and Alkaline-Earth Hydroxides. Starch/Staerke 2002, 54, 25–30. [Google Scholar] [CrossRef]
- Huang, J.; Schols, H.A.; Jin, Z.; Sulmann, E.; Voragen, A.G.J. Characterization of Differently Sized Granule Fractions of Yellow Pea, Cowpea and Chickpea Starches after Modification with Acetic Anhydride and Vinyl Acetate. Carbohydr. Polym. 2007, 67, 11–20. [Google Scholar] [CrossRef]
- Aadil, R.M.; Zeng, X.A.; Sun, D.W.; Wang, M.S.; Liu, Z.W.; Zhang, Z.H. Combined Effects of Sonication and Pulsed Electric Field on Selected Quality Parameters of Grapefruit Juice. LWT Food Sci. Technol. 2015, 62, 890–893. [Google Scholar] [CrossRef]
- Lidstrom, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave Assisted Organic Synthesis-A Review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Kumoro, A.C.; Amalia, R.; Budiyati, C.S.; Retnowati, D.S.; Ratnawati, R. Preparation and Characterization of Physicochemical Properties of Glacial Acetic Acid Modified Gadung (Diocorea Hispida Dennst) Flours. J. Food Sci. Technol. 2015, 52, 6615–6622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; Chen, Y.; Chen, Z.; Yang, Y.; Wang, Y. Preparation and Characteristics of Starch Esters and Its Effects on Dough Physicochemical Properties. J. Food Qual. 2018, 2018, 1395978. [Google Scholar] [CrossRef] [Green Version]
- Teodoro, A.P.; Mali, S.; Romero, N.; de Carvalho, G.M. Cassava Starch Films Containing Acetylated Starch Nanoparticles as Reinforcement: Physical and Mechanical Characterization. Carbohydr. Polym. 2015, 126, 9–16. [Google Scholar] [CrossRef]
- Otache, M.A.; Duru, R.U.; Achugasim, O.; Abayeh, O.J. Advances in the Modification of Starch via Esterification for Enhanced Properties. J. Polym. Environ. 2021, 29, 1365–1379. [Google Scholar] [CrossRef]
- Casas, J.; Persson, P.V.; Iversen, T.; Córdova, A. Direct Organocatalytic Ring-Opening Polymerizations of Lactones. Adv. Synth. Catal. 2004, 346, 1087–1089. [Google Scholar] [CrossRef]
- Imre, B.; Vilaplana, F. Organocatalytic Esterification of Corn Starches towards Enhanced Thermal Stability and Moisture Resistance. Green Chem. 2020, 22, 5017–5031. [Google Scholar] [CrossRef]
- Trela, V.D.; Ramallo, A.L.; Albani, O.A. Synthesis and Characterization of Acetylated Cassava Starch with Different Degrees of Substitution. Brazilian Arch. Biol. Technol. 2020, 63, e20180292. [Google Scholar] [CrossRef]
- Sodhi, N.S.; Singh, N. Characteristics of Acetylated Starches Prepared Using Starches Separated from Different Rice Cultivars. J. Food Eng. 2005, 70, 117–127. [Google Scholar] [CrossRef]
- Xu, Y.; Miladinov, V.; Hanna, M.A. Synthesis and Characterization of Starch Acetates with High Substitution. Cereal Chem. 2004, 81, 735–740. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhao, D.; Ma, X.; Guo, D.; Tong, X.; Zhang, Y.; Qu, L. Effect of Different Starch Acetates on the Quality Characteristics of Frozen Cooked Noodles. Food Sci. Nutr. 2022, 10, 678–688. [Google Scholar] [CrossRef] [PubMed]
- Babic, J.; Subaric, D.; Ackar, D.; Kovacevic, D.; Pilizota, V.; Kopjar, M. Preparation and Characterization of Acetylated Tapioca Starches. Dtsch. Leb. 2007, 103, 580–585. [Google Scholar]
- Huang, J.; Schols, H.A.; Klaver, R.; Jin, Z.; Voragen, A.G.J. Acetyl Substitution Patterns of Amylose and Amylopectin Populations in Cowpea Starch Modified with Acetic Anhydride and Vinyl Acetate. Carbohydr. Polym. 2007, 67, 542–550. [Google Scholar] [CrossRef]
- Singh, N.; Chawla, D.; Singh, J. Influence of Acetic Anhydride on Physicochemical, Morphological and Thermal Properties of Corn and Potato Starch. Food Chem. 2004, 86, 601–608. [Google Scholar] [CrossRef]
- Lee, H.L.; Yoo, B. Dynamic Rheological and Thermal Properties of Acetylated Sweet Potato Starch. Starch/Staerke 2009, 61, 407–413. [Google Scholar] [CrossRef]
- Pietrzyk, S.; Fortuna, T.; Łabanowska, M.; Juszczak, L.; Gałkowska, D.; Bączkowicz, M.; Kurdziel, M. The Effect of Amylose Content and Level of Oxidation on the Structural Changes of Acetylated Corn Starch and Generation of Free Radicals. Food Chem. 2018, 240, 259–267. [Google Scholar] [CrossRef]
- Ren, F.; Wang, J.; Yu, J.; Zhong, C.; Xie, F.; Wang, S. Green Synthesis of Acetylated Maize Starch in Different Imidazolium Carboxylate and Choline Carboxylate Ionic Liquids. Carbohydr. Polym. 2022, 288, 119353. [Google Scholar] [CrossRef]
- Nasseri, R.; Moresoli, C.; Yu, A.; Yuan, Z.; Xu, C. (Charles) Structural Dependence of the Molecular Mobility in Acetylated Starch. Polymer 2021, 215, 123371. [Google Scholar] [CrossRef]
- Chen, Z.; Schols, H.A.; Voragen, A.G.J. Differently Sized Granules from Acetylated Potato and Sweet Potato Starches Differ in the Acetyl Substitution Pattern of Their Amylose Populations. Carbohydr. Polym. 2004, 56, 219–226. [Google Scholar] [CrossRef]
- Mbougueng, P.D.; Tenin, D.; Scher, J.; Tchiégang, C. Influence of Acetylation on Physicochemical, Functional and Thermal Properties of Potato and Cassava Starches. J. Food Eng. 2012, 108, 320–326. [Google Scholar] [CrossRef]
- Mendoza, J.S.; RuyDíaz, J.H.; Quintero, A.F. Effect of the Acetylation Process on Native Starches of Yam (Dioscorea spp.). Rev. Fac. Nac. Agron. Medellin 2016, 69, 7997–8006. [Google Scholar] [CrossRef]
- Lawal, O.S. Composition, Physicochemical Properties and Retrogradation Characteristics of Native, Oxidised, Acetylated and Acid-Thinned New Cocoyam (Xanthosoma sagittifolium) Starch. Food Chem. 2004, 87, 205–218. [Google Scholar] [CrossRef]
- Luo, Z.G.; Shi, Y.C. Distribution of Acetyl Groups in Acetylated Waxy Maize Starches Prepared in Aqueous Solution with Two Different Alkaline Concentrations. Food Hydrocoll. 2018, 79, 491–497. [Google Scholar] [CrossRef]
- Ayucitra, A. Preparation and Characterisation of Acetylated Corn Starches. Int. J. Chem. Eng. Appl. 2012, 3, 156–159. [Google Scholar] [CrossRef] [Green Version]
- González, Z.; Pérez, E. Effect of Acetylation on Some Properties of Rice Starch. Starch-Stärke 2002, 54, 148–154. [Google Scholar] [CrossRef]
- Shah, A.; Masoodi, F.A.; Gani, A.; Ashwar, B.A. Physicochemical, Rheological and Structural Characterization of Acetylated Oat Starches. LWT Food Sci. Technol. 2017, 80, 19–26. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Afolabi, T.A.; Olu-Owolabi, B.I. Functional, Physicochemical and Retrogradation Properties of Sword Bean (Canavalia gladiata) Acetylated and Oxidized Starches. Carbohydr. Polym. 2006, 65, 93–101. [Google Scholar] [CrossRef]
- Lee, S.J.; Hong, J.Y.; Lee, E.J.; Chung, H.J.; Lim, S.T. Impact of Single and Dual Modifications on Physicochemical Properties of Japonica and Indica Rice Starches. Carbohydr. Polym. 2015, 122, 77–83. [Google Scholar] [CrossRef]
- Van Hung, P.; Morita, N. Effects of Granule Sizes on Physicochemical Properties of Cross-Linked and Acetylated Wheat Starches. Starch/Staerke 2005, 57, 413–420. [Google Scholar] [CrossRef]
- Rahim, A.; Mahfudz; Muhardi; Kadir, S.; Rostiati; Alam, N.; Hutomo, G.S.; Priyantono, E.; Salingkat, C.A.; Abdullah, R. Effect of PH and Acetic Anhydride Concentration on Physicochemical Characteristics of Acetylated Sago Starch. IOP Conf. Ser. Earth Environ. Sci. 2022, 1107, 12124. [Google Scholar] [CrossRef]
- Yu, S.X.; Mu, T.H.; Zhang, M.; Ma, M.M.; Zhao, Z.K. Effects of Retrogradation and Further Acetylation on the Digestibility and Physicochemical Properties of Purple Sweet Potato Flour and Starch. Starch/Staerke 2015, 67, 892–902. [Google Scholar] [CrossRef]
- Kapelko, M.; Zięba, T.; Gryszkin, A.; Styczyńska, M.; Wilczak, A. Properties of Retrograded and Acetylated Starch Produced via Starch Extrusion or Starch Hydrolysis with Pullulanase. Carbohydr. Polym. 2013, 97, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Castro, A.; Jacobo-Valenzuela, N.; Félix-Salazar, L.A.; Zazueta-Morales, J.d.J.; Martínez-Bustos, F.; Fitch-Vargas, P.R.; Carrillo-López, A.; Aguilar-Palazuelos, E. Optimization of Corn Starch Acetylation and Succinylation Using the Extrusion Process. J. Food Sci. Technol. 2019, 56, 3940–3950. [Google Scholar] [CrossRef] [PubMed]
- Sitanggang, A.; Sani, P.; Mastuti, T. Modification of Mung Bean Starch by Annealing Treatment and Acetylation. In Proceedings of the 2nd SEAFAST International Seminar—2nd SIS, Bogor, Indonesia, 4–5 September 2019; SciTePress: Setubal, Portugal, 2020; pp. 10–19, ISBN 978-989-758-466-4. [Google Scholar] [CrossRef]
- Rahim, A.; Siswo Huto, G.; Rahman, N.; Bohari, H.S.A. Structure and Functional Properties of Arenga Starch by Acetylation with Different Concentrations of Acetic Anhydride. Asian J. Sci. Res. 2019, 12, 220–228. [Google Scholar] [CrossRef] [Green Version]
- Otemuyiwa, I.O.; Aina, A.F. Physicochemical Properties and In-Vitro Digestibility Studies of Microwave Assisted Chemically Modified Breadfruit (Artocarpus altilis) Starch. Int. J. Food Prop. 2021, 24, 140–151. [Google Scholar] [CrossRef]
- Chi, H.; Xu, K.; Wu, X.; Chen, Q.; Xue, D.; Song, C.; Zhang, W.; Wang, P. Effect of Acetylation on the Properties of Corn Starch. Food Chem. 2008, 106, 923–928. [Google Scholar] [CrossRef]
- Vidal, N.P.; Bai, W.; Geng, M.; Martinez, M.M. Organocatalytic Acetylation of Pea Starch: Effect of Alkanoyl and Tartaryl Groups on Starch Acetate Performance. Carbohydr. Polym. 2022, 294, 119780. [Google Scholar] [CrossRef]
- Kemas, C.U.; Ngwuluka, N.C.; Ochekpe, N.A.; Nep, E.I. Starch-Based Xerogels: Effect of Acetylation on Physicochemical and Rheological Properties. Int. J. Biol. Macromol. 2017, 98, 94–102. [Google Scholar] [CrossRef]
- Chakraborty, I.; N, P.; Mal, S.S.; Paul, U.C.; Rahman, M.H.; Mazumder, N. An Insight into the Gelatinization Properties Influencing the Modified Starches Used in Food Industry: A Review. Food Bioprocess Technol. 2022, 15, 1195–1223. [Google Scholar] [CrossRef]
- Yadav, A.R.; Guha, M.; Reddy, S.Y.; Tharanathan, R.N.; Ramteke, R.S. Physical Properties of Acetylated and Enzyme-Modified Potato and Sweet Potato Flours. J. Food Sci. 2007, 72, E249–E253. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lv, Y. Structure, Functionality, and Digestibility of Acetylated Hulless Barley Starch. Int. J. Food Prop. 2017, 20, 1818–1828. [Google Scholar] [CrossRef]
- Wu, X.; Liu, P.; Ren, L.; Tong, J.; Zhou, J. Optimization of Corn Starch Succinylation Using Response Surface Methodology. Starch-Stärke 2014, 66, 508–514. [Google Scholar] [CrossRef]
- Debet, M.R.; Gidley, M.J. Three Classes of Starch Granule Swelling: Influence of Surface Proteins and Lipids. Carbohydr. Polym. 2006, 64, 452–465. [Google Scholar] [CrossRef]
- Vermeylen, R.; Derycke, V.; Delcour, J.A.; Goderis, B.; Reynaers, H.; Koch, M.H.J. Gelatinization of Starch in Excess Water: Beyond the Melting of Lamellar Crystallites. A Combined Wide- and Small-Angle X-Ray Scattering Study. Biomacromolecules 2006, 7, 2624–2630. [Google Scholar] [CrossRef]
- Song, M.-R.; Choi, S.-H.; Oh, S.-M.; Kim, H.; Bae, J.-E.; Park, C.-S.; Kim, B.-Y.; Baik, M.-Y. Characterization of Amorphous Granular Starches Prepared by High Hydrostatic Pressure (HHP). Food Sci. Biotechnol. 2017, 26, 671–678. [Google Scholar] [CrossRef]
- Tao, K.; Li, C.; Yu, W.; Gilbert, R.G.; Li, E. How Amylose Molecular Fine Structure of Rice Starch Affects Functional Properties. Carbohydr. Polym. 2019, 204, 24–31. [Google Scholar] [CrossRef]
- Nikolenko, M.V.; Myrhorodska-Terentieva, V.D.; Sakhno, Y.; Jaisi, D.P.; Likozar, B.; Kostyniuk, A. Hydrothermal Leaching of Amylose from Native, Oxidized and Heat-Treated Starches. Processes 2023, 11, 1464. [Google Scholar] [CrossRef]
- Sondari, D.; Amanda, P.; Suryaningrum, R.; Burhani, D.; Pramasari, D.A.; Septevani, A.A.; Restu, W.K.; Agustian, E.; Irawan, Y.; Oktaviani, M. Effect of Different Amount of Cross-Linker and Catalyst on Modified Cassava towards Its Chemical Characteristic. IOP Conf. Ser. Earth Environ. Sci. 2021, 759, 12007. [Google Scholar] [CrossRef]
- Li, W.; Shan, Y.; Xiao, X.; Luo, Q.; Zheng, J.; Ouyang, S. Physicochemical Properties of A- and B- Starch Granules Isolated from Hard Red and Soft Red Winter Wheat. Agric. Food Chem. 2013, 61, 6477–6484. [Google Scholar] [CrossRef]
- Waduge, R.N.; Hoover, R.; Vasanthan, T.; Gao, J.; Li, J. Effect of Annealing on the Structure and Physicochemical Properties of Barley Starches of Varying Amylose Content. Food Res. Int. 2006, 39, 59–77. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Shah, M.A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I.A. Rice Starch Diversity: Effects on Structural, Morphological, Thermal, and Physicochemical Properties-A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 417–436. [Google Scholar] [CrossRef]
- Ratnayake, W.S.; Hoover, R.; Warkentin, T. Pea Starch: Composition, Structure and Properties—A Review. Starch/Staerke 2002, 54, 217–234. [Google Scholar] [CrossRef]
- Bello, M.O.; Tolaba, M.P.; Suarez, C. Water Absorption and Starch Gelatinization in Whole Rice Grain during Soaking. LWT Food Sci. Technol. 2007, 40, 313–318. [Google Scholar] [CrossRef]
- Ulfa, G.M.; Putri, W.D.R.; Fibrianto, K.; Prihatiningtyas, R.; Widjanarko, S.B. The Influence of Temperature in Swelling Power, Solubility, and Water Binding Capacity of Pregelatinised Sweet Potato Starch. IOP Conf. Ser. Earth Environ. Sci. 2020, 475, 12036. [Google Scholar] [CrossRef]
- Wang, S.; Jin, F.; Yu, J. Pea Starch Annealing: New Insights. Food Bioprocess Technol. 2013, 6, 3564–3575. [Google Scholar] [CrossRef]
- Hoover, R.; Ratnayake, W.S. Starch Characteristics of Black Bean, Chick Pea, Lentil, Navy Bean and Pinto Bean Cultivars Grown in Canada. Food Chem. 2002, 78, 489–498. [Google Scholar] [CrossRef]
- Iuga, M.; Mironeasa, S. A Review of the Hydrothermal Treatments Impact on Starch Based Systems Properties. Crit. Rev. Food Sci. Nutr. 2020, 60, 3890–3915. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, N.; Xu, Y.; Huang, J.; Yuan, M.; Wu, D.; Shu, X. Physicochemical Properties of Hydroxypropylated and Cross-Linked Rice Starches Differential in Amylose Content. Int. J. Biol. Macromol. 2019, 128, 775–781. [Google Scholar] [CrossRef]
- Lan, H.; Hoover, R.; Jayakody, L.; Liu, Q.; Donner, E.; Baga, M.; Asare, E.K.; Hucl, P.; Chibbar, R.N. Impact of Annealing on the Molecular Structure and Physicochemical Properties of Normal, Waxy and High Amylose Bread Wheat Starches. Food Chem. 2008, 111, 663–675. [Google Scholar] [CrossRef]
- Sobini, N.; Darsiga, S.; Kananke, T.C.; Srivijeindran, S. Characterization of Modified Palmyrah Tuber Starch by Pre-Gelatinization, Acid and Dextrinization Processes and Its Applicability. Food Chem. Adv. 2022, 1, 100143. [Google Scholar] [CrossRef]
- Marta, H.; Cahyana, Y.; Djali, M.; Pramafisi, G. The Properties, Modification, and Application of Banana Starch. Polymers 2022, 14, 3092. [Google Scholar] [CrossRef] [PubMed]
- Sindhu, R.; Devi, A.; Khatkar, B.S. Morphology, Structure and Functionality of Acetylated, Oxidized and Heat Moisture Treated Amaranth Starches. Food Hydrocoll. 2021, 118, 106800. [Google Scholar] [CrossRef]
- Chen, Z.; Schols, H.A.; Voragen, A.G.J. The Use of Potato and Sweet Potato Starches Affects White Salted Noodle Quality. J. Food Sci. 2003, 68, 2630–2637. [Google Scholar] [CrossRef]
- Chibuzo, I.M. Physicochemical and Retrogradation Characteristics of Modified Sweet Potato (Ipomoea Batatas L. (Lam)) Sarch. J. Agric. Food. Tech 2012, 2, 49–55. [Google Scholar]
- Simsek, S.; Ovando-Martínez, M.; Whitney, K.; Bello-Pérez, L.A. Effect of Acetylation, Oxidation and Annealing on Physicochemical Properties of Bean Starch. Food Chem. 2012, 134, 1796–1803. [Google Scholar] [CrossRef]
- Abedi, E.; Pourmohammadi, K.; Abbasi, S. Dual-Frequency Ultrasound for Ultrasonic-Assisted Esterification. Food Sci. Nutr. 2019, 7, 2613–2624. [Google Scholar] [CrossRef] [Green Version]
- Van Hung, P.; Morita, N. Dough Properties and Bread Quality of Flours Supplemented with Cross-Linked Cornstarches. Food Res. Int. 2004, 37, 461–467. [Google Scholar] [CrossRef]
- Liu, H.; Corke, H.; Ramsden, L. Functional Properties and Enzymatic Digestibility of Cationic and Cross-Linked Cationic Ae, Wx, and Normal Maize Starch. J. Agric. Food Chem. 1999, 47, 2523–2528. [Google Scholar] [CrossRef]
- Bello-Pérez, L.A.; Agama-Acevedo, E.; Zamudio-Flores, P.B.; Mendez-Montealvo, G.; Rodriguez-Ambriz, S.L. Effect of Low and High Acetylation Degree in the Morphological, Physicochemical and Structural Characteristics of Barley Starch. LWT Food Sci. Technol. 2010, 43, 1434–1440. [Google Scholar] [CrossRef]
- Singh, J.; Kaur, L.; Singh, N. Effect of Acetylation on Some Properties of Corn and Potato Starches. Starch-Stärke 2004, 56, 586–601. [Google Scholar] [CrossRef]
- Kaushal, P.; Kumar, V.; Sharma, H.K. Comparative Study of Physicochemical, Functional, Antinutritional and Pasting Properties of Taro (Colocasia esculenta), Rice (Oryza sativa) Flour, Pigeonpea (Cajanus cajan) Flour and Their Blends. LWT Food Sci. Technol. 2012, 48, 59–68. [Google Scholar] [CrossRef]
- Han, F.; Liu, M.; Gong, H.; Lü, S.; Ni, B.; Zhang, B. Synthesis, Characterization and Functional Properties of Low Substituted Acetylated Corn Starch. Int. J. Biol. Macromol. 2012, 50, 1026–1034. [Google Scholar] [CrossRef]
- Ren, L.; Dong, Z.; Jiang, M.; Tong, J.; Zhou, J. Hydrophobization of Starch Nanocrystals through Esterification in Green Media. Ind. Crops Prod. 2014, 59, 115–118. [Google Scholar] [CrossRef]
- Suh, D.S.; Jane, J. Comparison of Starch Pasting Properties at Various Cooking Conditions Using the Micro Visco-Amylo-Graph and the Rapid Visco Analyser. Cereal Chem. 2003, 80, 745–749. [Google Scholar] [CrossRef]
- BeMiller, J.N. Pasting, Paste, and Gel Properties of Starch–Hydrocolloid Combinations. Carbohydr. Polym. 2011, 86, 386–423. [Google Scholar] [CrossRef]
- Collado, L.S.; Mabesa, L.B.; Oates, C.G.; Corke, H. Bihon-Type Noodles from Heat-Moisture-Treated Sweet Potato Starch. J. Food Sci. 2001, 66, 604–609. [Google Scholar] [CrossRef]
- Guo, K.; Liu, T.; Xu, A.; Zhang, L.; Bian, X.; Wei, C. Structural and Functional Properties of Starches from Root Tubers of White, Yellow, and Purple Sweet Potatoes. Food Hydrocoll. 2019, 89, 829–836. [Google Scholar] [CrossRef]
- Pranoto, Y.; Rahmayuni; Haryadi; Rakshit, S.K. Physicochemical Properties of Heat Moisture Treated Sweet Potato Starches of Selected Indonesian Varieties. Int. Food Res. J. 2014, 21, 2031–2038. [Google Scholar]
- Hutabarat, D.J.C.; Stevensen, J. Physicochemical Properties of Enzymatically Modified Starch: A Review. IOP Conf. Ser. Earth Environ. Sci. 2023, 1169, 12093. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, M.; Ma, S.; Wang, H. Physicochemical Characterization of Rice, Potato, and Pea Starches, Each with Different Crystalline Pattern, When Incorporated with Konjac Glucomannan. Food Hydrocoll. 2020, 101, 105499. [Google Scholar] [CrossRef]
- He, W.; Wei, C. Progress in C-Type Starches from Different Plant Sources. Food Hydrocoll. 2017, 73, 162–175. [Google Scholar] [CrossRef]
- Lewandowicz, J.; Le Thanh-Blicharz, J.; Szwengiel, A. The Effect of Chemical Modification on the Rheological Properties and Structure of Food Grade Modified Starches. Processes 2022, 10, 938. [Google Scholar] [CrossRef]
- Lewandowicz, G.; Blaszczak, W.; Fornal, J. Effect of Acetylation on Microstructure of Potato Starch. Polish J. Food Nutr. Sci. 1998, 7, 78–84. [Google Scholar]
- Iftikhar, S.A.; Chakraborty, S.; Dutta, H. Effect of Acetylation, Hydroxypropylation and Dual Acetylation-Hydroxypropylation on Physicochemical and Digestive Properties of Rice Starches with Different Amylose Content. Biointerface Res. Appl. Chem. 2022, 12, 6788–6803. [Google Scholar] [CrossRef]
- Perera, C.; Hoover, R.; Martin, A.M. The Effect of Hydroxypropylation on the Structure and Physicochemical Properties of Native, Defatted and Heat-Moisture Treated Potato Starches. Food Res. Int. 1997, 30, 235–247. [Google Scholar] [CrossRef] [Green Version]
- Wickramasinghe, M.; Yamamoto, K.; Yamauchi, H.; Noda, T. Effect of Low Level of Starch Acetylation on Physicochemical Properties of Potato Starch. Food Sci. Biotechnol. 2009, 18, 118–123. [Google Scholar]
- Subroto, E.; Filianty, F.; Indiarto, R.; Andita Shafira, A. Physicochemical and Functional Properties of Modified Adlay Starch (Coix Lacryma-Jobi) by Microwave and Ozonation. Int. J. Food Prop. 2022, 25, 1622–1634. [Google Scholar] [CrossRef]
- Marta, H.; Cahyana, Y.; Bintang, S.; Soeherman, G.P.; Djali, M. Physicochemical and Pasting Properties of Corn Starch as Affected by Hydrothermal Modification by Various Methods. Int. J. Food Prop. 2022, 25, 792–812. [Google Scholar] [CrossRef]
- Hoover, R. Composition, Molecular Structure, and Physicochemical Properties of Tuber and Root Starches: A Review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Trung, P.T.B.; Ngoc, L.B.B.; Hoa, P.N.; Tien, N.N.T.; Hung, P. Van Impact of Heat-Moisture and Annealing Treatments on Physicochemical Properties and Digestibility of Starches from Different Colored Sweet Potato Varieties. Int. J. Biol. Macromol. 2017, 105, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Vieira, F.C.; Sarmento, S.B.S. Heat-Moisture Treatment and Enzymatic Digestibility of Peruvian Carrot, Sweet Potato and Ginger Starches. Starch/Staerke 2008, 60, 223–232. [Google Scholar] [CrossRef]
- Chen, P.; Yu, L.; Simon, G.; Petinakis, E.; Dean, K.; Chen, L. Morphologies and Microstructures of Cornstarches with Different Amylose-Amylopectin Ratios Studied by Confocal Laser Scanning Microscope. J. Cereal Sci. 2009, 50, 241–247. [Google Scholar] [CrossRef]
- Glaring, M.A.; Koch, C.B.; Blennow, A. Genotype-Specific Spatial Distribution of Starch Molecules in the Starch Granule: A Combined CLSM and SEM Approach. Biomacromolecules 2006, 7, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Fornal, J.; Sadowska, J.; Błaszczak, W.; Jeliński, T.; Stasiak, M.; Molenda, M.; Hajnos, M. Influence of Some Chemical Modifications on the Characteristics of Potato Starch Powders. J. Food Eng. 2012, 108, 515–522. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, W.; Zhao, X.; Liu, Y.; Gao, W. Study on the Morphology, Crystalline Structure and Thermal Properties of Yellow Ginger Starch Acetates with Different Degrees of Substitution. Thermochim. Acta 2009, 495, 57–62. [Google Scholar] [CrossRef]
- El Halal, S.L.M.; Colussi, R.; Pinto, V.Z.; Bartz, J.; Radunz, M.; Carreño, N.L.V.; Dias, A.R.G.; Zavareze, E.D.R. Structure, Morphology and Functionality of Acetylated and Oxidised Barley Starches. Food Chem. 2015, 168, 247–256. [Google Scholar] [CrossRef] [Green Version]
- Ariyantoro, A.R.; Fitriyani, A.; Affandi, D.R.; Muhammad, D.R.A.; Yulviatun, A.; Nishizu, T. The Effect of Dual Modification with Annealing and Heat Moisture Treatment (HMT) on Physicochemical Properties of Jack Bean Starch (Canavalia ensiformis). Food Res. 2022, 6, 189–198. [Google Scholar] [CrossRef]
- Marboh, V.; Gayary, M.A.; Gautam, G.; Mahanta, C.L. Comparative Study of Heat-Moisture Treatment and Annealing on Morphology, Crystallinity, Pasting, and Thermal Properties of Sohphlang (Flemingia vestita) Starch. Starch-Stärke 2022, 74, 2100294. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical Properties, Modifications and Applications of Starches from Different Botanical Sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline Structures of the Main Components of Starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Dome, K.; Podgorbunskikh, E.; Bychkov, A.; Lomovsky, O. Changes in the Crystallinity Degree of Starch Having Different Types of Crystal Structure after Mechanical Pretreatment. Polymers 2020, 12, 641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukhchin, D.G.; Malkov, A.V.; Tyshkunova, I.V.; Mayer, L.V.; Novozhilov, E. V Diffractometric Method for Determining the Degree of Crystallinity of Materials. Crystallogr. Rep. 2016, 61, 371–375. [Google Scholar] [CrossRef]
- Cheetham, N.W.H.; Tao, L. Variation in Crystalline Type with Amylose Content in Maize Starch Granules: An X-Ray Powder Diffraction Study. Carbohydr. Polym. 1998, 36, 277–284. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch Granules: Structure and Biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef] [Green Version]
- Buléon, A.; Gallant, D.J.; Bouchet, B.; Mouille, G.; D’Hulst, C.; Kossmann, J.; Ball, S. Starches from A to C: Chlamydomonas Reinhardtii as a Model Microbial System to Investigate the Biosynthesis of the Plant Amylopectin Crystal. Plant Physiol. 1997, 115, 949–957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, S.; Wu, X.; Lin, S.; Zeng, H.; Lu, X.; Zhang, Y.; Zheng, B. Structural Characteristics and Physicochemical Properties of Lotus Seed Resistant Starch Prepared by Different Methods. Food Chem. 2015, 186, 213–222. [Google Scholar] [CrossRef]
- Ahn, J.H.; Baek, H.R.; Kim, K.M.; Han, G.J.; Choi, J.B.; Kim, Y.; Moon, T.W. Slowly Digestible Sweetpotato Flour: Preparation by Heat-Moisture Treatment and Characterization of Physicochemical Properties. Food Sci. Biotechnol. 2013, 22, 383–391. [Google Scholar] [CrossRef]
- Buléon, A.; Gérard, C.; Riekel, C.; Vuong, R.; Chanzy, H. Details of the Crystalline Ultrastructure of C-Starch Granules, Revealed by Synchrotron Microfocus Mapping. Macromolecules 1998, 31, 6605–6610. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Lin, L.; Wang, J.; Liu, Q.; Wei, C. Long Branch-Chains of Amylopectin with B-Type Crystallinity in Rice Seed with Inhibition of Starch Branching Enzyme I and IIb Resist in Situ Degradation and Inhibit Plant Growth during Seedling Development. BMC Plant Biol. 2018, 18, 9. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rubio, A.; Flanagan, B.M.; Gilbert, E.P.; Gidley, M.J. A Novel Approach for Calculating Starch Crystallinity and Its Correlation with Double Helix Content: A Combined XRD and NMR Study. Biopolymers 2008, 89, 761–768. [Google Scholar] [CrossRef] [PubMed]
- If’all, I.; Hasanuddin, A.; Rahim, A.; Kadir, S. Modification of Starch by Acetylation of the Acetyl Function Group and the Kristanility of Banggai Yam Starch Acetate. Rekayasa 2019, 12, 135–140. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Saleh, A.S.M.; Gong, B.; Li, B.; Jing, L.; Gou, M.; Jiang, H.; Li, W. The Effect of Repeated versus Continuous Annealing on Structural, Physicochemical, and Digestive Properties of Potato Starch. Food Res. Int. 2018, 111, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, A.; Hoover, R. Effect of Heat-Moisture Treatment on the Structure and Physicochemical Properties of Tuber and Root Starches. Carbohydr. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Miao, M.; Zhang, T.; Jiang, B. Characterisations of Kabuli and Desi Chickpea Starches Cultivated in China. Food Chem. 2009, 113, 1025–1032. [Google Scholar] [CrossRef]
- Wang, Y.J.; Den Truong, V.; Wang, L. Structures and Rheological Properties of Corn Starch as Affected by Acid Hydrolysis. Carbohydr. Polym. 2003, 52, 327–333. [Google Scholar] [CrossRef]

| Starch/Flour and Treatment | Products/Applications | Characteristics | References |
|---|---|---|---|
| Acetylated cassava starch | Starch-based nanoparticles for encapsulation of antioxidants | Acetylated starch interacts well with antioxidant compounds, especially BHT, and protects antioxidants from degradation. Acetylated starch can increase the thermal stability of nanoparticles. | [30] |
| Acetylated rice starch nanocrystals | Nanocrystal for protein delivery | BSA protein release is significantly slowed. Acetylated rice starch nanocrystals can be good for protein delivery. | [31] |
| Acetylated debranched waxy corn starch | Nanocarrier for curcumin | Acetylated starch to be amphiphilic, having polar and non-polar groups. Curcumin micelles were spherical with a particle size of about 50–100 nm. Micelles of acetylated starch can accommodate curcumin until the concentration of 0.45 mg/mL | [32] |
| Retrograded acetylated corn starch | Drug (budesonide) delivery | Acetylation increased the hydrophobicity and reduced the swelling power and granule porosity. Tablets from retrograded acetylated corn starch released the drug in ileocolonic by 81.38%. Tablets were potentially suitable for the treatment of ileocolonic diseases. | [33] |
| Acetylated cassava starch and Maltol Incorporated | Active biodegradable film/packaging | The film based on acetylated cassava starch, which was incorporated with 10% maltol reduced molecular mobility and hydrophilicity; elongation was reduced by 34%, while the tensile strength was reduced by 37%. The active film inhibited the fungal growth for up to 6 times longer and maintained the flavor of bakery products. | [34] |
| Acetylated corn starch | Starch-based bioplastics | Acetylated corn starch improved the homogeneity and mechanical properties of biocomposites. The solubility of starch-based bioplastics decreased to 24.9–28.2% | [35] |
| Acetylated corn starch | Biodegradable polymers poly(lactic acid) for packaging materials | Acetylated corn starch increased the thermal stability of biodegradable polymers. Acetylated corn starch improved mechanical properties such as toughness and tensile strength. | [36] |
| Acetylated cassava starch | Starch nanoparticles for emulsion stabilizer | Acetylated starch nanoparticles with DS of 0.53 improved the hydrophobicity by a contact angle of more than 89.56°. Acetylated starch nanoparticles of 1.5% improved storage stability for up to 35 days and emulsion capacity by improving the droplet size and homogeneity. | [37] |
| Cross-linked acetylated cassava starch | Set yogurt | Cross-linked acetylated cassava starch improved the stability, viscous modulus (G″), and elastic modulus (G′) of the set yogurt. | [38] |
| Acetylated corn starch | Fat replacer of beef patties | The use of 15% acetylated corn starch improved the acceptance of organoleptic, microstructure, and physicochemical properties in beef patties, Acetylated corn starch is a suitable fat replacer for meat products. | [16] |
| Acetylated arenga starch | Bread | The addition of acetylated arenga starch up to 50% improved the quality of the bread produced, including sensorial properties, oven spring, oil absorption, and oil holding capacity. | [39] |
| Acetylated corn starch | Noodles | Acetylated corn starch increased the brightness of noodles. Acetylated starch reduced the tensile properties, chewiness, adhesion, and hardness of noodles. Acetylated starch increased the resistant starch and slowly digestible starch of noodles. | [40] |
| Acetylated rice starch and potato starch | Gut microbiota fermentation | Acetylated starch produced more SCFA than native starch. Acetylated starches were easier to use and more quickly fermentable by the gut microbiota. | [41] |
| Acetylated-crosslinked corn starch | Wood-based panel adhesive | The adhesive had better water resistance up to 1 MPa, The adhesive was also heat resistant, so it can be used in high-temperature pressing. | [42] |
| Acetylated waxy corn starch | Wood adhesive | The adhesive resistance to water increased up to 61.1% The shear strength increased up to 321% in the wet state and 59.4% in the dry state. | [43] |
| Acetylated corn starch and potato starch | Coagulants for wastewater treatment | Acetylated starch from corn and potato starch was effective as a coagulant for wastewater treatment by significantly reducing pH, color, turbidity, and electrical conductivity. | [44] |
| Starches or Flours | Reagents/Acetylation Condition | Degree of Substitution (DS) | References | |
|---|---|---|---|---|
| One Step Acetylation | ||||
| Corn starch Waxy corn starch | Acetic anhydride, pH 8–9 using NaOH 2%. | Corn (0.05–0.07) Waxy corn (0.08–0.09) | [79] | |
| Maize starch | Choline carboxylate, imidazolium carboxylate, and imidazolium chloride | 0.26–2.63 | [80] | |
| Corn starch | Acetic anhydride using toluene sulfuric acid as the catalyst. | 1.5 and 3.0 | [81] | |
| Sweet potato starch Potato starch | Acetic anhydride | 0.041–0.076 | [82] | |
| Sweet potato starch | Acetic anhydride 0–8%, pH 8.1–8.3, NaOH 3% | 0.032–0.123 | [78] | |
| Potato starch Cassava starch | pH 8, NaOH 3%, 10–20 min | Potato starch (0.10–0.26) Cassava starch (0.10–0.18) | [83] | |
| Potato starch Corn starch | pH 8.0–8.4, NaOH 3%, 10 min | Potato (0.180–0.238) Corn (0.133–0.184) | [77] | |
| Purple yam (PY) starch White yam (WY) starch | pH 8.0–8.4, NaOH 3%, 10 min and 240 min | Purple yam (0.034–0.051) White yam (0.036–0.043) | [84] | |
| Cocoyam starch | pH 8.0–8.5, 1 M NaOH, 5 min | 0.30 | [85] | |
| Waxy maize starch | NaOH 20% for 40 min, and NaOH 2% for 120 min | 0.12 | [86] | |
| Waxy maize starch | pH 8.0–8.5, NaOH 1 N, KOH 1 N, and Ca(OH)2 1 N for 60 min | 0.077–0.085 | [61] | |
| Maize starch | pH 8, 1 M NaOH, 60 min | 0.080–0.210 | [87] | |
| High amylose maize starch | pH 8, NaOH 50%, 15–240 min | 0.57–2.23 | [73] | |
| Rice starch | pH 8, NaOH 3% | 0.03 | [88] | |
| Oat starch | pH 8–8.5, NaOH 1 M for 5 min | 0.02–0.05 | [89] | |
| Sword bean starch | pH 8.0–8.4, 1 M NaOH, 30 min | 0.14 | [90] | |
| Yellow pea starch Chickpea starch Cowpea starch | Acetic anhydride, pH 7.5–9.0, 1–2 h, 20–25 °C, vinyl acetate, pH 9–10, 1–2 h, 20–25 °C | Acetic anhydride (0.059–0.066) Vinyl acetate (0.064–0.071) | [62] | |
| Japonica rice starch Indica rice starch | NaOH 4%, pH 7.8–8.2, 5 min | Japonica rice (0.066) Indica rice (0.060) | [91] | |
| Small granule wheat starch Large granule wheat starch | Acetic anhydride 8%, 30 °C, pH 8, NaOH 1 M, 15–20 min | 0.039–0.043 | [92] | |
| Sago starch | Acetylation (acetic anhydride, pH 7–10, NaOH 3%, T = room temperature, t = 50 min) | 0.21–0.58 | [93] | |
| Oat starch | Acetic anhydride (6% and 8%), pH 8.0–8.4, NaOH 3%, T = 25 °C, t = 10 min. | 0.05–0.11 | [21] | |
| Two steps acetylation | ||||
| Second step | Starches or flours | Reagents/Acetylation Condition | Degree of Substitution (DS) | References |
| HMT | Buckwheat starch | Acetic anhydride, HMT= Moisture content 25%, temperature 110 °C, 4 h. | 0.0289 | [59] |
| Retrogradation | Purple sweet potato flour and starch | pH 8.5, NaOH 0,5 M, 15 min. | Purple sweet potato flour (0.08) Purple sweet potato starch (0.165) | [94] |
| Retrogradation | Potato starch | Acetic acid anhydride, pH 8–9, NaOH 3%, 15 min. Retrogradation using extruder, T = 60–65–70 °C, 100–110–120 °C or 150–160–170 °C. | 3.1–4.4 | [95] |
| Extrusion | Corn starch | Acetic anhydride 7.88%. Extrusion at Screw Speed (SS, 100 rpm) and Barrel Temperature (BT, 80 °C). | 0.2 | [96] |
| ANN | Mung bean starch | Acetic acid anhydride 20%, 15 min, pH 8 by NaOH 3%, 25 °C 25 min, ANN = 60 °C, 6 h. | 0.02–0.26 | [97] |
| ANN | Potato starch | Acetic acid anhydride, pH 8–9 by NaOH 0.5 M, 15 min, ANN = 51–61 °C, 48 h. | 0.07–0.1 | [26] |
| ANN | Waxy potato (WP) starch Waxy rice (WR) starch Waxy barley (WB) starch Waxy corn (WC) starch | Acetic anhydride 20 g/100 g, at pH 8, using NaOH 3%, 15 min, ANN = Moisture 75%, 10 °C below gelatinization temperature, 2–72 h. | WC (0.03–0.13) WB (0.06–0.24) WR (0.03–0.25) WP (0.01–0.12) | [10] |
| Treatments | Materials | Functional Properties | References |
|---|---|---|---|
| Acetylation by vinyl acetate | Amaranth starch | Swelling power (SP) increased while solubility, WAC, and OAC decreased. | [126] |
| Acetylation | Sweet potato starch and flour Potato starch and flour | Starch: swelling volume (SV) increased; whiteness decreased. Flour: SV increased; whiteness increased. The whiteness degree of starch was higher than flour | [127] |
| Acetylation (pH 8.0–8.5, NaOH 1 M, 5 min) | Sweet potato starch | An increase in WAC, OAC, swelling power (SP), solubility, starch clarity, and gel strength. More stable to low-temperature storage. | [128] |
| Acetylation (Acetic anhydride 0–8%, pH 8.1–8.3, NaOH 3%) | Sweet potato starch | Swelling (SV) and solubility increased with increasing DS | [78] |
| Acetylation (pH 8, NaOH 3%, 10–20 menit) | Potato starch Cassava starch | Water binding capacity (WBC), paste clarity, and solubility increased Whiteness was decreased Acetylated potato starch, which was reacted for 20 min, caused a decrease in WBC. | [83] |
| Acetylation (pH 8.0–8.4, NaOH 3%, 10 and 240 min) | Purple yam (PY) starch White yam (WY) starch | In PY, there is an increase in WAC, SV, and solubility, but in 240 min of acetylated PY, the changes were not significant. At 240 min of acetylated WY, the increase in WAC and SV was greater, but the solubility value was lower than at 10 min of acetylated WY. | [84] |
| Acetylation (pH 8.0–8.5, 1 M NaOH, 5 min) | Cocoyam starch | SV increased with increasing temperature and pH Solubility was decreased Improved WAC, OAC, and paste clarity | [85] |
| Acetylation (pH 8.0–8.4, NaOH 3%, 10 min) | Potato starch Maize starch | SV and paste clarity increased Paste clarity potato starch > corn starch Solubility increased until the concentration of 8%, then decreased at 10–12%. WAC decreased | [77] |
| Acetylation (pH 8–8.5, 1 M NaOH, 5 min) | Oat starch | WAC and OAC increased Frozen storage stability increased | [89] |
| Acetylation (pH 8, NaOH 3%) | Rice starch | Aggregation and deformation of starch granules occurred Size of starch granules reduced Granules become perforated | [88] |
| Acetylation (pH 8.0–8.4, 1 M NaOH, 30 min) | Sword bean starch | WAC and SV increased Solubility decreased | [90] |
| Acetylation (acetic anhydride, pH 7.5–9.0, 20–25 °C, 1–2 h,) and (vinyl acetate, pH 9–10, 20–25 °C, 1–2 h) | Yellow pea starch Chickpea starch Cowpea starch | Acetylation by vinyl acetate had a greater swelling ability Acetylation by acetic anhydride in cowpea reduced the swelling ability | [62] |
| Acetylation (acetic anhydride, pH 7–10, NaOH 3%, T = room temperature, t = 50 min) | Sago starch | There was an increase in solubility, SP, oil absorption capacity, water absorption capacity, and clarity. | [93] |
| Acetylation and ANN | Black bean starch Pinto bean starch | Acetylation: SV increased ANN: SV decreased | [129] |
| Acetylation and HMT | Buckwheat seed starch | Acetylation: OAC, WAC, solubility, SV, paste clarity, and whiteness increased. HMT: WAC, OAC, and paste clarity increased, while solubility, SV, and whiteness decreased | [59] |
| Acetylation (8% Acetic anhydride, 30 °C, pH 8, 1 M NaOH, 15–20 min) | Small-sized granule wheat starch Large-sized granule wheat starch | SV and paste clarity increased, while FTS decreased SV in large granules was greater than in small granules | [92] |
| Acetylation (4% NaOH, pH 7.8–8.2, 5 min) | Japonica rice starch Indica rice starch | SV and solubility deceased | [91] |
| Acetylation (Acetic anhydride 6% and 8%, pH 8.0–8.4, NaOH 3%, T = 25°C, t = 10 min.) | Oat starch | Swelling factor increased Swelling temperature decreased Synaeresis decreased | [21] |
| Sonication-Acetylation (25, 40, and 25 + 40 Hz, 5 min, 45–75 °C) | Wheat starch | WAC and solubility increased frozen storage stability increased | [130] |
| Acetylation-ANN | Potato starch | Swelling power (SP) was higher than native starch and annealed starch | [26] |
| Acetylation-ANN | Mung beans starch | SV and solubility decreased | [97] |
| Acetylation and Acetylation-ANN | Waxy potato (WP) starch Waxy rice (WR) starch Waxy barley (WB) starch Waxy corn (WC) starch | Acetylation caused an increase in SV. Dual modification caused an increase in the SV of cereal starch, but there was no significant change in WP starch. Acetylation and Dual modified stable to low-temperature storage and paste clarity increased. | [10] |
| Acetylation-retrogradation (pH 8.5; NaOH 0.5 M, 15 min) | Purple sweet potato flour and starch | Solubility increased WAC and SP decreased | [94] |
| Acetylation-retrogradation (pH 8–9; NaOH 3%, 15 min) | Potato starch | Solubility increased water absorbability increased | [95] |
| Acetylation-Extrusion (Acetic anhydride 7.88%, Extrusion at BT = 80 °C, SS = 100 rpm) | Corn starch | Water absorption index decreased Water solubility index decreased | [96] |
| Treatments | Starch/Flour | Pasting Properties | References |
|---|---|---|---|
| Acetylation (pH 8.0–8.5, NaOH 1 M, 5 min) | Sweet potato starch | Peak viscosity (PV), setback viscosity (SB), breakdown viscosity (BD), and pasting temperature (PT) decreased. | [128] |
| Acetylation by vinyl acetate | Amaranth starch | PV and BD increased, while PT and final viscosity (FV) decreased. | [126] |
| Acetylation-Enzymatic | Sweet potato flour Potato flour | PV, BD, and SB decreased. | [104] |
| Acetylation | Sweet potato starch Potato starch | PT and SB of potato starch did not change significantly, but PV and BD decreased. Whereas in sweet potato starch, there was a decrease in PT, PV, BD, and SB. There was a decreased PT, PV, BD, and SB in acetylated flour. | [127] |
| Acetylation | Commercial potato starch | PT, PV, BD, SB, and final viscosity (FV) decreased. | [146] |
| Acetylation | Potato starch | PT, PV, and SB decreased, while BD increased. | [147] |
| Acetylation (pH 8.0–8.4, NaOH 3%, 10 and 240 min) | Purple yam (PY) starch White yam (WY) starch | The initial gelatinization temperature decreased, but heating to 95 °C increased the viscosity. PV and SB in purple yam increased, while PV and BD in white yam decreased. | [84] |
| Acetylation (pH 8.0–8.5, NaOH 1 M, 5 min) | Cocoyam starch | PT, PV, hot paste viscosity (HPv), cold paste viscosity (CPv), and SB decreased. BD increased. | [85] |
| Acetylation, NaOH (pH 8.0–8.5, NaOH 1 N, KOH 1 N, Ca(OH)2 1 N, 60 min) | Waxy maize starch | PV increased, while PT decreased | [61] |
| Acetylation and HMT | Buckwheat seed starch | PV, FV, trough viscosity (TV), and PT for acetylated starch increased, while BD and SB decreased. PT in HMT-starch increased, while PV, FV, TV, BD, and SB decreased. | [59] |
| Acetylation (pH 8, NaOH 3%) | Rice starch | PV and TV increased, while PT and SB decreased. | [88] |
| Acetylation and ANN | Black bean starch Pinto bean starch | Acetylation-ANN reduces PV, HPV, CPV, BD, and SB Acetylation lowers PT and faster gelatinization time ANN increases PT and longer gelatinization time | [129] |
| Acetylation (pH 8.0–8.4, NaOH 1 M, 30 min) | Sword bean starch | PV, BD, and PT increased, while SB decreased | [90] |
| Cross-linked Acetylation starch (Acetic anhydride, pH 8, and sodium trimetaphosphate 0.7–0.9%) | Maize starch | PV and SB increased, while PT and BD decreased | [60] |
| Acetylation (acetic anhydride, pH 7.5–9.0, 1–2 h, 20–25 °C) and (vinyl acetate, pH 9–10, 1–2 h, 20–25 °C) | Yellow pea starch Chickpea starch Cowpea starch | The smaller the particle size of the chickpea and yellow pea, the greater the viscosity of the paste, while the particle size of the cowpea does not affect the viscosity. Acetylation using vinyl acetate produces a paste with a greater viscosity. | [62] |
| Acetylation (8% Acetic anhydride, 30 °C, pH 8, NaOH 1 M, 15–20 min) | Small granule wheat starch Large granule wheat starch | Acetylation increased paste viscosity, but PT decreased | [92] |
| Acetylation (4% NaOH, pH 7.8–8.2, 5 min) | Japonica rice starch Indica rice starch | PV and PT decreased; the reduction was greater with the single acetylation modification. BD and SB increased. | [91] |
| Acetylation-sonication (25, 40, and 25 + 40 Hz, 5 min, 45–75 °C) | Wheat starch | PV and BD increased, while PT and SB decreased | [130] |
| Acetylation-ANN | Mung bean starch | PV, BD, HPv, and CPv decreased, while PT and SB increased | [97] |
| Acetylation-retrogradation (pH 8.5, NaOH 0.5 M, 15 min) | Purple sweet potato flour and starch | Gelatinization occurs more quickly PV, BD, TV, FV, and SB decreased drastically | [94] |
| Treatments | Starch/Flour | Granule Morphology | References |
|---|---|---|---|
| Acetylated-Enzymatic | Sweet potato flour Potato flour | Aggregation of starch granules was formed, and the surface became irregular/rough. | [104] |
| Acetylation by vinyl acetate | Amaranth starch | The surface of the granules was smooth, showing, and no significant changes occurred. | [126] |
| Acetylated (pH 8, NaOH 3%, 10–20 min) | Potato starch Cassava starch | No significant changes occurred Slight granule fusion occurred | [83] |
| Acetylation (acetic acid, pH 8.0–8.5 by NaOH 0.5 N) | Corn starch Potato starch | The surface of starch granules in corn and potato starch becomes rough due to breakdown and erosion. | [44] |
| Acetylation | Potato starch | Fragmentation of starch granules | [147] |
| Acetylated (pH 8.0–8.4, NaOH 3%, 10 min) | Potato starch Corn starch | Fusion of starch granules occurred where potato starch granules were more susceptible than corn starch. The greater the concentration of reactants and DS, the greater the damage that occurred. | [77] |
| Acetylated (acetic anhydride) and acetylated distarch adipate (acetic anhydride and adipic acid) | Potato starch | Hole formation occurred in the middle of the granules (doughnut-like forms) in acetylated starch. There was no significant change, but the modified acetylated distarch adipate starch had a more compact surface. | [158] |
| Acetylated (pH 8.0–8.4, NaOH 3%, 10 and 240 min) | Purple yam (PY) starch White yam (WY) starch | Holes appeared, but starch granules tended to retain their shape | [84] |
| Acetylated (pH 8, NaOH 1 M, 60 min) | Corn starch | Granule aggregation occurred; the aggregation between granules was getting bigger along with the greater concentration of acetic anhydride. | [87] |
| Acetylated (pH 8, NaOH 50%, 15–240 min) | High-amylose maize starch | In the largest DS (2.23), granule fusion occurred, which caused the surface to become rougher and shaped like fibers. Granular damage was getting bigger along with the bigger the DS value. | [73] |
| Acetylated (pH 8, NaOH 3%) | Rice starch | Aggregation and deformation of starch granules occurred The size of the starch granules became smaller Starch granules became hollow | [88] |
| Acetylated (pH 8–8.5, NaOH 1 M, 5 min) | Oat starch | The texture of the starch granules becomes coarser, and small pores appear on the surface. | [89] |
| Acetylated (pH 8.0–8.4, NaOH 1 M, 30 min) | Sword bean starch | Some of the starch granules were broken (<10%) and caused the surface texture to become rough. | [90] |
| Acetylated (8% Acetic anhydride, 30 °C, pH 8, NaOH 1 M, 15–20 min) | Small granule wheat starch Large granule wheat starch | Starch granule damaged Starch with a larger granule size was easily damaged | [92] |
| Acetylation (Acetic anhydride 6% and 8%, pH 8.0–8.4, NaOH 3%, T = 25 °C, t = 10 min.) | Oat starch | The whole surface of starch granules was slightly damaged. | [21] |
| Acetylated and HMT | Buckwheat seed starch | The granules decreased slightly in size and became more separated from each other. Combination with HMT caused some starch granules to perforate. | [59] |
| Cross-linked Acetylation starch (Acetic anhydride, pH 8, and sodium trimetaphosphate 0.7–0.9%) | Maize starch | No significant changes occurred Starch granules have smooth surfaces and clear edges. | [60] |
| Acetylated and retrogradation (pH 8.5, NaOH 0.5 M, 15 min) | Purple sweet potato starch and flour | Acetylation caused granule aggregation and an increase in size Modification of acetylation + retrogradation resulted in a more compact structure, where the granule structure of starch is more compact than that of flour | [94] |
| Acetylated -sonication (25, 40, 65 Hz, 5 min, 45–75 °C) | Wheat starch | Starch granule fusion occurred There were holes and cracks on the surface of the starch granules | [130] |
| Acetylated and ANN | Mung bean starch | The granules became weaker and had a rougher surface The distance between granules became more tenuous, especially in dual-modified starch | [97] |
| Acetylated and ANN | Waxy potato (WP) starch Waxy rice (WR) starch Waxy barley (WB) starch Waxy corn (WC) starch | Acetylated starch had a rougher surface than native starch; the greater the damage, the greater the DS. The combination of acetylation + ANN caused a slight change in the granule surface in the form of a rougher surface. | [10] |
| Treatments | Starch/Flour | Crystallinity | References |
|---|---|---|---|
| Acetylation by acetic anhydride and toluene sulfuric acid | Corn starch | Acetylation reduced the degree of crystallinity of corn starch by increasing the amorphous regions. Acetylated starch with a DS of 3 had a slightly higher Tg than acetylated starch with a DS of 1.5. | [81] |
| Acetylation | Sweet potato Potato | There was no significant change in the crystalline region | [82] |
| Acetylation by vinyl acetate | Amaranth starch | There was no change in the crystalline diffraction pattern, but the relative crystallinity (RC) decreased. | [126] |
| Acetylation (pH 8, NaOH 3%, 10–20 min) | Potato starch Cassava starch | There was no change in the crystalline diffraction pattern, but the crystallinity index increased. | [83] |
| Acetylation | Potato starch | The degree of crystallinity increased | [147] |
| Acetylation (pH 8, NaOH 3%, 30–55 min) | Banggai yam starch | The crystalline diffraction pattern remained the same, but the degree of crystallinity increased. Acetylation for 50 min had the highest degree of crystallinity | [175] |
| Acetylation (pH 8.0–8.4, NaOH 3%, 10 and 240 min) | Purple yam (PY) starch White yam (WY) starch | The type of crystallinity remained the same, but the degree of crystallinity decreased with increasing DS. | [84] |
| Acetylation (pH 8.0–8.5, NaOH 1 M, 5 min) | Cocoyam | The crystalline diffraction pattern and degree of crystallinity did not change significantly. | [85] |
| Acetylation (NaOH 20%, 40 min, and NaOH 2%, 120 min) | Waxy maize starch | The crystalline diffraction pattern remained the same, but there was a decrease in the degree of crystallinity. | [86] |
| Acetylation (pH 8.0–8.5, NaOH 1 N, KOH 1 N, and Ca(OH)2 1 N, 60 min) | Waxy maize starch | The crystalline diffraction pattern remained the same. | [61] |
| Acetylation (pH 8, NaOH 50%, 15–240 min) | High amylose maize starch | The crystalline diffraction pattern remained the same, but an increase in the degree of crystallinity. | [73] |
| Acetylation (pH 8–8.5, NaOH 1 M, 5 min) | Oat starch | The crystalline diffraction pattern remained the same, but there was a decrease in the degree of crystallinity. | [89] |
| Acetylation (pH 8.0–8.4, NaOH 1 M, 30 min) | Sword bean starch | There was a change in the type of starch crystallinity from type B to type C | [90] |
| Acetylation (acetic anhydride, pH 7.5–9.0, 1–2 h, 20–25 °C) and (vinyl acetate, pH 9–10, 1–2 h, 20–25 °C) | Yellow pea starch Chickpea starch Cowpea starch | The crystalline diffraction pattern and the degree of crystallinity of the starch did not change significantly. | [62] |
| Acetylation (acetic acid, pH 8.0–8.5 by NaOH 0.5 N) | Corn starch Potato starch | There was a similar diffraction pattern of starch crystallinity but a slight loss in the degree of starch crystallinity. | [44] |
| Acetylation | Corn starch Waxy corn starch | The crystalline diffraction pattern remained the same, but there was a decrease in the degree of starch crystallinity. | [79] |
| ANN-Acetylation | Mung bean starch | The crystalline diffraction pattern remained the same, The crystalline index increased, where the crystallinity of ANN was greater than the crystallinity of the dual modification. | [97] |
| ANN-Acetylation | Waxy corn (WC) starch Waxy barley (WB) starch Waxy rice (WR) starch Waxy potato (WP) starch | The degree of crystallinity decreased in acetylated starch, where the greater the DS, the greater the decrease. The greatest decrease in crystallinity occurred in the dual modification (ANN- Acetylation) | [10] |
| Sonication- Acetylation (25, 40, and 25 + 40 Hz, 5 min, 45–75 °C) | Wheat starch | There was a change in the type of starch crystallinity and a decrease in the degree of starch crystallinity as the sonication frequency increased in the dual modification (sonication-acetylation). | [130] |
| Parameters | Acetylated Starch | Oxidized Starch | Crosslinked Starch | Hydrothermal Starch (HMT and Others) |
|---|---|---|---|---|
| Energy consumption for process modification | Low | Low | Low | High |
| Environmental friendliness | Less environmentally friendly | Less environmentally friendly | Less environmentally friendly | Environmentally friendly |
| Starch paste clarity | Paste clarity increases | Paste clarity increases | Paste clarity increases | Paste clarity decreases |
| Starch solubility | Solubility increases | Solubility increases | Solubility decreases | Solubility decreases |
| Swelling power (SP) | SP increases | SP increases | SP decreases | SP increases |
| Water absorption capacity (WAC) | WAC increases | WAC increases | WAC increases | WAC increases |
| Stability at high temperatures | The stability decreases | The stability increases | The stability increases | The stability increases |
| Stability against retrogradation | The stability increases | The stability increases | The stability decreases | The stability decreases |
| Degree of crystallinity | The degree of crystallinity decreases | The degree of crystallinity decreases | The degree of crystallinity increases | The degree of crystallinity increases |
| Starch granule morphology | The starch granules are rougher | The starch granules are rougher and more porous | The starch granules are rougher | The starch granules are rougher |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Subroto, E.; Cahyana, Y.; Indiarto, R.; Rahmah, T.A. Modification of Starches and Flours by Acetylation and Its Dual Modifications: A Review of Impact on Physicochemical Properties and Their Applications. Polymers 2023, 15, 2990. https://doi.org/10.3390/polym15142990
Subroto E, Cahyana Y, Indiarto R, Rahmah TA. Modification of Starches and Flours by Acetylation and Its Dual Modifications: A Review of Impact on Physicochemical Properties and Their Applications. Polymers. 2023; 15(14):2990. https://doi.org/10.3390/polym15142990
Chicago/Turabian StyleSubroto, Edy, Yana Cahyana, Rossi Indiarto, and Tiara Aray Rahmah. 2023. "Modification of Starches and Flours by Acetylation and Its Dual Modifications: A Review of Impact on Physicochemical Properties and Their Applications" Polymers 15, no. 14: 2990. https://doi.org/10.3390/polym15142990
APA StyleSubroto, E., Cahyana, Y., Indiarto, R., & Rahmah, T. A. (2023). Modification of Starches and Flours by Acetylation and Its Dual Modifications: A Review of Impact on Physicochemical Properties and Their Applications. Polymers, 15(14), 2990. https://doi.org/10.3390/polym15142990






