Structural and Theoretical Study of Copper(II)-5-fluoro Uracil Acetate Coordination Compounds: Single-Crystal to Single-Crystal Transformation as Possible Humidity Sensor
Abstract
:1. Introduction
2. Materials and Methods
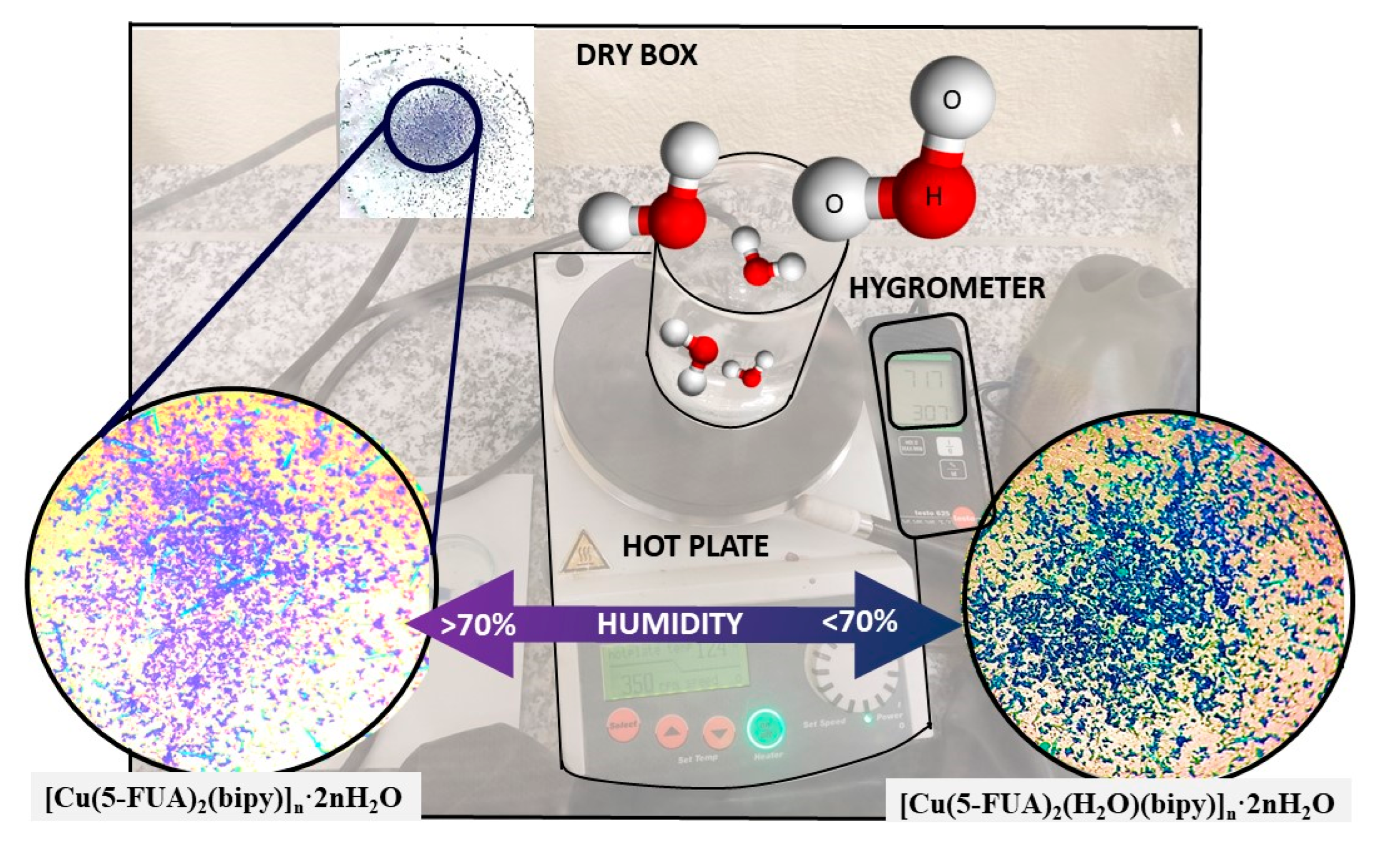
3. Results and Discussion
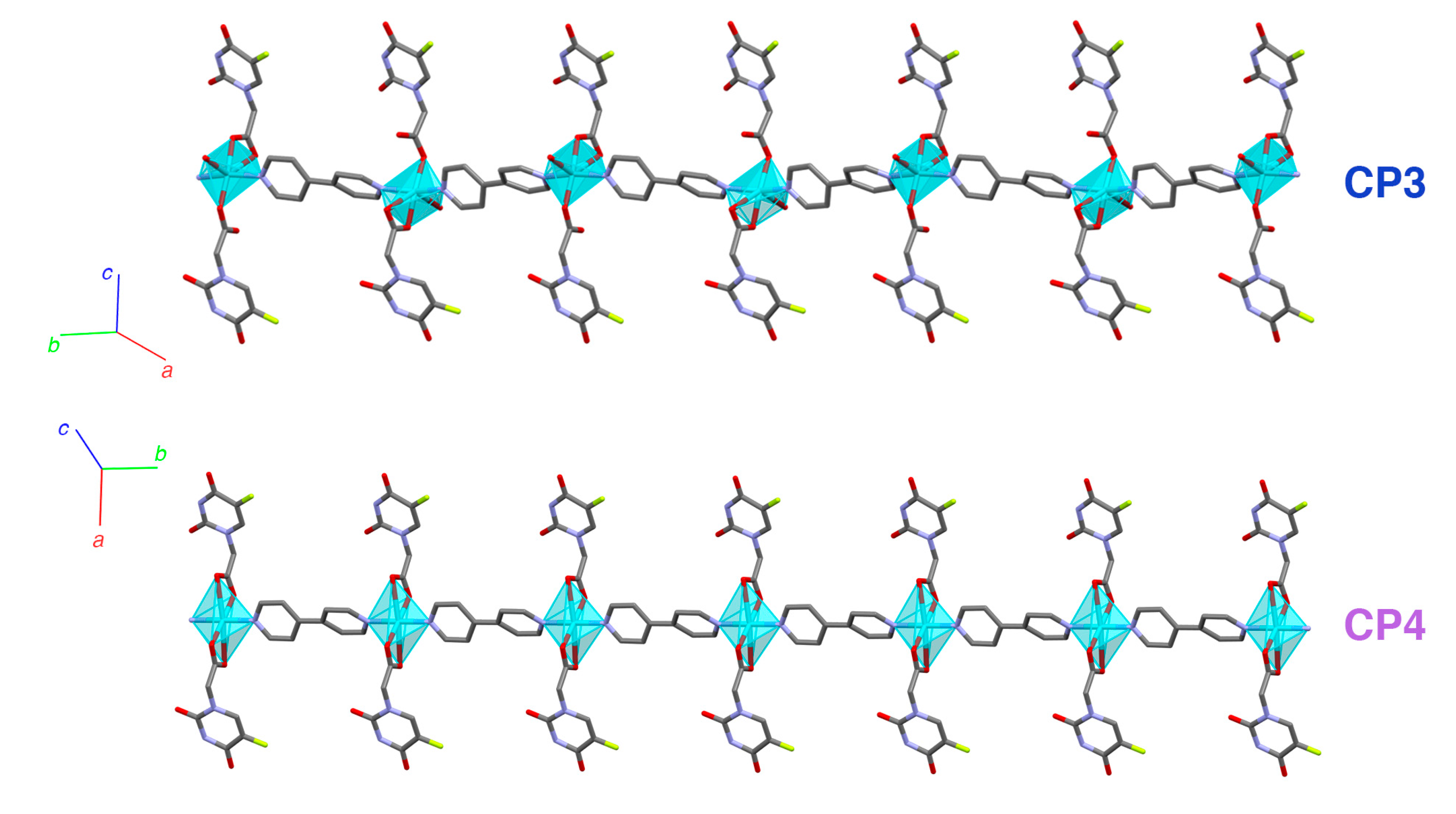
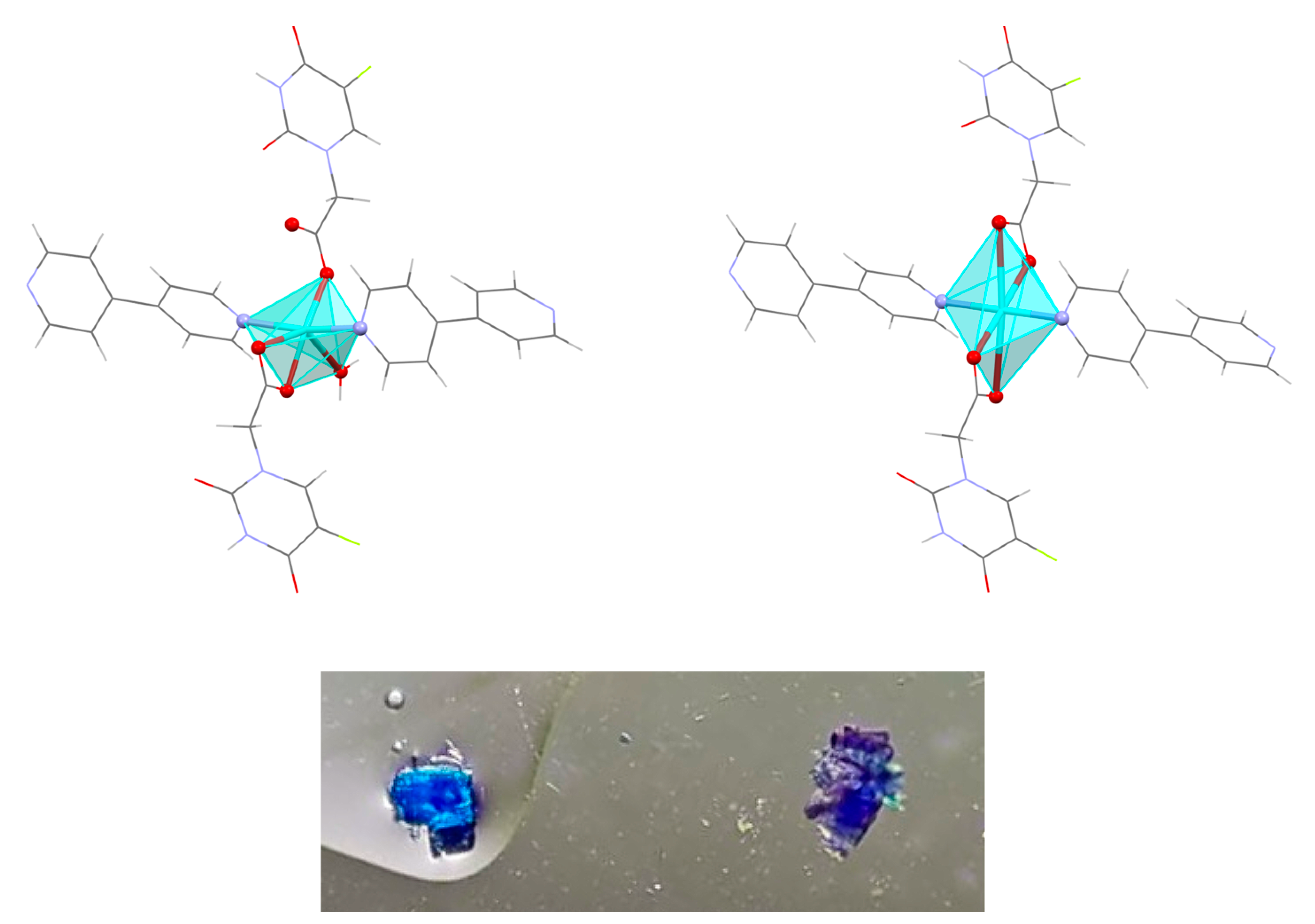
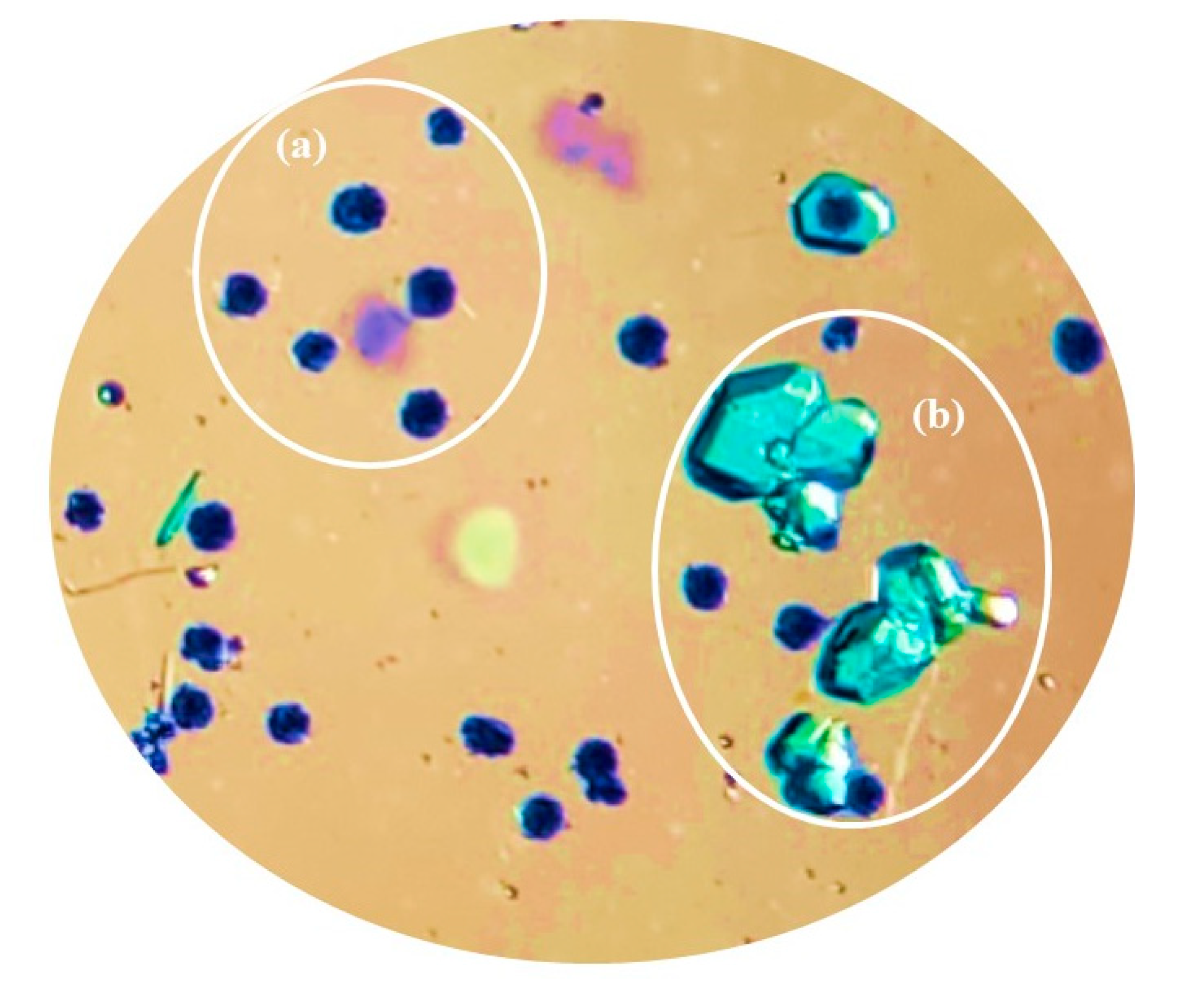
Structural Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Batten, S.R.N.; Neville, S.M.; Turner, D. Coordination Polymers: Design, Analysis and Applications; RSC Publishing: Cambridge, UK, 2009; p. 7. [Google Scholar]
- Batten, S.R.; Chen, B.; Vittal, J.J. Coordination Polymers/MOFs: Structures, Properties and Applications. Chempluschem 2016, 81, 669–670. [Google Scholar] [CrossRef] [PubMed]
- Krasnovskaya, O.; Naumov, A.; Guk, D.; Gorelkin, P.; Erofeev, A.; Beloglazkina, E.; Majouga, A. Copper Coordination Compounds as Biologically Active Agents. Int. J. Mol. Sci. 2020, 21, 3965. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; AlAjmi, M.F.; Rehman, M.T.; Amir, S.; Husain, F.M.; Alsalme, A.; Siddiqui, M.A.; AlKhedhairy, A.A.; Khan, R.A. Copper(II) complexes as potential anticancer and Nonsteroidal anti-inflammatory agents: In vitro and in vivo studies. Sci. Rep. 2019, 9, 5237–5254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.-Y.; Zhou, P.; Wang, Y.; Duan, R.; Chen, C.; Song, W.; Zhao, J. Copper-Based Coordination Polymer Nanostructure for Visible Light Photocatalysis. Adv. Mater. 2016, 28, 9657–9868. [Google Scholar] [CrossRef] [PubMed]
- Troyano, J.; Perles, J.; Amo-Ochoa, P.; Martínez, J.I.; Zamora, F.; Delgado, S. Reversible recrystallization process of copper and silver thioacetamide–halide coordination polymers and their basic building blocks. Crystengcomm 2014, 16, 8224–8231. [Google Scholar] [CrossRef]
- Vegas, V.G.; Villar-Alonso, M.; Gómez-García, C.J.; Zamora, F.; Amo-Ochoa, P. Direct Formation of Sub-Micron and Nanoparticles of a Bioinspired Coordination Polymer Based on Copper with Adenine. Polymers 2017, 9, 565. [Google Scholar] [CrossRef] [Green Version]
- Amo-Ochoa, P.; Castillo, O.; Gómez-García, C.J.; Hassanein, K.; Verma, S.; Kumar, J.; Zamora, F. Semiconductive and Magnetic One-Dimensional Coordination Polymers of Cu(II) with Modified Nucleobases. Inorg. Chem. 2013, 52, 11428–11437. [Google Scholar] [CrossRef]
- Usman, M.; Mendiratta, S.; Lu, K.-L. Semiconductor Metal-Organic Frameworks: Future Low-Bandgap Materials. Adv. Mater. 2017, 29, 1605071. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, M.; Amo-Ochoa, P.; Freire, E.; Suárez, S.; Halac, E.; Baggio, R. Structure and electrical properties of a one-dimensional polymeric silver thiosaccharinate complex with argentophilic interactions. Acta Crystallogr. 2018, 74, 186–193. [Google Scholar] [CrossRef]
- Amo-Ochoa, P.; Alexandre, S.S.; Hribesh, S.; Galindo, M.A.; Castillo, O.; Gómez-García, C.J.; Pike, A.R.; Soler, J.M.; Houlton, A.; Harrington, R.W. Coordination Chemistry of 6-Thioguanine Derivatives with Cobalt: Toward Formation of Electrical Conductive One-Dimensional Coordination Polymers. Inorg. Chem. 2013, 52, 7306. [Google Scholar] [CrossRef]
- Rowsell, J.L.C.; Yaghi, O.M. Metal–organic frameworks: A new class of porous materials. Microporous Mesoporous Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 974–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; McHale, R. Metal-Containing Polymers: Building Blocks for Functional (Nano)Materials. Macromol. Rapid Commun. 2010, 31, 331–350. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, Z.-O.; Ren, Z.-G.; Li, H.-X.; Li, D.-X.; Liu, D.; Zhang, Y.; Lang, J.-P. Solvothermal Stepwise Formation of Cu/I/S-Based Semiconductors from a Three-Dimensional Net to One-Dimensional Chains. Cryst. Growth Des. 2009, 9, 4963–4968. [Google Scholar] [CrossRef]
- Chen, X.-M.; Tong, M.-L. Solvothermal in Situ Metal/Ligand Reactions: A New Bridge between Coordination Chemistry and Organic Synthetic Chemistry. Acc. Chem. Res. 2007, 40, 162–170. [Google Scholar] [CrossRef]
- Han, Z.-P.; Li, Y. Solvothermal synthesis, structure and catalytic activity of a mixed-valence CuI/CuII complex with 1-D chain structure. Inorg. Chem. Commun. 2012, 22, 73–76. [Google Scholar] [CrossRef]
- Murillo, M.; García-Hernan, A.; López, J.; Perles, J.; Brito, I.; Amo-Ochoa, P. The flexibility of CuI chains and the functionality of pyrazine-2-thiocarboxamide keys to obtaining new Cu(I)-I coordination polymers with potential use as photocatalysts for organic dye degradation. Catal. Today 2023, 418, 114072–114081. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, W.-X.; Ye, B.-H.; Lin, J.-B.; Chen, X.-M. In Situ Solvothermal Generation of 1,2,4-Triazolates and Related Compounds from Organonitrile and Hydrazine Hydrate: A Mechanism Study. Inorg. Chem. 2007, 46, 1135–1143. [Google Scholar] [CrossRef]
- Das, M.C.; Bharadwaj, P.K. A Porous Coordination Polymer Exhibiting Reversible Single-Crystal to Single-Crystal Substitution Reactions at Mn(II) Centers by Nitrile Guest Molecules. J. Am. Chem. Soc. 2009, 131, 10942–10949. [Google Scholar] [CrossRef]
- Kobayashi, A.; Kato, M. Stimuli-responsive Luminescent Copper(I) Complexes for Intelligent Emissive Devices. Chem. Lett. 2017, 46, 154–162. [Google Scholar] [CrossRef]
- Chen, W.-H.; Liao, W.-C.; Sohn, Y.S.; Fadeev, M.; Cecconello, A.; Nechushtai, R.; Willner, I. Stimuli-Responsive Nucleic Acid-Based Polyacrylamide Hydrogel-Coated Metal-Organic Framework Nanoparticles for Controlled Drug Release. Adv. Funct. Mater. 2018, 28, 1705137–1705146. [Google Scholar] [CrossRef]
- Nishikawa, M.; Kume, S.; Nishihara, H. Stimuli-responsive pyrimidine ring rotation in copper complexes for switching their physical properties. Phys. Chem. Chem. Phys. 2013, 15, 10549–10565. [Google Scholar] [CrossRef] [PubMed]
- Burneo, I.; Stylianou, K.C.; Rodríguez-Hermida, S.; Juanhuix, J.; Fontrodona, X.; Imaz, I.; Maspoch, D. Two New Adenine-Based Co(II) Coordination Polymers: Synthesis, Crystal Structure, Coordination Modes, and Reversible Hydrochromic Behavior. Cryst. Growth Des. 2015, 15, 3182–3189. [Google Scholar] [CrossRef] [Green Version]
- Ke, S.-Y.; Chang, Y.-F.; Wang, H.-Y.; Yang, C.-C.; Ni, C.-W.; Lin, G.-Y.; Chen, T.-T.; Ho, M.-L.; Lee, G.-H.; Chuang, Y.-C.; et al. Self-Assembly of Four Coordination Polymers in Three-Dimensional Entangled Architecture Showing Reversible Dynamic Solid-State Structural Transformation and Color-Changing Behavior upon Thermal Dehydration and Rehydration. Cryst. Growth Des. 2014, 14, 4011–4018. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, B.; Xue, J.-P.; Xie, J.; Yao, Z.-S.; Tao, J. Giant single-crystal-to-single-crystal transformations associated with chiral interconversion induced by elimination of chelating ligands. Nat. Commun. 2021, 12, 6908–6917. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, G.; Morsali, A. Crystal-to-Crystal Transformation from a Weak Hydrogen-Bonded Two-Dimensional Network Structure to a Two-Dimensional Coordination Polymer on Heating. Cryst. Growth Des. 2008, 8, 391–394. [Google Scholar] [CrossRef]
- Sarma, D.; Natarajan, S. Usefulness of in Situ Single Crystal to Single Crystal Transformation (SCSC) Studies in Understanding the Temperature-Dependent Dimensionality Cross-over and Structural Reorganization in Copper-Containing Metal–Organic Frameworks (MOFs). Cryst. Growth Des. 2011, 11, 5415–5423. [Google Scholar] [CrossRef]
- Aríñez-Soriano, J.; Albalad, J.; Vila-Parrondo, C.; Pérez-Carvajal, J.; Rodríguez-Hermida, S.; Cabeza, A.; Juanhuix, J.; Imaz, I.; Maspoch, D. Single-crystal and humidity-controlled powder diffraction study of the breathing effect in a metal–organic framework upon water adsorption/desorption. Chem. Commun. 2016, 52, 7229–7232. [Google Scholar] [CrossRef] [Green Version]
- Daszkiewicz, M.; Puszyńska-Tuszkanow, M.; Staszak, Z.; Chojnacka, I.; Fałtynowicz, H.; Cieślak-Golonka, M. Single crystal-to-single crystal transformations induced by ammonia–water equilibrium changes. Crystengcomm 2018, 20, 2907–2911. [Google Scholar] [CrossRef]
- Vegas, V.G.; Latorre, A.; Marcos, M.L.; Gómez-García, C.J.; Castillo, O.; Zamora, F.; Gómez, J.; Martínez-Costas, J.; López, M.V.; Somoza, A.; et al. Rational Design of Copper(II)–Uracil Nanoprocessed Coordination Polymers to Improve Their Cytotoxic Activity in Biological Media. ACS Appl. Mater. Interfaces 2021, 13, 36948–36957. [Google Scholar] [CrossRef]
- Rao, X.; Zhao, L.; Xu, L.; Wang, Y.; Liu, K.; Wang, Y.; Chen, G.Y.; Liu, T.; Wang, Y. Review of Optical Humidity Sensors. Sensors 2021, 21, 8049. [Google Scholar] [CrossRef] [PubMed]
- Montes-García, V.; Samorì, P. Humidity Sensing with Supramolecular Nanostructures. Adv. Mater. 2022, 2208766. [Google Scholar] [CrossRef]
- Chou, K.-S.; Lee, T.-K.; Liu, F.-J. Sensing mechanism of a porous ceramic as humidity sensor. Sens. Actuators B Chem. 1999, 56, 106–111. [Google Scholar] [CrossRef]
- Li, Y.; Yang, M.; Camaioni, N.; Casalbore-Miceli, G. Humidity sensors based on polymer solid electrolytes: Investigation on the capacitive and resistive devices construction. Sens. Actuators B Chem. 2001, 77, 625–631. [Google Scholar] [CrossRef]
- Coleman, J.R.; Meggers, F. Sensing of Indoor Air Quality—Characterization of Spatial and Temporal Pollutant Evolution Through Distributed Sensing. Front. Built Environ. 2018, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- McMullan, R. Environmental Science in Building; Bloomsbury Publishing: London, UK, 2017. [Google Scholar]
- Masao, T. Antineoplastic Agents. The Preparation of 5-Fluorouracil-1-acetic Acid Derivatives. Bull. Chem. Soc. Jpn. 1975, 48, 3427–3428. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SADABS, Program for Area Detector Adsorption Correction; Institute for Inorganic Chemistry, University of Gottingen: Göttingen, Germany, 1996. [Google Scholar]
- Bruker; AXS Inc.: Madison, WI, USA, 2012.
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. SHELXL-97, Program for Crystal Structure Refinement; University Gottingen: Gottingen, Germany, 1997. [Google Scholar]
- Frisch, M.; Cahill, C.L. In situ ligand synthesis with the UO22+ cation under hydrothermal conditions. J. Solid State Chem. 2007, 180, 2597–2602. [Google Scholar] [CrossRef]
- Jin, H.-G.; Wang, M.-F.; Hong, X.-J.; Yang, J.; Li, T.; Ou, Y.-J.; Zhao, L.-Z.; Cai, Y.-P. 2D pillar-chained lanthanide(III)-copper(I) metal–organic frameworks based on isonicotinate and in situ generated oxalate. Inorg. Chem. Commun. 2013, 36, 236–240. [Google Scholar] [CrossRef]
- Vegas, V.G.; Maldonado, N.; Castillo, O.; Gómez-García, C.J.; Amo-Ochoa, P. Multifunctional coordination polymers based on copper with modified nucleobases, easily modulated in size and conductivity. J. Inorg. Biochem. 2019, 200, 110805–110814. [Google Scholar] [CrossRef]
- Scano, A.; Mereu, E.; Cabras, V.; Mannias, G.; Garau, A.; Pilloni, M.; Orrù, G.; Scano, A.; Ennas, G. Green Preparation of Antimicrobial 1D-Coordination Polymers: [Zn(4,4′-bipy)Cl2]∞ and [Zn(4,4′-bipy)2(OAc)2]∞ by Ultrasonication of Zn(II) Salts and 4,4′-Bipyridine. Molecules 2022, 27, 6677. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.K.; Branton, A.; Trivedi, D.; Nayak, G.; Bairwa, K.; Jana, S. Spectroscopic Characterization of Disodium Hydrogen Orthophosphate and Sodium Nitrate after Biofield Treatment. J. Chromatogr. Sep. Tech. 2015, 6, 5. [Google Scholar] [CrossRef] [Green Version]
- Nara, M.; Torii, H.; Tasumi, M. Correlation between the Vibrational Frequencies of the Carboxylate Group and the Types of Its Coordination to a Metal Ion: An ab Initio Molecular Orbital Study. J. Phys. Chem. 1996, 100, 19812–19817. [Google Scholar] [CrossRef]
- Maldonado, N.; Vegas, V.G.; Halevi, O.; Martínez, J.I.; Lee, P.S.; Magdassi, S.; Wharmby, M.T.; Platero-Prats, A.E.; Moreno, C.; Zamora, F.; et al. 3D Printing of a Thermo- and Solvatochromic Composite Material Based on a Cu(II)–Thymine Coordination Polymer with Moisture Sensing Capabilities. Adv. Funct. Mater. 2019, 29, 1808424–1808435. [Google Scholar] [CrossRef]
- Jimenez-Bescos, C.; Prewett, R. Monitoring IAQ and thermal comfort in a conservation area low energy retrofit. In Proceedings of the International Scientific Conference on Environmental and Climate Technologies (CONECT), Riga, Latvia, 16–18 May 2018; Volume 147, pp. 195–201. [Google Scholar]
- Silva, H.E.; Coelho, G.B.A.; Henriques, F.M.A. Climate monitoring in World Heritage List buildings with low-cost data loggers: The case of the Jerónimos Monastery in Lisbon (Portugal). J. Build. Eng. 2020, 28, 101029–1011046. [Google Scholar] [CrossRef]
- Gu, H.-T.; Yang, Z.-H.; Fan, Z.; Jiang, W. Real-time in situ visualization of internal relative humidity in fluorescence embedded cement-based materials. J. Cent. South Univ. 2021, 28, 3790–3799. [Google Scholar] [CrossRef]
- Qabbal, L.; Younsi, Z.; Naji, H. An indoor air quality and thermal comfort appraisal in a retrofitted university building via low-cost smart sensor. Indoor Built Environ. 2022, 31, 586–606. [Google Scholar] [CrossRef]
- Castillo, O.; Alonso, J.; García-Couceiro, U.; Luque, A.; Román, P. A 2D polymer constructed through bridging oxalato and 4,4′-bipyridine ligands: Crystal structure and magnetic behavior of [Cu3(μ-ox)3(μ-4,4′-bpy)2(4,4′-bpy)2]n. Inorg. Chem. Commun. 2003, 6, 803–806. [Google Scholar] [CrossRef]
- Mantasha, I.; Hussain, S.; Ahmad, M.; Shahid, M. Two dimensional (2D) molecular frameworks for rapid and selective adsorption of hazardous aromatic dyes from aqueous phase. Sep. Purif. Technol. 2020, 238, 116413. [Google Scholar] [CrossRef]
- Huang, J.; Li, Y.-Z.; Sun, G.-C.; Dai, R.-B.; Li, Q.-X.; Wang, L.-F.; Xia, C.-G. Tetraaqua(5-fluorouracil-1-acetato-O)copper(II) tetrahydrate. Acta Crystallogr. Sect. C 2000, 56, 489–490. [Google Scholar] [CrossRef]
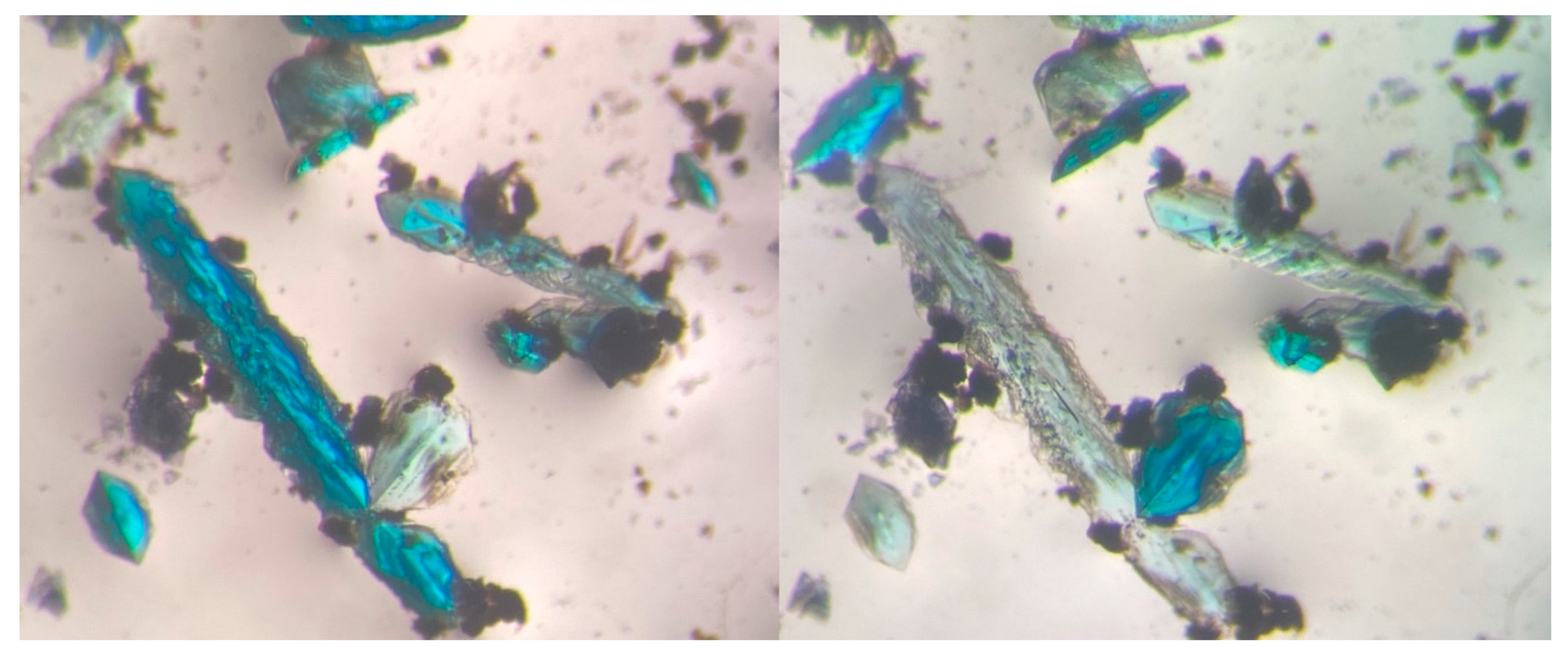


| Time | 0 (Initial) | ½ Day | 6 Days | 30 Days |
|---|---|---|---|---|
| CCs and concentration variation | [(H2bipy)+2 2NO3−] (1) | Stable | Decrease | Decrease |
| [Cu2(5-FUA)2(ox)(bipy)]n ·2n H2O (CP2) | Stable | Decrease | [Cu3(ox)3(bipy)4]n [Cu(ox)(bipy)]n | |
| [Cu(5-FUA)2(H2O)(bipy)]n ·2n H2O (CP3) | Increase | Increase | Decrease | |
| [Cu(5-FUA)2(bipy)]n ·3.5n H2O | Increase | Increase |
| Time | ½ Day | 6 Days | 30 Days |
|---|---|---|---|
| CCs and concentration variation | [Cu(5-FUA)2(H2O)4]·4H2O | Increase | |
| [Cu(5-FUA)2(H2O)(bipy)]n ·2n H2O (CP3) | Increase | Decrease | |
| [Cu(5-FUA)2(bipy)]n ·3.5n H2O | Increase | Increase |
| Compound | Relative Energy (eV) |
|---|---|
| [Cu(5-FUA)2(H2O)(bipy)]n·2nH2O | 0.00 |
| [Cu(5-FUA)2(bipy)]n·2nH2O + 8H2O (isolated) | 4.86 |
| [Cu(5-FUA)2(bipy)]n·2nH2O + 8H2O (condensed phase) | −0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vegas, V.G.; García-Hernán, A.; Aguilar-Galindo, F.; Perles, J.; Amo-Ochoa, P. Structural and Theoretical Study of Copper(II)-5-fluoro Uracil Acetate Coordination Compounds: Single-Crystal to Single-Crystal Transformation as Possible Humidity Sensor. Polymers 2023, 15, 2827. https://doi.org/10.3390/polym15132827
Vegas VG, García-Hernán A, Aguilar-Galindo F, Perles J, Amo-Ochoa P. Structural and Theoretical Study of Copper(II)-5-fluoro Uracil Acetate Coordination Compounds: Single-Crystal to Single-Crystal Transformation as Possible Humidity Sensor. Polymers. 2023; 15(13):2827. https://doi.org/10.3390/polym15132827
Chicago/Turabian StyleVegas, Verónica G., Andrea García-Hernán, Fernando Aguilar-Galindo, Josefina Perles, and Pilar Amo-Ochoa. 2023. "Structural and Theoretical Study of Copper(II)-5-fluoro Uracil Acetate Coordination Compounds: Single-Crystal to Single-Crystal Transformation as Possible Humidity Sensor" Polymers 15, no. 13: 2827. https://doi.org/10.3390/polym15132827






