Influence of Waste Material Additives on the Performance of a Novel Hybrid Sol-Gel Coating on Mild Steel in 3.5% NaCl Medium
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the Parent Hybrid Coating
2.3. Preparation of the Waste Additives-Loaded Hybrid Coatings
2.4. Surface Preparation of the MS Substrates
2.5. Deposition of Functional Coatings
2.6. Characterization of Coatings
3. Results and Discussion
3.1. FTIR Analysis of the Neat and Modified Hybrid Polymers
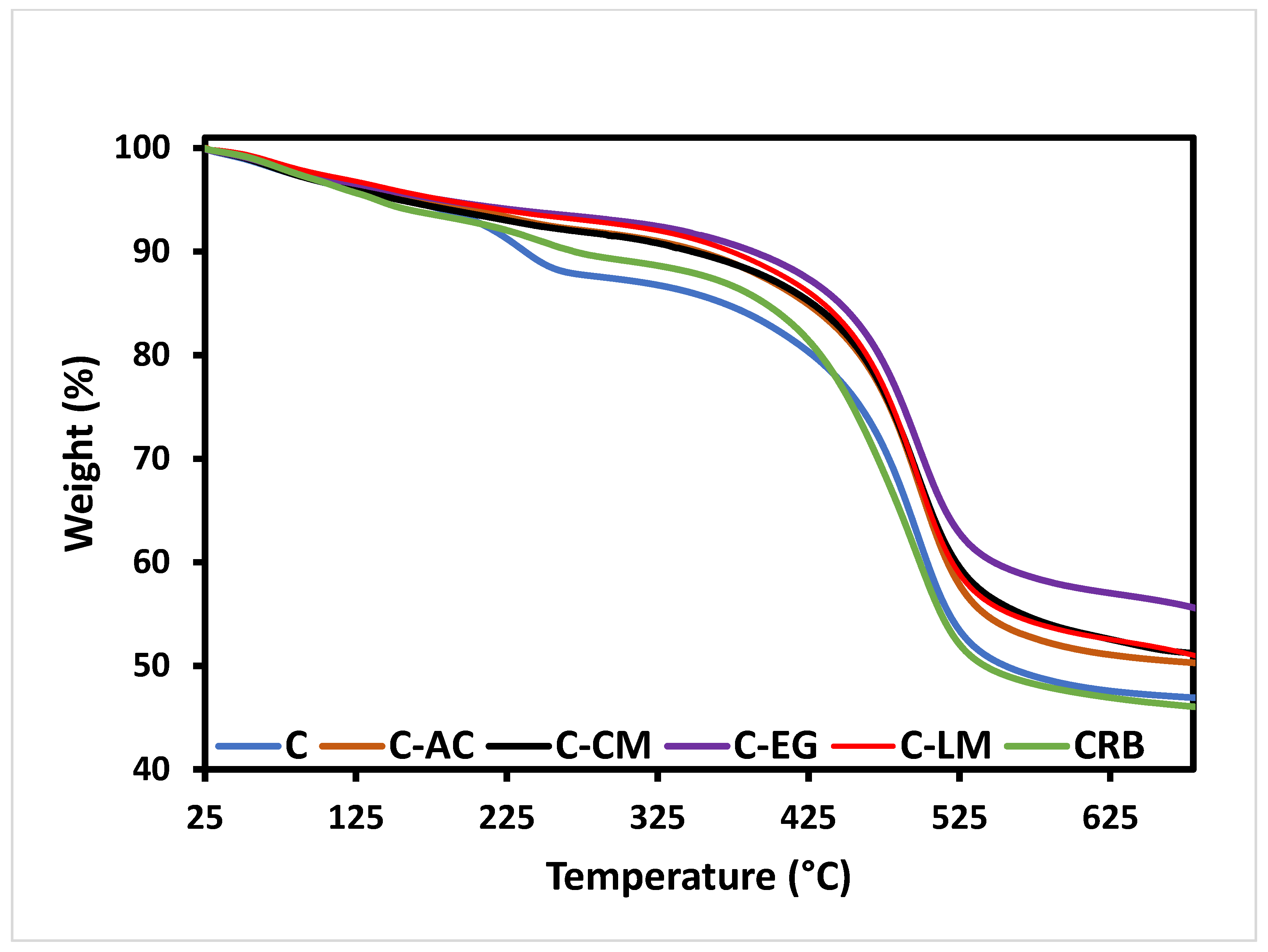
3.2. Thermal Analysis of the Coatings Matrices
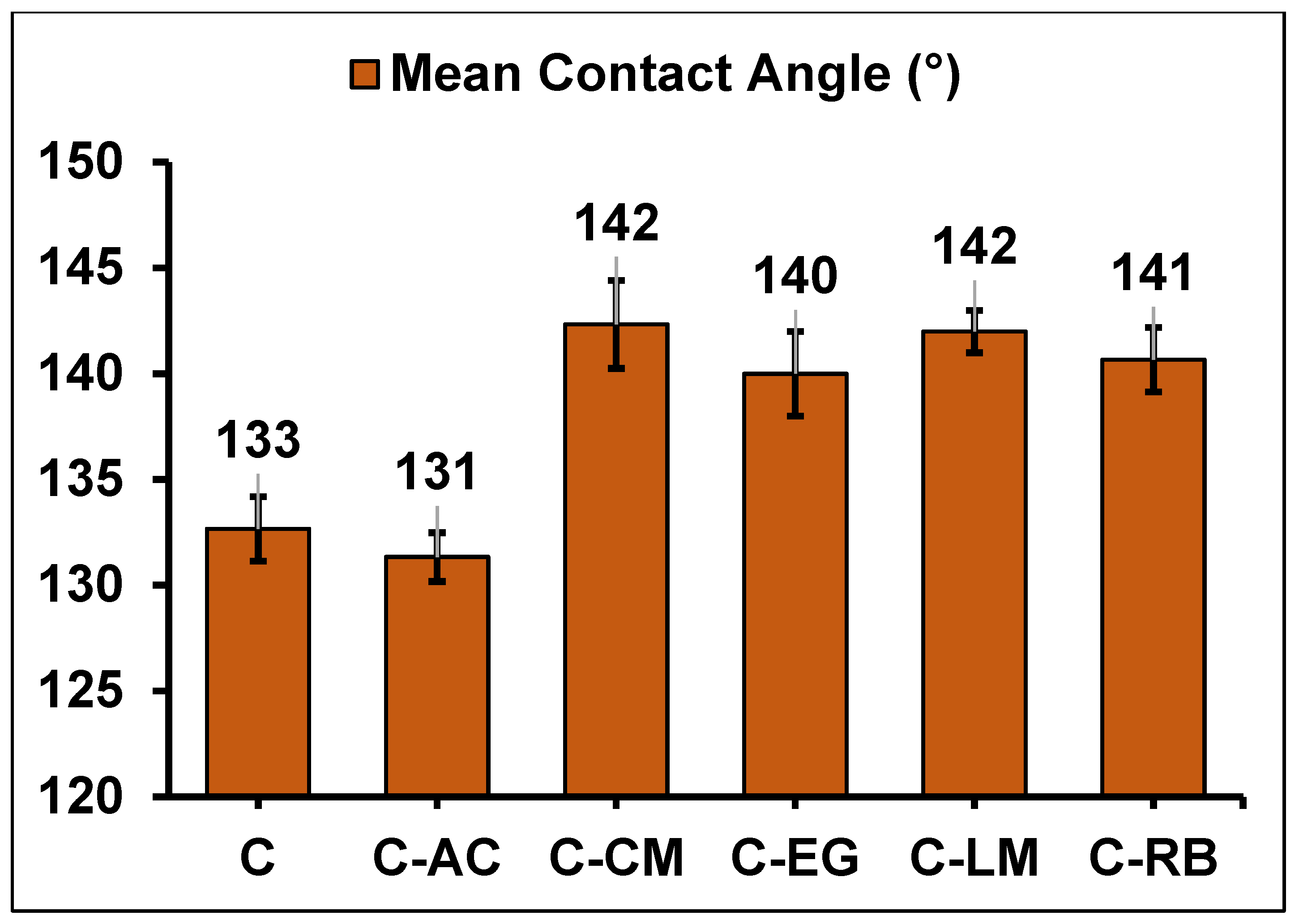
3.3. Morphological Analyses of the Coating Formulations on Steel
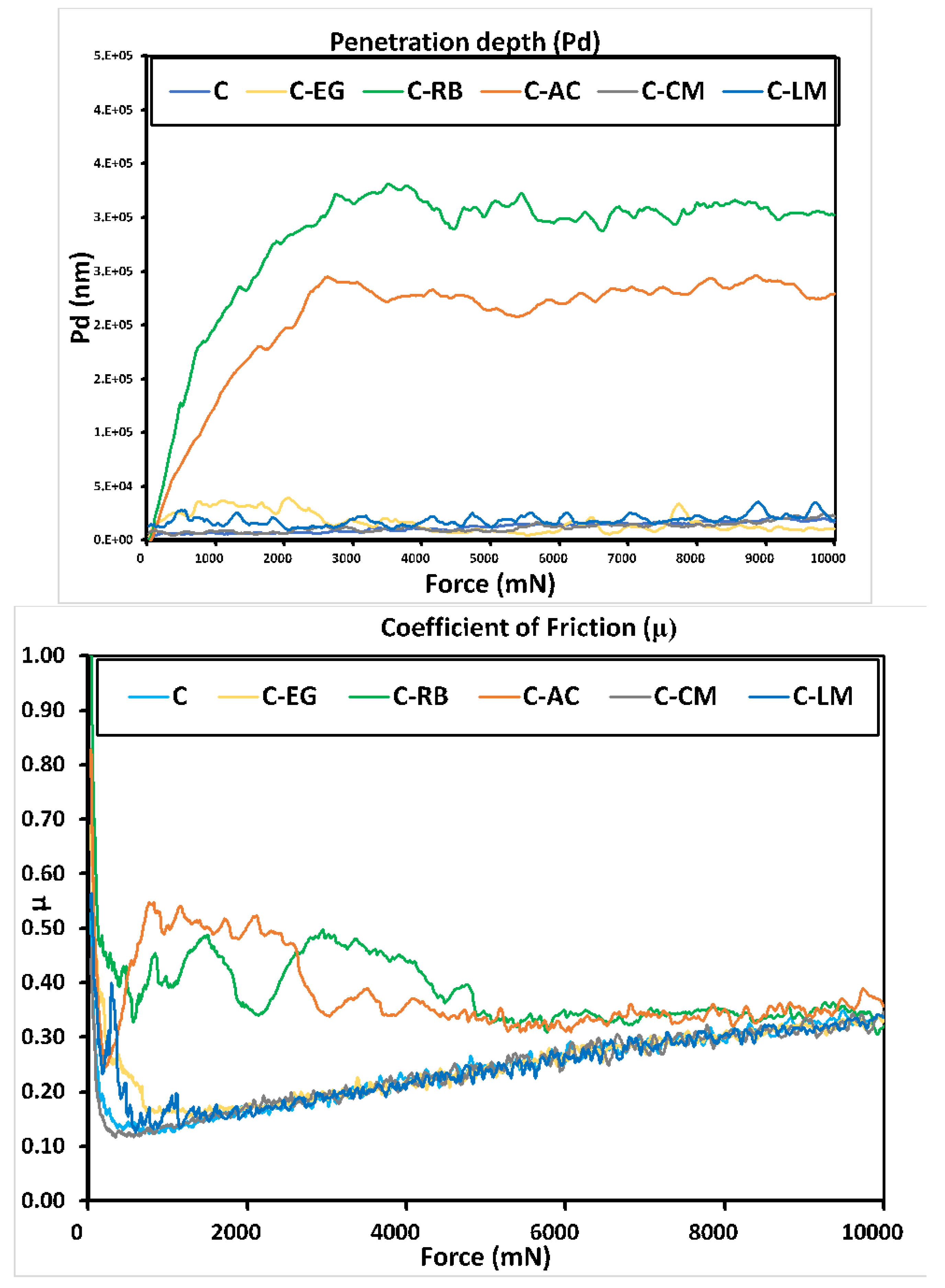
3.4. Mechanical Testing
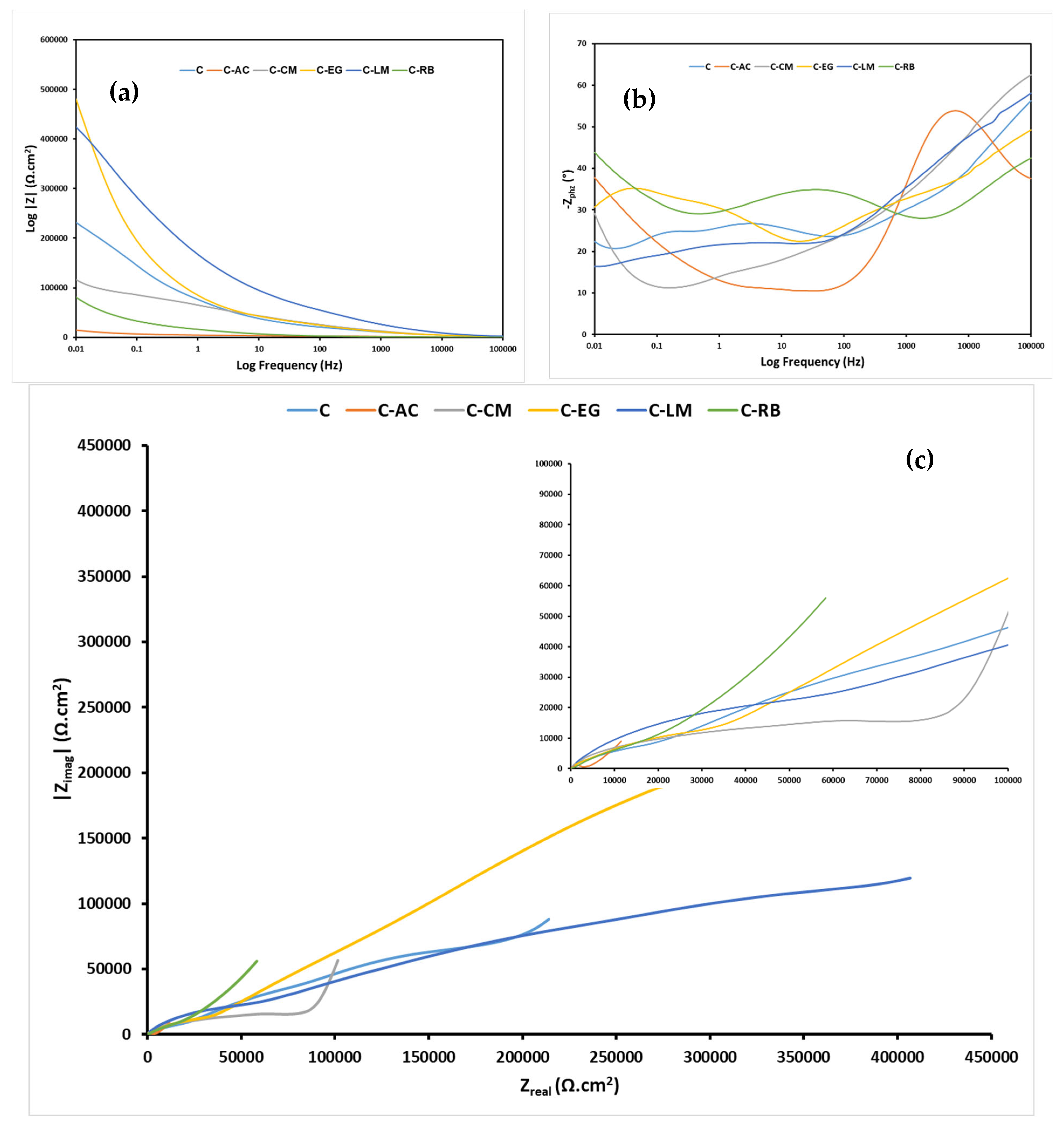
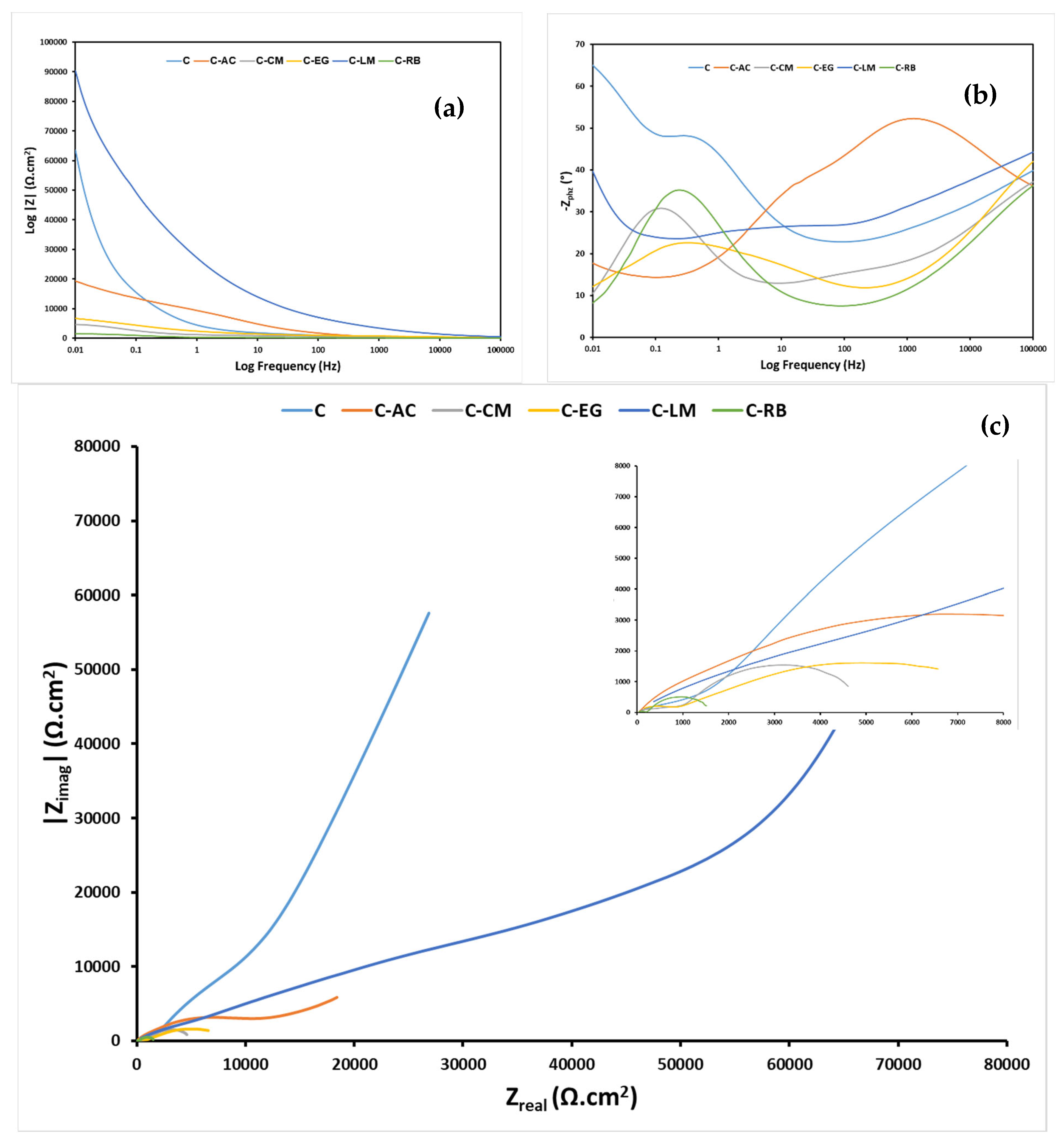
3.5. Electrochemical Assessment of the Anticorrosion Performance of the Hybrid Coating Systems on Steel Substrate
4. Conclusions
- Provide a low-cost and eco-friendly source of fillers, pigments, or modifiers for the hybrid sol-gel coatings;
- Enhance the roughness, hydrophobicity, or adhesion of the hybrid sol-gel coatings and;
- Improve the mechanical strength, electrical conductivity, or thermal stability of the hybrid sol-gel coatings by forming a percolating network or acting as a catalyst within the matrix.
- Negatively affect the corrosion resistance, barrier properties, or homogeneity of the hybrid sol-gel coatings by introducing defects, impurities, or incompatibilities in the network, and;
- Increase the complexity and variability of the hybrid sol-gel system, making it more difficult to optimize and control the coating parameters and properties.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perez, T.E. Corrosion in the Oil and Gas Industry: An Increasing Challenge for Materials. JOM 2013, 65, 1033–1042. [Google Scholar] [CrossRef] [Green Version]
- Refait, P.; Grolleau, A.-M.; Jeannin, M.; Rémazeilles, C.; Sabot, R. Corrosion of Carbon Steel in Marine Environments: Role of the Corrosion Product Layer. Corros. Mater. Degrad. 2020, 1, 198–218. [Google Scholar] [CrossRef]
- Melchers, R.E.; Jeffrey, R. Early corrosion of MS in seawater. Corros. Sci. 2005, 47, 1678–1693. [Google Scholar] [CrossRef]
- Milošev, I.; Frankel, G.S. Review—Conversion Coatings Based on Zirconium and/or Titanium. J. Electrochem. Soc. 2018, 165, C127–C144. [Google Scholar] [CrossRef]
- Figueira, R.B. Hybrid Sol–gel Coatings for Corrosion Mitigation: A Critical Review. Polymers 2020, 12, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coatings Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Balgude, D.; Sabnis, A. Sol–gel derived hybrid coatings as an environment friendly surface treatment for corrosion protection of metals and their alloys. J. Sol-Gel Sci. Technol. 2012, 64, 124–134. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 5102014. [Google Scholar] [CrossRef]
- Yilmaz, E.; Soylak, M. Functionalized nanomaterials for sample preparation methods. In Handbook of Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 375–413. [Google Scholar]
- Suleiman, R.K. Improved mechanical and anticorrosion properties of metal oxide-loaded hybrid sol–gel coatings for MS in a saline medium. J. Adhes. Sci. Technol. 2020, 34, 1315–1330. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Zheludkevich, M.; Yasakau, K.; Lamaka, S.; Ferreira, M.G.S.; Möhwald, H. Layer-by-Layer Assembled Nanocontainers for Self-Healing Corrosion Protection. Adv. Mater. 2006, 18, 1672–1678. [Google Scholar] [CrossRef]
- Mohammadzadeh, M.A.; Es’haghi, M. Novel Additive-Included Hybrid Sol–Gel Coatings for Practical Corrosion Protection of Carbon Steel in a Saline Medium. J. Mater. Eng. Perform. 2021, 30, 8395–8401. [Google Scholar] [CrossRef]
- Santana, I.; Pepe, A.; Schreiner, W.; Pellice, S.; Ceré, S. Hybrid sol-gel coatings containing clay nanoparticles for corrosion protection of MS. Electrochim. Acta 2016, 203, 396–403. [Google Scholar] [CrossRef] [Green Version]
- Kamanina, O.A.; Saverina, E.A.; Rybochkin, P.V.; Arlyapov, V.A.; Vereshchagin, A.N.; Ananikov, V.P. Preparation of Hybrid Sol-Gel Materials Based on Living Cells of Microorganisms and Their Application in Nanotechnology. Nanomaterials 2022, 12, 1086. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, R.K.; Saleh, T.A.; Hamouz, O.C.S.A.; Ibrahim, M.B.; Sorour, A.A.; Ali, B. El Corrosion and fouling protection performance of biocide-embedded hybrid organosiloxane coatings on MS in a saline medium. Surf. Coat. Technol. 2017, 324, 526–535. [Google Scholar] [CrossRef]
- Moutarlier, V.; Neveu, B.; Gigandet, M.P. Evolution of corrosion protection for sol-gel coatings doped with inorganic inhibitors. Surf. Coat. Technol. 2008, 202, 2052–2058. [Google Scholar] [CrossRef] [Green Version]
- Krzak, J.; Szczurek, A.; Babiarczuk, B.; Gąsiorek, J.; Borak, B. Sol–gel surface functionalization regardless of form and type of substrate. In Handbook of Nanomaterials for Manufacturing Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 111–147. [Google Scholar]
- Adesina, A.Y.; Zainelabdeen, I.H.; Dalhat, M.A.; Mohammed, A.S.; Sorour, A.A.; Al-Badour, F.A. Influence of micronized waste tire rubber on the mechanical and tribological properties of epoxy composite coatings. Tribol. Int. 2020, 146, 106244. [Google Scholar] [CrossRef]
- Naderi-Samani, H.; Shoja Razavi, R.; Loghman-Estarki, M.R.; Ramazani, M.; Barekat, M.; Mishra, A.K.; Fattahi, H. The effects of Cloisite 20A content on the adhesion strength and corrosion behavior of poly (amide-imide)/cloisite 20A nanocomposite coatings. Compos. Part B Eng. 2019, 175, 107154. [Google Scholar] [CrossRef]
- Morshed-Behbahani, K.; Najafisayar, P.; Hessam, R.; Zakerin, N. Characterization of Anodic Films Produced on Anodized AA1050 Aluminum Alloy: Effect of Bio-additive. Metall. Mater. Trans. A 2020, 51, 5475–5483. [Google Scholar] [CrossRef]
- Suleiman, R.K.; Adesina, A.Y.; Kumar, A.M.; Rahman, M.M.; Al-Badour, F.A.; El Ali, B. Anticorrosion Properties of a Novel Hybrid Sol–Gel Coating on Aluminum 3003 Alloy. Polymers 2022, 14, 1798. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Kumar, N.; Jyothirmayi, A.; Raju, K.R.C.S.; Uma, V.; Subasri, R. One-Step Anodization/Sol-Gel Deposition of -Doped Silica-Zirconia Self-Healing Coating on Aluminum. ISRN Corros. 2013, 2013, 424805. [Google Scholar] [CrossRef] [Green Version]
- Eduok, U.; Suleiman, R.; Khaled, M.; Akid, R. Enhancing water repellency and anticorrosion properties of a hybrid silica coating on MS. Prog. Org. Coat. 2016, 93, 97–108. [Google Scholar] [CrossRef]
- Milošev, I.; Kapun, B.; Rodič, P.; Iskra, J. Hybrid sol–gel coating agents based on zirconium(IV) propoxide and epoxysilane. J. Sol-Gel Sci. Technol. 2015, 74, 447–459. [Google Scholar] [CrossRef]
- Qu, H.; Bhattacharyya, S.; Ducheyne, P. 4.34 Sol–Gel Processed Oxide Controlled Release Materials. In Comprehensive Biomaterials II; Elsevier: Amsterdam, The Netherlands, 2017; pp. 617–643. [Google Scholar]
- Buasri, A.; Liangraksa, K.; Sirisom, T.; Tangkachalakul, N. Characterization and Thermal Properties of Sol-Gel Processed PMMA/SiO2 Hybrid Materials. Adv. Mater. Res. 2008, 55–57, 749–752. [Google Scholar] [CrossRef]
- Catauro, M.; Piccolella, S.; Leonelli, C. FT-IR Characterization of Antimicrobial Hybrid Materials through Sol-Gel Synthesis. Appl. Sci. 2020, 10, 1180. [Google Scholar] [CrossRef] [Green Version]
- Almeida, R.M.; Marques, A.C. Characterization of Sol-Gel Materials by Infrared Spectroscopy. In Handbook of Sol-Gel Science and Technology; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 1121–1151. [Google Scholar]
- Catauro, M.; Vecchio Ciprioti, S. Sol-Gel Synthesis and Characterization of Hybrid Materials for Biomedical Applications. In Thermodynamics and Biophysics of Biomedical Nanosystems; Springer Verlag: Berlin, Germany, 2019; pp. 445–475. [Google Scholar]
- Heiman-Burstein, D.; Dotan, A.; Dodiuk, H.; Kenig, S. Hybrid Sol–Gel Superhydrophobic Coatings Based on Alkyl Silane-Modified Nanosilica. Polymers 2021, 13, 539. [Google Scholar] [CrossRef]
- Rodič, P.; Korošec, R.C.; Kapun, B.; Mertelj, A.; Milošev, I. Acrylate-Based Hybrid Sol-Gel Coating for Corrosion Protection of AA7075-T6 in Aircraft Applications: The Effect of Copolymerization Time. Polymers 2020, 12, 948. [Google Scholar] [CrossRef] [Green Version]
- Juan-Díaz, M.J.; Martínez-Ibáñez, M.; Hernández-Escolano, M.; Cabedo, L.; Izquierdo, R.; Suay, J.; Gurruchaga, M.; Goñi, I. Study of the degradation of hybrid sol–gel coatings in aqueous medium. Prog. Org. Coat. 2014, 77, 1799–1806. [Google Scholar] [CrossRef]
- Danish, M. Contact Angle Studies of Hydrophobic and Hydrophilic Surfaces. In Handbook of Magnetic Hybrid Nanoalloys and their Nanocomposites; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 1–22. [Google Scholar]
- Kalidindi, R.S.R.; Subasri, R. Sol-gel nanocomposite hard coatings. In Anti-Abrasive Nanocoatings; Elsevier: Amsterdam, The Netherlands, 2015; pp. 105–136. [Google Scholar]
- Sakai, R.T.; da Cruz, F.M.D.L.; de Melo, H.G.; Benedetti, A.V.; Santilli, C.V.; Suegama, P.H. Electrochemical study of TEOS, TEOS/MPTS, MPTS/MMA and TEOS/MPTS/MMA films on tin coated steel in 3.5% NaCl solution. Prog. Org. Coat. 2012, 74, 288–301. [Google Scholar] [CrossRef]
- Al-Badour, F.A.; Adesina, A.Y.; Ibrahim, A.B.; Suleiman, R.K.; Merah, N.; Sorour, A.A. Electrochemical Investigation of the Effect of Process Parameters on the Corrosion Behavior of Aluminum-Cladded Pressure Vessel Steel using a Friction Stir Diffusion Cladding Process. Metals 2020, 10, 623. [Google Scholar] [CrossRef]
- Merino, E.; Durán, A.; Ceré, S.; Castro, Y. Hybrid Epoxy-Alkyl Sol–Gel Coatings Reinforced with SiO2 Nanoparticles for Corrosion Protection of Anodized AZ31B Mg Alloy. Gels 2022, 8, 242. [Google Scholar] [CrossRef] [PubMed]
- Yasakau, K.A.; Starykevich, M.; Ferreira, M.G.S.; Zheludkevich, M.L. A critical look at interpretation of electrochemical impedance spectra of sol-gel coated aluminium. Electrochim. Acta 2021, 378, 138091. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Wu, M.; Huang, R. Study of depassivation of carbon steel in simulated concrete pore solution using different equivalent circuits. Constr. Build. Mater. 2017, 157, 357–362. [Google Scholar] [CrossRef]





| Sample Code | Additive (Amount in g) | Sonication Time (in min.) | Curing Methodology (Time in h) |
|---|---|---|---|
| C | - | - | RT (2) |
| C-AC | Activated carbon (0.25) | 0.5 | Oven (24) |
| C-CM | Cement (0.2) | 2.5 | RT (6) |
| C-EG | Eggshell (0.5) | 5 | Oven (24) |
| C-LM | Limestone (0.2) | 2.5 | RT (6) |
| C-RB | Tires rubber (0.5) | 5 | Oven (24) |
| Sample | Ra (μm) | Rq (μm) | Rp (μm) | Rv (μm) | Rpv (μm) |
|---|---|---|---|---|---|
| C | 1.891 ± 0.263 | 2.415 ± 0.247 | 13.655 ± 1.01 | 32.270 ± 3.286 | 45.925 ± 16.746 |
| C-AC | 5.408 ± 0.817 | 7.524 ± 0.111 | 10.801 ± 1.678 | 35.100 ± 6.616 | 45.901 ± 8.297 |
| C-CM | 2.214 ± 0.456 | 2.982 ± 0.702 | 6.467 ± 0.837 | 19.260 ± 5.073 | 25.727 ± 8.339 |
| C-EG | 2.103 ± 0.326 | 2.866 ± 0.356 | 6.876 ± 0.902 | 21.950 ± 2.510 | 28.826 ± 2.385 |
| C-LM | 2.136 ± 0.313 | 2.719 ± 0.357 | 7.724 ± 1.553 | 13.637 ± 1.724 | 21.360 ± 10.044 |
| C-RB | 2.596 ± 0.512 | 3.370 ± 0.621 | 8.221 ± 0.702 | 26.518 ± 6.422 | 34.738 ± 6.002 |
| Samples | EIT [GPa] | HIT [MPa] | ||
|---|---|---|---|---|
| Average | STD | Average | STD | |
| C | 0.554 | 0.049 | 4.460 | 0.389 |
| C-AC | 0.426 | 0.063 | 13.137 | 2.5413 |
| C-CM | 1.351 | 0.101 | 5.1005 | 1.654 |
| C-EG | 1.255 | 0.171 | 4.044 | 1.184 |
| C-LM | 2.373 | 0.105 | 15.357 | 3.435 |
| C-RB | 0.202 | 0.030 | 3.351 | 0.691 |
| Immersion Time | Parameter | Sample | |||||
|---|---|---|---|---|---|---|---|
| C | C-AC | C-CM | C-EG | C-LM | C-RB | ||
| 24 h | Rsoln (Ω) | 46.1 | 10.0 | 19.1 | 10.0 | 5.0 | 10.0 |
| Rcoat (kΩ) | 39.91 | 4.83 | 72.58 | 4.05 | 35.36 | 0.67 | |
| Qcoat (μF cm−2 s−(1−αc)) | 3.27 | 90.04 | 2.92 | 0.045 | 0.075 | 1.39 | |
| acoat | 0.5 | 0.3 | 0.5 | 0.7 | 0.6 | 0.6 | |
| Rint (MΩ) | 14.93 | 3.77 | 0.19 | 0.024 | 0.9 | 13.9 | |
| Qint (μF cm−2 s−(1−αc)) | 7.11 | 257.90 | 170.30 | 1.16 | 3.29 | 77.19 | |
| aint | 0.2 | 0.6 | 0.9 | 0.5 | 0.3 | 0.6 | |
| Rct (kΩ) | 10.93 | 1.79 | 16.79 | 2.03 × 103 | 2.16 × 103 | 22.42 | |
| Qdl (μF cm−2 s−(1−αc)) | 0.25 | 0.12 | 0.16 | 6.68 | 139.00 | 18.82 | |
| adl | 0.6 | 0.9 | 0.7 | 0.5 | 0.8 | 0.5 | |
| χ2 (×103) | 1.68 | 0.22 | 0.067 | 0.14 | 0.21 | 0.059 | |
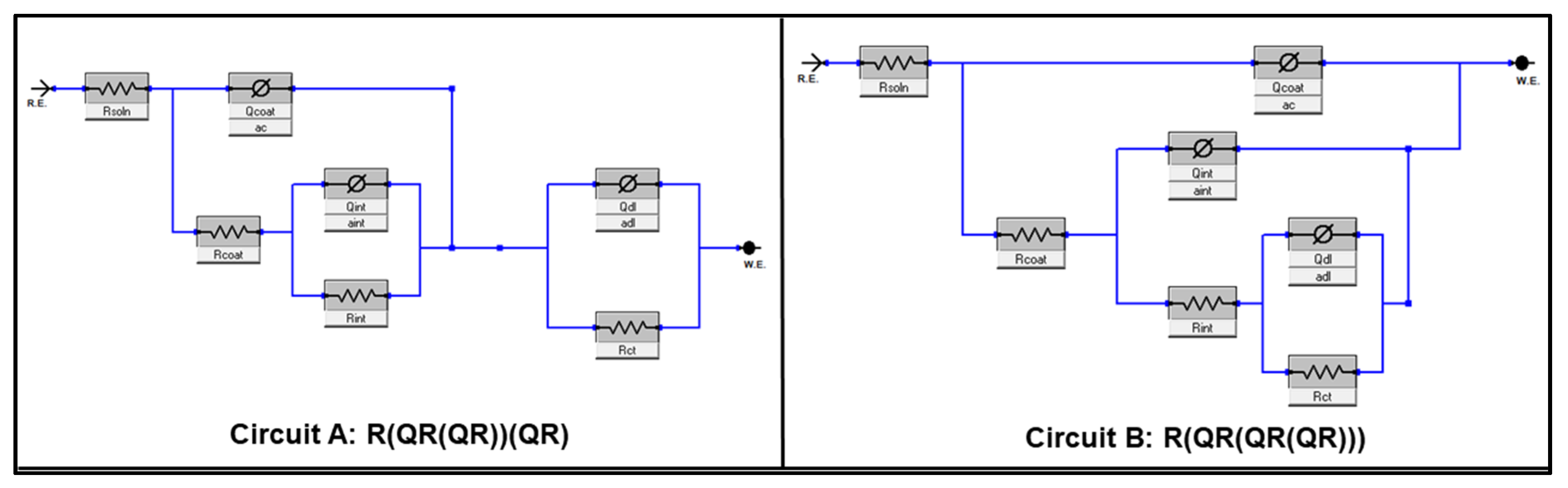
| Circuit | A | A | A | A | B | A | |
| 4 Weeks | Rsoln (Ω) | 14.0 | 15.0 | 20.0 | 15.0 | 15.0 | 10.6 |
| Rcoat (kΩ) | 1.19 | 0.30 | 0.24 | 0.92 | 4.92 | 0.11 | |
| Qcoat (μF cm−2 s−(1−αc)) | 20.02 | 2.09 | 1.31 | 2.74 | 1.68 | 2.25 | |
| acoat | 0.5 | 0.9 | 0.7 | 0.6 | 0.5 | 0.7 | |
| Rint (kΩ) | 10.37 × 103 | 132.90 | 1.06 | 0.12 | 129.20 | 0.13 | |
| Qint (μF cm−2 s−(1−αc)) | 225.90 | 349.2 | 160.80 | 3.07 | 16.33 | 435.90 | |
| aint | 0.9 | 0.4 | 0.4 | 0.9 | 0.4 | 0.4 | |
| Rct (kΩ) | 20.94 | 11.29 | 3.900 | 8.39 | 2.51 × 103 | 1.44 | |
| Qdl (μF cm−2 s−(1−αc)) | 88.48 | 17.43 | 715.00 | 232.10 | 99.11 | 1.26 × 103 | |
| adl | 0.6 | 0.6 | 0.8 | 0.5 | 0.9 | 0.8 | |
| χ2 (×103) | 1.32 | 0.28 | 0.021 | 0.28 | 0.15 | 0.16 | |
| Circuit | A | A | A | B | B | A | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suleiman, R.K.; Adesina, A.Y.; Olalekan, O.N.; Kumar, A.M.; Al-Badour, F.A.; Subbaiah, S. Influence of Waste Material Additives on the Performance of a Novel Hybrid Sol-Gel Coating on Mild Steel in 3.5% NaCl Medium. Polymers 2023, 15, 2842. https://doi.org/10.3390/polym15132842
Suleiman RK, Adesina AY, Olalekan ON, Kumar AM, Al-Badour FA, Subbaiah S. Influence of Waste Material Additives on the Performance of a Novel Hybrid Sol-Gel Coating on Mild Steel in 3.5% NaCl Medium. Polymers. 2023; 15(13):2842. https://doi.org/10.3390/polym15132842
Chicago/Turabian StyleSuleiman, Rami K., Akeem Y. Adesina, Ogunlakin Nasirudeen Olalekan, Arumugam Madhan Kumar, Fadi A. Al-Badour, and Sowrirajan Subbaiah. 2023. "Influence of Waste Material Additives on the Performance of a Novel Hybrid Sol-Gel Coating on Mild Steel in 3.5% NaCl Medium" Polymers 15, no. 13: 2842. https://doi.org/10.3390/polym15132842









