Experimental Advances in the Real-Time Recording of Cross-Linking Alginate In Situ Gelation: A Review
Abstract
:1. Introduction
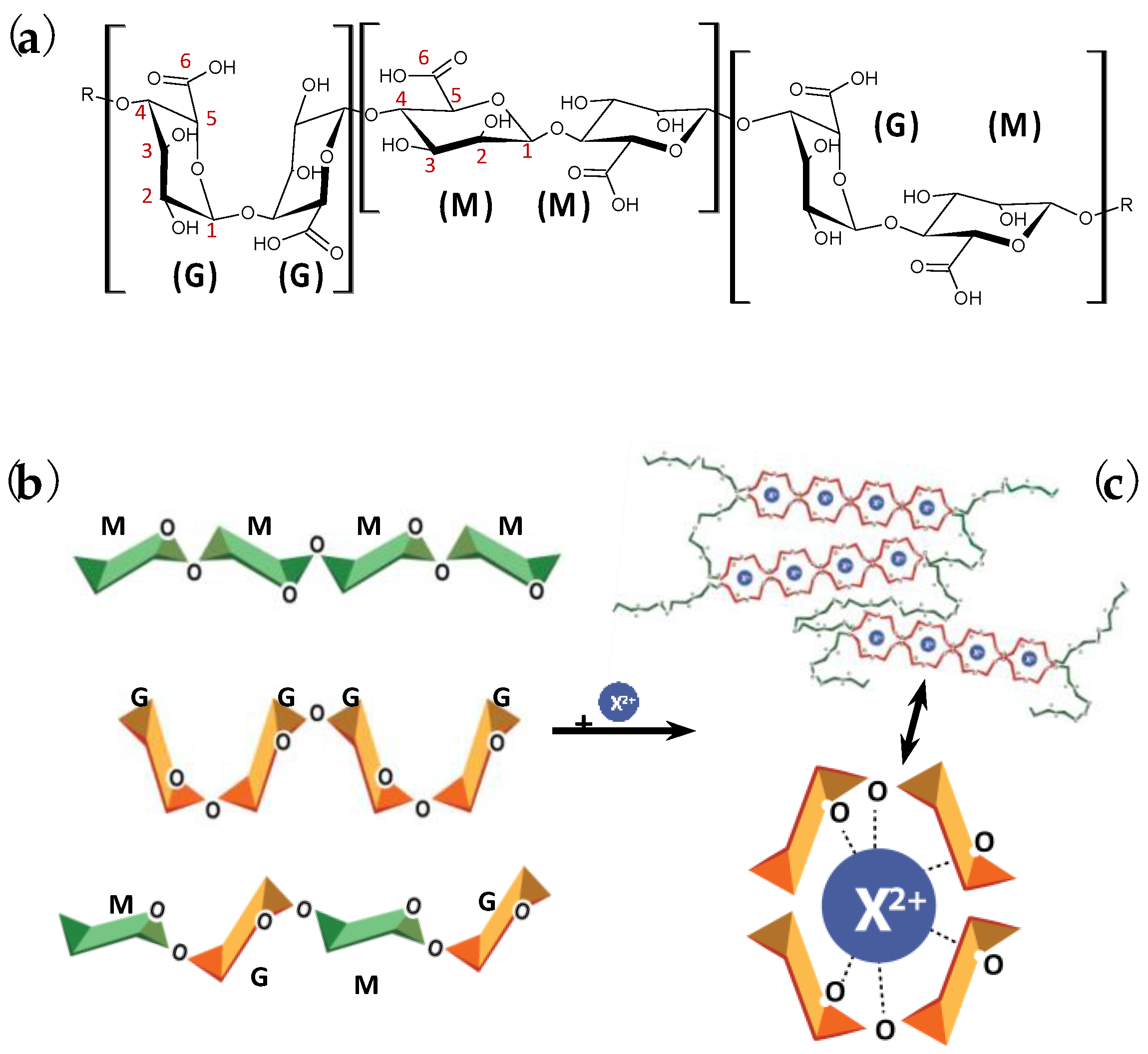

2. Introduction to In Situ Gelation Methods
3. In Situ Real-Time Recording of Alginate Hydrogel Formation
3.1. Conventional Rheometric Setups
3.2. Advanced Rheometric Setups
3.3. Alternative Techniques beyond Rheology
3.4. In Situ Alginate Hydrogels in Membranes
4. In Situ Real-Time Recording of Alginate Bead Formation
| Technique | Experimental Details | Research Objectives | Year of Publication | Reference |
|---|---|---|---|---|
| Rheology | In situ release of cross-linker on alginate/gelatin mixture | Rheological investigation of gelation | 2009 | [63] |
| Incubation of alginate in internal cross-linker on rheometer | In situ evaluation of gelation behavior | [66] | ||
| LED bottom rheometric plate for UV cross-linking gelation | Real-time evaluation of mechanical properties | 2011 | [77] | |
| Rheological characterization upon direct mixing of reactants | Evaluation of gelation speed | 2012 | [116] | |
| In situ rheological characterization of fouled alginate membrane | Assessment of the parameters manifesting alginate fouling layer | 2013 | [107] | |
| Fluid hydrogel formation on rheometer | Controlling the shearing profile on gelation kinetics | 2014 | [68] | |
| UV irradiation on rheometer for photo-activated ionic gelation | Monitoring of the two-step gelation mechanism | [78] | ||
| Rheological investigation upon ex situ mixing of reactants | Recording of gelation kinetics | 2015 | [69] | |
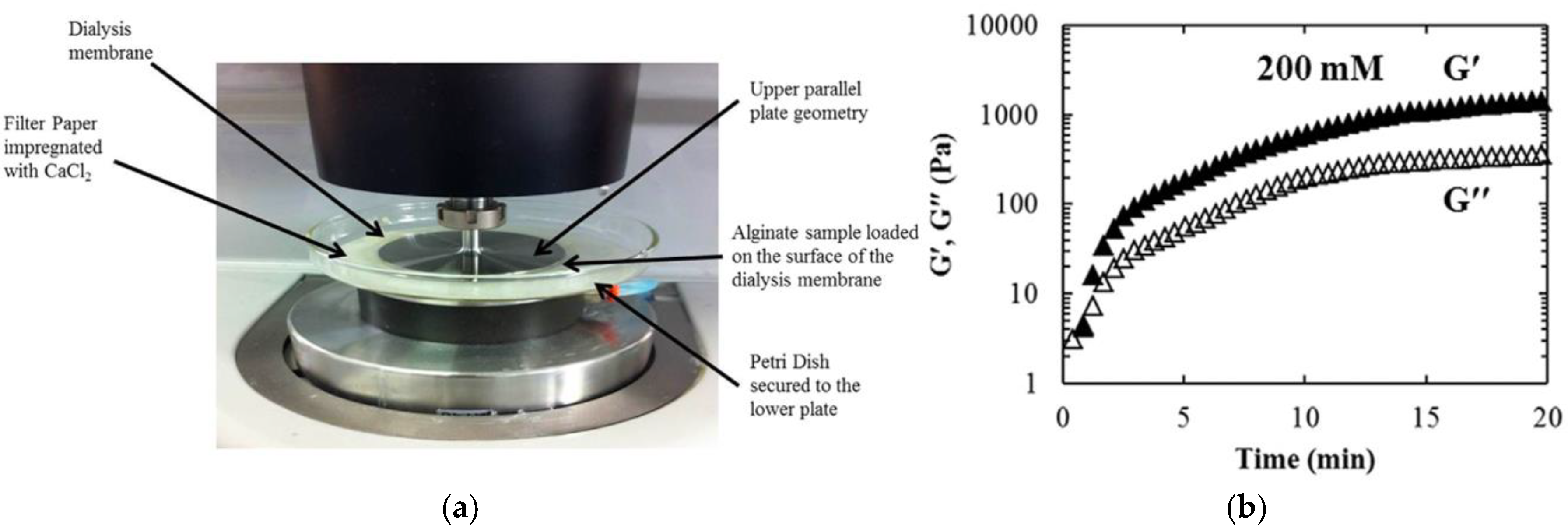
| Petri dish with filter paper and a dialysis membrane on the rheometer | Recording of alginate-Ca2+ kinetics | 2016 | [79] | |
| Visible light-curable alginate | Control of furfuryl alginate gel formation | 2020 | [76] | |
| Rheometric setup for injection of Ca2+ into alginate through micro-holes | Detailed recording of cross-linking gelation | [82] | ||
| In situ micro-rheology of foulant membrane surface | Study on foulant mechanical response for effective cleaning | 2021 | [108] | |
| CO2 in alginate solution to initiate the gelation on the rheometer | Generation and characterization of hydrogel foams | 2022 | [86] | |
| Microfluidics | Microfabrication of alginate hydrogels | Controlling of Ca2+ diffusion into alginate | 2010 | [99,100] |
| Microfluidic flow-focusing device | Size and shape of the alginate-Ca2+ microparticles | 2013 | [117] | |
| Visualization methods | Visualized extrusion-dripping method | Prediction models for alginate-Ca2+macrobeads | 2009 | [118] |
| Visualization of gelation on a petri dish | Determination of sol–gel transition and gelling time | 2011 | [101] | |
| Photo correlation imaging during alginate gelation | Investigation of gelling kinetics formation | 2013 | [102] | |
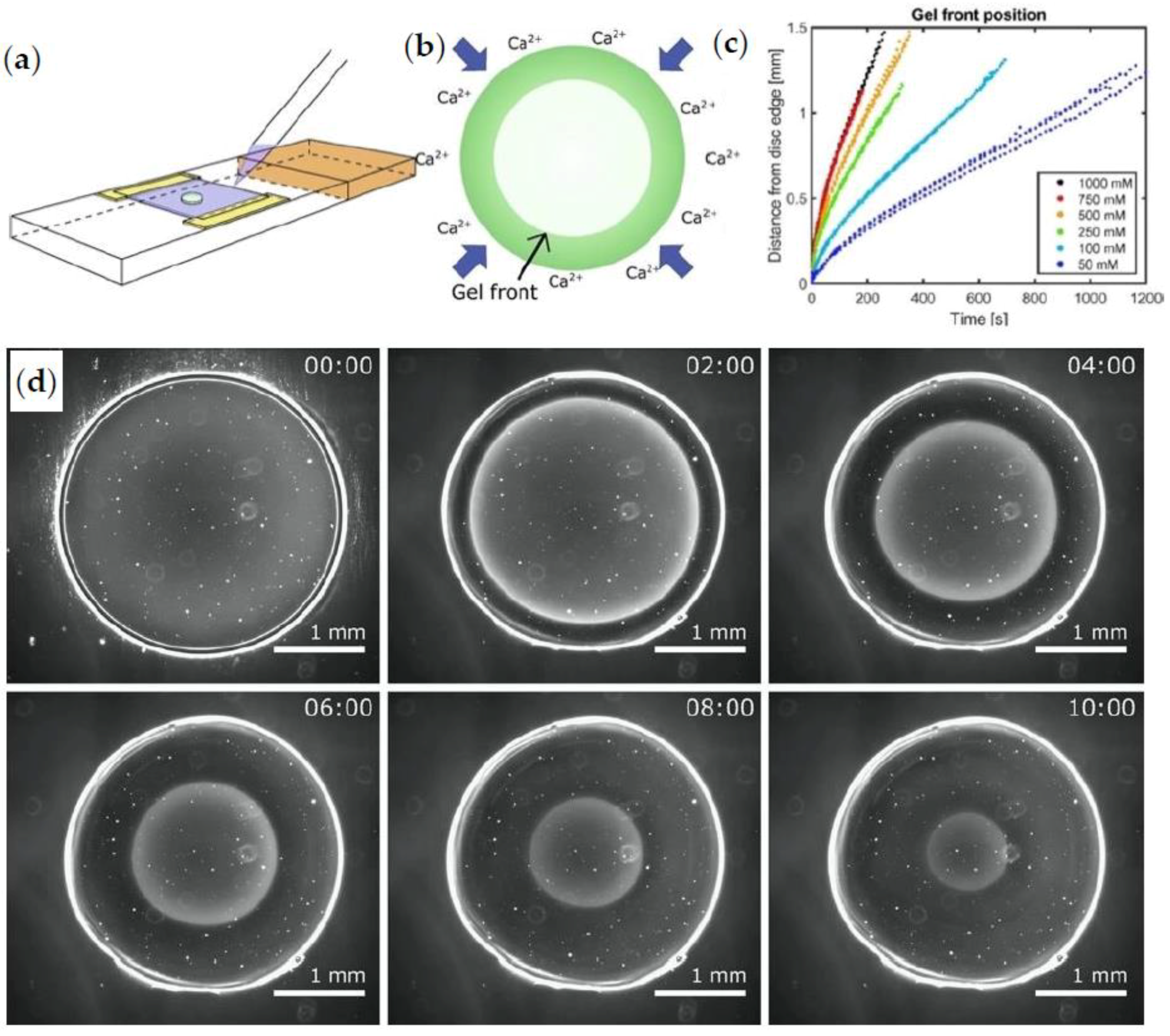
| Flow cell between two microscope slides | Real-time studying of gel front | 2016 | [103,104] | |
| Microscopy on a drop-wise method | Microscopical characterization of alginate-Ca2+ beads | 2017 | [119] | |
| High-speed imaging system on drop impact gelation | Effect of drop kinetic energy on gelation | 2018 | [120,121] | |
| Custom setup for optical video microscopy | Measurement of alginate bead volume change over time | 2021 | [124] | |
| Light-triggered methods | UV light in microchannels | Progress of UV-triggered gelation | 2010 | [98] |
| Capillary columns | Capillary formation of ionotropic alginate membranes | Propose equation for gelation kinetics | 2013 | [106] |
| Cantilever sensors | Dynamic-mode cantilever sensors on alginate gelation | Real-time monitoring of gelation | 2020 | [87,88] |
| Texture analyzer | Customized bead formation on a texture analyzer | In situ mechanical texture evaluation of alginate beads | 2019 | [123] |
| SAXS | Small-angle X-ray scattering oncompetitive ligand exchangegelation | Determination of the local structure of hydrogels | 2019 | [92] |
| Time-induced methods | FTIR, Uv-vis, and Raman spectroscopy on alginate–acrylamide gelation | In situ mapping of gel structure | 2021 | [95] |
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- de Castro Spadari, C.C.; de Bastiani, F.W.M.S.; Lopes, L.B.; Ishida, K. Alginate nanoparticles as non-toxic delivery system for miltefosine in the treatment of candidiasis and cryptococcosis. Int. J. Nanomed. 2019, 14, 5187–5199. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, B.J.; Bezerra, A.C.; Oliveira, L.L.; Arroyo, S.J.; de Melo, E.A.; Santos, A.M.P. Antimicrobial active edible coating of alginate and chitosan add ZnO nanoparticles applied in guavas (Psidium guajava L.). Food Chem. 2020, 309, 125566. [Google Scholar] [CrossRef] [PubMed]
- Lv, C.; Zhou, X.; Wang, P.; Li, J.; Wu, Z.; Jaio, Z.; Guo, M.; Wang, Z.; Wang, Y.; Wang, L.; et al. Biodegradable alginate-based sponge with antibacterial and shape memory properties for penetrating wound hemostasis. Compos. B Eng. 2022, 247, 110263. [Google Scholar] [CrossRef]
- Iravani, S.; Varma, R.S. Alginate-based micro- and nanosystems for targeted cancer therapy. Mar. Drugs 2022, 20, 598. [Google Scholar] [CrossRef]
- Puscaselu, R.G.; Lobiuc, A.; Dimian, M.; Covasa, M. Alginate: From food industry to biomedical applications and management of metabolic disorders. Polymers 2022, 12, 2417. [Google Scholar] [CrossRef]
- Wang, B.; Wan, Y.; Zheng, Y.; Lee, X.; Liu, T.; Yu, Z.; Huang, J.; Ok, Y.S.; Chen, J.; Gao, B. Alginate-based composites for environmental applications: A critical review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 318–356. [Google Scholar] [CrossRef]
- Trica, B.; Delattre, C.; Gros, F.; Ursu, A.V.; Dobre, T.; Djelveh, G.; Michaud, P.; Oancea, F. Extraction and characterization of alginate from an edible brown seaweed (Cystoseira barbata) harvested in the Romanian Black Sea. Mar. Drugs 2019, 17, 405. [Google Scholar] [CrossRef]
- Chee, S.Y.; Wong, P.K.; Wong, C.L. Extraction and characterisation of alginate from brown seaweeds (Fucales, Phaeophyceae) collected from Port Dickson, Peninsular Malaysia. J. Appl. Phycol. 2011, 23, 191–196. [Google Scholar] [CrossRef]
- Sabra, W.; Zeng, A.P.; Deckwer, W.D. Bacterial alginate: Physiology, product quality and process aspects. Appl. Microbiol. Biotechnol. 2001, 56, 315–325. [Google Scholar] [CrossRef]
- Hay, I.D.; Rehman, Z.U.; Moradali, M.F.; Wang, Y.; Rehm, B.H.A. Microbial alginate production, modification and its applications. Microb. Biotechnol. 2013, 6, 637–650. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjåk-Braek, G.; Smidsrød, O. Alginate based new materials. Int. J. Biol. Macromol. 1997, 21, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. J. Mater. 2013, 6, 1285–1309. [Google Scholar] [CrossRef] [PubMed]
- Papajová, E.; Bujdoš, M.; Chorvát, D.; Stach, M.; Lacík, I. Method for preparation of planar alginate hydrogels by external gelling using an aerosol of gelling solution. Carbohydr. Polym. 2012, 90, 472–482. [Google Scholar] [CrossRef]
- Pham, L.H.P.; Ly, K.L.; Colon-Ascanio, M.; Ou, J.; Wang, H.; Lee, S.W.; Wang, Y.; Choy, J.S.; Phillips, K.S.; Luo, X. Dissolvable alginate hydrogel-based biofilm microreactors for antibiotic susceptibility assays. Biofilm 2023, 5, 100103. [Google Scholar] [CrossRef]
- Draget, K.I.; Skjåk-Bræk, G.; Stokke, B.T. Similarities and differences between alginic acid gels and ionically crosslinked alginate gels. Food Hydrocoll. 2006, 20, 170–175. [Google Scholar] [CrossRef]
- Ma, W.; Ling, S.; Zhang, J.; Chen, Z.; Xu, J. Microfluidic fabrication of calcium alginate helical microfibers for highly stretchable wound dressing. J. Polym. Sci. 2022, 60, 1741–1749. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A.; Li, L. Multiple steps and critical behaviors of the binding of calcium to alginate. J. Phys. Chem. B 2007, 111, 2456–2462. [Google Scholar] [CrossRef]
- Supriya; Pal, C.K.; Sengupta, S.; Basu, J.K. One pot synthesis of nano Ag in calcium alginate beads and its catalytic application in p-Nitrophenol reduction with kinetic parameter estimation and model fitting. Can. J. Chem. Eng. 2021, 99, 359–373. [Google Scholar] [CrossRef]
- Merakchi, A.; Bettayeb, S.; Drouiche, N.; Adour, L.; Lounici, H. Cross-linking and modification of sodium alginate biopolymer for dye removal in aqueous solution. Polym. Bull. 2019, 76, 3535–3554. [Google Scholar] [CrossRef]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horský, J.; Vetchy, D.; et al. Structure and dynamics of alginate gels cross-linked by polyvalent ions probed via solid state NMR spectroscopy. Biomacromolecules 2017, 18, 2478–2488. [Google Scholar] [CrossRef]
- Song, E.H.; Shang, J.; Ratner, D.M. 9.08—Polysaccharides. Ιn Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Oxford, UK, 2012; Volume 9, pp. 137–155. [Google Scholar]
- Hecht, H.; Srebnik, S. Structural characterization of sodium alginate and calcium alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Smidsrød, O. Selectivity of some anionic polymers for divalent metal ions. Acta Chem. Scand. 1970, 24, 843–854. [Google Scholar] [CrossRef]
- Mørch, Ý.A.; Donati, I.; Strand, B.L.; Skjåk-Bræk, G. Effect of Ca2+, Ba2+, and Sr2+ on alginate microbeads. Biomacromolecules 2006, 7, 1471–1480. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qian, L.; Shu, T.; Tong, Z. Rheology characterization of sol–gel transition in aqueous alginate solutions induced by calcium cations through in situ release. Polymer 2003, 44, 407–412. [Google Scholar] [CrossRef]
- Lu, L.; Liu, X.; Qian, L.; Shu, T.; Tong, Z. Sol–gel transition in aqueous alginate solutions induced by cupric cations observed with viscoelasticity. Polym. J. 2003, 35, 804–809. [Google Scholar] [CrossRef]
- Huang, X.; Zhang, Y.; Li, F.; Zhang, J.; Dai, Y.; Shi, M.; Zhao, Y. Highly efficient alginate-based macromolecular photoinitiator for crosslinking and toughening gelatin hydrogels. J. Polym. Sci. 2020, 58, 1439–1449. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Tavakolizadeh, M.; Hosseini, S.H.; Rabiee, N.; Bagherzadeh, M. Highly stretchable, self-adhesive, and self-healable double network hydrogel based on alginate/polyacrylamide with tunable mechanical properties. J. Polym. Sci. 2020, 58, 2062–2073. [Google Scholar] [CrossRef]
- Guo, B.; Ma, Z.; Pan, L.; Shi, Y. Properties of conductive polymer hydrogels and their application in sensors. J. Polym. Sci. B Polym. Phys. 2019, 57, 1606–1621. [Google Scholar] [CrossRef]
- Mei, X.; Ye, D.; Zhang, F.; Di, C. Implantable application of polymer-based biosensors. J. Polym. Sci. 2022, 60, 328–347. [Google Scholar] [CrossRef]
- Radebe, N.W.; Fengler, C.; Klein, C.O.; Figuli, R.; Wilhelm, M. A combined setup for correlating chemical changes via FTIR spectroscopy and rheological properties in a strain-controlled rheometer. J. Rheol. 2021, 65, 681–693. [Google Scholar] [CrossRef]
- Meng, Z.; Zhou, X.; Xu, J.; Han, X.; Dong, Z.; Wang, H.; Zhang, Y.; She, J.; Xu, L.; Wang, C.; et al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv. Mater. 2019, 31, 1900927. [Google Scholar] [CrossRef] [PubMed]
- Rhein-Knudsen, N.; Ale, M.T.; Ajalloueian, F.; Meyer, A.S. Characterization of alginates from Ghanaian brown seaweeds: Sargassum spp. and Padina spp. Food Hydrocoll. 2017, 71, 236–244. [Google Scholar] [CrossRef]
- Kopplin, G.; Lervik, A.; Draget, K.I.; Aachmann, F.L. Alginate gels crosslinked with chitosan oligomers—A systematic investigation into alginate block structure and chitosan oligomer interaction. RSC Adv. 2021, 11, 13780–13798. [Google Scholar] [CrossRef]
- Mikula, K.; Skrzypczak, D.; Ligas, B.; Witek-Krowiak, A. Preparation of hydrogel composites using Ca2+ and Cu2+ ions as crosslinking agents. SN Appl. Sci. 2019, 1, 643. [Google Scholar] [CrossRef]
- Milocco, A.; Scuor, N.; Lughi, V.; Lamberti, G.; Barba, A.A.; Divittorio, R.; Grassi, G.; Perkan, A.; Grassi, M.; Abrami, M. Thermal gelation modeling of a pluronic-alginate blend following coronary angioplasty. J. Appl. Polym. Sci. 2020, 137, 48539. [Google Scholar] [CrossRef]
- Urbanova, I.M.; Pavelkova, M.; Czernek, J.; Kubova, K.; Vyslouzil, J.; Pechova, A.; Molinkova, D.; Vyslouzil, J.; Vetchy, D.; Brus, J. Interaction pathways and structure−chemical transformations of alginate gels in physiological environments. Biomacromolecules 2019, 20, 4158–4170. [Google Scholar] [CrossRef]
- Jadach, B.; Świetlik, W.; Froelich, A. Sodium Alginate as a Pharmaceutical Excipient: Novel Applications of a Well-known Polymer. J. Pharm. Sci. 2022, 111, 1250–1261. [Google Scholar] [CrossRef]
- Zhao, J.; Li, B.; Liu, Z.; Dai, D.; Li, Y.; Shi, R.; Zhang, H. A novel solar-triggered MIL-125(Ti)/g-C3N4/SA composite aerogel with high catalytic activity for degradation of organic contaminants. Sep. Purif. Technol. 2021, 279, 119696. [Google Scholar] [CrossRef]
- Ul-Islam, M.; Ahmad, F.; Fatima, A.; Shah, N.; Yasir, S.; Ahmad, M.W.; Manan, S.; Ullah, M.W. Ex situ synthesis and characterization of high strength multipurpose bacterial cellulose-aloe vera hydrogels. Front. Bioeng. Biotechnol. 2021, 9, 601988. [Google Scholar] [CrossRef]
- Kuang, L.; Ma, X.; Ma, Y.; Yao, Y.; Tariq, M.; Yuan, Y.; Liu, C. Self-assembled injectable nanocomposite hydrogels coordinated by in situ generated CaP nanoparticles for bone regeneration. ACS Appl. Mater. Interfaces 2019, 11, 17234–17246. [Google Scholar] [CrossRef] [PubMed]
- Martin- Saldaña, S.; Al Waeel, M.; Alsharabasy, A.M.; Daly, A.; Pandit, A. An interdisciplinary framework for the characterization of extracellular matrix-hydrogels for biomedical applications. Matter 2022, 5, 3659–3705. [Google Scholar] [CrossRef]
- Holly, E.E.; Venkataraman, S.K.; Chambon, F.; Winter, H.H. Fourier transform mechanical spectroscopy of viscoelastic materials with transient structure. J. Nonnewton. Fluid Mech. 1988, 27, 17–26. [Google Scholar] [CrossRef]
- Fu, S.; Thacker, A.; Sperger, D.M.; Boni, R.L.; Buckner, I.S.; Velankar, S.; Munson, E.J.; Block, L.H. Relevance of rheological properties of sodium alginate in solution to calcium alginate gel properties. AAPS PharmSciTech 2011, 12, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Puguan, J.M.C.; Yu, X.; Kim, H. Characterization of structure, physico-chemical properties and diffusion behavior of Ca-Alginate gel beads prepared by different gelation methods. J. Colloid Interface Sci. 2014, 432, 109–116. [Google Scholar] [CrossRef]
- Chan, L.W.; Lee, H.Y.; Heng, P.W.S. Mechanisms of external and internal gelation and their impact on the functions of alginate as a coat and delivery system. Carbohydr. Polym. 2006, 63, 176–187. [Google Scholar] [CrossRef]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Choi, B.Y.; Park, H.J.; Hwang, S.J.; Park, J.B. Preparation of alginate beads for floating drug delivery system: Effects of CO2 gas-forming agents. Int. J. Pharm. 2002, 239, 81–91. [Google Scholar] [CrossRef]
- Liu, X.D.; Yu, W.Y.; Zhang, Y.; Xue, W.M.; Yu, W.T.; Xiong, Y.; Ma, X.J.; Chen, Y.; Yuan, Q. Characterization of structure and diffusion behaviour of Ca-alginate beads prepared with external or internal calcium sources. J. Microencapsul. 2002, 16, 775–782. [Google Scholar] [CrossRef]
- Ramdhan, T.; Ching, S.H.; Prakash, S.; Bhandari, B. Time dependent gelling properties of cuboid alginate gels made by external gelation method: Effects of alginate-CaCl2 solution ratios and pH. Food Hydrocoll. 2019, 90, 232–240. [Google Scholar] [CrossRef]
- Kaklamani, G.; Cheneler, D.; Grover, L.M.; Adams, M.J.; Bowen, J. Mechanical properties of alginate hydrogels manufactured using external gelation. J. Mech. Behav. Biomed. Mater. 2014, 36, 135–142. [Google Scholar] [CrossRef]
- Liu, X.D.; Bao, D.C.; Xue, W.M.; Xiong, Y.; Yu, W.T.; Yu, X.J.; Ma, X.J.; Yuan, Q. Preparation of uniform calcium alginate gel beads by membrane emulsification coupled with internal gelation. J. Appl. Polym. Sci. 2003, 87, 848–852. [Google Scholar] [CrossRef]
- Sangroniz, L.; Fernández, M.; Santamaria, A. Polymers and rheology: A tale of give and take. Polymer 2023, 271, 125811. [Google Scholar] [CrossRef]
- Papageorgiou, M.; Kasapis, S.; Gothard, M.G. Structural and textural properties of calcium induced, hot-made alginate gels. Carbohydr. Polym. 1994, 24, 199–207. [Google Scholar]
- Dalheim, M.Ø.; Omtvedt, L.A.; Bjørge, I.M.; Akbarzadeh, A.; Mano, J.F.; Aachmann, F.L.; Strand, B.L. Mechanical properties of Ca-saturated hydrogels with functionalized alginate. Gels 2019, 5, 23. [Google Scholar] [CrossRef]
- Adibnia, V.; Hill, R.J. Universal aspects of hydrogel gelation kinetics, percolation and viscoelasticity from PA-hydrogel rheology. J. Rheol. 2016, 60, 541–548. [Google Scholar] [CrossRef]
- Sun, X.; Li, Z.; Cui, Z.; Wu, S.; Zhu, S.; Liang, Y.; Yang, X. Preparation and physicochemical properties of an injectable alginate-based hydrogel by the regulated release of divalent ions via the hydrolysis of D-glucono- δ-lactone. J. Biomater. Appl. 2020, 34, 891–901. [Google Scholar] [CrossRef]
- Kim, H.; Song, D.; Ngo, H.V.; Jin, G.; Park, C.; Park, J.-B.; Lee, B.-J. Modulation of the clinically accessible gelation time using glucono-d-lactone and pyridoxal 5′-phosphate for long-acting alginate in situ forming gel injectable. Carbohydr. Polym. 2021, 272, 118453. [Google Scholar] [CrossRef]
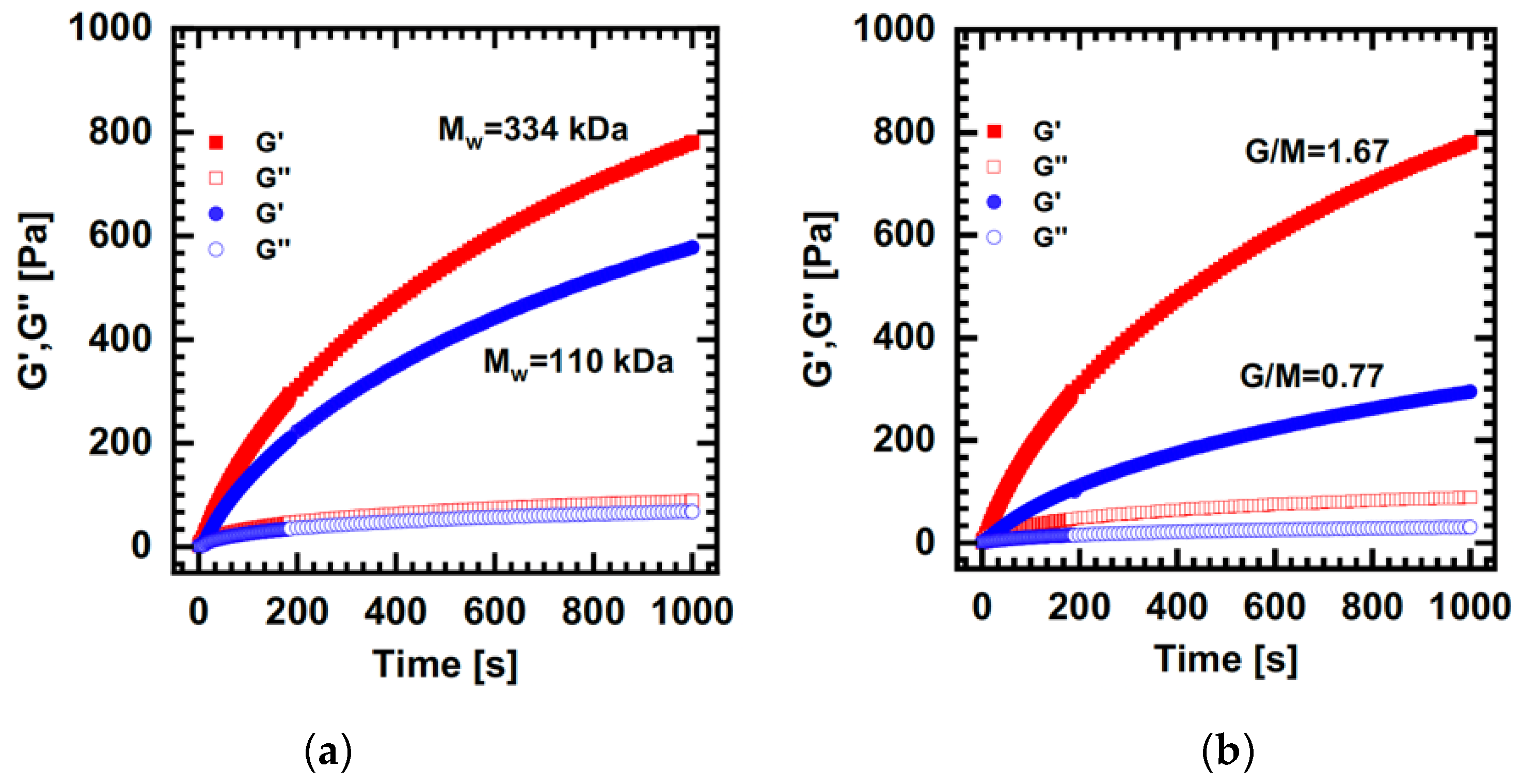
- Draget, K.I.; Skjåk Bræk, G.; Smidsrød, O. Alginic acid gels: The effect of alginate chemical composition and molecular weight. Carbohydr. Polym. 1994, 25, 31–38. [Google Scholar] [CrossRef]
- Nobile, M.R.; Pirozzi, V.; Somma, E.; Gomez D’Ayala, G.; Laurienzo, P. Development and rheological investigation of novel Alginate/N-Succinylchitosan hydrogels. J. Polym. Sci. B Polym. Phys. 2008, 46, 1167–1182. [Google Scholar] [CrossRef]
- Li, P.; Guo, C.; Li, X.; Yuan, K.; Yang, X.; Guo, Y.; Yang, X. Preparation and structural characteristics of composite alginate/casein emulsion gels: A microscopy and rheology study. Food Hydrocoll. 2021, 118, 106792. [Google Scholar] [CrossRef]
- Panouillé, M.; Larreta-Garde, V. Gelation behaviour of gelatin and alginate mixtures. Food Hydrocoll. 2009, 23, 1074–1080. [Google Scholar] [CrossRef]
- Li, A.; Gong, T.; Yang, X.; Guo, Y. Interpenetrating network gels with tunable physical properties: Glucono-δ-lactone induced gelation of mixed Alg/gellan sol systems. Int. J. Biol. Macromol. 2020, 151, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Lu, W.; Sun, C.; Zhao, Y.; Zhang, Y.; Fang, Y. Gelation behavior and mechanism of alginate with calcium: Dependence on monovalent counterions. Carbohydr. Polym. 2022, 294, 119788. [Google Scholar] [CrossRef]
- Funami, T.; Fang, Y.; Noda, S.; Ishihara, S.; Nakauma, M.; Draget, K.I.; Nishinari, K.; Phillips, G.O. Rheological properties of sodium alginate in an aqueous system during gelation in relation to supermolecular structures and Ca2+ binding. Food Hydrocoll. 2009, 23, 1746–1755. [Google Scholar] [CrossRef]
- Liao, H.; Ai, W.; Zhang, K.; Nakauma, M.; Funami, T.; Fang, Y.; Nishinari, K.; Draget, K.I.; Phillips, G.O. Mechanisms of oligoguluronate modulating the calcium-induced gelation of alginate. Polymer 2015, 74, 166–175. [Google Scholar] [CrossRef]
- Fernández Farrés, I.; Norton, I.T. Formation kinetics and rheology of alginate fluid gels produced by in-situ calcium release. Food Hydrocoll. 2014, 40, 76–84. [Google Scholar] [CrossRef]
- Larsen, B.E.; Bjørnstad, J.; Pettersen, E.O.; Tønnesen, H.H.; Melvik, J.E. Rheological characterization of an injectable alginate gel system. BMC Biotechnol. 2015, 15, 29. [Google Scholar] [CrossRef]
- Iatridi, Z.; Saravanou, S.-F.; Tsitsilianis, C. Injectable self-assembling hydrogel from alginate grafted by P(N-isopropylacrylamide-co-N-tert-butylacrylamide) random copolymers. Carbohydr. Polym. 2019, 219, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Theodorakis, N.; Saravanou, S.-F.; Kouli, N.-P.; Iatridi, Z.; Tsitsilianis, C. pH/thermo-responsive grafted alginate-based SiO2 hybrid nanocarrier/hydrogel drug delivery systems. Polymers 2021, 13, 1228. [Google Scholar] [CrossRef]
- Goudoulas, T.B.; Germann, Ν. Phase transition kinetics and rheology of gelatin-alginate mixtures. Food Hydrocoll. 2017, 66, 49–60. [Google Scholar] [CrossRef]
- Li, X.; Weng, Y.; Kong, X.; Zhang, B.; Li, M.; Diao, K.; Zhang, Z.; Wang, X.; Chen, H. A covalently crosslinked polysaccharide hydrogel for potential applications in drug delivery and tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2857–2865. [Google Scholar] [CrossRef]
- Yang, J.; Steck, J.; Suo, Z. Gelation kinetics of alginate chains through covalent bonds. Extreme Mech. Lett. 2020, 40, 100898. [Google Scholar] [CrossRef]
- Wan, L.Q.; Jiang, J.; Arnold, D.E.; Guo, X.E.; Lu, H.H.; Mow, V.C. Calcium concentration effects on the mechanical and biochemical properties of chondrocyte-alginate constructs. Cell. Mol. Bioeng. 2008, 1, 93–102. [Google Scholar] [CrossRef]
- Heo, Y.; Akimoto, J.; Kobatake, E.; Yoshihiro, I. Gelation and release behavior of visible light-curable alginate. Polym. J. 2020, 52, 323–332. [Google Scholar] [CrossRef]
- Bonino, C.A.; Samorezov, J.E.; Jeon, O.; Alsberg, E.; Khan, S.A. Real-time in situ rheology of alginate hydrogel photocrosslinking. Soft Matter 2011, 7, 11510. [Google Scholar] [CrossRef]
- Higham, A.K.; Bonino, C.A.; Raghava, S.R.; Khan, S.A. Photo-activated ionic gelation of alginate hydrogel: Real-time rheological monitoring of the two-step crosslinking mechanism. Soft Matter 2014, 10, 4990–5002. [Google Scholar] [CrossRef]
- Mahdi, M.H.; Diryak, R.; Kontogiorgos, V.; Morris, G.A.; Smith, A.M. In situ rheological measurements of the external gelation of alginate. Food Hydrocoll. 2016, 55, 77–80. [Google Scholar] [CrossRef]
- Diryak, R.; Kontogiorgos, V.; Ghori, M.U.; Bills, P.; Tawfik, A.; Morris, G.A.; Smith, A.M. Behavior of in situ cross-linked hydrogels with rapid gelation kinetics on contact with physiological fluids. Macromol. Chem. Phys. 2018, 219, 1700584. [Google Scholar] [CrossRef]
- Senjoti, F.G.; Ghori, M.U.; Diryak, R.; Conway, B.R.; Morris, G.A.; Smith, A.M. Rheo-Dissolution: A new platform for the simultaneous measurement of rheology and drug release. Carbohydr. Polym. 2020, 229, 115541. [Google Scholar] [CrossRef] [PubMed]
- Besiri, I.N.; Goudoulas, T.B.; Germann, N. Custom-made rheological setup for in situ real-time fast alginate-Ca2+ gelation. Carbohydr. Polym. 2020, 246, 116615. [Google Scholar] [CrossRef]
- Besiri, I.N.; Goudoulas, T.B.; Germann, N. Impact of CaCl2 concentration and in situ rheometric setup configuration on fast alginate–Ca2+ reaction. Phys. Fluids 2022, 34, 053104. [Google Scholar] [CrossRef]
- Didonaki, A. Comparison of Hydrogels Using In Situ Characterization. Master’s Thesis, Technical University of Munich School of Life Sciences, Forschungsdepartment Life Science Engineering, Freising, Germany, 2022. [Google Scholar]
- Besiri, I.N.; Goudoulas, T.B.; Fattahi, E.; Becker, T. In situ evaluation of alginate-Ca2+ gelation kinetics. J. Appl. Polym. Sci. 2023, e54252. [Google Scholar] [CrossRef]
- Ben Djemaa, I.; Andrieux, S.; Auguste, S.; Jacomine, L.; Tarnowska, M.; Drenckhan-Andreatta, W. One-step generation of alginate-based hydrogel foams using CO2 for simultaneous foaming and gelation. Gels 2022, 8, 444. [Google Scholar] [CrossRef]
- Cesewski, E.; Singh, M.; Liu, Y.; Zhang, J.; Haring, A.P.; Johnson, B.N. Real-time monitoring of hydrogel rheological property changes and gelation processes using high-order modes of cantilever sensors. J. Appl. Phys. 2020, 128, 174502. [Google Scholar] [CrossRef]
- Haring, A.P.; Singh, M.; Koh, M.; Cesewski, E.; Dillard, D.A.; Kong, Z.J.; Johnson, B.N. Real-time characterization of hydrogel viscoelastic properties and sol-gel phase transitions using cantilever sensors. J. Rheol. 2020, 64, 837–850. [Google Scholar] [CrossRef]
- Sharma, H.; Lakshmanan, R.S.; Johnson, B.N.; Mutharasan, R. Piezoelectric cantilever sensors with asymmetric anchor exhibit picogram sensitivity in liquids. Sens. Actuators B Chem. 2011, 153, 64–70. [Google Scholar] [CrossRef]
- Ghernaouti, F.; Perrin, A.; Causse, J.; Chandre, F.; Cornu, D.; Cambedouzou, J. Small angle X-ray scattering to investigate the specific surface of hydrated alginate microbeads. Food Hydrocoll. 2022, 127, 107498. [Google Scholar] [CrossRef]
- Sikorski, P.; Mo, F.; Skjåk-Bræk, G.; Stokke, B.T. Evidence for egg-box-compatible interactions in calcium-alginate gels from fiber X-ray diffraction. Biomacromolecules 2007, 8, 2098–2103. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yuguchi, Y.; Stokke, B.T.; Sikorski, P.; Bassett, D.C. Local structure of Ca2+ alginate hydrogels gelled via competitive ligand exchange and measured by small angle X-ray scattering. Gels 2019, 5, 3. [Google Scholar] [CrossRef]
- Bassett, D.C.; Håti, A.G.; Melø, T.B.; Stokkea, B.T.; Sikorski, P. Competitive ligand exchange of crosslinking ions for ionotropic hydrogel formation. J. Mater. Chem. B 2016, 4, 6175. [Google Scholar] [CrossRef]
- Håti, A.G.; Bassett, D.C.; Ribe, J.M.; Sikorski, P.; Weitz, D.A.; Stokke, B.T. Versatile, cell and chip friendly method to gel alginate in microfluidic devices. Lab Chip 2016, 16, 3718–3727. [Google Scholar] [CrossRef] [PubMed]
- Pragya, A.; Mutalik, S.; Younas, M.W.; Pang, S.-K.; So, P.-K.; Wang, F.; Zheng, Z.; Noor, N. Dynamic cross-linking of an alginate–acrylamide tough hydrogel system: Time-resolved in situ mapping of gel self-assembly. RSC Adv. 2021, 11, 10710. [Google Scholar] [CrossRef]
- LaGrow, A.P.; Lloyd, D.C.; Schebarchov, D.; Gai, P.L.; Boyes, E.D. In situ visualization of site-dependent reaction kinetics in shape-controlled nanoparticles: Corners vs edges. J. Phys. Chem. C 2019, 123, 14746–14753. [Google Scholar] [CrossRef]
- Potter, K.; Balcom, B.J.; Carpenter, T.A.; Hall, L.D. The gelation of sodium alginate with calcium ions studied by magnetic resonance imaging (MRI). Carbohydr. Res. 1994, 257, 117–126. [Google Scholar] [CrossRef]
- Chueh, B.; Zheng, Y.; Torisawa, Y.; Hsiao, A.Y.; Ge, C.; Hsiong, S.; Huebsch, N.; Franceschi, R.; Mooney, D.J.; Takayama, S. Patterning alginate hydrogels using light-directed release of caged calcium in a microfluidic device. Biomed. Microdevices 2010, 12, 145–151. [Google Scholar] [CrossRef]
- Braschler, T.; Valero, A.; Colella, L.; Pataky, K.; Brugger, J.; Renaud, P. Fluidic microstructuring of alginate hydrogels for the single cell niche. Lab Chip 2010, 10, 2771–2777. [Google Scholar] [CrossRef]
- Braschler, T.; Valero, A.; Colella, L.; Pataky, K.; Brugger, J.; Renaud, P. Link between alginate reaction front propagation and general reaction diffusion theory. Anal. Chem. 2011, 83, 2234–2242. [Google Scholar] [CrossRef]
- Nunamaker, E.A.; Otto, K.J.; Kipke, D.R. Investigation of the material properties of alginate for the development of hydrogel repair of dura mater. J. Mech. Behav. Biomed. Mater. 2011, 4, 16–33. [Google Scholar] [CrossRef]
- Secchi, E.; Roversi, Τ.; Buzzaccaro, S.; Piazza, L.; Piazza, R. Biopolymer gels with “physical” cross-links: Gelation kinetics, aging, heterogeneous dynamics, and macroscopic mechanical properties. Soft Matter 2013, 9, 3931. [Google Scholar] [CrossRef]
- Bjørnøy, S.H.; Bassett, D.C.; Ucar, S.; Andreassen, J.-P.; Sikorski, P. A correlative spatiotemporal microscale study of calcium phosphate formation and transformation within an alginate hydrogel matrix. Acta Biomater. 2016, 44, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Bjørnøy, S.H.; Mandaric, S.; Bassett, D.C.; Åslund, A.K.O.; Ucar, S.; Andreassen, J.-P.; Strand, B.L.; Sikorski, P. Gelling kinetics and in situ mineralization of alginate hydrogels: A correlative spatiotemporal characterization toolbox. Acta Biomater. 2016, 15, 243–253. [Google Scholar] [CrossRef]
- Pfaff, N.-M.; Kleijn, J.M.; van Loosdrecht, M.C.M.; Kemperman, A.J.B. Formation and ripening of alginate-like exopolymer gel layers during and after membrane filtration. Water Res. 2021, 195, 116959. [Google Scholar] [CrossRef] [PubMed]
- Hassan, R.; Gobourib, A.; Zaafarany, I. Kinetics and mechanism of sol-gel transformation between sodium alginate anionic polyelectrolyte and some alkaline earth metal ions with formation of coordination biopolymer ionotropic polymembrane hydrogels of capillary structures. Adv. Biosens. Bioelectron. 2013, 2, 47–56. [Google Scholar]
- Sioutopoulos, D.C.; Goudoulas, T.B.; Kastrinakis, E.G.; Nychas, S.G.; Karabelas, A.J. Rheological and permeability characteristics of alginate fouling layers developing on reverse osmosis membraneσ during desalination. J. Membr. Sci. 2013, 434, 74–84. [Google Scholar] [CrossRef]
- Epstein, J.A.; Ramon, G.Z. In-situ micro-rheology of a foulant layer at a membrane surface. J. Membr. Sci. 2021, 640, 119747. [Google Scholar] [CrossRef]
- Gunatilake, U.B.; Venkatesan, M.; Basabe-Desmonts, L.; Benito-Lopez, F. Ex situ and in situ magnetic phase synthesised magneto-driven alginate beads. J. Colloid Interface Sci. 2022, 610, 741–750. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, M.; Koh, K.Y.; Neo, W.; Ong, C.N.; Chen, J.P. An optimized CaO2-functionalized alginate bead for simultaneous and efficient removal of phosphorous and harmful cyanobacteria. Sci. Total Environ. 2022, 806, 150382. [Google Scholar] [CrossRef]
- Zhao, Y.; Carvajal, M.T.; Won, Y.-Y.; Harris, M.T. Preparation of calcium alginate microgel beads in an electrodispersion reactor using an internal source of calcium carbonate nanoparticles. Langmuir 2007, 23, 12489–12496. [Google Scholar] [CrossRef]
- Vicini, S.; Castellano, M.; Mauri, M.; Marsano, E. Gelling process for sodium alginate: New technical approach by using calcium rich micro-spheres. Carbohydr. Polym. 2015, 134, 767–774. [Google Scholar] [CrossRef]
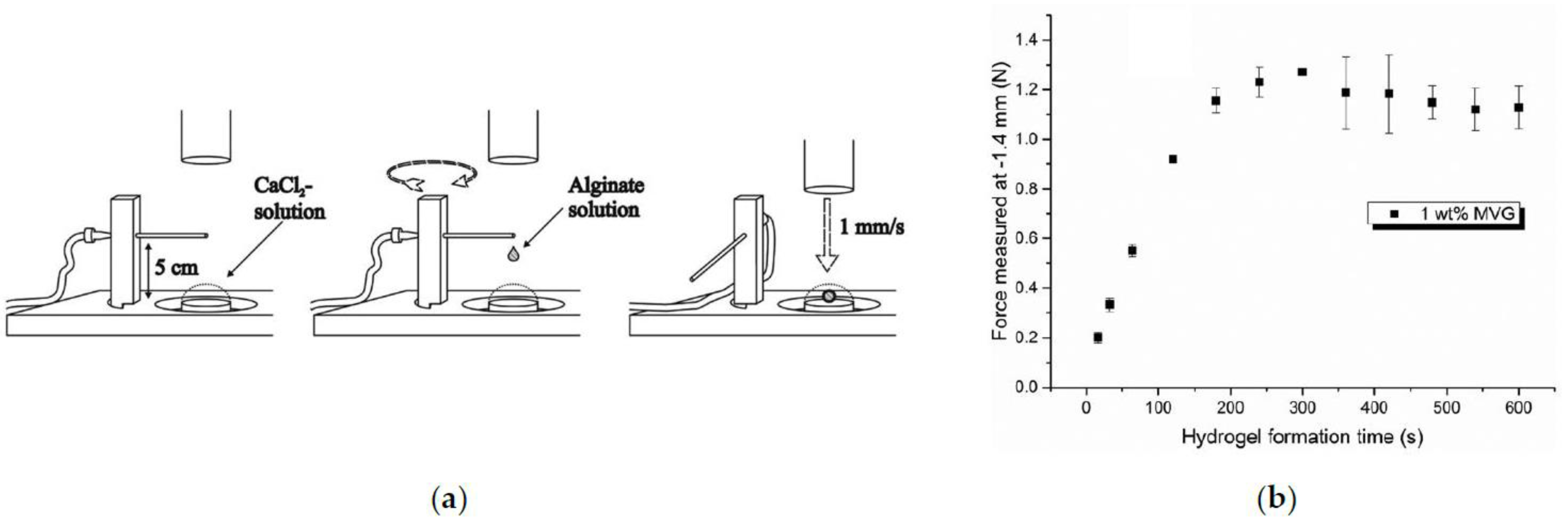
- Blandino, A.; Macías, M.; Cantero, D. Formation of calcium alginate gel capsules: Influence of sodium alginate and CaCl2 concentration on gelation kinetics. J. Biosci. Bioeng. 1999, 88, 686–689. [Google Scholar] [CrossRef]
- Chan, A.W.; Whitney, R.A.; Neufeld, R.J. Kinetic controlled synthesis of pH-responsive network alginate. Biomacromolecules 2008, 9, 2536–2545. [Google Scholar] [CrossRef] [PubMed]
- Abdellaoui, Y.; Celaya, C.A.; Elhoudi, M.; Boualou, R.; Agalit, H.; Reina, M.; Gamero-Melo, P.; Abou Oualid, H. Understanding of vibrational and thermal behavior of bio-based doped alginate@nickel cross-linked beads: A combined experimental and theoretical study. J. Mol. Struct. 2022, 1249, 131524. [Google Scholar] [CrossRef]
- Lee, P.; Rogers, M.A. Effect of calcium source and exposure-time on basic caviar spherification using sodium alginate. Int. J. Gastron. Food Sci. 2012, 1, 96–100. [Google Scholar] [CrossRef]
- Dang, T.D.; Joo, S.W. Preparation of tadpole-shaped calcium alginate microparticles with sphericity control. Colloids Surf. B 2013, 102, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.-S.; Lee, B.-B.; Ravindra, P.; Poncelet, D. Prediction models for shape and size of ca-alginate macrobeads produced through extrusion–dripping method. J. Colloid Interface Sci. 2009, 338, 63–72. [Google Scholar] [CrossRef]
- Swioklo, S.; Ding, P.; Pacek, A.W.; Connon, C.J. Process parameters for the high-scale production of alginate-encapsulated stem cells for storage and distribution throughout the cell therapy supply chain. Process Biochem. 2017, 59, 289–296. [Google Scholar] [CrossRef]
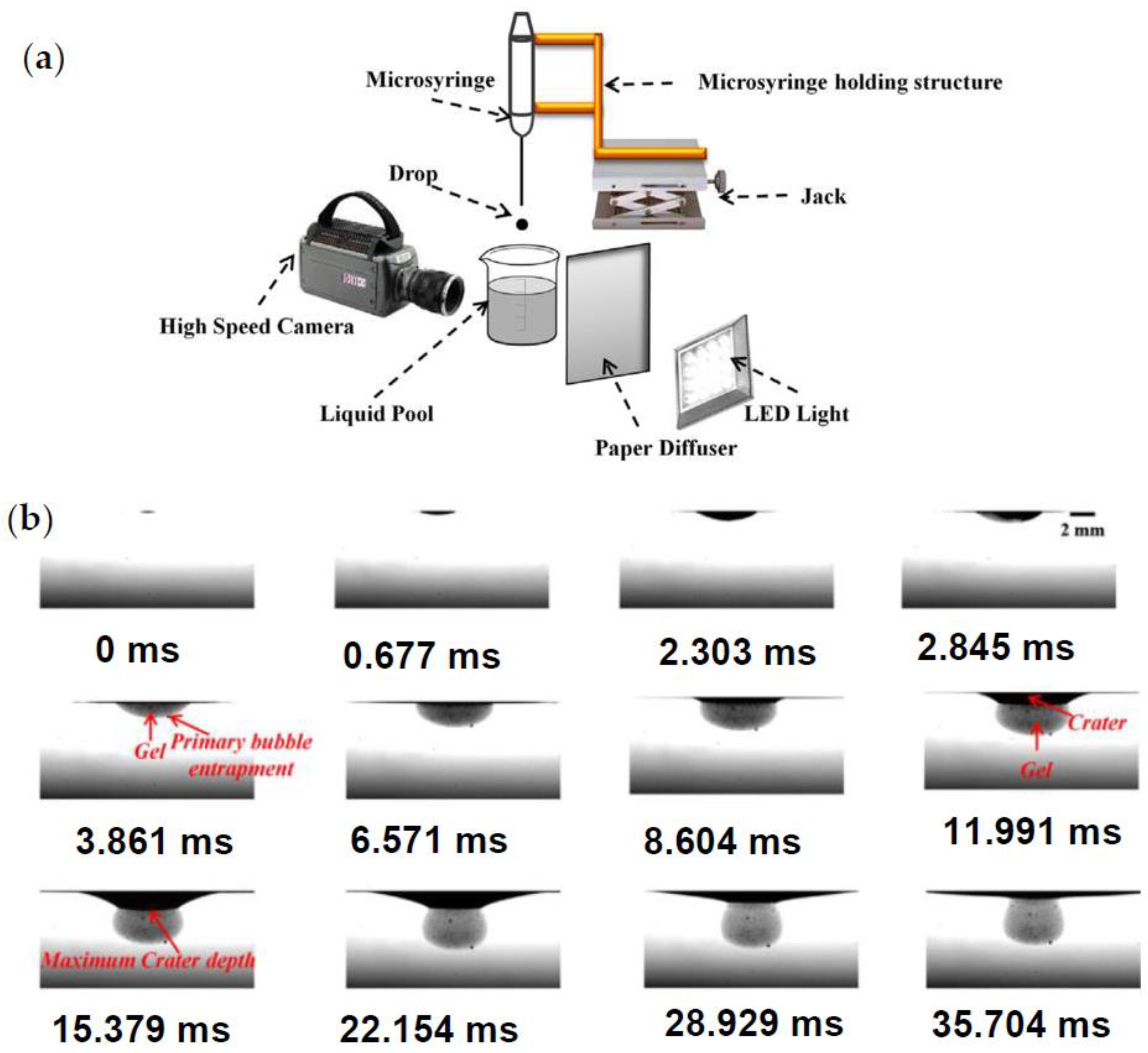
- Haldar, K.; Chakraborty, S. Effect of liquid pool concentration on chemically reactive drop impact gelation process. J. Colloid Interface Sci. 2018, 528, 156–165. [Google Scholar] [CrossRef]
- Haldar, K.; Chakraborty, S. Role of chemical reaction and drag force during drop impact gelation process. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 401–409. [Google Scholar] [CrossRef]
- Haldar, K.; Chakraborty, S. Investigation of chemical reaction during sodium alginate drop impact on calcium chloride film. Phys. Fluids 2019, 31, 072102. [Google Scholar] [CrossRef]
- Stößlein, S.; Grunwald, I.; Stelten, J.; Hartwig, A. In-situ determination of time-dependent alginate-hydrogel formation by mechanical texture analysis. Carbohydr. Polym. 2019, 205, 287–294. [Google Scholar] [CrossRef] [PubMed]
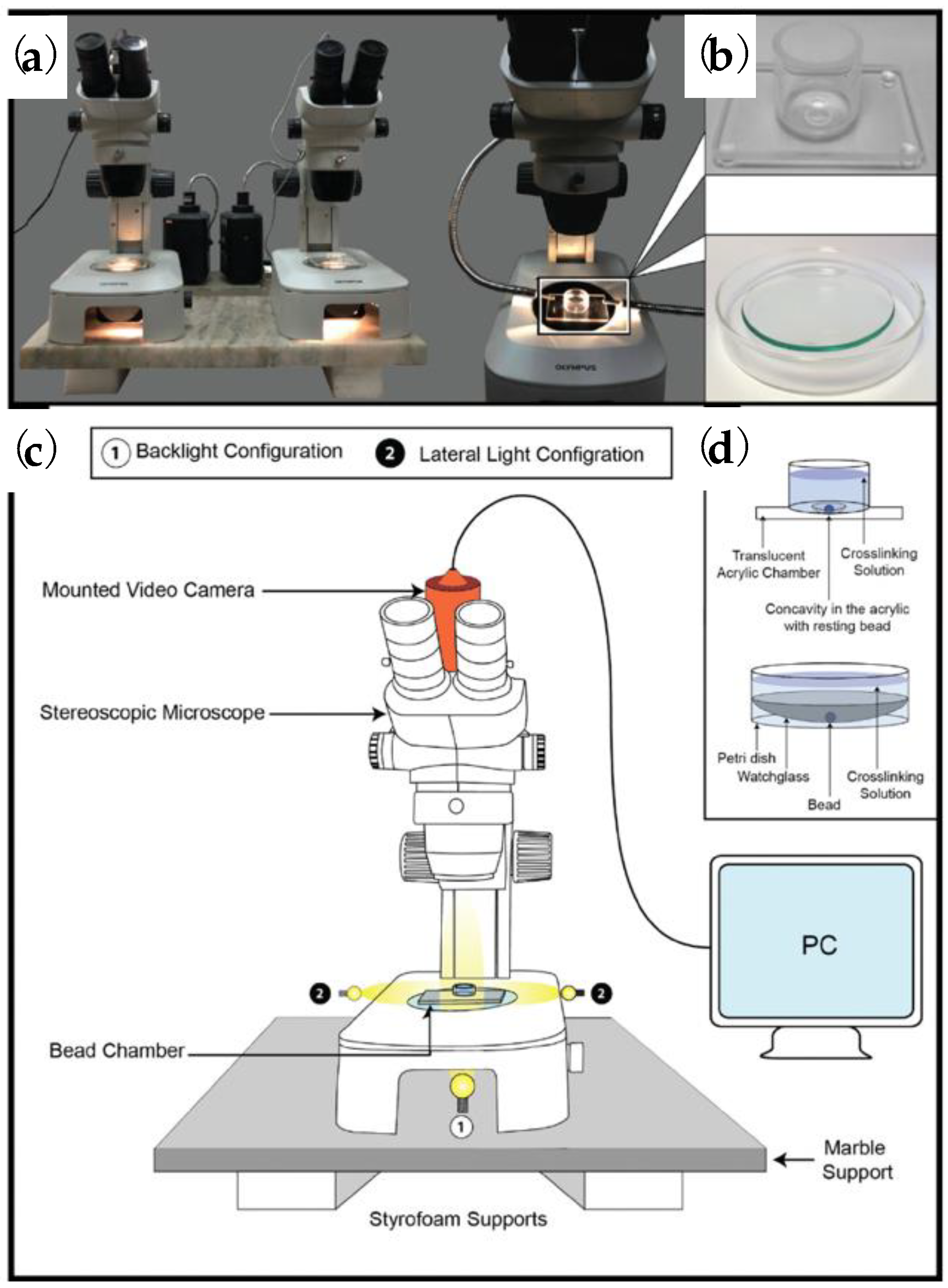
- Posbeyikian, A.; Tubert, E.; Bacigalupe, A.; Escobar, M.M.; Santagapita, P.R.; Amodeo, G.; Perullini, M. Evaluation of calcium alginate bead formation kinetics: An integrated analysis through light microscopy, rheology and microstructural SAXS. Carbohydr. Polym. 2021, 269, 118293. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Kelly, A.L.; Maidannyk, V.; Miao, S. Effect of concentrations of alginate, soy protein isolate and sunflower oil on water loss, shrinkage, elastic and structural properties of alginate-based emulsion gel beads during gelation. Food Hydrocoll. 2020, 108, 105998. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Besiri, I.N.; Goudoulas, T.B.; Fattahi, E.; Becker, T. Experimental Advances in the Real-Time Recording of Cross-Linking Alginate In Situ Gelation: A Review. Polymers 2023, 15, 2875. https://doi.org/10.3390/polym15132875
Besiri IN, Goudoulas TB, Fattahi E, Becker T. Experimental Advances in the Real-Time Recording of Cross-Linking Alginate In Situ Gelation: A Review. Polymers. 2023; 15(13):2875. https://doi.org/10.3390/polym15132875
Chicago/Turabian StyleBesiri, Ioanna N., Thomas B. Goudoulas, Ehsan Fattahi, and Thomas Becker. 2023. "Experimental Advances in the Real-Time Recording of Cross-Linking Alginate In Situ Gelation: A Review" Polymers 15, no. 13: 2875. https://doi.org/10.3390/polym15132875






