Elastomer Nanocomposites: Effect of Filler–Matrix and Filler–Filler Interactions
Abstract
:1. Introduction
2. Basic Mechanisms of Filler Reinforcement in Conventional Composites
3. Nanospherical Particles
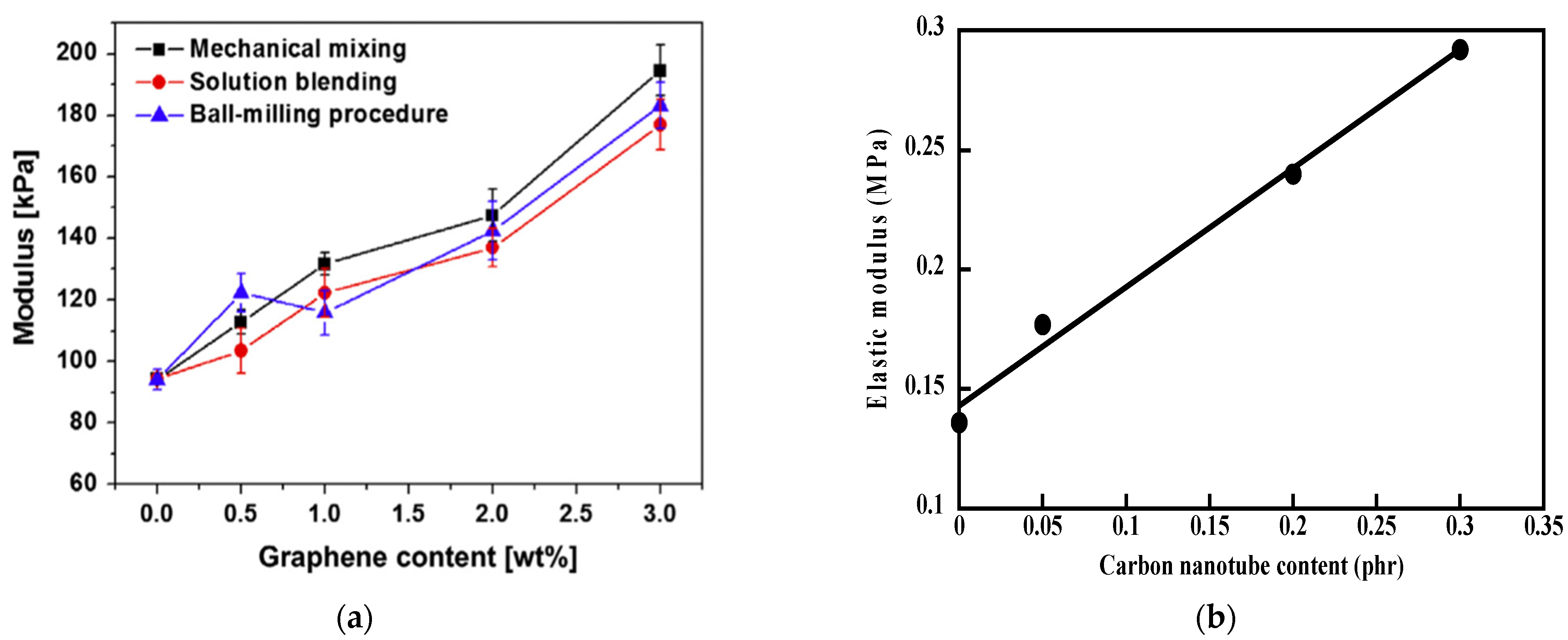
4. Rod-Shaped Particles
5. Layered Fillers
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Payne, A.R.; Whittaker, R.E. Reinforcement of rubber with carbon black. Composites 1970, 1, 203–214. [Google Scholar] [CrossRef]
- Kraus, G. Reinforcement of elastomers by carbon black. Adv. Polym. Sci. 1971, 8, 155–237. [Google Scholar]
- Wagner, M.P. Reinforcing silicas and silicates. Rubber Chem. Technol. 1976, 49, 703–774. [Google Scholar] [CrossRef]
- Dannenberg, E.M. The effects of surface chemical interactions on the properties of filler-reinforced rubbers. Rubber Chem. Technol. 1975, 48, 410–444. [Google Scholar] [CrossRef]
- Voet, A. Reinforcement of elastomers by fillers: Review of period 1967–1976. J. Polym. Sci. Macromol. Rev. 1980, 15, 327–373. [Google Scholar] [CrossRef]
- Edwards, D.C. Polymer-filler interactions in rubber reinforcement. J. Mater. Sci. 1990, 25, 4175–4185. [Google Scholar] [CrossRef]
- Ahmed, S.; Jones, F.R. A review of particulate reinforcement theories for polymer composites. J. Mater. Sci. 1990, 25, 4933–4942. [Google Scholar] [CrossRef]
- Bokobza, L. The reinforcement of elastomeric networks by fillers. Macromol. Mater. Eng. 2004, 289, 607–621. [Google Scholar] [CrossRef]
- Roland, C.M. Reinforcement of elastomers. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Robertson, C.G.; Hardman, N.J. Nature of carbon black reinforcement of rubber: Perspective on the original polymer nanocomposite. Polymers 2021, 13, 538. [Google Scholar] [CrossRef] [PubMed]
- Guth, E. Theory of filler reinforcement. J. Appl. Phys. 1945, 16, 20–25. [Google Scholar] [CrossRef]
- Bokobza, L. Filled elastomers:a new approach based on measurements of chain orientation. Polymer 2001, 42, 5415–5423. [Google Scholar] [CrossRef]
- Huber, G.; Vilgis, T.A. Universal properties of filled rubbers: Mechanisms for reinforcement on different length scales. KGK Kautsch. Gummi Kunstst. 1999, 52, 102–107. [Google Scholar]
- Payne, A.R. The dynamic properties of carbon black-loaded natural rubber vulcanizates. Part I. J. Appl. Polym. Sci. 1962, 6, 57–63. [Google Scholar] [CrossRef]
- Wang, M.-J. Effect of polymer-filler and filler-filler interactions on dynamics properties of filled vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Mooney, M. A theory of large elastic deformation. J. Appl. Phys. 1940, 11, 582–592. [Google Scholar] [CrossRef]
- Rivlin, R.S.; Saunders, D.W. Large elastic deformations of isotropic materials. VII. Experiments on the deformation of rubber. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 1951, 243, 251–288. [Google Scholar]
- Bueche, F. Mullins effect and rubber-filler interactions. J. Appl. Polym. Sci. 1961, 5, 271–281. [Google Scholar] [CrossRef]
- Lapra, A.; Clément, F.; Bokobza, L.; Monnerie, L. Straining effects in silica-filled elastomers investigated by atomic force microscopy: From macroscopic stretching to nanoscale strainfield. Rubber Chem. Technol. 2003, 76, 60–81. [Google Scholar] [CrossRef]
- Bokobza, L.; Gaulliard, V.; Ladouce, L. Silica reinforcement of styrene-butadiene rubbers. Kautsch. Gummi Kunstst. 2001, 54, 177–180. [Google Scholar]
- Bokobza, L.; Rapoport, O. Reinforcement of natural rubber. J. Appl. Polym. Sci. 2002, 85, 2301–2316. [Google Scholar] [CrossRef]
- Bernal-Ortega, P.; Anyska, R.; Morishita, Y.; di Ronza, R.; Blume, A. Comparison between SBR compounds filled in-situ and ex-situ silanized silica. Polymers 2021, 13, 281. [Google Scholar] [CrossRef] [PubMed]
- Huneau, B.; Masquelier, I.; Marco, Y.; Le Saux, V.; Noizet, S.; Schiel, C.; Charrier, P. Fatigue crack initiation in a carbon black-filled natural rubber. Rubber Chem. Technol. 2016, 89, 126–141. [Google Scholar] [CrossRef] [Green Version]
- Gehling, T.; Schieppati, J.; Balasooriya, W.; Kerschbaumer, R.C.; Pinter, G. Fatigue behavior of elastomeric components: A review of the key factors of rubber formulation, manufacturing, and service conditions. Polym. Rev. 2023. [Google Scholar] [CrossRef]
- Maxwell, A.S.; Broughton, W.R.; Lodeiro, M.J. Review of Techniques for Characterization of Residual Stress in Polymer Composites; National Physical Laboratory Report; DEPC MPR 056; National Physical Laboratory: London, UK, 2006. [Google Scholar]
- Maxwell, A.S.; Broughton, W.R.; Lodeiro, M.J.; Shaw, R. Measurement of Residual Stresses and Strains in Carbon Fibre Composites; Report MN7; National Physical Laboratory: London, UK, 2009. [Google Scholar]
- Shokrieh, M.M. Residual Stresses in Composite Materials; Woodhead Publishing Series in Composites Science and Engineering: Number 48; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Gong, L.; Yang, H.; Ke, Y.; Wang, S.; Yao, X. Investigation on vulcanization degree and residual stress on fabric rubber composites. Compos. Struct. 2019, 209, 472–480. [Google Scholar] [CrossRef]
- McCarthy, D.W.; Mark, J.E.; Schaefer, D.W. Synthesis, structure and properties of hybrid organic-inorganic composites based on polysiloxanes. I. Poly(dimethylsiloxane) elastomers containing silica. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1167–1189. [Google Scholar] [CrossRef]
- McCarthy, D.W.; Mark, J.E.; Clarson, S.J.; Schaefer, D.W. Synthesis, structure and properties of hybrid organic-inorganic composites based on polysiloxanes. II. Comparisons between poly(methylphenylsiloxane) and poly(dimethylsiloxane), and between titania and silica. J. Polym. Sci. Part B Polym. Phys. 1998, 36, 1191–1200. [Google Scholar] [CrossRef]
- Breiner, J.M.; Mark, J.E.; Beaucage, G. Dependence of silica particle sizes on network chain lengths, silica contents, and catalyst concentrations in in situ-reinforced polysiloxane elastomers. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1421–1427. [Google Scholar] [CrossRef]
- Dewimille, L.; Bresson, B.; Bokobza, L. Synthesis, structure and morphology of poly(dimethylsiloxane) networks filled with in situ generated silica particles. Polymer 2005, 46, 4135–4143. [Google Scholar] [CrossRef]
- Bokobza, L.; Diop, A.L. Reinforcement of poly(dimethylsiloxane) by sol-gel in situ generated silica and titania particles. Express Polym. Lett. 2010, 4, 355–363. [Google Scholar] [CrossRef]
- Bokobza, L.; Diop, A.L. Reinforcement of silicone rubbers by sol-gel in situ generated filler particles. In Rubber Nanocomposites: Preparation, Properties, and Applications; Chapter 3; Thomas, S., Stephen, R., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2010. [Google Scholar]
- Klonos, P.; Panagopoulou, A.; Bokobza, L.; Kyritsis, A.; Peoglos, V.; Pissis, P. Comparative studies on effects of silica and titania nanoparticles on crystallization and complex segmental dynamics in poly(dimethylsiloxane). Polymer 2010, 54, 5490–5499. [Google Scholar] [CrossRef]
- Klonos, P.; Panagopoulou, A.; Kyritsis, A.; Bokobza, L.; Pissis, P. Dielectric studies of segmental dynamics in poly(dimethylsiloxane)/titania nanocomposites. J. Non-Cryst. Solids 2011, 357, 610–614. [Google Scholar] [CrossRef]
- Klonos, P.; Kyritsis, A.; Bokobza, L.; Gun’ko, V.M.; Pissis, P. Interfacial effects in PDMS/titania nanocomposites studied by thermal and dielectric studies. Colloids Surf. A Physicochem. Eng. Asp. 2017, 519, 212–222. [Google Scholar] [CrossRef]
- Gong, J.P. Why are double network hydrogels so tough? Soft Matter 2010, 6, 2583–2590. [Google Scholar] [CrossRef]
- Murai, J.; Nakajima, T.; Matsuda, T.; Tsunoda, K.; Nonoyama, T.; Kurokawa, T.; Gong, J.P. Tough double network elastomers reinforced by the amorphous cellulose network. Polymer 2019, 178, 121686. [Google Scholar] [CrossRef]
- Yang, J.; Li, K.; Tang, C.; Liu, Z.; Fan, J.; Qin, G.; Cui, W.; Zhu, L.; Chen, Q. Recent progress in double network elastomers: One plus is greater than two. Adv. Funct. Mater. 2022, 32, 2110244. [Google Scholar] [CrossRef]
- Strohmeier, L.; Schrittesser, B.; Schlögl, S. Approaches toward in situ reinforcement of organic rubbers: Strategy and recent progress. Polym. Rev. 2022, 62, 142–174. [Google Scholar] [CrossRef]
- Das, C.; Bansod, N.D.; Kapgate, B.P.; Reuter, U.; Heinrich, G.; Das, A. Development of highly reinforced acrylonitrile butadiene rubber composites via controlled loading of sol-gel titania. Polymer 2017, 109, 25–37. [Google Scholar] [CrossRef]
- Das, C.; Bansod, N.D.; Kapgate, B.P.; Rajkumar, K.; Das, A. Incorporation of titania nanoparticles in elastomer matrix to develop highly reinforced multifunctional solution styrene butadiene rubber composites. Polymer 2019, 162, 1–10. [Google Scholar] [CrossRef]
- Bokobza, L.; Chauvin, J.-P. Reinforcement of natural rubber: Use of in situ generated silicas and nanofibers of sepiolite. Polymer 2005, 46, 4144–4151. [Google Scholar] [CrossRef]
- Ambilkar, S.C.; Bansod, N.D.; Kapgate, B.P.; Das, A.; Formanek, P.; Rajkumar, K.; Das, C. In situ zirconia: A superior reinforcing filler for high-performance nitrile rubber composites. ACS Omega 2020, 5, 7751–7761. [Google Scholar] [CrossRef] [Green Version]
- De, B. Carbon dots and their polymeric nanocomposites. In Nanomaterials and Polymer Nanocomposites, Raw Materials to Applications; Karak, N., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 217–260. [Google Scholar]
- Kausar, A. Polymer/carbon-based quantum dot nanocomposite: Forthcoming materials for technical application. J. Macromol. Sci. Part A Pure Appl. Chem. 2019, 56, 341–356. [Google Scholar] [CrossRef]
- De, B.; Karak, N. Recent progress on carbon dot-metal based nanohybrids for photochemical and electrochemical applications. J. Mater. Chem. A 2017, 5, 1826–1859. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Y.; Dong, P.; Huang, J. A mini review on carbon quantum dots: Preparation, properties, and electrocatalytic application. Front. Chem. 2019, 7, 671. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.S.; Fen, Y.W.; Irmawati, R.; Hashim, H.S.; Ramdzan, N.S.M.; Fauzi, N.I.M. A review on carbon dots: Synthesis, characterization and its application in optical sensor for environmental monitoring. Nanomaterials 2022, 12, 2365. [Google Scholar] [CrossRef] [PubMed]
- Sreenath, P.R.; Singh, S.; Satyanarayana, M.S.; Das, P.; Kumar, K.D. Carbon dot-Unique reinforcing filler for polymer with special reference to physico-mechanical properties. Polymer 2017, 112, 189–200. [Google Scholar] [CrossRef]
- Sreenath, P.R.; Mandal, S.; Panigrahi, H.; Das, P.; Kumar, K.D. Carbon dots: Fluorescence active, covalently conjugated and strong reinforcing nanofiller for polymer latex. Nano-Struct. Nano-Objects 2020, 23, 100477. [Google Scholar] [CrossRef]
- Fernandes, D.; Heslop, K.A.; Kelarakis, A.; Krysmann, M.J.; Estevez, L. In situ generation of carbon dots within a polymer matrix. Polymer 2020, 188, 122159. [Google Scholar] [CrossRef]
- Yu, P.; Jin, G.; Wang, W.; Wang, R.; Guo, B.; Zhang, L. Dispersing carbon dots in non-polar rubber by slurry compounding and in situ compatibilizing. Compos. Commun. 2020, 22, 100429. [Google Scholar] [CrossRef]
- Jin, G.; Lu, Y.; Yu, P.; Zhang, L. Simple method to prepare fluorescent silicon rubber by melt-compounding with crude carbon dots fluid. Mater. Today Commun. 2021, 27, 102413. [Google Scholar] [CrossRef]
- Shauloff, N.; Bhattacharya, S.; Jelinek, R. Elastic carbon dot/polymer films for fluorescent tensile sensing and mechano-optical tuning. Carbon 2019, 152, 363–371. [Google Scholar] [CrossRef]
- Desai, A.V.; Haque, M.A. Mechanics of the interface for carbon nanotube-polymer composites. Thin-Walled Struct. 2005, 43, 1787–1803. [Google Scholar] [CrossRef]
- Bokobza, L. Enhanced electrical and mechanical properties of multiwall carbon nanotube rubber composites. Polym. Adv. Technol. 2012, 23, 1543–1549. [Google Scholar] [CrossRef]
- Bokobza, L.; Bresson, B.; Garnaud, G.; Zhang, J. Mechanical and AFM investigations of elastomers filled with multiwall carbon nanotubes. Compos. Interfaces 2012, 19, 285–295. [Google Scholar] [CrossRef]
- Nakaramontri, Y.; Kummerlöwe, C.; Nakason, C.; Vennemann, N. The effect of surface functionalization of carbon nanotubes on properties of natural rubber/carbon nanotube composites. Polym. Compos. 2015, 36, 2113–2122. [Google Scholar] [CrossRef]
- Sahakaro, K. Mechanism of reinforcement using nanofillers in rubber nanocomposites. In Progress in Rubber Nanocomposites; Woodhead Publishing: Cambridge, UK, 2017; pp. 81–113. [Google Scholar]
- Bokobza, L. Mechanical and electrical properties of elastomer nanocomposites based on different carbon nanomaterials. C J. Carbon Res. 2017, 3, 10. [Google Scholar] [CrossRef] [Green Version]
- Bokobza, L. Natural rubber nanocomposites: A review. Nanomaterials 2019, 9, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Rennhofer, H.; Zanghellini, B. Dispersion state and damage of carbon nanotubes and carbon nanofillers by ultrasonic dispersion: A review. Nanomaterials 2021, 11, 1469. [Google Scholar] [CrossRef]
- Sahoo, N.G.; Rana, S.; Cho, J.W.; Li, L.; Chan, S.H. Polymer nanocomposites based on functionalized carbon nanotubes. Prog. Polym. Sci. 2010, 35, 837–867. [Google Scholar] [CrossRef]
- Ponnamma, D.; Sadasivuni, K.K.; Grohens, Y.; Guo, Q.; Thomas, S. Carbon nanotube based elastomer composites-an approach towards multifunctional materials. J. Mater. Chem. C 2014, 2, 8446–8485. [Google Scholar] [CrossRef]
- Setaro, A. Advanced carbon nanotubes functionalization. J. Phys. Condens. Matter 2017, 29, 423003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speranza, G. The role of functionalization in the applications of carbon materials: An overview. C J. Carbon Res. 2019, 4, 84. [Google Scholar] [CrossRef] [Green Version]
- Speranza, G. Carbon nanomaterials: Synthesis, functionalization and sensing applications. Nanomaterials 2021, 11, 967. [Google Scholar] [CrossRef] [PubMed]
- Singaravel, D.K.; Sharma, S.; Kumar, P. Recent progress in experimental and molecular dynamics study of carbon nanotube reinforced rubber composites: A review. Polym.-Plast. Technol. Mater. 2022, 61, 1792–1825. [Google Scholar] [CrossRef]
- Staudinger, U.; Janke, A.; Steinbach, C.; Reuter, U.; Ganb, M.; Voigt, O. Influence of CNT length on dispersion, localization, and electrical percolation in a styrene-butadiene-based star block copolymer. Polymers 2022, 14, 2715. [Google Scholar] [CrossRef]
- Fukushima, T.; Kosaka, A.; Ishimura, Y.; Yamamoto, T.; Takigawa, T.; Ishii, N.; Aida, T. Molecular ordering of organic molten salts triggered by single-walled carbon nanotubes. Science 2003, 300, 2072–2074. [Google Scholar] [CrossRef] [Green Version]
- Polo-Luque, M.L.; Simonet, B.M.; Valcárcel, M. Functionalization and dispersion of carbon nanotubes in ionic liquids. Trends Anal. Chem. 2013, 47, 99–110. [Google Scholar] [CrossRef]
- Das, A.; Stöckelhuber, K.W.; Jurk, R.; Fritzsche, J.; Klüppel, M.; Heinrich, G. Coupling activity of ionic liquids between diene elastomers and multi-walled carbon nanotubes. Carbon 2009, 47, 3313–3321. [Google Scholar] [CrossRef]
- Krainoi, A.; Kummerlöwe, C.; Nakaramontri, Y.; Wisunthorn, S.; Vennemann, N.; Pichaiyut, S.; Kiatkamjornwong, S.; Nakason, C. Influence of carbon nanotube and ionic liquid on properties of natural rubber composites. Express Polym. Lett. 2019, 13, 327–348. [Google Scholar] [CrossRef]
- Ponnamma, D.; Sung, S.H.; Hong, J.S.; Ahn, K.H.; Varughese, K.T.; Thomas, S. Influence of non-covalent functionalization of carbon nanotubes on the rheological behavior of natural rubber latex nanocomposites. Eur. Polym. J. 2014, 53, 147–159. [Google Scholar] [CrossRef]
- Galimberti, M.; Coombs, M.; Riccio, P.; Riccò, T.; Passera, S.; Pandini, S.; Conzatti, L.; Ravasio, A.; Tritto, I. The role of CNTs in promoting hybrid filler networking and synergism with carbon black in the mechanical behavior of filled polyisoprene. Macromol. Mater. Eng. 2013, 298, 241–251. [Google Scholar] [CrossRef]
- Galimberti, M.; Infortuna, G.; Guerra, S.; Barbera, V.; Agnelli, S.; Pandini, S. sp2 carbon allotropes in elastomer matrix: From master curves for the mechanical reinforcement to lightweight materials. Express Polym. Lett. 2018, 12, 265–283. [Google Scholar] [CrossRef]
- Bernal-Ortega, P.; Bernal, M.M.; Blume, A.; González-Jiménez, A.; Posadas, P.; Navarro, R.; Valentín, J.L. Sulfur-modified carbon nanotubes for the development of advanced elastomeric materials. Polymers 2021, 13, 821. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, A.; Omelan, M.C.V.; Giese, U. Influence of carbon nanotube-pretreatment on the properties of polydimethylsiloxane/carbon nanotube-nanocomposites. Polymers 2021, 13, 1355. [Google Scholar] [CrossRef]
- Nakaramontri, Y.; Nakason, C.; Kummerlöwe, C.; Vennemann, N. Influence of modified natural rubber properties of natural rubber-carbon nanotube composites. Rubber Chem. Technol. 2015, 88, 199–218. [Google Scholar] [CrossRef]
- Krainoi, A.; Johns, J.; Kalkornsurapranee, E.; Nakaramontri, Y. Carbon nanotubes reinforced natural rubber composites. In Carbon Nanotubes—Redefining the World of Electronics; Chapter 4; Ghosh, P., Rushi, A., Datta, K., Eds.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Bokobza, L.; Rahmani, M.; Belin, C.; Bruneel, J.-L.; El Bounia, N.-E. Blends of carbon blacks and multiwall carbon nanotubes as reinforcing fillers for hydrocarbon rubbers. J. Polym. Sci. Part B Polym. Phys. 2008, 46, 1939–1951. [Google Scholar] [CrossRef]
- Bokobza, L.; Leroy, E.; Lalanne, V. Effect of filling mixtures of sepiolite and a surface modified fumed silica on the mechanical and swelling behavior of a styrene-butadiene rubber. Eur. Polym. J. 2009, 45, 996–1001. [Google Scholar] [CrossRef]
- Haghgoo, M.; Ansari, R.; Hassanzadeh-Aghdam, M.K. The effect of nanoparticle conglomeration on the overall conductivity of nanocomposites. Int. J. Eng. Sci. 2020, 157, 103392. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Maiti, M.; Bhowmick, A.K. Influence of different nanofillers and their dispersion methods on the properties of natural rubber nanocomposites. Rubber Chem. Technol. 2008, 81, 782–808. [Google Scholar] [CrossRef]
- Satyanarayana, M.S.; Sreenath, P.R.; Bhowmick, A.K.; Kumar, K.D. Selective orientation of needlelike sepiolite nanoclay in polymer blend for controlled properties. ACS Omega 2018, 3, 11691–11702. [Google Scholar] [CrossRef]
- Bokobza, L. Infrared linear dichroism for the analysis of molecular orientation in polymers and in polymer composites. Polymers 2022, 14, 1257. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef] [Green Version]
- George, S.C.; Rajan, R.; Aprem, A.S.; Thomas, S.; Kim, S.S. The fabrication and properties of natural rubber-clay nanocomposites. Polym. Test. 2016, 51, 165–173. [Google Scholar] [CrossRef]
- Sookyung, U.; Nakason, C.; Venneman, N.; Thaijaroend, W. Influence of modifying agent on properties of natural rubber/organoclay nanocomposites. Polym. Test. 2016, 54, 223–232. [Google Scholar] [CrossRef]
- Siririttikrai, N.; Thanavan, S.; Suchiva, K.; Amornsakchai, T. Comparative study of natural rubber/clay nanocomposites prepared from fresh or concentrated latex. Polym. Test. 2017, 63, 244–250. [Google Scholar] [CrossRef]
- Perera, S.J.; Egodage, S.M.; Walpalage, S. Enhancement of mechanical properties of natural rubber-clay nanocomposites through incorporation of silanated organoclay into natural rubber latex. e-Polymers 2020, 20, 144–153. [Google Scholar] [CrossRef]
- Kausar, A.; Ahmad, I.; Maaza, M.; Eisa, M.H. State-of-the-Art nanoclay reinforcement in green polymeric nanocomposite: From design to new opportunities. Minerals 2022, 12, 1495. [Google Scholar] [CrossRef]
- Wang, M.; Yan, C.; Ma, L. Graphene nanocomposites. In Composites and Their Properties; Hu, N., Ed.; Chapter 2; IntechOpen: London, UK, 2012. [Google Scholar]
- Dhand, V.; Rhee, K.Y.; Kim, H.J.; Jung, D.H. A comprehensive review of graphene nanocomposites: Research status and trends. J. Nanomater. 2013, 2013, 763953. [Google Scholar] [CrossRef] [Green Version]
- Young, R.J.; Kinloch, I.A.; Gong, L.; Novoselov, K.S. The mechanics of graphene nanocomposites: A review. Compos. Sci. Technol. 2012, 72, 1459–1476. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Ibrahim, A.; Klopocinska, A.; Horvat, K.; Hamid, Z.A. Graphene-based nanocomposites: Synthesis, mechanical properties, and characterizations. Polymers 2021, 13, 2869. [Google Scholar] [CrossRef] [PubMed]
- Díez-Pascual, A.M. Development of graphene-based polymeric nanocomposites: A brief overview. Polymers 2021, 13, 2978. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhang, C.; Chen, Y.; Nie, Z. Research progress on the preparation and applications of laser-induced graphene technology. Nanomaterials 2022, 12, 2336. [Google Scholar] [CrossRef] [PubMed]
- Pham Duy, L.N.; Bui, C.; Nguyen, L.T.; Nguyen, T.H.; Nguyen, T.T.; La, D.D. Dioctyl phthalate-modified graphene nanoplatelets: An effective additive for enhanced mechanical properties of natural rubber. Polymers 2022, 14, 2541. [Google Scholar]
- Al-Harthi, M.A.; Hussain, M. Effect on the surface functionalization of graphene and MWCNT on the thermodynamic, mechanical and electrical properties of the graphene/MWCNT-PVDF nanocomposites. Polymers 2022, 14, 2976. [Google Scholar] [CrossRef]
- Sarath, P.S.; Thomas, S.; Haponiuk, J.T.; George, S.C. Fabrication, characterization and properties of silane functionalized graphene oxide/silicone rubber nanocomposites. J. Appl. Polym. Sci. 2022, 139, e52299. [Google Scholar] [CrossRef]
- Fu, D.H.; Zhan, Y.H.; Yan, N.; Xia, H.S. A comparative investigation on strain induced crystallization for graphene and carbon nanotubes filled natural rubber composites. Express Polym. Lett. 2015, 9, 597–607. [Google Scholar] [CrossRef]
- Wu, X.; Lin, T.F.; Tang, Z.H.; Cuo, B.C.; Huang, G.S. Natural rubber/graphene oxide composites: Effect of sheet size on mechanical properties and strain-induced crystallization behavior. Express Polym. Lett. 2015, 9, 672–685. [Google Scholar] [CrossRef]
- She, X.; He, C.; Peng, Z.; Kong, L. Molecular-level dispersion of graphene into epoxized natural rubber: Morphology, interfacial interaction and mechanical reinforcement. Polymer 2014, 55, 6803–6810. [Google Scholar] [CrossRef]
- Bokobza, L.; Rahmani, M. Carbon nanotubes: Exceptional reinforcing fillers for silicone rubbers. KGK Kautsch. Gummi Kunstst. 2009, 62, 112–117. [Google Scholar]
- Xu, H.; Gong, L.-X.; Wang, X.; Zhao, L.; Pei, Y.-B.; Wang, G.; Liu, Y.-J.; Wu, L.-B.; Jiang, J.-X.; Tang, L.-C. Influence of processing conditions on dispersion, electrical and mechanical properties of graphene-filled-silicone rubber composites. Compos. Part A 2016, 91, 53–64. [Google Scholar] [CrossRef]
- Liang, W.; Ge, X.; Ge, J.; Li, T.; Zhao, T.; Chen, X.; Song, Y.; Cui, Y.; Khan, M.; Ji, J.; et al. Silicon resin nano-aggregates for silicone rubber composites with enhanced thermal conductivity and mechanical performance. Polymers 2018, 10, 1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.; Wang, Z.; Zeng, H.; Yang, T.; Wang, X. Effects of graphene on various properties and applications of silicone rubber and silicone resin. Compos. Part A 2021, 142, 106240. [Google Scholar] [CrossRef]
- Deng, B.; Shi, Y.; Zhang, X.; Ma, W.; Liu, H.; Gong, C. Thermally conductive and electrically insulated silicone rubber composites incorporated with boron nitride—Multilayer graphene hybrid nanofiller. Nanomaterials 2022, 12, 2335. [Google Scholar] [CrossRef]
- Kumar, V.; Mandal, T.K.; Kumar, A.; Alam, M.N.; Parvin, N.; Joo, S.W.; Lee, D.J.; Park, S.-S. Multifunctional properties of chemically reduced graphene oxide and silicone rubber composite: Facile synthesis and application as soft composites for piezoelectric energy harvesting. Express Polym. Lett. 2022, 16, 978–985. [Google Scholar] [CrossRef]
- Beigbeder, A.; Linares, M.; Devalckenaere, M.; Degée, P.; Claes, M.; Beljonne, D.; Lazzaroni, R.; Dubois, P. CH-π interactions as the driving force for silicone-based nanocomposites with exceptional properties. Adv. Mater. 2008, 20, 1003–1007. [Google Scholar] [CrossRef]
- Paduvilan, J.K.; Velayudhan, P.; Amanulla, A.; Maria, H.J.; Saiter-Fourcin, A.; Thomas, S. Assessment of graphene oxide and nanoclay based hybrid filler in chlorobutyl-natural rubber blend for advanced gas barrier applications. Nanomaterials 2021, 11, 1098. [Google Scholar] [CrossRef]
- Barshutina, M.N.; Volkov, V.S.; Arsenin, A.V.; Nasibulin, A.G.; Barshutin, S.N.; Tkachev, A.G. Silicone composites with CNT/graphene hybrid fillers: A review. Materials 2021, 14, 2418. [Google Scholar] [CrossRef]
- Chen, L.; Yu, G.; Zhuang, T.; Pang, X. Reinforcing effects of carbon nanotubes on graphene/trans-polyisoprene/natural rubber composites. J. Polym. Res. 2022, 29, 387. [Google Scholar] [CrossRef]
- Sreenath, P.R.; Mandal, S.; Singh, S.; Das, P.; Bhowmick, A.N.; Kumar, K.D. Remarkable synergetic effect by in-situ covalent hybridization of carbon dots with graphene oxide and carboxylated acrylonitrile butadiene rubber. Polymer 2019, 175, 283–293. [Google Scholar] [CrossRef]
- Rigbi, Z. Reinforcement of rubber by carbon black. Adv. Polym. Sci. 1980, 36, 21–68. [Google Scholar]
- Donnet, J.-B.; Vidal, A. Carbon black: Surface properties and interactions with elastomers. Adv. Polym. Sci. 1986, 76, 103–127. [Google Scholar]
- Salvetat, J.-P.; Briggs, G.A.D.; Bonard, J.-M.; Bacsa, R.R.; Kulik, A.J.; Stöckli, T.; Burnham, N.A.; Forró, L. Elastic and shear moduli of single-walled carbon nanotubes ropes. Phys. Rev. Lett. 1999, 82, 944–947. [Google Scholar] [CrossRef] [Green Version]
- Peigney, A.; Laurent, C.; Flahaut, E.; Bacsa, R.R.; Rousset, A. Specific surface area of carbon nanotubes and bundles of carbon nanotubes. Carbon 2001, 39, 507–514. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Park, H.; Yamamoto, G.; Lee, C.; Suk, J.W. Measurements of the electrical conductivity of monolayer graphene flakes using conductive atomic force microscopy. Nanomaterials 2021, 11, 2575. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Hasan, M.R.; Sharma, P.; Narang, J. Graphene nanomaterials: The wondering material from synthesis to applications. Sens. Int. 2022, 3, 100190. [Google Scholar] [CrossRef]
- Bergaya, F.; Jaber, M.; Lambert, J.-F. Clays and clay minerals. In Rubber-Clay Nanocomposites: Science, Technology, and Applications, 1st ed.; Galimberti, M., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Zare, Y.; Rhee, K.Y. Simulation of Young’s modulus for clay-reinforced nanocomposites assuming mechanical percolation, clay-interphase and interfacial linkage. J. Mater. Res. Technol. 2020, 9, 12473–12483. [Google Scholar] [CrossRef]
- Ikeda, Y.; Kameda, Y. Preparation of “green” composites by the sol-gel process: In situ silica filled natural rubber. J. Sol-Gel Sci. Technol. 2004, 31, 137–142. [Google Scholar] [CrossRef]
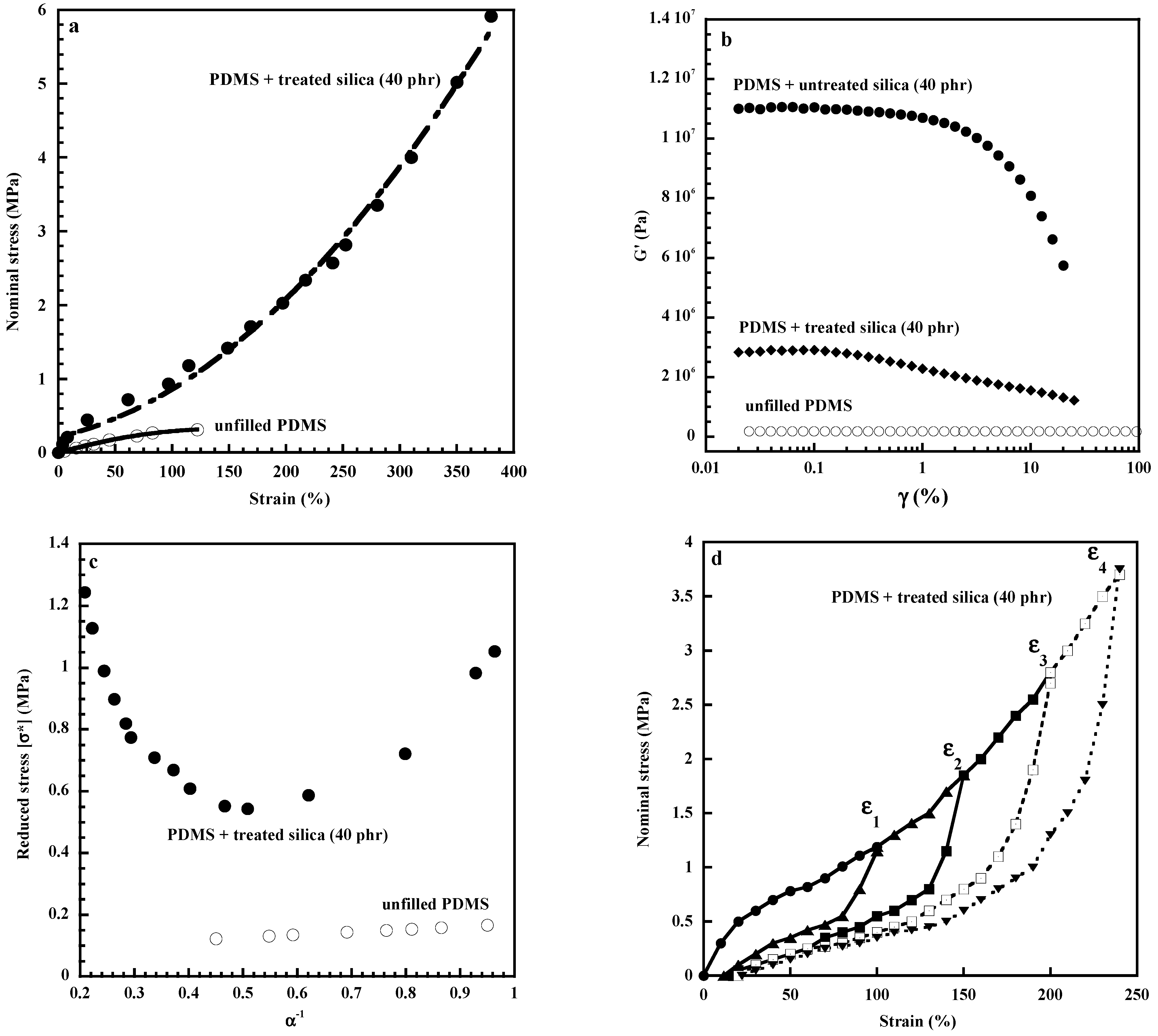
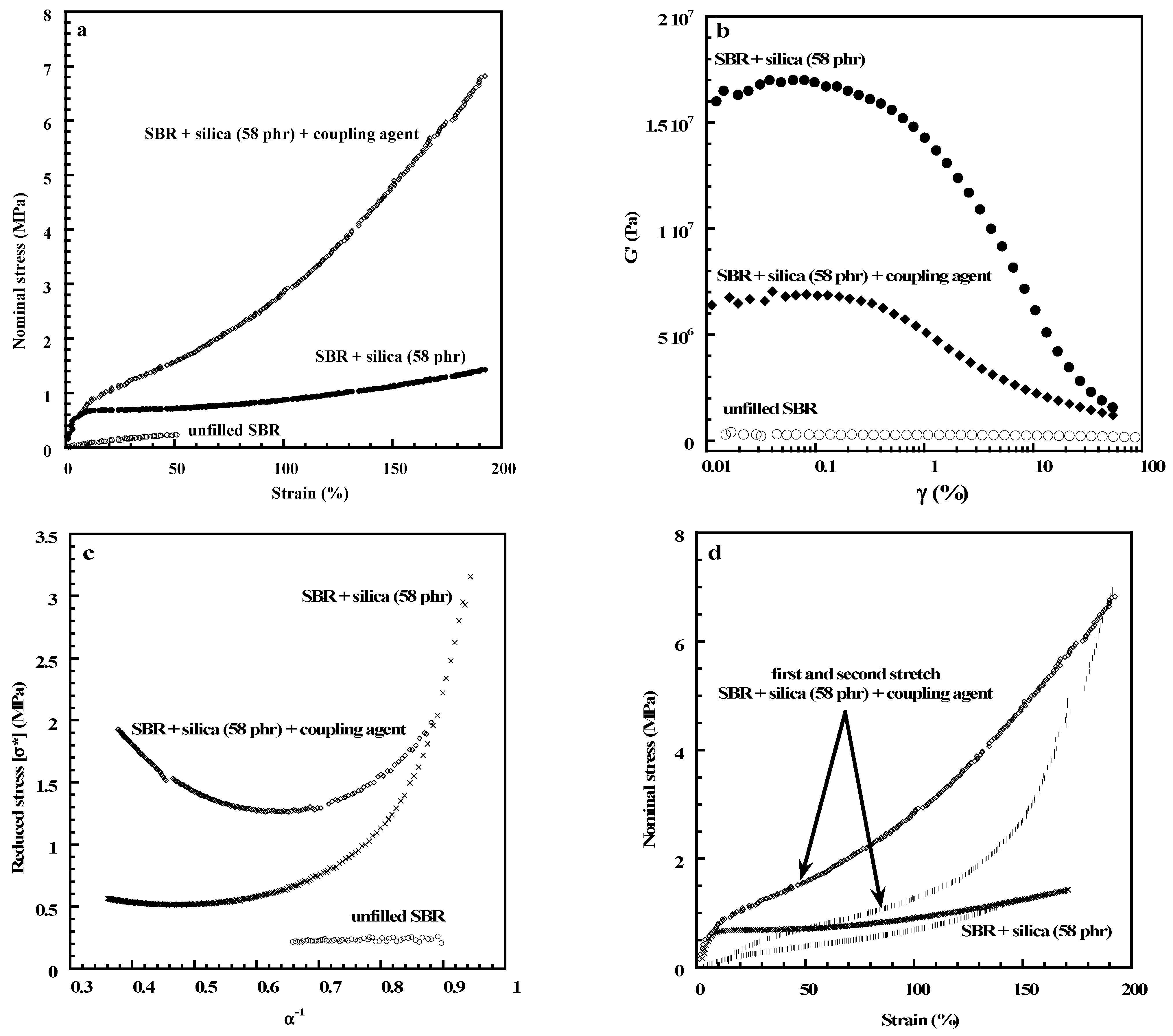

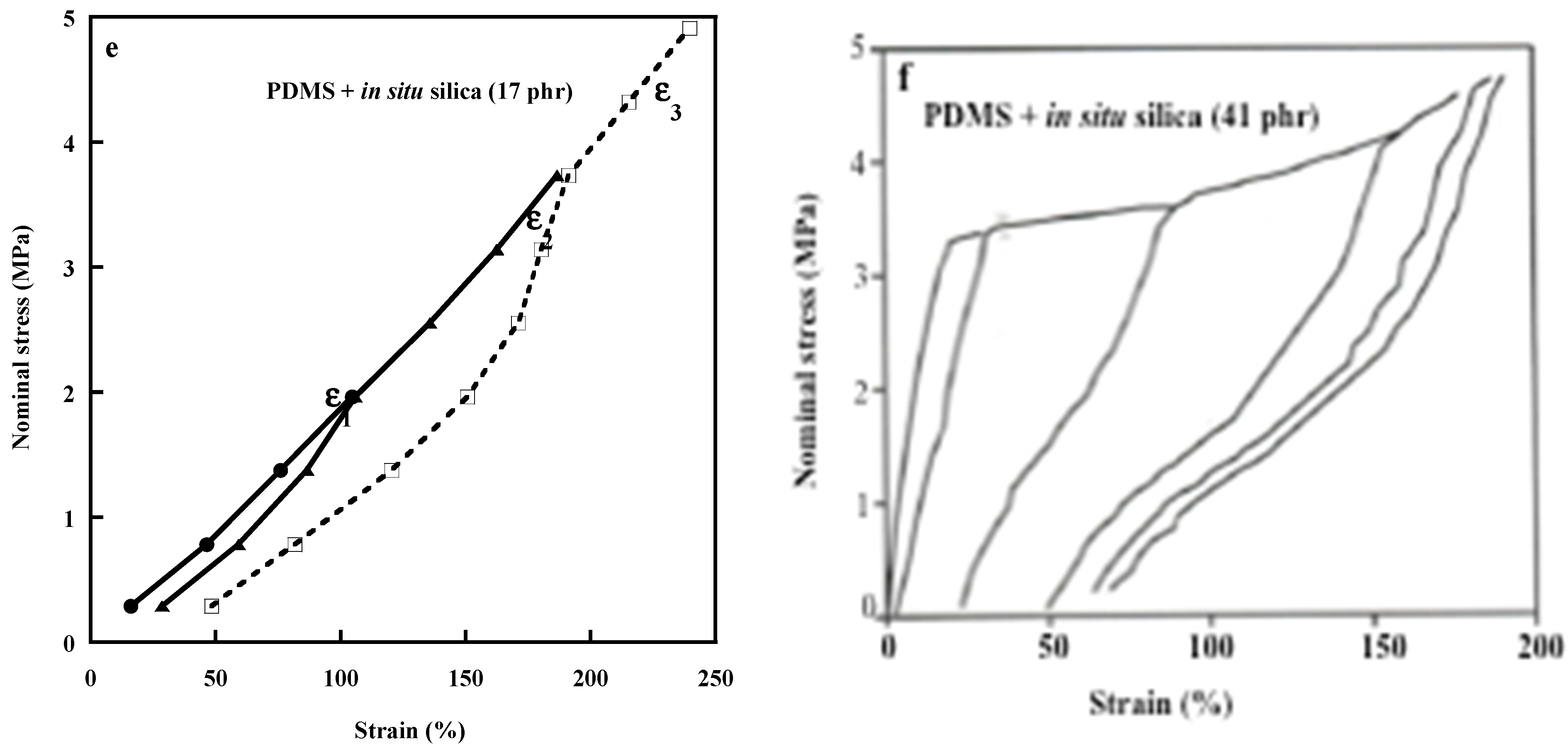
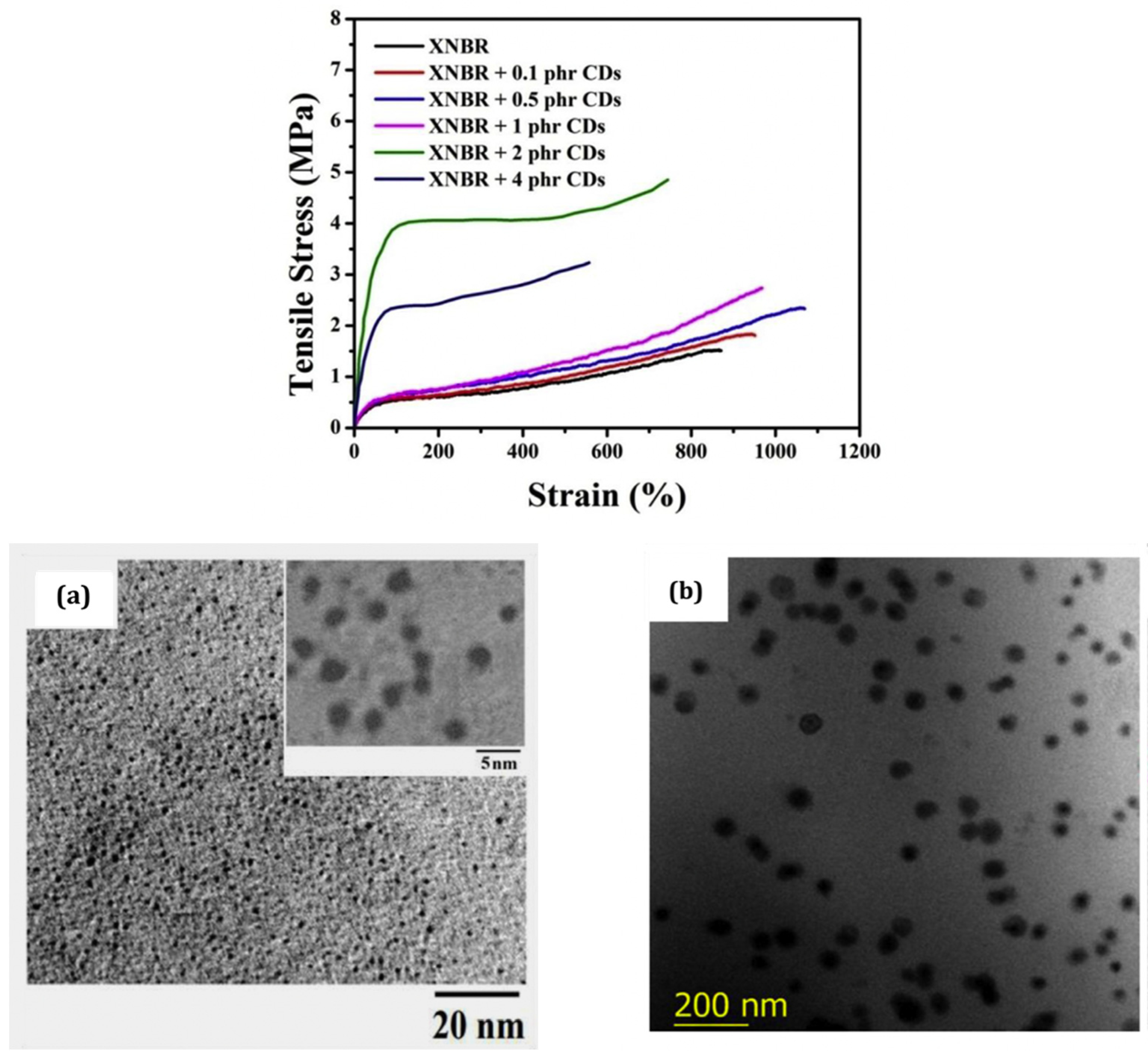

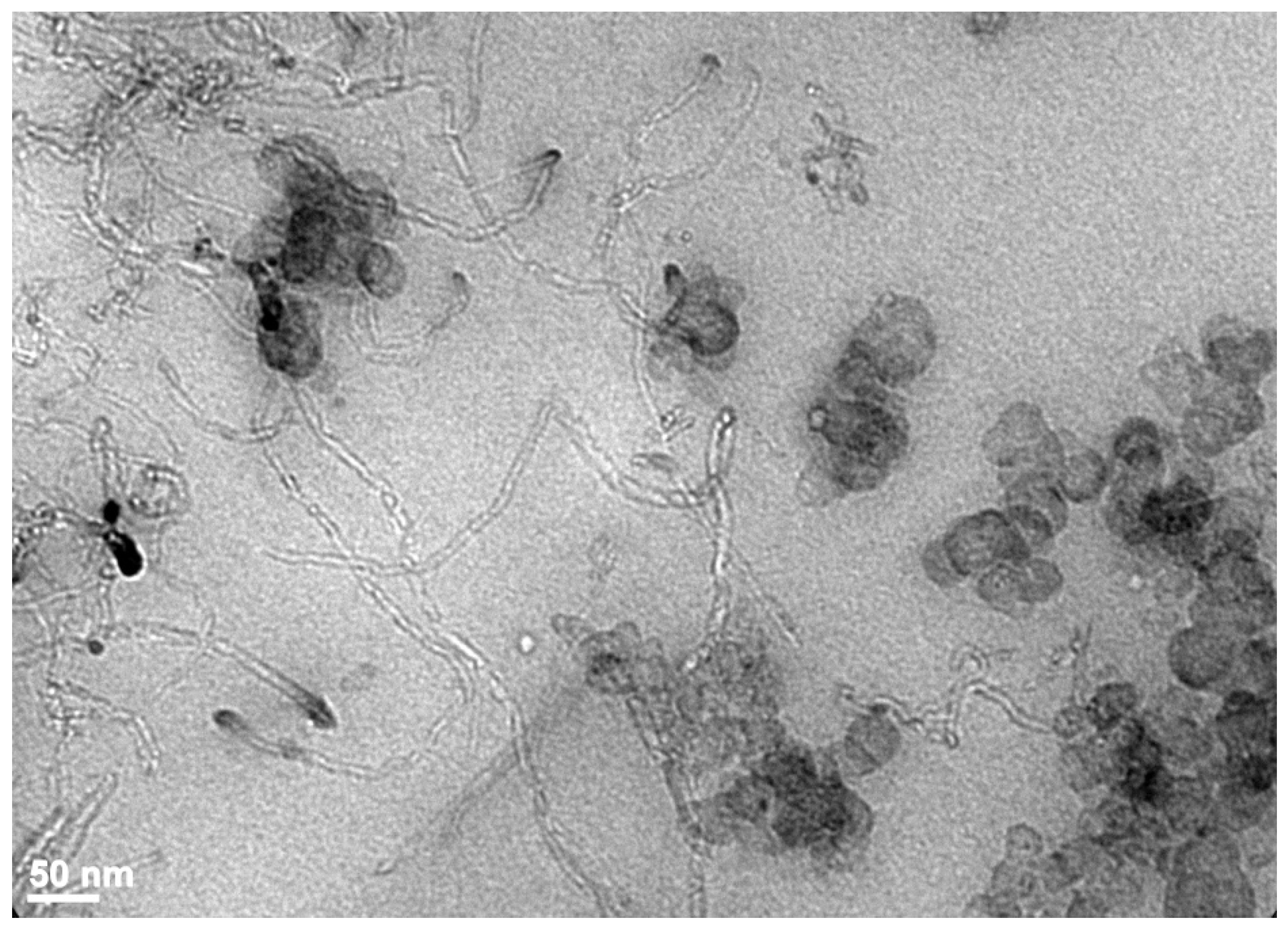
| Properties | Carbon Black [120,121] | Carbon Nanotubes MWCNTs [122,123] | Graphene [124,125] | Layered Clays Ex: Montmorillonite [126,127] | Silica [3] | Nano Silica [32,33,34,128] | Nano Titania [33,34,42,43] |
|---|---|---|---|---|---|---|---|
| Dimensions | 10–500 nm | Diameter: 10–50 nm Length: micrometers to millimeters | Lateral size greater than 100 nm | Lateral dimensions: 100–1000 nm Layers 1 nm thick | 10–100 nm | Diameter: 5–40 nm | Diameter: 20–150 nm |
| Modulus | ~10 MPa | ~1 TPa | ~1 TPa | 178 GPa | 73 GPa | ||
| Tensile strength | 2–20 MPa | ~60 GPa | 130 GPa | 36 MPa | ~50 MPa | ||
| Surface area (m2/g) | 10–300 | >50 a | 2630 | Up to 800 if complete exfoliation | 100–400 | ||
| Electrical conductivity (S/cm) | 10–104 | 103–105 | ~1.5 × 104 (monolayer) |
| Carbon black |
|
| Carbon nanotubes MWCNTs |
|
| Graphene |
|
| Layered clays Ex: Montmorillonite |
|
| Silica |
|
| Nano silica |
|
| Nano titania |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokobza, L. Elastomer Nanocomposites: Effect of Filler–Matrix and Filler–Filler Interactions. Polymers 2023, 15, 2900. https://doi.org/10.3390/polym15132900
Bokobza L. Elastomer Nanocomposites: Effect of Filler–Matrix and Filler–Filler Interactions. Polymers. 2023; 15(13):2900. https://doi.org/10.3390/polym15132900
Chicago/Turabian StyleBokobza, Liliane. 2023. "Elastomer Nanocomposites: Effect of Filler–Matrix and Filler–Filler Interactions" Polymers 15, no. 13: 2900. https://doi.org/10.3390/polym15132900
APA StyleBokobza, L. (2023). Elastomer Nanocomposites: Effect of Filler–Matrix and Filler–Filler Interactions. Polymers, 15(13), 2900. https://doi.org/10.3390/polym15132900





