Sericin from Fibroin-Deficient Silkworms Served as a Promising Resource for Biomedicine
Abstract
:1. Introduction
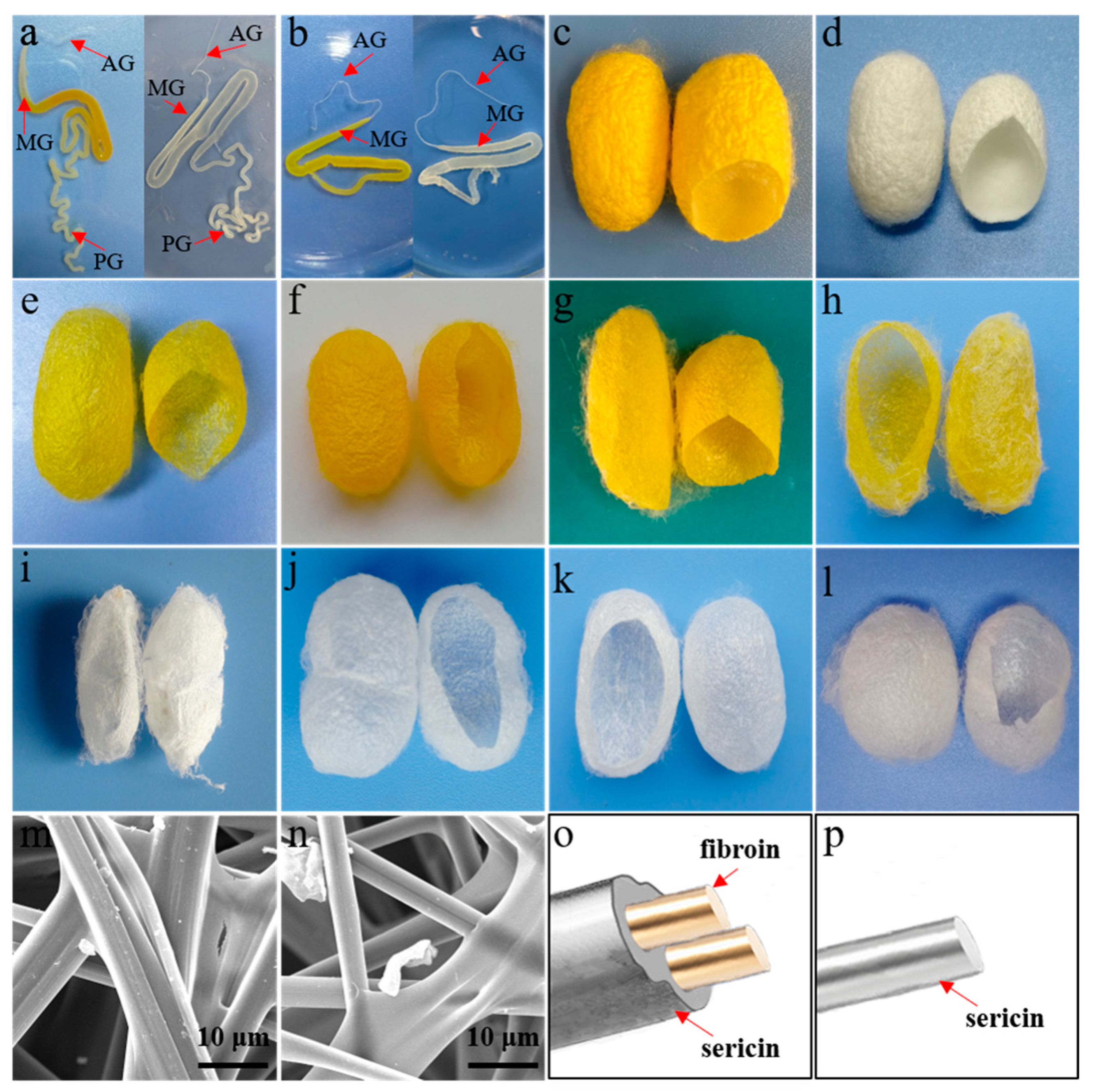
2. Resources of Sericin and Cultivation of Silkworm Varieties
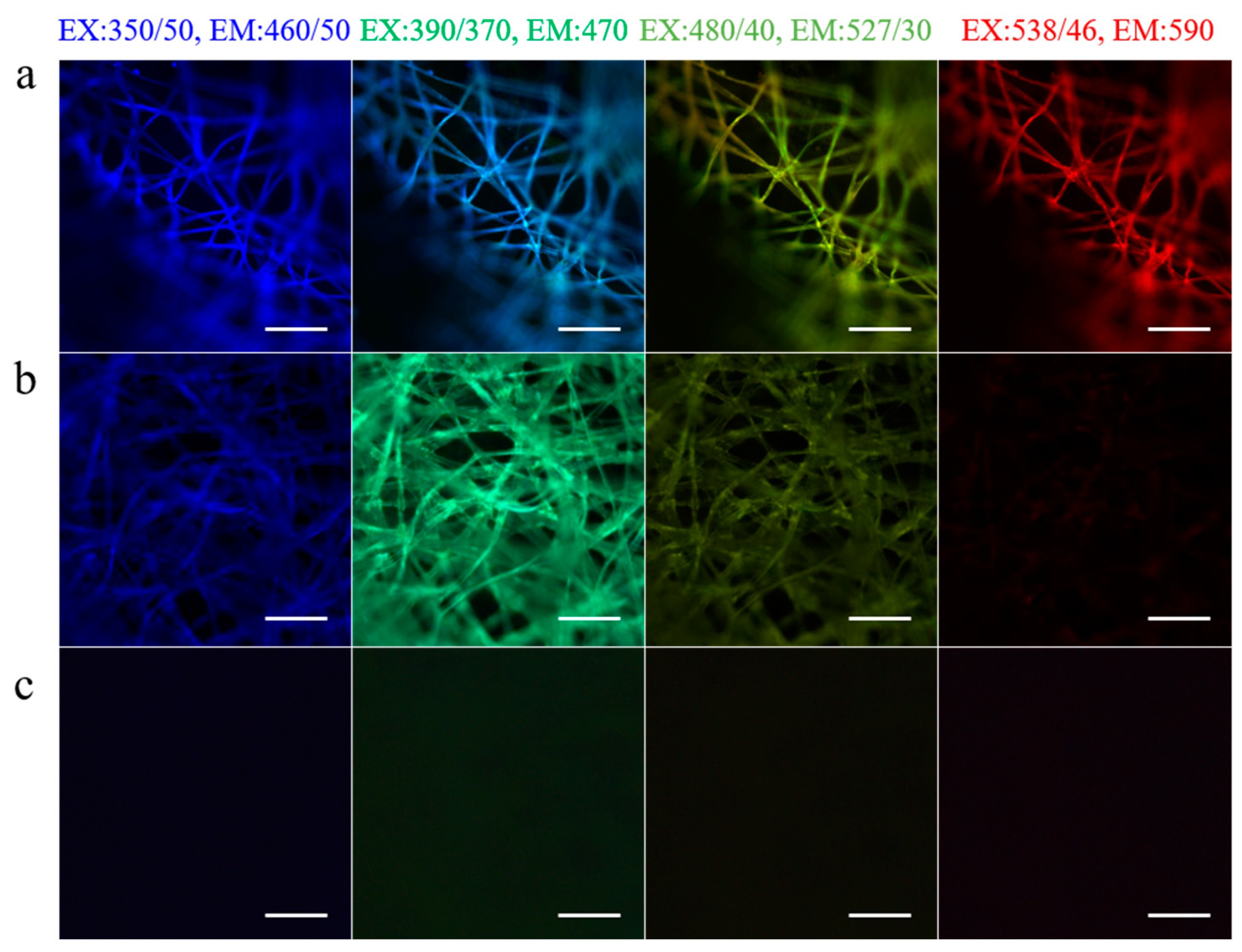
3. Characteristics and Advantages of Sericin from Fibroin-Deficient Mutant Silkworms
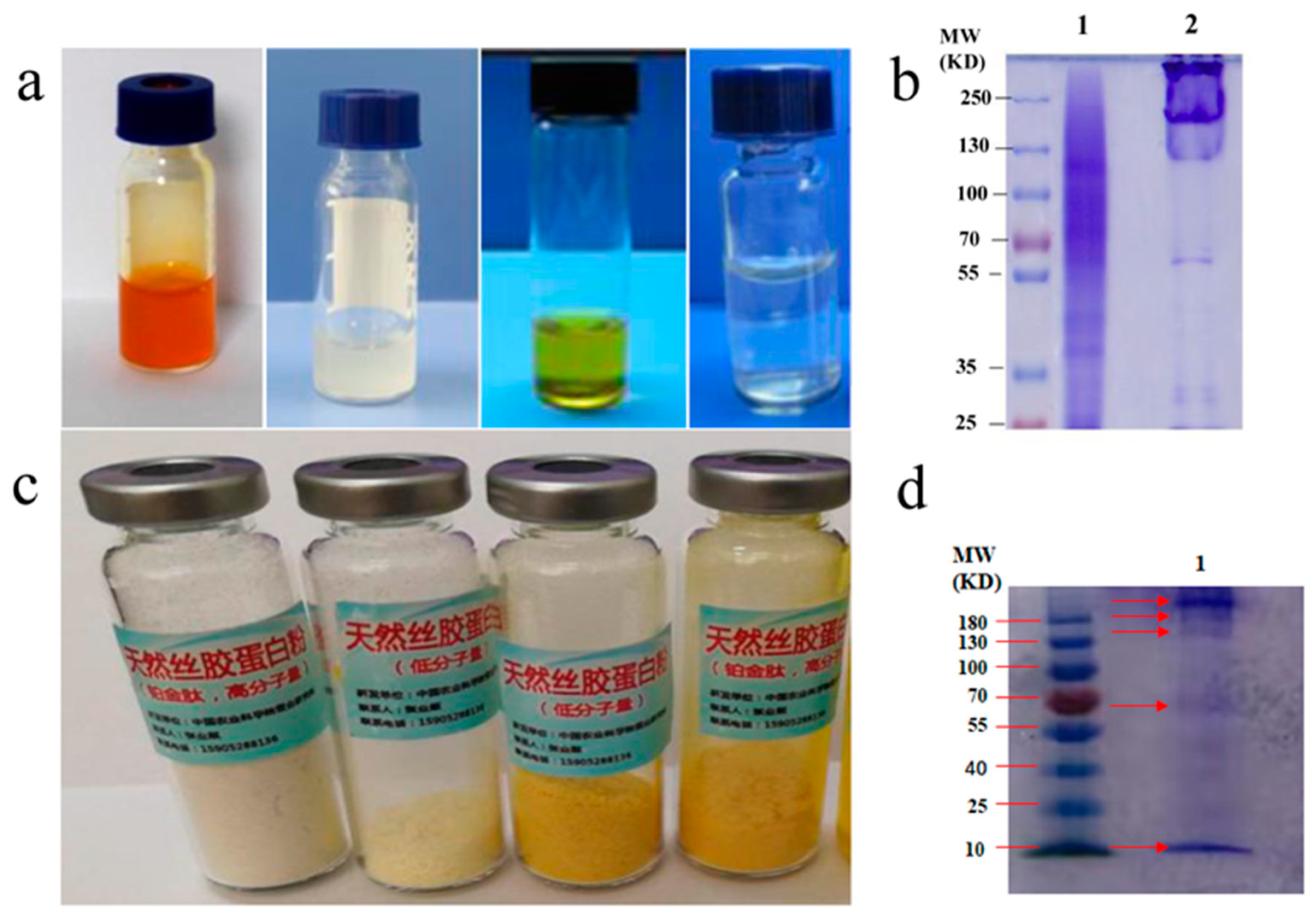
3.1. Extraction Processes of Sericin
- (1)
- The cocoons were crushed at a low temperature and then sieved to 100 mesh, which could accelerate the hydration of sericin, shorten the time of sericin dissolution, and further reduce the degradation of sericin.
- (2)
- Sericin with a low degradation degree could be extracted from cocoons using a relatively mild method of LiBr [16].
- (3)
- (4)
- The concentration of sericin reached 16% (w/v) by extracting sericin from silk glands of silkworms directly at the mature stage in the 5th instar.
3.2. Advantages of Sericin from Fibroin-Deficient Mutant Silkworms
4. Preparation and Application of Sericin-Based Biomaterials
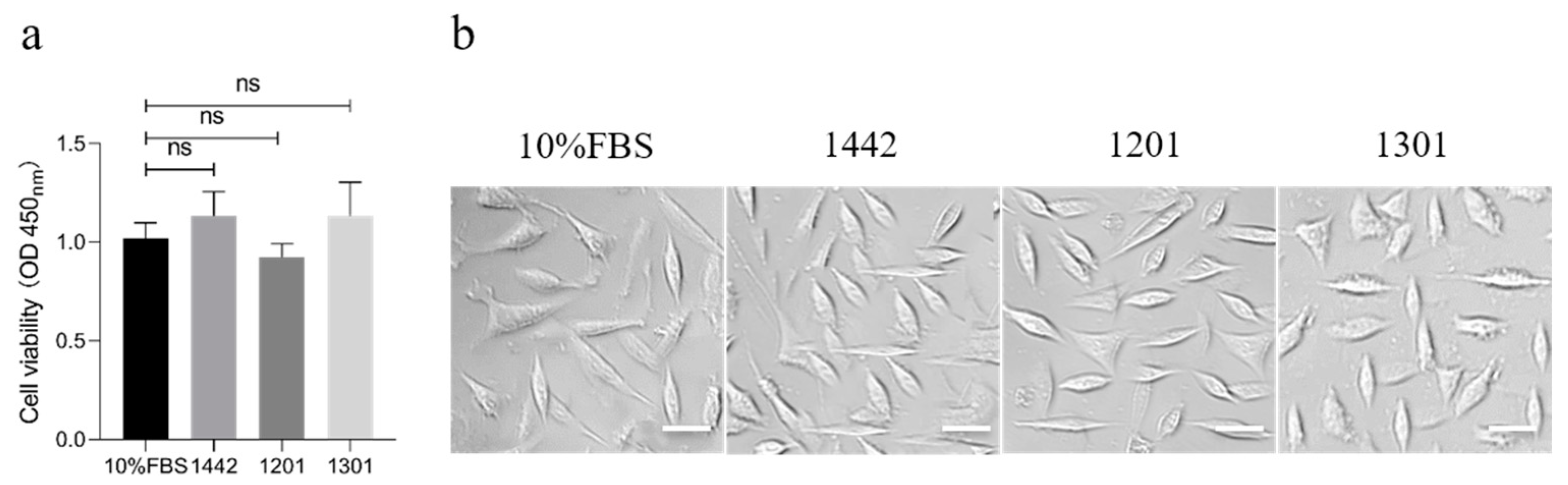
4.1. Cell Culture
4.2. Tissue Engineering
4.3. Drug Delivery
4.4. Cosmetics
5. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nouara, A.; Lü, P.; Chen, K. Silkworm, bombyx mori, as an alternative model organism in toxicological research. Environ. Sci. Pollut. Res. 2018, 25, 35048–35054. [Google Scholar]
- Chen, X.; Wang, Y.; Wang, Y.; Li, Q.; Liang, X.; Wang, G.; Li, J.; Peng, R.; Sima, Y.; Xu, S. Ectopic expression of sericin enables efficient production of ancient silk with structural changes in silkworm. Nat. Commun. 2022, 13, 6295. [Google Scholar] [CrossRef]
- Du, S.; Zhang, J.; Zhou, W.; Li, Q.; Wang, X. Interactions between fibroin and sericin proteins from antheraea pernyi and bombyx mori silk fibers. J. Colloid Interface Sci. 2016, 478, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Rajkhowa, R.; Kundu, S.C.; Wang, X. Silk fibroin biomaterials for tissue regenerations. Adv. Drug Deliv. Rev. 2013, 65, 457–470. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, T.; Liang, W.; Wang, Y.; Feng, M.; Sun, J. Silk sericin as building blocks of bioactive materials for advanced therapeutics. J. Control. Release 2023, 353, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, W.; Wang, F.; Zhang, Y. Using of hydrated lime water as a novel degumming agent of silk and sericin recycling from wastewater. J. Clean. Prod. 2018, 172, 2090–2096. [Google Scholar] [CrossRef]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y. Greener degumming production of layered sericin peptides from a silkworm cocoon and their physicochemical characteristics and bioactivities in vitro. J. Clean. Prod. 2020, 261, 121080. [Google Scholar] [CrossRef]
- Ahsan, F.; Ansari, T.; Usmani, S.; Bagga, P. An insight on silk protein sericin: From processing to biomedical application. Drug Res. 2018, 68, 317–327. [Google Scholar] [CrossRef]
- Yun, H.; Oh, H.; Kim, M.; Kwak, H.; Lee, J.; Um, I.; Vootla, S.; Lee, K. Extraction conditions of antheraea mylitta sericin with high yields and minimum molecular weight degradation. Int. J. Biol. Macromol. 2013, 52, 59–65. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suryawanshi, R.; Kanoujia, J.; Parashar, P.; Saraf, A. Sericin: A versatile protein biopolymer with therapeutic significance. Curr. Pharm. Des. 2020, 26, 5414–5429. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, M. A new silkworm race for sericin production. Indian Silk 2007, 46, 28–29. [Google Scholar]
- Mase, K.; Iizuka, T.; Okada, E.; Miyajima, T.; Yamamoto, T. A new silkworm race for sericin production, “sericin hope” and its product, “virgin sericin”. J. Insect Biotechnol. Sericology 2006, 75, 85–88. [Google Scholar]
- Mase, K.; Iizuka, T.; Okada, E.; Miyajima, T.; Yamamoto, T. A new silkworm race for the production of sericin cocoon with the higher function. Bio Ind. 2007, 24, 53–59. [Google Scholar]
- Wang, Z.; Zhang, Y.; Zhang, J.; Huang, L.; Liu, J.; Li, Y.; Zhang, G.; Kundu, S.; Wang, L. Exploring natural silk protein sericin for regenerative medicine: An injectable, photoluminescent, cell-adhesive 3d hydrogel. Sci. Rep. 2014, 4, 7064. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Liu, J.; Huang, L.; Wang, Z.; Wang, L. Design and performance of a sericin-alginate interpenetrating network hydrogel for cell and drug delivery. Sci. Rep. 2015, 5, 12374. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Guo, C.; Yang, Q.; Li, C.; Zhao, P.; Xia, Q.; Kaplan, D. Protein composites from silkworm cocoons as versatile biomaterials. Acta Biomater. 2021, 121, 180–192. [Google Scholar] [CrossRef]
- Perteghella, S.; Rassu, G.; Gavini, E.; Obinu, A.; Bari, E.; Mandracchia, D.; Bonferoni, M.; Giunchedi, P.; Torre, M. Crocetin as new cross-linker for bioactive sericin nanoparticles. Pharmaceutics 2021, 13, 680. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Jin, Y.; Luo, Z.; Yang, W.; Xie, H.; Huang, K.; Wang, L. A neuroprotective sericin hydrogel as an effective neuronal cell carrier for the repair of ischemic stroke. ACS Appl. Mater. Interfaces 2015, 7, 24629–24640. [Google Scholar] [CrossRef]
- Xie, H.; Yang, W.; Chen, J.; Zhang, J.; Lu, X.; Zhao, X.; Huang, K.; Li, H.; Chang, P.; Wang, Z.; et al. A silk sericin/silicone nerve guidance conduit promotes regeneration of a transected sciatic nerve. Adv. Healthc. Mater. 2015, 4, 2195–2205. [Google Scholar] [CrossRef] [PubMed]
- Parker, B.; Rhodes, D.; O’Brien, C.; Rodda, A.; Cameron, N. Nerve guidance conduit development for primary treatment of peripheral nerve transection injuries: A commercial perspective. Acta Biomater. 2021, 135, 64–86. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Duan, S.; Chen, L.; Xiang, H.; Dong, Y.; Wang, W. Systematic evaluation of sericin protein as a substitute for fetal bovine serum in cell culture. Sci. Rep. 2016, 6, 31516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yang, W.; Xie, H.; Wang, H.; Wang, J.; Su, Q.; Li, X.; Song, Y.; Wang, G.; Wang, L.; et al. Sericin nerve guidance conduit delivering therapeutically repurposed clobetasol for functional and structural regeneration of transected peripheral nerves. ACS Biomater. Sci. Eng. 2019, 5, 1426–1439. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, S.; Li, Y.; Zhang, G.; Wu, T.; Wei, Y.; Cao, X.; Yan, H.; Liang, P.; Yan, Z.; et al. Resveratrol loaded native silk fiber-sericin hydrogel double interpenetrating bioactive wound dressing facilitates full-thickness skin wound healing. Biomed. Mater. 2023, 18, 045007. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Liu, J.; Jin, Y.; Xu, L.; Wang, G.; Wang, Z.; Wang, L. Photo-crosslinkable, injectable sericin hydrogel as 3d biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials 2018, 163, 89–104. [Google Scholar] [CrossRef]
- Nardini, M.; Perteghella, S.; Mastracci, L.; Grillo, F.; Marrubini, G.; Bari, E.; Formica, M.; Gentili, C.; Cancedda, R.; Torre, M.; et al. Growth factors delivery system for skin regeneration: An advanced wound dressing. Pharmaceutics 2020, 12, 120. [Google Scholar] [CrossRef] [Green Version]
- Nishida, A.; Yamada, M.; Kanazawa, T.; Takashima, Y.; Ouchi, K.; Okada, H. Sustained-release of protein from biodegradable sericin film, gel and sponge. Int. J. Pharm. 2011, 407, 44–52. [Google Scholar] [CrossRef]
- Liu, J.; Qi, C.; Tao, K.; Zhang, J.; Zhang, J.; Xu, L.; Jiang, X.; Zhang, Y.; Huang, L.; Li, Q.; et al. Sericin/dextran injectable hydrogel as an optically trackable drug delivery system for malignant melanoma treatment. ACS Appl. Mater. Interfaces 2016, 8, 6411–6422. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, W.; Tao, K.; Song, Y.; Xie, H.; Wang, J.; Li, X.; Shuai, X.; Gao, J.; Chang, P.; et al. Sustained local release of ngf from a chitosan–sericin composite scaffold for treating chronic nerve compression. ACS Appl. Mater. Interfaces 2017, 9, 3432–3444. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, C.; Zhang, J.; Sun, N.; Huang, K.; Li, H.; Wang, Z.; Huang, K.; Wang, L. An injectable silk sericin hydrogel promotes cardiac functional recovery after ischemic myocardial infarction. Acta Biomater. 2016, 41, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Giovannelli, L.; Milanesi, A.; Ugazio, E.; Fracchia, L.; Segale, L. Effect of methyl–β–cyclodextrin and trehalose on the freeze–drying and spray–drying of sericin for cosmetic purposes. Pharmaceuticals 2021, 14, 262. [Google Scholar] [CrossRef] [PubMed]
- Tengattini, S.; Orlandi, G.; Perteghella, S.; Bari, E.; Amadio, M.; Calleri, E.; Massolini, G.; Torre, M.L.; Temporini, C. Chromatographic profiling of silk sericin for biomedical and cosmetic use by complementary hydrophylic, reversed phase and size exclusion chromatographic methods. J. Pharm. Biomed. Anal. 2020, 186, 113291. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Deng, J.; Li, W.; Malyi, O.; Zhang, Y.; Zhou, X.; Pan, S.; Wei, J.; Cai, Y.; Chen, Z.; et al. Water-soluble sericin protein enabling stable solid–electrolyte interphase for fast charging high voltage battery electrode. Adv. Mater. 2017, 29, 1701828. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ji, X.; Li, Z.; Huang, F. Fluorescent supramolecular polymeric materials. Adv. Mater. 2017, 29, 1606117. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, C.; Gai, F.; Cirrincione, S.; Giribaldi, M.; Purrotti, M.; Manfredi, M.; Marengo, E.; Sicuro, B.; Saviane, A.; Cappellozza, S. Investigation of the protein profile of silkworm (bombyx mori) pupae reared on a well-calibrated artificial diet compared to mulberry leaf diet. PeerJ 2019, 7, e6723. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Liu, X.; Xia, L.; Zhang, C.; Xu, W. Effects of iron oxide content on the growth of silkworm and properties of silk. J. Nat. Fibers 2022, 19, 319–328. [Google Scholar] [CrossRef]
- Dong, H.; Zhang, S.; Tao, H.; Chen, Z.; Li, X.; Qiu, J.; Cui, W.; Sima, Y.; Cui, W.; Xu, S. Metabolomics differences between silkworms (bombyx mori) reared on fresh mulberry (morus) leaves or artificial diets. Sci. Rep. 2017, 7, 10972. [Google Scholar] [CrossRef] [Green Version]
- Offord, C.; Vollrath, F.; Holland, C. Environmental effects on the construction and physical properties of bombyx mori cocoons. J. Mater. Sci. 2016, 51, 10863–10872. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, S.; Yadav, U.; Bhouraskar, J. Effects of temperature and relative humidity on rearing performances of eri silkworm (philosami aricini). Environ. Conserv. J. 2014, 15, 189–196. [Google Scholar] [CrossRef]
- Biswal, B.; Dan, A.; Sengupta, A.; Das, M.; Bindhani, B.; Das, D.; Parhi, P. Extraction of silk fibroin with several sericin removal processes and its importance in tissue engineering: A review. J. Polym. Environ. 2022, 30, 2222–2253. [Google Scholar] [CrossRef]
- Li, Y.; Huang, Y.; Pan, P.; Che, X.; Zhang, Y.; Zhang, Y.; Amal, A.; Li, X.; Niu, W.; Luo, N.; et al. Sericin and sericin-derived peptide alleviate viral pathogenesis in mice though inhibiting lactate production and facilitating antiviral response. Appl. Mater. Today 2021, 25, 101256. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; He, X.; Fang, A.; Jiang, R.; Wu, T.; Chen, H.; Cao, X.; Liang, P.; Xia, D.; et al. A sterile self-assembled sericin hydrogel via a simple two-step process. Polym. Test. 2019, 80, 106016. [Google Scholar] [CrossRef]
- Mahmoodi, N.; Arami, M.; Mazaheri, F.; Rahimi, S. Degradation of sericin (degumming) of persian silk by ultrasound and enzymes as a cleaner and environmentally friendly process. J. Clean. Prod. 2010, 18, 146–151. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, T.; Shen, C.; Xu, G.; Chen, H.; Yan, H.; Xiong, M.; Zhang, G. A robust sericin hydrogel formed by a native sericin from silkworm bodies. Fibers Polym. 2022, 23, 1826–1833. [Google Scholar] [CrossRef]
- Yang, C.; Yao, L.; Zhang, L. Silk sericin-based biomaterials shine in food and pharmaceutical industries. Smart Mater. Med. 2023, 4, 447–459. [Google Scholar] [CrossRef]
- Das, G.; Shin, H.; Campos, E.; Fraceto, L.; del Pilar Rodriguez-Torres, M.; Mariano, K.; de Araujo, D.; Fernández-Luqueño, F.; Grillo, R.; Patra, J. Sericin based nanoformulations: A comprehensive review on molecular mechanisms of interaction with organisms to biological applications. J. Nanobiotechnology 2021, 19, 30. [Google Scholar] [CrossRef]
- Sano, K.; Ishida, Y.; Aida, T. Synthesis of anisotropic hydrogels and their applications. Angew. Chem. Int. Ed. 2018, 57, 2532–2543. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, R.; Fang, A.; Zhao, Y.; Wu, T.; Cao, X.; Liang, P.; Xia, D.; Zhang, G. A highly transparent, elastic, injectable sericin hydrogel induced by ultrasound. Polym. Test. 2019, 77, 105890. [Google Scholar] [CrossRef]
- Gu, Z.; Chen, L.; Xu, Y.; Liu, Y.; Zhao, Z.; Zhao, C.; Lei, W.; Rong, Q.; Fang, R.; Zhao, T.; et al. General strategy to fabricate highly filled microcomposite hydrogels with high mechanical strength and stiffness. ACS Appl. Mater. Interfaces 2018, 10, 4161–4167. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Li, Y.; Fang, A.; Wu, T.; Shen, C.; Zhao, Y.; Zhang, G. A transparent sericin-polyacrylamide interpenetrating network hydrogel as visualized dressing material. Polym. Test. 2020, 87, 106517. [Google Scholar] [CrossRef]
- Qi, C.; Xu, L.; Deng, Y.; Wang, G.; Wang, Z.; Wang, L. Retracted article: Sericin hydrogels promote skin wound healing with effective regeneration of hair follicles and sebaceous glands after complete loss of epidermis and dermis. Biomater. Sci. 2018, 6, 2859–2870. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Deng, Y.; Xu, L.; Yang, C.; Zhu, Y.; Wang, G.; Wang, Z.; Wang, L. A sericin/graphene oxide composite scaffold as a biomimetic extracellular matrix for structural and functional repair of calvarial bone. Theranostics 2020, 10, 741–756. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, H.; Kameda, T.; Tamada, Y. Preparation of gel film from bombyx mori silk sericin and its characterization as a wound dressing. Biosci. Biotechnol. Biochem. 2008, 72, 3189–3196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wu, T.; Zhang, G.; Fang, A.; Li, Y.; Wang, S.; Yan, H.; Liang, P.; Lian, J.; Zhang, Y. A native sericin wound dressing spun directly from silkworms enhances wound healing. Colloids Surf. B 2023, 225, 113228. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Song, Y.; Su, Q.; Xiaohalati, X.; Yang, W.; Xu, L.; Cai, B.; Wang, G.; Wang, Z.; et al. Injectable silk sericin scaffolds with programmable shape-memory property and neuro-differentiation-promoting activity for individualized brain repair of severe ischemic stroke. Bioact. Mater. 2021, 6, 1988–1999. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, W.; Xie, H.; Wang, J.; Zhang, L.; Wang, Z.; Wang, L. Cnt/sericin conductive nerve guidance conduit promotes functional recovery of transected peripheral nerve injury in a rat model. ACS Appl. Mater. Interfaces 2020, 12, 36860–36872. [Google Scholar] [CrossRef]
- Terada, S.; Nishimura, T.; Sasaki, M.; Yamada, H.; Miki, M. Sericin, a protein derived from silkworms, accelerates the proliferation of several mammalian cell lines including a hybridoma. Cytotechnology 2002, 40, 3–12. [Google Scholar] [CrossRef]
- Terada, S.; Sasaki, M.; Yanagihara, K.; Yamada, H. Preparation of silk protein sericin as mitogenic factor for better mammalian cell culture. J. Biosci. Bioeng. 2005, 100, 667–671. [Google Scholar] [CrossRef]
- Sahu, N.; Pal, S.; Sapru, S.; Kundu, J.; Talukdar, S.; Singh, N.; Yao, J.; Kundu, S. Non-mulberry and mulberry silk protein sericins as potential media supplement for animal cell culture. BioMed Res. Int. 2016, 2016, 7461041. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, A.; Terada, S.; Miki, M.; Kimura, T.; Yamaguchi, A.; Sasaki, M.; Yamada, H. Supplementation of sericin is effective in maintenance of islet survival and function under serum-free culture. In Animal Cell Technology: Basic & Applied Aspects; Springer: Dordrecht, The Netherlands, 2006; Volume 14, pp. 107–112. [Google Scholar]
- Deng, T.; Gao, D.; Song, X.; Zhou, Z.; Zhou, L.; Tao, M.; Jiang, Z.; Yang, L.; Luo, L.; Zhou, A.; et al. A natural biological adhesive from snail mucus for wound repair. Nat. Commun. 2023, 14, 396. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Hou, K.; Chen, W.; Wang, Y.; Wang, R.; Tian, C.; Xu, S.; Ji, Y.; Yang, Q.; Zhao, P.; et al. Transgenic pdgf-bb/sericin hydrogel supports for cell proliferation and osteogenic differentiation. Biomater. Sci. 2020, 8, 657–672. [Google Scholar] [CrossRef] [PubMed]
- El-Samad, L.M.; Hassan, M.A.; Basha, A.A.; El-Ashram, S.; Radwan, E.H.; Abdul Aziz, K.K.; Tamer, T.M.; Augustyniak, M.; El Wakil, A. Carboxymethyl cellulose/sericin-based hydrogels with intrinsic antibacterial, antioxidant, and anti-inflammatory properties promote re-epithelization of diabetic wounds in rats. Int. J. Pharm. 2022, 629, 122328. [Google Scholar] [CrossRef] [PubMed]
- Padamwar, M.; Pawar, A.; Daithankar, A.; Mahadik, K. Silk sericin as a moisturizer: An in vivo study. J. Cosmet. Dermatol. 2005, 4, 250–257. [Google Scholar] [CrossRef]
- Rahimpour, S.; Jabbari, H.; Yousofi, H.; Fathi, A.; Mahmoodi, S.; Jafarian, M.; Shomali, N.; Shotorbani, S. Regulatory effect of sericin protein in inflammatory pathways; a comprehensive review. Pathol. Res. Pract. 2023, 243, 154369. [Google Scholar] [CrossRef]
- Kato, N.; Sato, S.; Yamanaka, A.; Yamada, H.; Fuwa, N.; Nomura, M. Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci. Biotechnol. Biochem. 1998, 62, 145–147. [Google Scholar] [CrossRef] [Green Version]
- Jena, K.; Pandey, J.; Kumari, R.; Sinha, A.; Gupta, V.; Singh, G. Free radical scavenging potential of sericin obtained from various ecoraces of tasar cocoons and its cosmeceuticals implication. Int. J. Biol. Macromol. 2018, 120, 255–262. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, S.; Yin, X.; Wang, H.; Wei, Z.; Zhang, Y. Silk sericin has significantly hypoglycaemic effect in type 2 diabetic mice via anti-oxidation and anti-inflammation. Int. J. Biol. Macromol. 2020, 150, 1061–1071. [Google Scholar] [CrossRef]
- Manesa, K.; Kebede, T.; Dube, S.; Nindi, M. Profiling of silk sericin from cocoons of three southern african wild silk moths with a focus on their antimicrobial and antioxidant properties. Materials 2020, 13, 5706. [Google Scholar] [CrossRef]
- Kunz, R.; Brancalhão, R.; Ribeiro, L.; Natali, M. Silkworm sericin: Properties and biomedical applications. BioMed Res. Int. 2016, 2016, 8175701. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhong, Z.; Weng, Y.; Wei, Z.; Zhang, Y. Degraded sericin significantly regulates blood glucose levels and improves impaired liver function in t2d rats by reducing oxidative stress. Biomolecules 2021, 11, 1255. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, D.; Chen, S.; Hu, G. Protective effects of sericin protein on alcohol-mediated liver damage in mice. Alcohol Alcohol. 2008, 43, 246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watkins, S.; Thomas, D.; Clifford, M.; Tidmarsh, S.; Sweeney, A.; Ah, E.; Dickerson, J.; Cowie, V.; Shaw, D. Plasma amino acids in patients with senile dementia and in subjects with down’s syndrome at an age vulnerable to alzheimer changes. J. Intellect. Disabil. Res. 1989, 33, 159–166. [Google Scholar] [CrossRef] [PubMed]



| Materials | Applications | Reference |
|---|---|---|
| Hydrogels | ||
| Sericin | Tissue engineering | [43,45,49] |
| Sericin | Wound dressing | [25] |
| Sericin | Ischemic Stroke | [20] |
| Sericin | Ischemic myocardial infarction | [31] |
| Sericin | Cartilage regeneration | [26] |
| Sericin/Methacrylate | Skin wound healing | [52] |
| Sericin/Graphene oxide | Calvarial bone regeneration | [53] |
| Sericin/Alginate | Cell culture and drug delivery | [17] |
| Sericin/Glutaraldehyde | Cell culture and drug delivery | [16] |
| Sericin/Polyacrylamide | Visualized dressing | [51] |
| Films | ||
| Sericin | Wound dressing | [54] |
| Sericin | Drug delivery | [28] |
| Sponges | ||
| Sericin | Drug delivery | [28] |
| Scaffolds | ||
| Sericin | Wound dressing | [55] |
| Sericin/Chitosan | Chronic nerve compression treatment | [30] |
| Sericin/Carbon-Nanotubes (CNTs) | Ischemic stroke damage treatment | [56] |
| Conduits | ||
| Sericin | Peripheral nerve regeneration | [22] |
| Sericin/Silicone | Peripheral nerve regeneration | [21] |
| Sericin/Clobetasol | Peripheral nerve regeneration | [24] |
| Sericin/CNTs | Peripheral nerve regeneration | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wei, Y.; Zhang, G.; Zhang, Y. Sericin from Fibroin-Deficient Silkworms Served as a Promising Resource for Biomedicine. Polymers 2023, 15, 2941. https://doi.org/10.3390/polym15132941
Li Y, Wei Y, Zhang G, Zhang Y. Sericin from Fibroin-Deficient Silkworms Served as a Promising Resource for Biomedicine. Polymers. 2023; 15(13):2941. https://doi.org/10.3390/polym15132941
Chicago/Turabian StyleLi, Yurong, Yongkang Wei, Guozheng Zhang, and Yeshun Zhang. 2023. "Sericin from Fibroin-Deficient Silkworms Served as a Promising Resource for Biomedicine" Polymers 15, no. 13: 2941. https://doi.org/10.3390/polym15132941
APA StyleLi, Y., Wei, Y., Zhang, G., & Zhang, Y. (2023). Sericin from Fibroin-Deficient Silkworms Served as a Promising Resource for Biomedicine. Polymers, 15(13), 2941. https://doi.org/10.3390/polym15132941







