Pendant Modification of Poly(methyl methacrylate) to Enhance Its Stability against Photoirradiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General
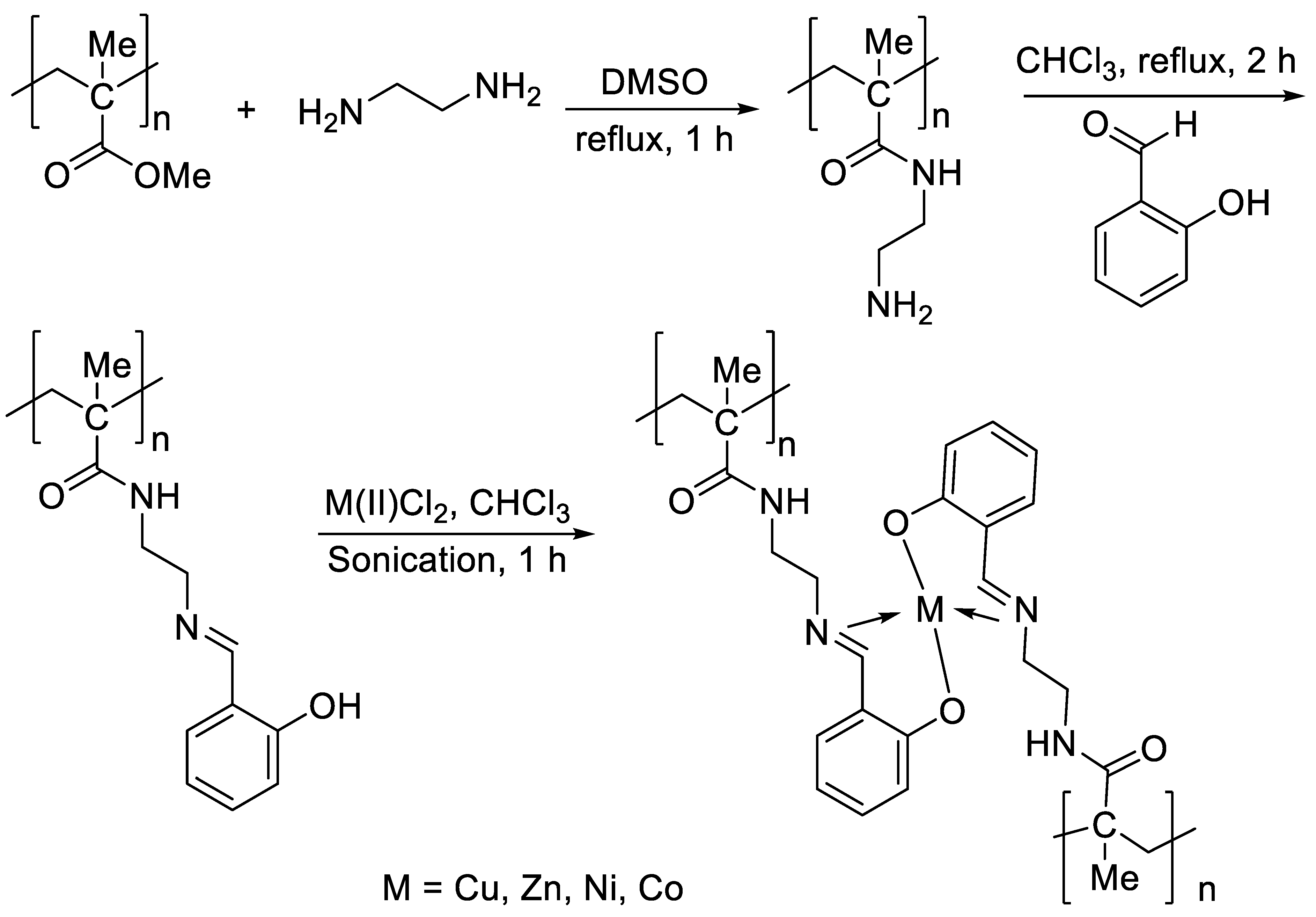
2.2. Preparation of PMMA Containing Ethylene Amine
2.3. Preparation of PMMA Containing Schiff Base
2.4. Preparation of PMMA Containing Schiff Base and Metal
2.5. Irradiation of PMMA Films
2.6. FTIR Spectroscopy of PMMA Films
2.7. Weight Loss of Irradiated PMMA Films
2.8. Photodecomposition Rate Constant of PMMA
2.9. Surface Morphology of Irradiated PMMA Films
3. Results and Discussion
3.1. Pendant Modifications of PMMA
3.2. FTIR Spectroscopy of Irradiated PMMA Films
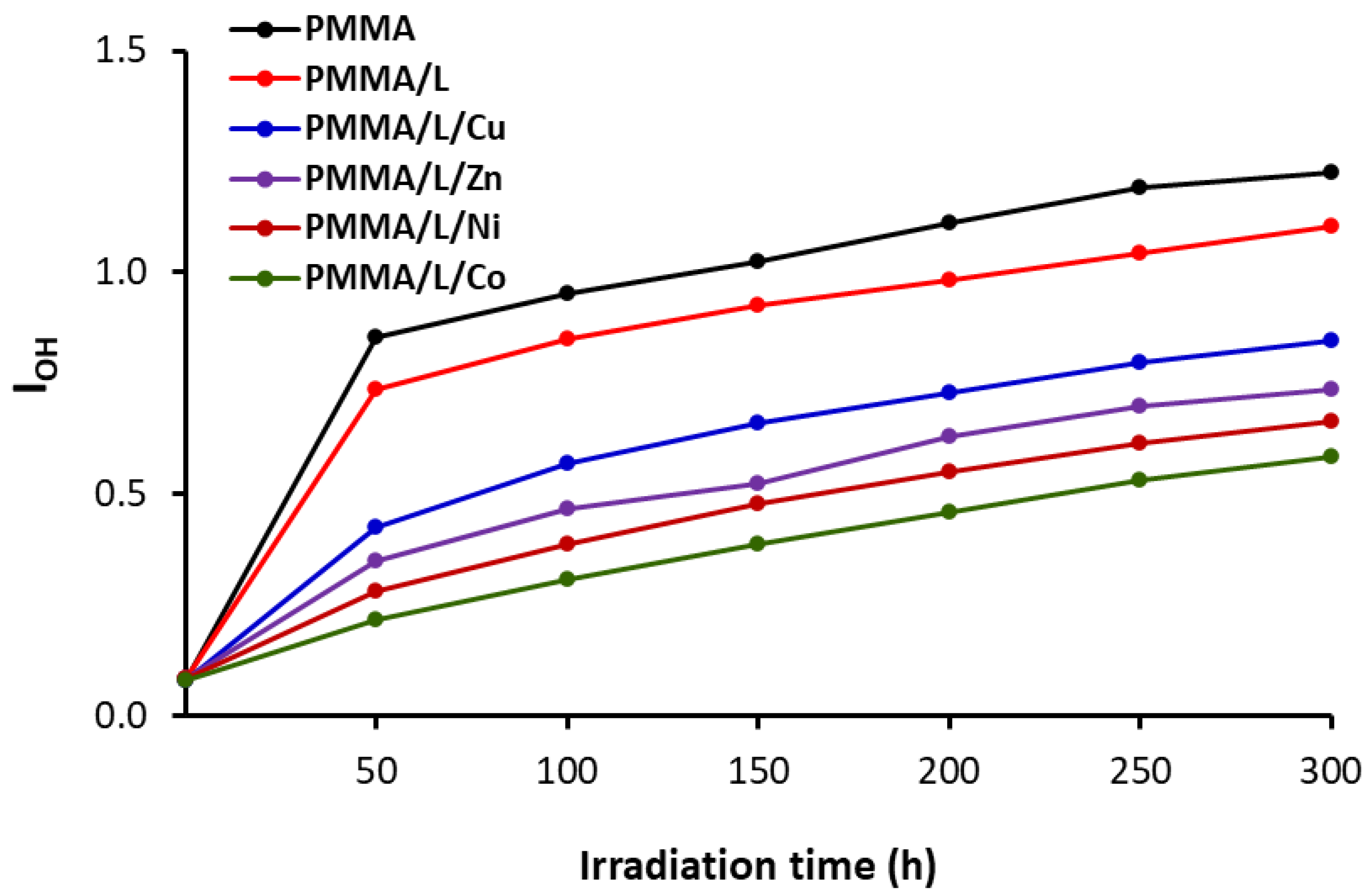
3.3. Weight Loss of Irradiated PMMA Films
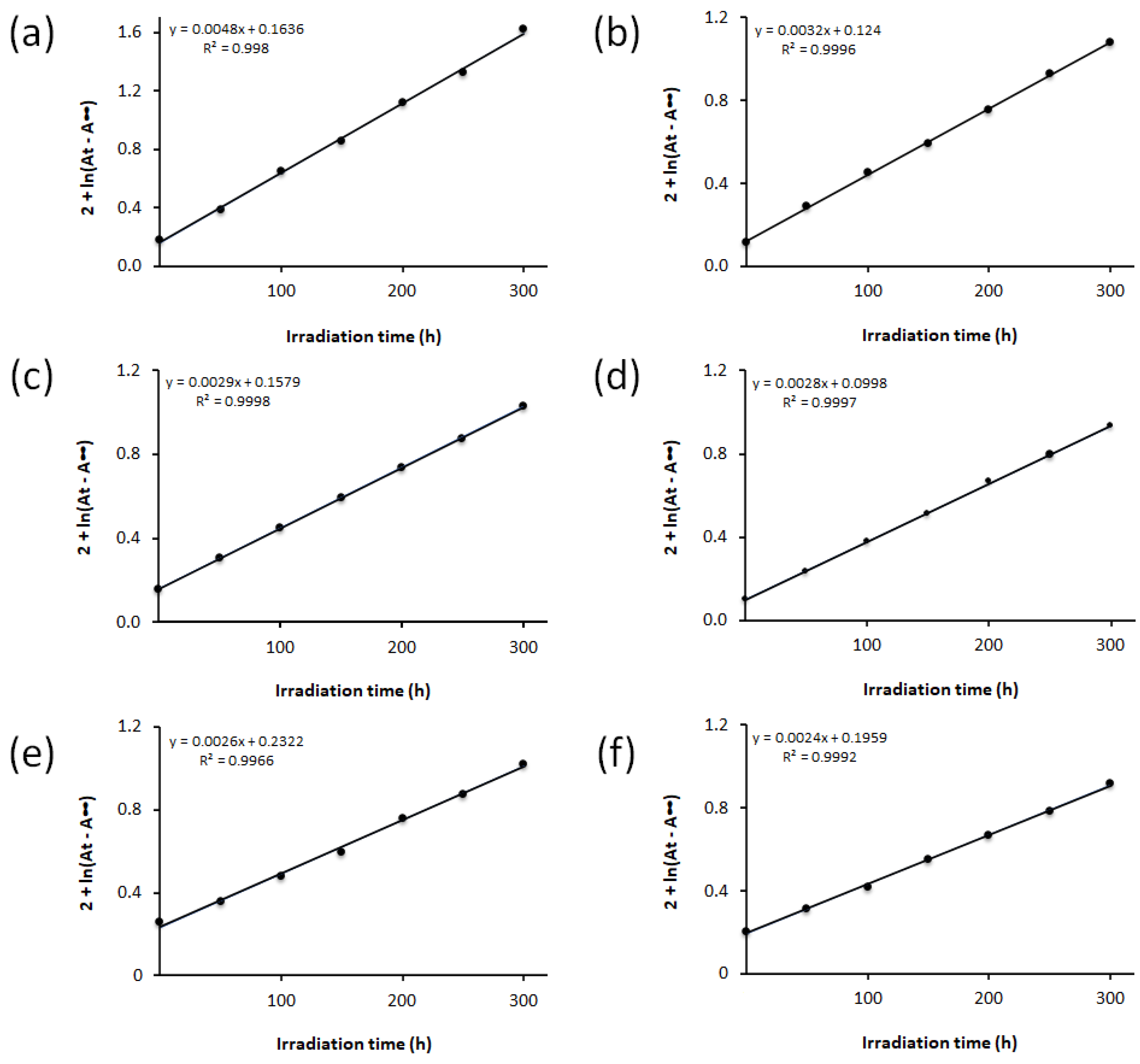
3.4. Photodecomposition Rate Constant of PMMA
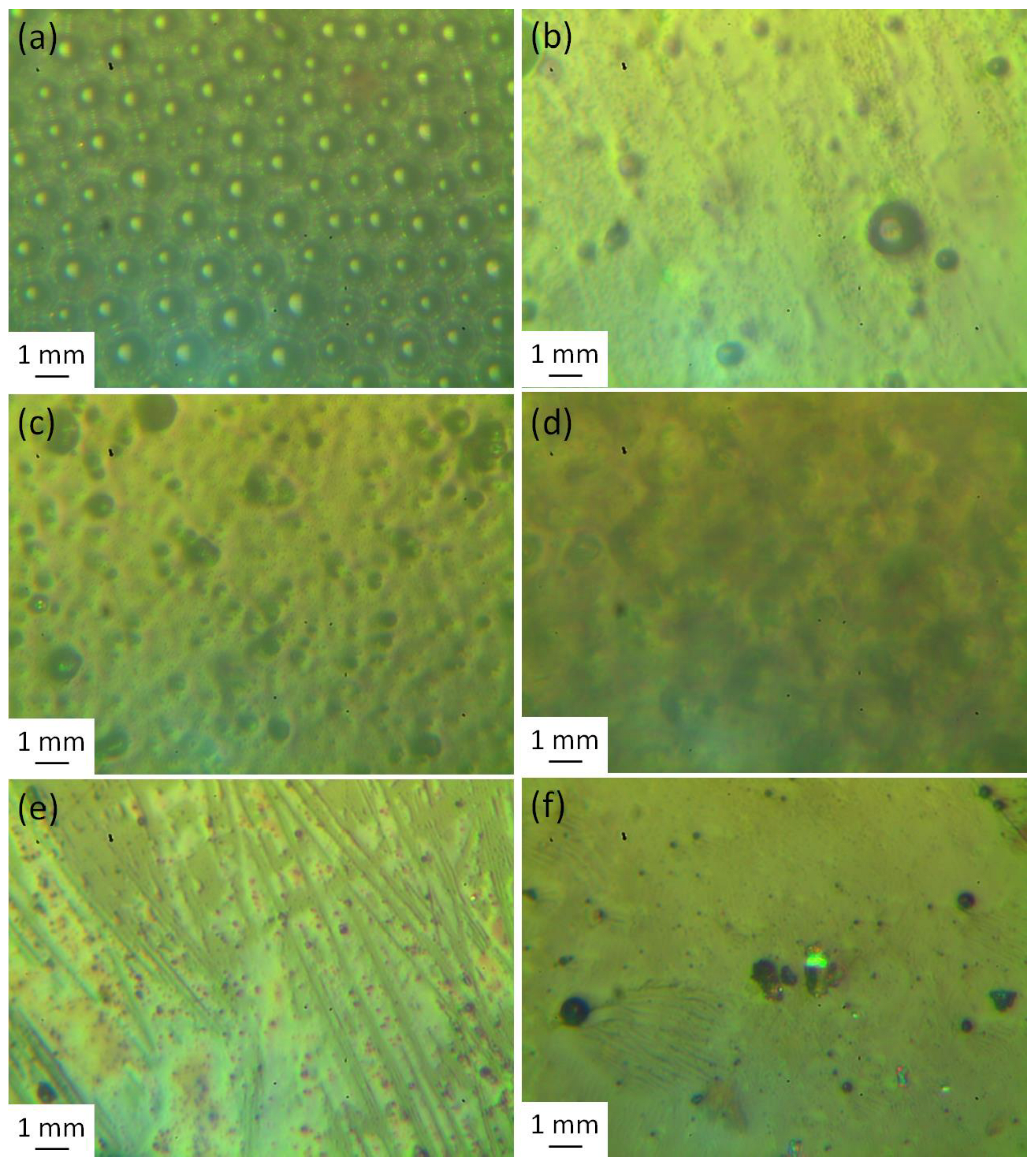
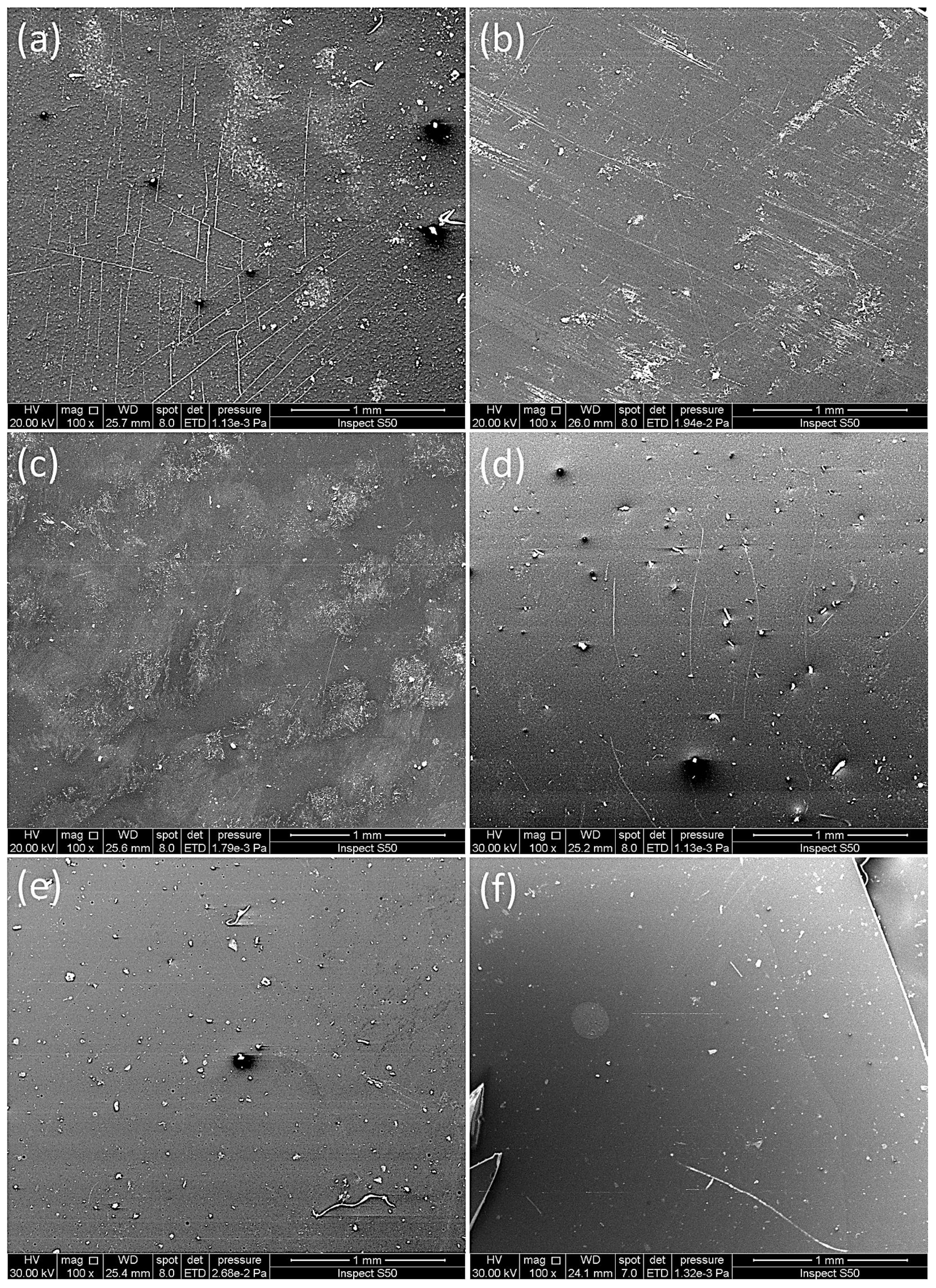
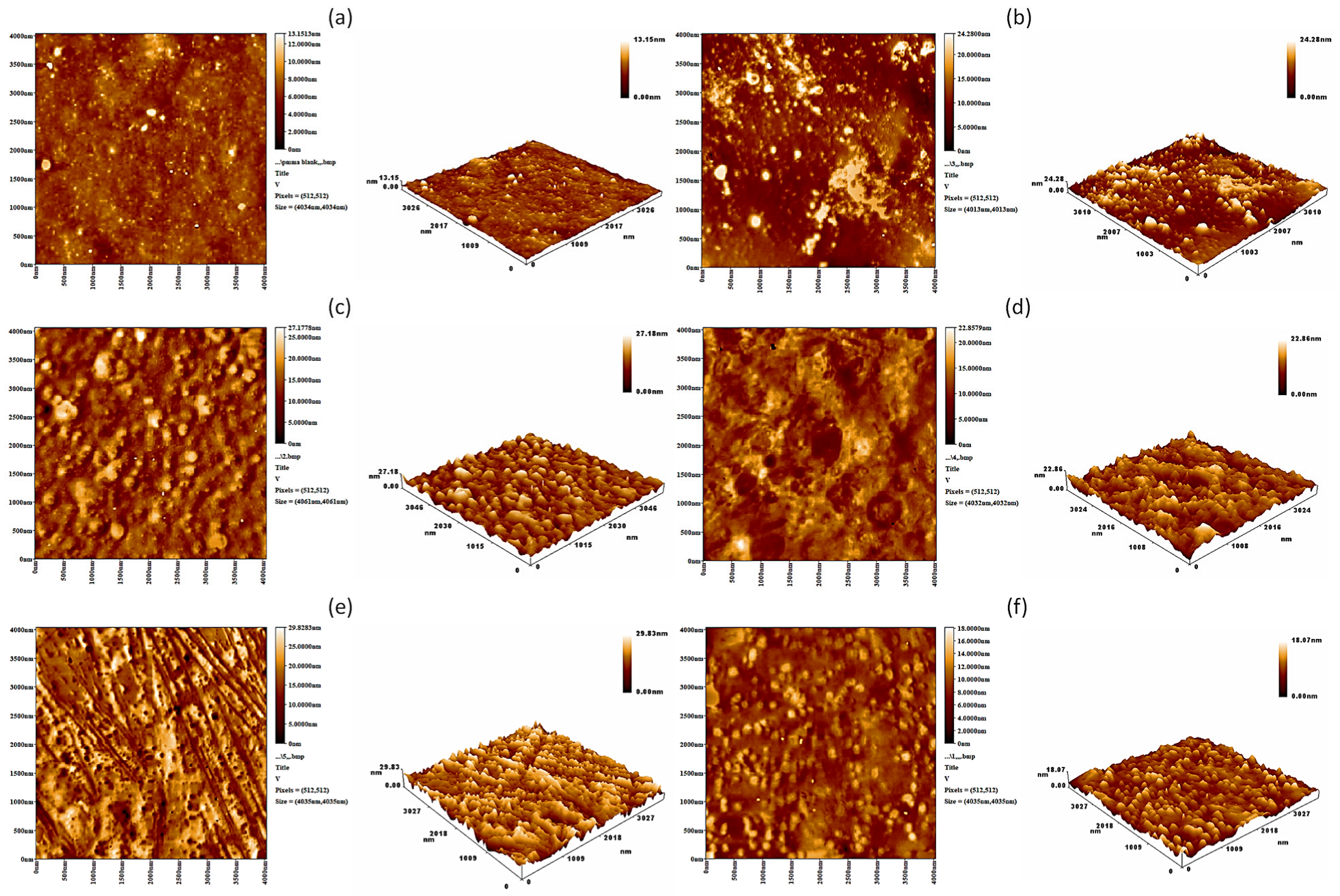
3.5. Surface Morphology Analysis
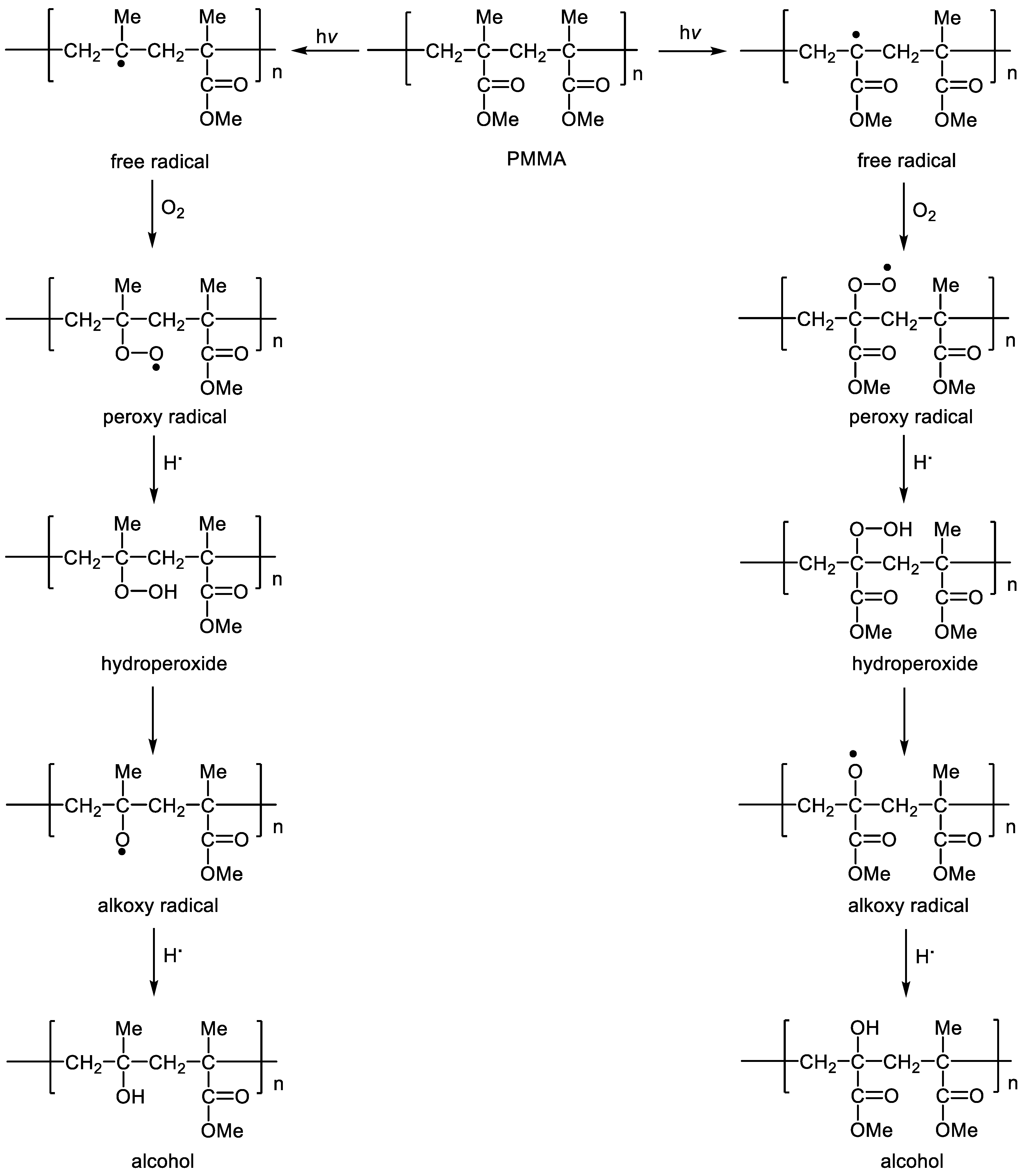
3.6. Photostabilization Proposed Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanda, M.; Nishi, Y. Effects of water absorption on impact value of aluminum dispersed composite nylon6. Mater. Trans. 2009, 50, 177–181. [Google Scholar] [CrossRef] [Green Version]
- Blasco, E.; Sims, M.B.; Goldmann, A.S.; Sumerlin, B.S.; Barner-Kowollik, C. 50th Anniversary perspective: Polymer functionalization. Macromolecules 2017, 50, 5215–5252. [Google Scholar] [CrossRef]
- Zyoud, S.H.; Almoadi, A.; Alabdulaal, T.H.; Alqahtani, M.S.; Harraz, F.A.; Al-Assiri, M.S.; Yahia, I.S.; Zahran, H.Y.; Mohammed, M.I.; Abdel-Wahab, M.S. Structural, optical, and electrical investigations of Nd2O3-doped PVA/PVP polymeric composites for electronic and optoelectronic applications. Polymers 2023, 15, 1351. [Google Scholar] [CrossRef]
- Chen, Q.; Yang, C.Q.; Zhao, T. The chemical bonding and fire performance of the nylon/cotton blend fabrics treated with a hydroxy-functional organophosphorus oligomer. Polym. Degrad. Stab. 2016, 128, 237–244. [Google Scholar] [CrossRef]
- Fan, J.; Njuguna, J. An introduction to lightweight composite materials and their use in transport structures. In Lightweight Composite Structures in Transport: Design, Manufacturing, Analysis and Performance; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 3–34. [Google Scholar]
- Amin, E.M.; Karmakar, N.C.; Winther-Jensen, B. Polyvinyl-alcohol (PVA)-based rf humidity sensor in microwave frequency. Prog. Electromagn. Res. 2013, 54, 149–166. [Google Scholar] [CrossRef] [Green Version]
- Teodorescu, M.; Bercea, M.; Morariu, S. Biomaterials of PVA and PVP in medical and pharmaceutical applications: Perspectives and challenges. Biotechnol. Adv. 2018, 37, 109–131. [Google Scholar] [CrossRef]
- Ibrahim, B.A.; Kadum, K.M. Influene of polymer blending on mechanical and thermal properties. Mod. Appl. Sci. 2010, 4, 157–161. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic applications of polymethyl methacrylate (PMMA): An update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef]
- Ali, U.; Abd Karim, K.J.B.; Buang, N.A. A Review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym. Rev. 2015, 55, 678–705. [Google Scholar] [CrossRef]
- Hassan, M.; Asghar, M.; Din, S.U.; Zafar, M.S. Chapter 8. In Thermoset Polymethacrylate-Based Materials for Dental Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 273–308. [Google Scholar]
- Nejatian, T.; Pezeshki, S.; Yaqin Syed, A.U. Acrylic denture base materials. In 5 Advanced Dental Biomaterials; Khurshid, Z., Najeeb, S., Zafar, M.S., Sefat, F., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 79–104. [Google Scholar]
- Deb, S. Polymers in dentistry. J. Eng. Med. 1998, 212, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Shanti, R.; Hadia, A.N.; Salimb, Y.S.; Cheec, S.Y.; Ramesh, S.; Ramesh, K. Degradation of ultra-high molecular weight poly(methyl methacrylate-co-butyl acrylate-co-acrylic acid) under ultra violet irradiation. RSC Adv. 2017, 7, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Beauvois, S.; Renaut, D.; Lazzaroni, R.; Laude, L.D.; Bredas, J.L. Physico-chemical characterization of the effect of excimer laser irradiation on PMMA thin films. Appl. Surf. Sci. 1997, 109–110, 218–221. [Google Scholar] [CrossRef]
- Ahmed, B.; Raghuvanshi, S.K.; Sharma, N.P.S.; Krishna, B.M.J.; Wahab, M.A. Gamma irradiated induced physical and chemical changes in poly vinylidene fluoride (PVDF) polymer. Prog. Nanotechnol. Nanomater. 2013, 2, 42–46. [Google Scholar]
- Samperi, F.; Puglisi, C.; Alicata, R.; Montaudo, G. Thermal degradation of poly(ethylene terephthalate) at the processing temperature. Polym. Degrad. Stab. 2004, 83, 3–10. [Google Scholar] [CrossRef]
- Duquesne, S.; Lefebvre, J.; Delobel, R.; Camino, G.; LeBras, M.; Seeley, G. Vinyl acetate/butyl acrylate copolymers—Part 1: Mechanism of degradation. Polym. Degrad. Stab. 2004, 83, 19–28. [Google Scholar] [CrossRef]
- Rebollar, E.; Bounos, G.; Oujia, M.; Domingo, C.; Georgiou, S.; Castillejo, M. Influence of polymer molecular weight on the chemical modifications induced by UV laser ablation. J. Phys. Chem. B 2006, 110, 14215–14220. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, A.K.; Achilias, D.S. Thermal degradation kinetics and viscoelastic behavior of poly(methyl methacrylate)/organomodified montmorillonite nanocomposites prepared via in situ bulk radical polymerization. Polymers 2018, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Gałka, P.; Kowalonek, J.; Kaczmarek, H. Thermogravimetric analysis of thermal stability of poly(methyl methacrylate) films modified with photoinitiators. J. Therm. Anal. Calorim. 2014, 115, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Dakka, S.M. TG/DTA/MS of poly(methyl methacrylate). J. Therm. Anal. Calorim. 2003, 74, 729–734. [Google Scholar] [CrossRef]
- Zhang, B.; Blum, F.D. Thermogravimetric study of ultrathin PMMA films on silica: Effect of tacticity. Thermochim. Acta 2003, 396, 211–217. [Google Scholar] [CrossRef]
- Lomonaco, D.; Cangane, F.Y.; Mazzetto, S.E. Thiosphate esters of cashew nutshell liquid derivatives as new antioxidants for poly(methyl methacrylate). J. Therm. Anal. Calorim. 2011, 104, 1177–1183. [Google Scholar] [CrossRef]
- Holland, B.J.; Hay, J.N. The effect of polymerization conditions on the kinetics and mechanisms of thermal degradation of PMMA. Polym. Degrad. Stab. 2002, 77, 435–439. [Google Scholar] [CrossRef]
- Bae, G.; Park, T.; Song, I.-H. Surface modification of polymethylmethacrylate (PMMA) by ultraviolet (UV) irradiation and IPA rinsing. Micromachines 2022, 13, 1952. [Google Scholar] [CrossRef] [PubMed]
- Lomonaco, D.; Maia, F.J.N.; Mazzetto, S.E. Thermal evaluation of cashew nutshell liquid as new bioadditives for poly(metylmethacrylate). J. Therm. Anal. Calorim. 2013, 111, 619–626. [Google Scholar] [CrossRef]
- Pal, M.K.; Singh, B.; Guatam, J. Thermal stability and UV-shielding of polymethyl methacrylate and polystyrene modified with calcium carbonate nanoparticles. J. Therm. Anal. Calorim. 2012, 107, 85–96. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Li, J. Synergistic flame retarded poly(metylmethacrylate) by nano-ZrO2 and triphenylphosphate. J. Therm. Anal. Calorim. 2011, 103, 741–746. [Google Scholar] [CrossRef]
- Barrick, A.; Champeau, O.; Chatel, A.; Manier, N.; Northcott, G.; Tremblay, L.A. Plastic additives: Challenges in ecotox hazard assessment. PeerJ. 2021, 9, e11300. [Google Scholar] [CrossRef]
- Hahladakis, J.N.; Velis, C.A.; Weber, R.; Iacovidou, E.; Purnell, P. An overview of chemical additives present in plastics: Migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard. Mater. 2018, 344, 179–199. [Google Scholar] [CrossRef] [PubMed]
- Arantes, T.M.; Sala, R.L.; Leite, E.R.; Longo, E.; Camargo, E.R. Comparison of the nanoparticles performance in the photocatalytic degradation of a styrene–butadiene rubber nanocomposite. Appl. Polym. Sci. 2013, 128, 2368–2374. [Google Scholar] [CrossRef]
- Zhao, Y.; Dan, Y. Preparation and characterization of a high molecular weight UV-stabilizer based on a derivative of 2,4-dihydroxybenzophenone and its application in polymer aterials. J. Appl. Polym. Sci. 2006, 102, 2203–2211. [Google Scholar] [CrossRef]
- Salayová, A.; Bedlovičová, Z.; Daneu, N.; Baláž, M.; Lukáčová Bujňáková, Z.; Balážová, Ľ.; Tkáčiková, Ľ. Green synthesis of silver nanoparticles with antibacterial activity using various medicinal plant extracts: Morphology and antibacterial efficacy. Nanomaterials 2021, 11, 1005. [Google Scholar] [CrossRef] [PubMed]
- Abdellatif, A.A.H.; Alhathloul, S.S.; Aljohani, A.S.M.; Maswadeh, H.; Abdallah, E.M.; Musa, K.H.; El Hamd, M.A. Green synthesis of silver nanoparticles incorporated aromatherapies utilized for their antioxidant and antimicrobial activities against some clinical bacterial isolates. Bioinorg. Chem. Appl. 2022, 2022, 2432758. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Xu, B.; Liu, F.; Li, W.; Sy, N.; Zhou, X.; Yan, B. Effects of chemical and natural ageing on the release of potentially toxic metal additives in commercial PVC microplastics. Chemosphere 2021, 283, 131274. [Google Scholar] [CrossRef]
- Fu, M.; Li, D.; Liu, H.; Ai, H.; Zhang, Y.; Zhang, L. Synergistic effects of zinc-mannitol alkoxide with calcium/zinc stearates and with _-diketone on thermal stability of rigid poly(vinyl chloride). J. Polym. Res. 2016, 23, 13. [Google Scholar] [CrossRef]
- Li, D.; Xie, L.; Fu, M.; Zhang, J.; Indrawirawan, S.; Zhang, Y.; Tang, S. Synergistic effects of lanthanum-pentaerythritol alkoxide with zinc stearates and with beta-diketone on the thermal stability of poly(vinyl chloride). Polym. Degrad. Stab. 2015, 114, 52–59. [Google Scholar] [CrossRef]
- Yang, T.C.; Noguchi, T.; Isshiki, M.; Wu, J.H. Effect of titanium dioxide particles on the surface morphology and the mechanical properties of PVC composites during QUV accelerated weathering. Polym. Compos. 2016, 37, 3391–3397. [Google Scholar] [CrossRef]
- Schiller, M. PVC Additives: Performance, Chemistry, Developments, and Sustainability; Carl Hanser Verlag: Munich, Germany, 2015. [Google Scholar]
- Yang, T.C.; Noguchi, T.; Isshiki, M.; Wu, J.H. Effect of titanium dioxide on chemical and molecular changes in PVC sidings during QUV accelerated weathering. Polym. Degrad. Stab. 2014, 104, 33–39. [Google Scholar] [CrossRef]
- Arraq, R.R.; Hadi, A.G.; Ahmed, D.S.; El-Hiti, G.A.; Kariuki, B.M.; Husain, A.A.; Bufaroosha, M.; Yousif, E. Enhancement of photostabilization of poly(vinyl chloride) in the presence of tin–cephalexin complexes. Polymers 2023, 15, 550. [Google Scholar] [CrossRef]
- Naoom, N.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Synthesis of methyldopa–tin complexes and their applicability as photostabilizers for the protection of polyvinyl chloride against photolysis. Polymers 2022, 14, 4590. [Google Scholar] [CrossRef]
- Fadhil, M.; Yousif, E.; Ahmed, D.S.; Kariuki, B.M.; El-Hiti, G.A. Synthesis and application of levofloxacin–tin complexes as new photostabilizers for polyvinyl chloride. Polymers 2022, 14, 3720. [Google Scholar] [CrossRef]
- Fadhil, M.; Yousif, E.; Ahmed, D.S.; Mohammed, A.; Hashim, H.; Ahmed, A.; Kariuki, B.M.; El-Hiti, G.A. Synthesis of new norfloxacin–tin complexes to mitigate the effect of ultraviolet-visible irradiation in polyvinyl chloride films. Polymers 2022, 14, 2812. [Google Scholar] [CrossRef]
- Emad, N.; El-Hiti, G.A.; Yousif, E.; Kariuki, B.M. Metal oxide nanoparticles containing clotrimazole to suppress photodegradation of poly(Vinyl chloride) thin films. Polymers 2023, 15, 1632. [Google Scholar] [CrossRef]
- Yousif, E.; El-Hiti, G.A.; Haddad, R.; Balakit, A.A. Photochemical stability and photostabilizing efficiency of poly(methyl methacrylate) based on 2-(6-methoxynaphthalen-2-yl)propanoate metal ion complexes. Polymers 2015, 7, 1005–1019. [Google Scholar] [CrossRef] [Green Version]
- Gaumet, S.; Gardette, J.-L. Photo-oxidation of poly(vinyl chloride): Part 2—A comparative study of the carbonylated products in photo-chemical and thermal oxidations. Polym. Degrad. Stab. 1991, 33, 17–34. [Google Scholar] [CrossRef]
- Pospíšil, J.; Nešpurek, S. Photostabilization of coatings. Mechanisms and performance. Prog. Polym. Sci. 2000, 25, 1261–1335. [Google Scholar] [CrossRef]
- Khan, S.R.; Tawakkul, M.; Sayeed, V.A.; Faustino, P.; Khan, M.A. Stability characterization, kinetics and mechanism of degradation of dantrolene in aqueous solution: Effect of pH and temperature. Pharmacol. Pharm. 2012, 3, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Mujbil, H.H.; Al Jebur, L.A.; Yousif, E.; Kadhom, M.; Mohammed, A.; Ahmed, D.S.; Ali, M.; Hashim, H. Utilization of metal oxides nanoparticles in modulating polyvinyl chloride films to resist ultraviolet light. Metals 2022, 12, 1413. [Google Scholar] [CrossRef]
- Hasan, A.A.; Al-Mashhadani, M.H.; Al-Dahhan, W.H.; Hashim, H.; Yousif, E. Synthesized and designed new modified poly(vinyl chloride) structures to enhance their photo-resistance characteristics. Chemistry 2022, 4, 1101–1122. [Google Scholar] [CrossRef]
- Soghli, I.; Khalaji, A.D.; Grivani, G. Copper(II) and vanadium(IV) complexes of new modified poly(vinyl chloride) schiff base for catalytic studies in Knoevenagel condensation. Inorg. Chem. Res. 2021, 5, 163–172. [Google Scholar]
- Brown, L.; Koerner, T.; Horton, J.H.; Oleschuk, R.D. Fabrication and characterization of poly(methylmethacrylate) microfluidic devices bonded using surface modifications and solvents. Lab Chip 2006, 6, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Tarafder, M.T.H.; Chew, K.-B.; Crouse, K.A.; Ali, M.A.; Yamin, B.M.; Fun, H.-K. Synthesis and characterization of Cu(II), Ni(II) and Zn(II) metal complexes of bidentate NS isomeric Schiff bases derived from S-methyldithiocarbazate (SMDTC): Bioactivity of the bidentate NS isomeric Schiff bases, some of their Cu(II), Ni(II) and Zn(II) complexes and the X-ray structure of the bis[S-methyl-β-N-(2-furyl-methyl)methylenedithiocarbazato]zinc(II) complex. Polyhedron 2002, 21, 2683–2690. [Google Scholar] [CrossRef]
- Morozov, O.S.; Vyshinskii, N.N.; Rudnevskii, N.K. Investigation of some organotin compounds and their complexes by IR spectroscopy. J. Appl. Spectrosc. 1981, 35, 1019–1023. [Google Scholar] [CrossRef]
- Vinu, R.; Madras, G. Photocatalytic degradation of methyl methacrylate copolymers. Polym. Degrad. Stab. 2008, 93, 1440–1449. [Google Scholar] [CrossRef]
- Silva, T.F.; Soares, B.G.; Ferreira, S.C.; Livi, S. Silylated montmorillonite as nanofillers for plasticized PVC nanocomposites: Effect of the plasticizer. Appl. Clay Sci. 2014, 99, 93–99. [Google Scholar] [CrossRef]
- Korobeinichev, O.P.; Paletsky, A.A.; Gonchikzhapov, M.B.; Glaznev, R.K.; Gerasimov, I.E.; Naganovsky, Y.K.; Shundrina, I.K.; Snegirev, A.Y.; Vinu, R. Kinetics of thermal decomposition of PMMA at different heating rates and in a wide temperature range. Thermochim. Acta 2019, 671, 17–25. [Google Scholar] [CrossRef]
- Venkateshaiah, A.; Padil, V.V.T.; Nagalakshmaiah, M.; Waclawek, S.; Černík, M.; Varma, R.S. Microscopic techniques for the analysis of micro and nanostructures of biopolymers and their derivatives. Polymers 2020, 12, 512. [Google Scholar] [CrossRef] [Green Version]
- Mehmood, N.; Andreasson, E.; Kao-Walter, S. SEM observations of a metal foil laminated with a polymer film. Procedia Mater. Sci. 2014, 3, 1435–1440. [Google Scholar] [CrossRef] [Green Version]
- See, C.H.; O’Haver, J. Atomic force microscopy characterization of ultrathin polystyrene films formed by admicellar polymerization on silica disks. J. Appl. Polym. Sci. 2003, 89, 36–46. [Google Scholar] [CrossRef]
- Nikafshar, S.; Zabihi, O.; Ahmadi, M.; Mirmohseni, A.; Taseidifar, M.; Naebe, M. The effects of UV light on the chemical and mechanical properties of a transparent epoxy-diamine system in the presence of an organic UV absorber. Materials 2017, 10, 180. [Google Scholar] [CrossRef]
- Shi, W.; Zhang, J.; Shi, X.M.; Jiang, G.D. Different photo-degradation processes of PVC with different average degrees of polymerization. J. Appl. Polym. Sci. 2008, 107, 528–540. [Google Scholar] [CrossRef]
- Kara, F.; Aksoy, E.; Yuksekdag, Z.; Hasirci, N.; Aksoy, S. Synthesis and surface modification of polyurethanes with chitosan for antibacterial properties. Carbohydr. Polym. 2014, 112, 39–47. [Google Scholar] [CrossRef]
- El-Hiti, G.A.; Ahmed, D.S.; Yousif, E.; Alotaibi, M.H.; Star, H.A.; Ahmed, A.A. Influence of polyphosphates on the physicochemical properties of poly(vinyl chloride) after irradiation with ultraviolet light. Polymers 2020, 12, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krehula, L.K.; Papi´c, A.; Krehula, S.; Gilja, V.; Foglar, L.; Hrnjak-Murgi´c, Z. Properties of UV protective films of poly(vinylchloride)/TiO2 nanocomposites for food packaging. Polym. Bull. 2017, 74, 1387–1404. [Google Scholar] [CrossRef]
- Xu, H.; Chen, R.; Sun, Q.; Lai, W.; Su, Q.; Huang, W.; Liu, X. Recent progress in metal-organic complexes for optoelectronic applications. Chem. Soc. Rev. 2014, 43, 3259–3302. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yang, J.; Fan, F.; Qing, B.; Zhu, C.; Shi, Y.; Fan, J.; Deng, X. Effect of divalent metals on the UV-shielding properties of MII/MgAl layered double hydroxides. ACS Omega 2019, 4, 10151–10159. [Google Scholar] [CrossRef] [PubMed]

| Polymer | Color | Conversion (%) | M.P (°C) |
|---|---|---|---|
| PMMA | White | 77 | 160–162 |
| PMMA/Schiff base | Pale yellow | 70 | 120–122 |
| PMMA/Schiff base/Cu | Brown | 72 | 280–282 |
| PMMA/Schiff base/Zn | Yellow | 74 | 264–266 |
| PMMA/Schiff base/Ni | Yellow | 71 | 280–282 |
| PMMA/Schiff base/Co | Brown | 70 | 220–222 |
| Film | kd (s−1) |
|---|---|
| PMMA | 4.8 × 10–3 |
| PMMA/Schiff base | 3.2 × 10–3 |
| PMMA/Schiff base/Cu | 2.9 × 10–3 |
| PMMA/Schiff base/Zn | 2.8 × 10–3 |
| PMMA/Schiff base/Ni | 2.6 × 10–3 |
| PMMA/Schiff base/Co | 2.4 × 10–3 |
| Film | Rq (nm) |
|---|---|
| PMMA | 285.1 |
| PMMA/Schiff base | 115.8 |
| PMMA/Schiff base/Cu | 93.6 |
| PMMA/Schiff base/Zn | 90.4 |
| PMMA/Schiff base/Ni | 75.5 |
| PMMA/Schiff base/Co | 64.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sansul, S.; Yousif, E.; Ahmed, D.S.; El-Hiti, G.A.; Kariuki, B.M.; Hashim, H.; Ahmed, A. Pendant Modification of Poly(methyl methacrylate) to Enhance Its Stability against Photoirradiation. Polymers 2023, 15, 2989. https://doi.org/10.3390/polym15142989
Sansul S, Yousif E, Ahmed DS, El-Hiti GA, Kariuki BM, Hashim H, Ahmed A. Pendant Modification of Poly(methyl methacrylate) to Enhance Its Stability against Photoirradiation. Polymers. 2023; 15(14):2989. https://doi.org/10.3390/polym15142989
Chicago/Turabian StyleSansul, Shaymaa, Emad Yousif, Dina S. Ahmed, Gamal A. El-Hiti, Benson M. Kariuki, Hassan Hashim, and Ahmed Ahmed. 2023. "Pendant Modification of Poly(methyl methacrylate) to Enhance Its Stability against Photoirradiation" Polymers 15, no. 14: 2989. https://doi.org/10.3390/polym15142989









