PMMA-Based Composite Gel Polymer Electrolyte with Plastic Crystal Adopted for High-Performance Solid ECDs
Abstract
:1. Introduction
2. Experimental Details
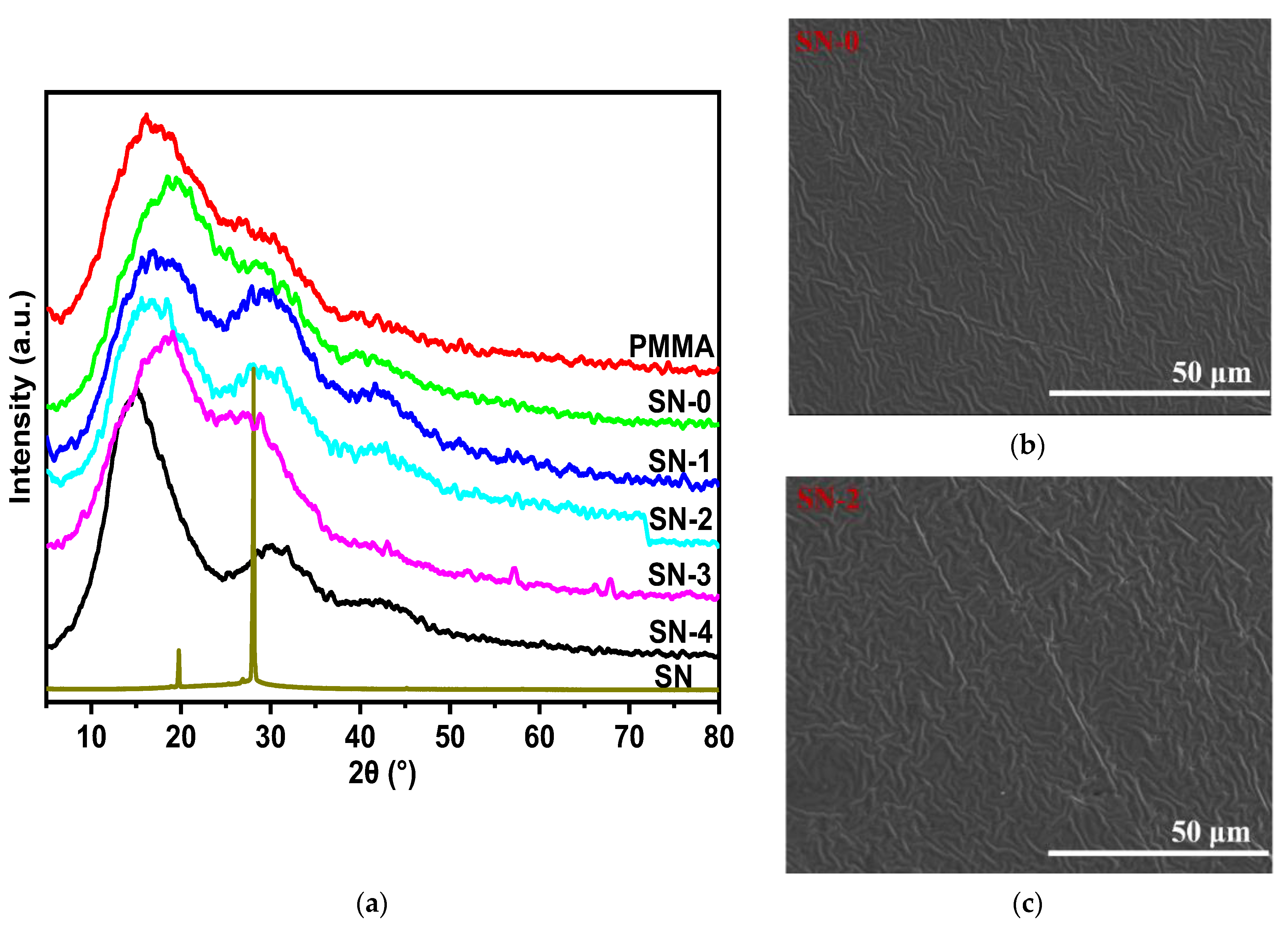
3. Results and Discussion
transparent dark blue
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yu, P.; Zeng, Y.; Zhang, H.; Yu, M.; Tong, Y.; Lu, X. Flexible Zn-Ion Batteries: Recent Progresses and Challenges. Small 2019, 15, 1804760. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zhu, Y.; Cheng, W.; Chen, W.; Wu, Y.; Yu, H. Cellulose-Based Flexible Functional Materials for Emerging Intelligent Electronics. Adv. Mater. 2021, 33, 2000619. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Q.; Mo, F.; Liang, G.; Liu, Z.; Tang, Z.; Ma, L.; Liu, J.; Shi, Z.; Zhi, C. MoS2 nanosheets with expanded interlayer spacing for rechargeable aqueous Zn-ion batteries. Energy Storage Mater. 2019, 19, 94–101. [Google Scholar] [CrossRef]
- Yun, T.; Park, M.; Kim, D.; Cheong, J.; Bae, J.; Han, S.; Kim, I. All Transparent-Stretchable Electrochromic-Supercapacitor Wearable Patch Device. ACS Nano 2019, 13, 3141–3150. [Google Scholar] [CrossRef]
- Liu, S.; Liu, W.; Ba, D.; Zhao, Y.; Ye, Y.; Li, Y.; Liu, J. Filler-Integrated Composite Polymer Electrolyte for Solid-State Lithium Batteries. Adv. Mater. 2023, 35, 2110423. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, Z.; Gray, J.L.; He, X.; Wang, D.; Chen, T.; Huang, Q.; Li, Y.; Wang, H.; Kim, S.; et al. Polymer–inorganic solid–electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 2019, 18, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, Q.; Luo, J.; Liang, J.; Liu, Y.; Zhang, L.; Wang, J.; Deng, S.; Lin, X.; Yang, X.; et al. 3D Porous Garnet/Gel Polymer Hybrid Electrolyte for Safe Solid-State LiO2 Batteries with Long Lifetimes. Chem. Mater. 2020, 32, 10113–10119. [Google Scholar] [CrossRef]
- Amici, J.; Torchio, C.; Versaci, D.; Dessantis, D.; Marchisio, A.; Caldera, F.; Bella, F.; Francia, C.; Bodoardo, S. Nanosponge-Based Composite Gel Polymer Electrolyte for Safer Li-O2 Batteries. Polymers 2021, 13, 1625. [Google Scholar] [CrossRef]
- Forsyth, M.; Porcarelli, L.; Wang, X.; Goujon, N.; Mecerreyes, D. Innovative Electrolytes Based on Ionic Liquids and Polymers for Next-Generation Solid-State Batteries. Acc. Chem. Res. 2019, 52, 686–694. [Google Scholar] [CrossRef]
- Isaac, J.A.; Devaux, D.; Bouchet, R. Dense inorganic electrolyte particles as a lever to promote composite electrolyte conductivity. Nat. Mater. 2022, 21, 1412–1418. [Google Scholar] [CrossRef]
- Simon, P.; Gogotsi, Y. Perspectives for electrochemical capacitors and related devices. Nat. Mater. 2020, 19, 1151–1163. [Google Scholar] [CrossRef]
- Muzaffar, A.; Ahamed, M.B.; Deshmukh, K.; Thirumalai, J. A review on recent advances in hybrid supercapacitors: Design, fabrication and applications. Renew. Sustain. Energy Rev. 2019, 101, 123–145. [Google Scholar] [CrossRef]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J.; Zhang, L.; Zhang, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef] [PubMed]
- Dubal, D.P.; Chodankar, N.R.; Kim, D.H.; Romero, P. Towards flexible solid-state supercapacitors for smart and wearable electronics. Chem. Soc. Rev. 2018, 47, 2065–2129. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Shanmukaraj, D.; Tkacheva, A.; Armand, M.; Wang, G. Polymer Electrolytes for Lithium-Based Batteries: Advances and Prospects[J]. Chem 2019, 5, 2326–2352. [Google Scholar] [CrossRef]
- Bocharova, V.; Sokolov, A.P. Perspectives for Polymer Electrolytes: A View from Fundamentals of Ionic Conductivity. Macromolecules 2020, 53, 4141–4157. [Google Scholar] [CrossRef]
- Zheng, J.; Hu, Y. New Insights into the Compositional Dependence of Li-Ion Transport in Polymer-Ceramic Composite Electrolytes. ACS Appl. Mater. Interfaces 2018, 10, 4113–4120. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Shi, F.; Lin, D.; Liu, Y.; Liu, W.; Pei, A.; Gong, Y.; Wang, H.; Liu, K. Vertically Aligned and Continuous Nanoscale Ceramic-Polymer Interfaces in Composite Solid Polymer Electrolytes for Enhanced Ionic Conductivity. Nano Let. 2018, 18, 3829–3838. [Google Scholar] [CrossRef]
- Khan, S.; Fang, C.; Ma, Y.; Haq, M.; Nisar, M.; Xu, G.; Liu, Y.; Han, G. High-Performance PVDF-HFP Based Gel Polymer Electrolyte Modified by Core-Shell SiO2-PMMA for Electrochromic Devices. J. Electrochem. Soc. 2021, 168, 022504. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, H.; Qie, B.; Cheng, Q.; Li, A.; Borovilas, J.; Xu, B.; Shi, C.; Jin, T.; Liao, X.; et al. Rechargeable solid-state lithium metal batteries with vertically aligned ceramic nanoparticle/polymer composite electrolyte. Nano Energy 2019, 60, 205–212. [Google Scholar] [CrossRef]
- Zachariah, M.; Romanini, M.; Tripathi, P.; Barrio, M.; Tamarit, J.L.; Macovez, R. Self-Diffusion, Phase Behavior, and Li+ Ion Conduction in Succinonitrile-Based Plastic Cocrystals. J. Phys. Chem. C 2015, 119, 27298–27306. [Google Scholar] [CrossRef] [Green Version]
- Fan, L.Z.; Hu, Y.S.; Bhattacharyya, A.J.; Maier, J. Succinonitrile as a Versatile Additive for Polymer Electrolytes. Adv. Funct. Mater. 2010, 17, 2800–2807. [Google Scholar] [CrossRef]
- Wang, A.; Geng, S.; Zhao, Z.; Hu, Z.; Luo, J. In Situ Cross-Linked Plastic Crystal Electrolytes for Wide-Temperature and High-Energy-Density Lithium Metal Batteries. Adv. Funct. Mater. 2022, 32, 2201861. [Google Scholar] [CrossRef]
- Wang, J.Y.; Wang, M.C.; Jan, D.J. Synthesis of poly(methyl methacrylate)-succinonitrile composite polymer electrolyte and its application for flexible electrochromic devices. Sol. Energy Mater. Sol. Cells 2017, 160, 476–483. [Google Scholar] [CrossRef]
- Rajendran, S.; Sivakumar, M.; Subadevi, R. Effect of salt concentration in poly(vinyl alcohol)-based solid polymer electrolytes. J. Power Sources 2003, 124, 225–230. [Google Scholar] [CrossRef]
- Echeverri, M.; Kim, N.; Kyu, T. Ionic Conductivity in Relation to Ternary Phase Diagram of Poly(ethylene oxide), Succinonitrile, and Lithium Bis(trifluoromethane)sulfonimide Blends. Macromolecules 2012, 45, 6068–6077. [Google Scholar] [CrossRef]
- Shukla, N.; Thakur, A.K. Role of salt concentration on conductivity optimization and structural phase separation in a solid polymer electrolyte based on PMMA-LiClO4. Ionics 2008, 15, 357–367. [Google Scholar] [CrossRef]
- Seo, D.M.; Reininger, S.; Kutcher, M.; Redmond, K.; Euler, W.B.; Lucht, B.L. Role of Mixed Solvation and Ion Pairing in the Solution Structure of Lithium Ion Battery Electrolytes. J. Phys. Chem. C 2015, 25, 14038–14046. [Google Scholar] [CrossRef]
- Chen, H.W.; Lin, T.P.; Chang, F.C. Ionic conductivity enhancement of the plasticized PMMA/LiClO4 polymer nanocomposite electrolyte containing clay. Polymer 2002, 43, 5281–5288. [Google Scholar] [CrossRef]
- Xuan, X.; Zhang, H.; Wang, J.; Wang, H. Vibrational spectroscopic and density functional studies on ion solvation and association of lithium tetrafluoroborate in acetonitrile. J. Phys. Chem. A 2004, 108, 7513–7521. [Google Scholar] [CrossRef]
- Loewenschuss, A.; Yellin, N.; Gabai, A. Infrared and Raman spectra of hexanitroethane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1974, 30, 371–378. [Google Scholar] [CrossRef]
- Alarco, P.J.; Abu-Lebdeh, Y.; Abouimrane, A.; Armand, M. The plastic-crystalline phase of succinonitrile as a universal matrix for solid-state ionic conductors. Nat Mater 2004, 3, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Lee, C.H.; Yu, J.H.; Doh, C.H.; Lee, S.M. Enhancement of ionic conductivity of composite membranes for all-solid-state lithium rechargeable batteries incorporating tetragonal Li7La3Zr2O12 into a polyethylene oxide matrix. J. Power Sources 2015, 274, 458–463. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, H.; Dong, S.; Hu, Z.; Hu, R.; Guo, Z.; Wang, T.; Cui, G.; Chen, L. High Polymerization Conversion and Stable High-Voltage Chemistry Underpinning an In Situ Formed Solid Electrolyte. Chem. Mater. 2020, 32, 9167–9175. [Google Scholar] [CrossRef]
- Chandra, S. Superionic Solids: Principles and Applications. 1981. Available online: https://www.osti.gov/etdeweb/biblio/6872169 (accessed on 1 January 1981).
- Dueramae, I.; Okhawilai, M.; Kasemsiri, P.; Uyama, H. High electrochemical and mechanical performance of zinc conducting-based gel polymer electrolytes. Sci. Rep. 2021, 11, 13268. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-H.; Choi, K.-H.; Cho, S.-J.; Park, J.-S.; Cho, K.; Lee, C.; Lee, S.; Shim, J.; Lee, S.-Y. A shape-deformable and thermally stable solid-state electrolyte based on a plastic crystal composite polymer electrolyte for flexible/safer lithium-ion batteries. J. Mater. Chem. A 2014, 2, 10854–10861. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.Y.; Chen, X.N.; Wan, L.S.; Xu, Z.K. Interactions between polyacrylonitrile and solvents: Density functional theory study and two-dimensional infrared correlation analysis. J. Phys. Chem. B 2012, 116, 8321–8330. [Google Scholar] [CrossRef]
- Stephan, A.M.; Nahm, K.S.; Kulandainathan, M.A.; Ravi, G.; Wilson, J. Poly(vinylidene fluoride-hexafluoropropylene) (PVdF-HFP) based composite electrolytes for lithium batteries. Eur. Polym. J. 2006, 42, 1728–1734. [Google Scholar] [CrossRef]
- Zhao, X.; Tao, C.; Li, Y.; Chen, X.; Wang, J.; Gong, H. Preparation of gel polymer electrolyte with high lithium ion transference number using GO as filler and application in lithium battery. Ionics 2020, 26, 4299–4309. [Google Scholar] [CrossRef]
- Puguan, J.M.C.; Chinnappan, A.; Ntiamoah, R.; Kim, H. Enhanced Ionic conductivity and optical transmissivity of functionalized ZrO2/PVdF-HFP hybrid electrolyte for energy efficient windows. Sol. Energy Mater. Sol. Cells 2015, 137, 265–273. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, B.; Huang, Y.; Song, A.; Lin, Y.; Wang, M.; Li, X.; Cao, H. Gel polymer electrolyte based on polymethyl methacrylate matrix composited with methacrylisobutyl-polyhedral oligomeric silsesquioxane by phase inversion method. Electrochim. Acta 2018, 278, 1–12. [Google Scholar] [CrossRef]
- Randles, J.E.B. A Cathode Ray Polarograph. Part II.-the Current-Voltage Curves. Trans. Faraday Soc. 1948, 44, 327–338. [Google Scholar] [CrossRef]



| Matrix | Salt/Fillers | Ionic Conductivity (S·cm−1) | Optic | Ref. |
|---|---|---|---|---|
| PEO | LiTFSI/Li1.5Al0.5Ge1.5(PO4)3 | 1.67 × 10−4 | opaque | [20] |
| PVdF-HFP | LiN(CF3SO2)2/AlO[OH]n | ~10−4 | - | [39] |
| PVdF-HFP | LiTFSI/graphene oxide | 1.3 × 10−3 | opaque | [40] |
| PVdF-HFP | LiCF3SO3/ZrO2 | 3.42 × 10−3 | opaque | [41] |
| PMMA | LiPF6/MA-POSS* | 2.77 × 10−3 | opaque | [42] |
| PVdF-HFP | LiClO4/SiO2-MMA | 2.22 × 10−3 | transparent | [19] |
| PMMA | LiClO4/SN | 2.02 × 10−3 | transparent | this work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Tang, Y.; Li, G.; Xu, G.; Liu, Y.; Han, G. PMMA-Based Composite Gel Polymer Electrolyte with Plastic Crystal Adopted for High-Performance Solid ECDs. Polymers 2023, 15, 3008. https://doi.org/10.3390/polym15143008
Zhou Z, Tang Y, Li G, Xu G, Liu Y, Han G. PMMA-Based Composite Gel Polymer Electrolyte with Plastic Crystal Adopted for High-Performance Solid ECDs. Polymers. 2023; 15(14):3008. https://doi.org/10.3390/polym15143008
Chicago/Turabian StyleZhou, Zhou, Yongkang Tang, Gang Li, Gang Xu, Yong Liu, and Gaorong Han. 2023. "PMMA-Based Composite Gel Polymer Electrolyte with Plastic Crystal Adopted for High-Performance Solid ECDs" Polymers 15, no. 14: 3008. https://doi.org/10.3390/polym15143008





