New Polyvinyl Alcohol/Succinoglycan-Based Hydrogels for pH-Responsive Drug Delivery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of SG
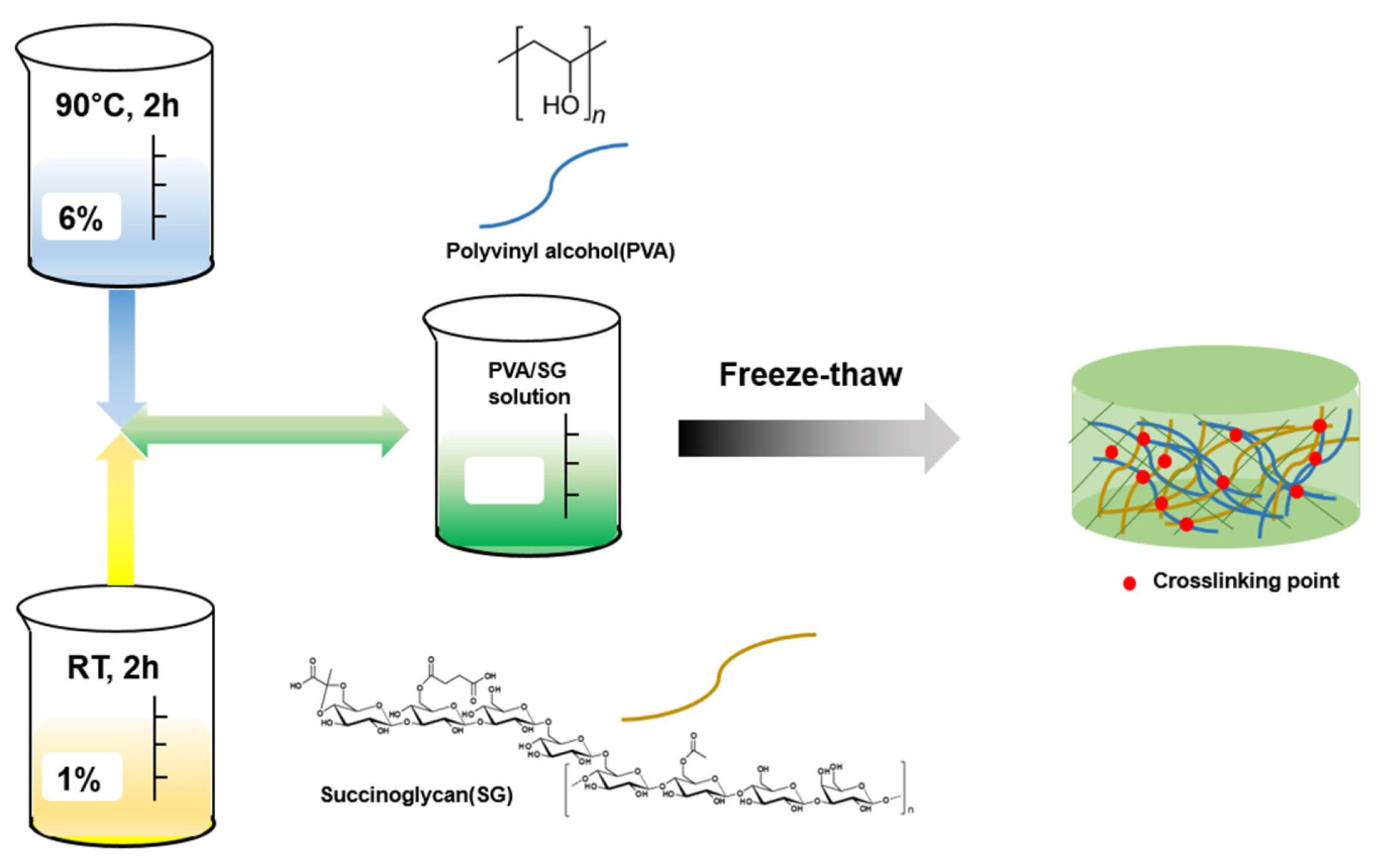
2.3. Synthesis of the PVA/SG Hydrogel
2.4. Fourier Transform Infrared Spectrum (FTIR)
2.5. Field Emission Scanning Electron Microscopy (FE-SEM)
2.6. X-ray Diffraction (XRD) Measurements
2.7. Thermogravimetric Analysis (TGA)
2.8. Differential Scanning Calorimetry (DSC)
2.9. Rheological Measurements
2.10. Compressive Tests
2.11. Swelling Behavior
2.12. Drug Release
2.13. In Vitro Cytotoxicity Test
3. Results
3.1. Optimization of the PVA/SG Hydrogel
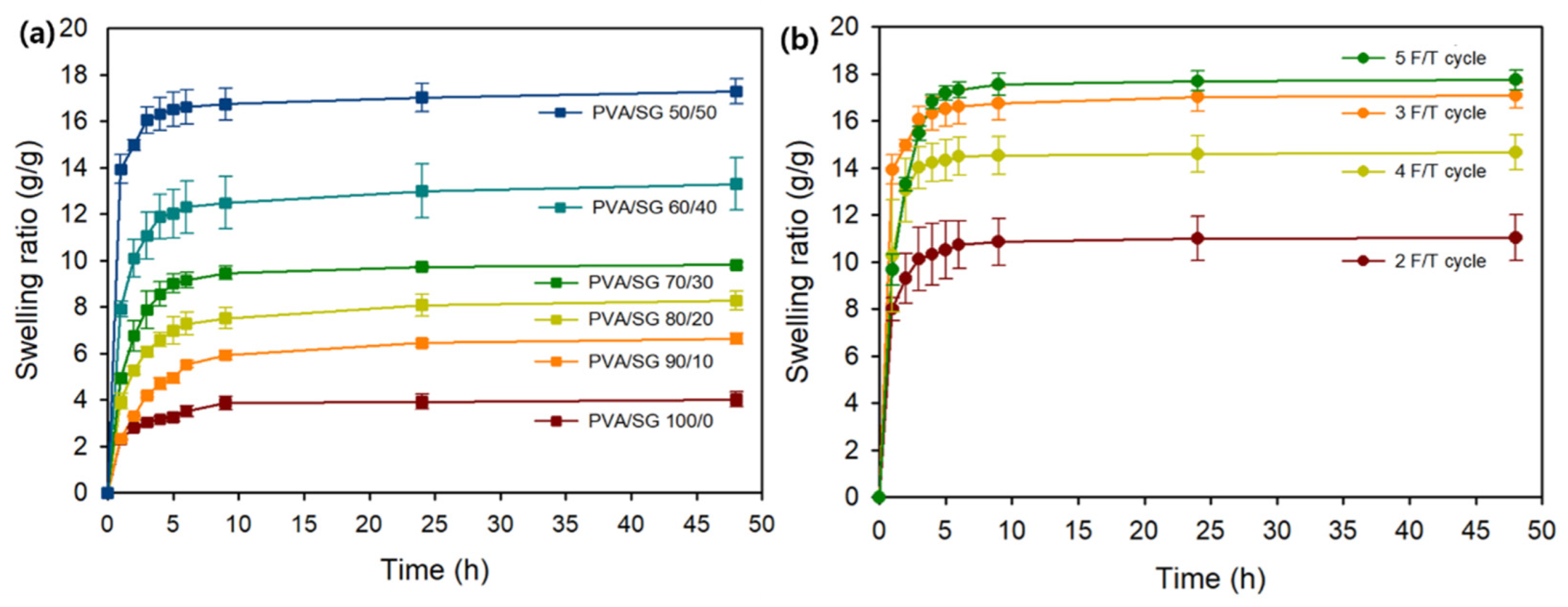
3.1.1. Swelling Studies
3.1.2. FE-SEM Analysis
3.1.3. Rheological Analysis
3.1.4. Compressive Test
3.2. Characterization of the PVA/SG Hydrogel
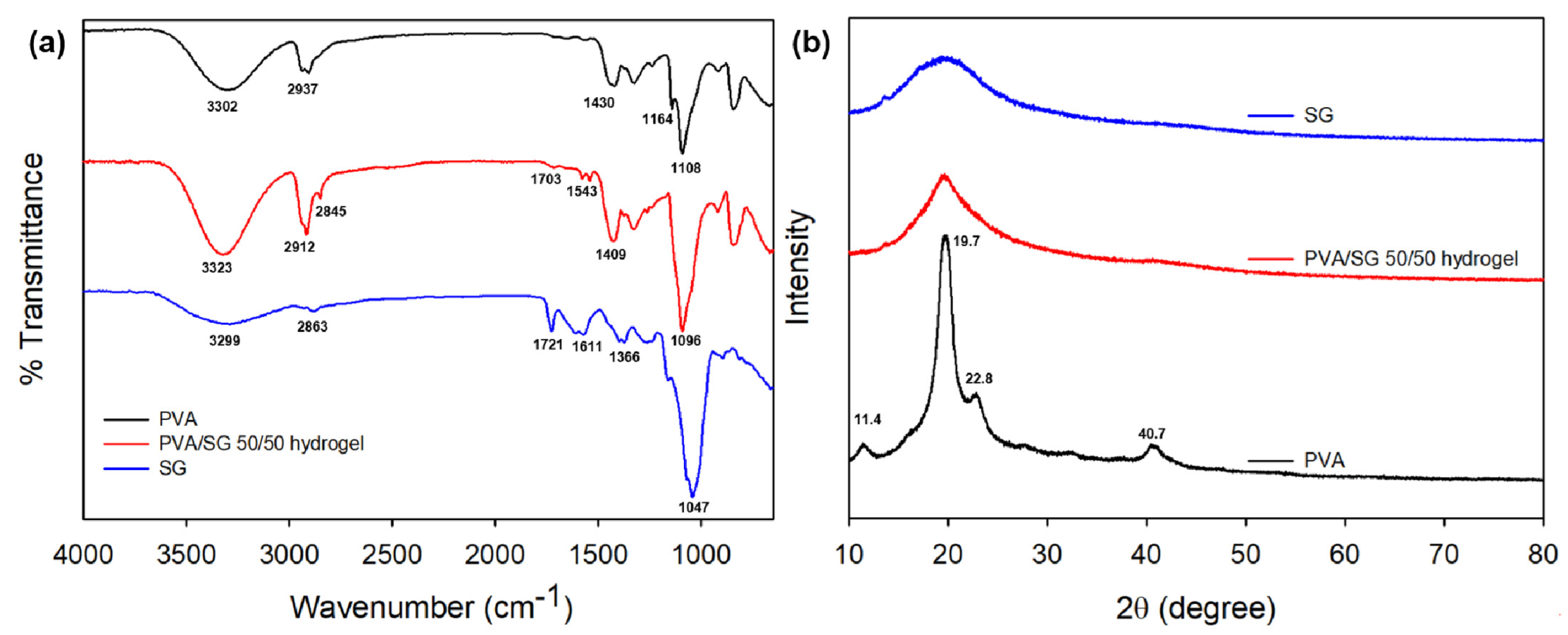
3.2.1. FTIR Analysis
3.2.2. XRD Studies
3.2.3. TGA
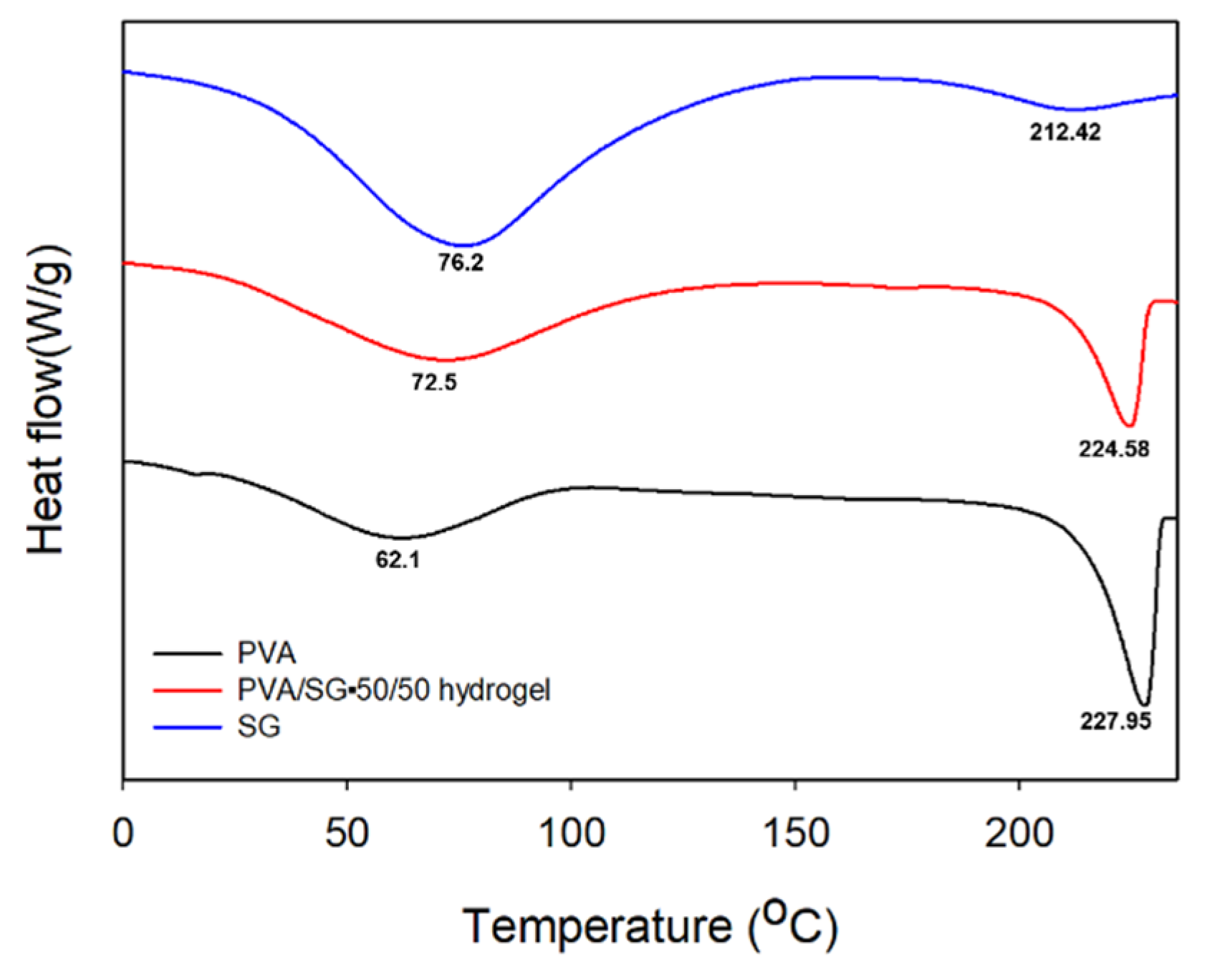
3.2.4. DSC
3.3. In Vitro Drug Release and Cell Viability
3.3.1. pH-Dependent Drug Release of 5-FU
3.3.2. Cell Viability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [PubMed]
- Vigata, M.; Meinert, C.; Hutmacher, D.W.; Bock, N. Hydrogels as drug delivery systems: A review of current characterization and evaluation techniques. Pharmaceutics 2020, 12, 1188. [Google Scholar] [CrossRef] [PubMed]
- Paradossi, G.; Cavalieri, F.; Chiessi, E.; Spagnoli, C.; Cowman, M.K. Poly (vinyl alcohol) as versatile biomaterial for potential biomedical applications. J. Mater. Sci. Mater. Med. 2003, 14, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Taufiq, A.; Mufti, N.; Hidayat, N.; Rugmai, S.; Soontaranon, S.; Putra, E. Analysis of Distribution of Polyvinyl Alcohol Hydrogel Nanocrystalline by using SAXS Synchrotron. In Proceedings of IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; p. 012041. [Google Scholar]
- Morandim-Giannetti, A.d.A.; Rubio, S.R.; Nogueira, R.F.; Ortega, F.d.S.; Magalhães Junior, O.; Schor, P.; Bersanetti, P.A. Characterization of PVA/glutaraldehyde hydrogels obtained using Central Composite Rotatable Design (CCRD). J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 1558–1566. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, P.C.; Edgren, D. Crosslinking reaction of poly (vinyl alcohol) with glyoxal. J. Polym. Res. 2010, 17, 725–730. [Google Scholar] [CrossRef]
- Redy Keisar, O.; Nahum, V.; Yehezkel, L.; Marcovitch, I.; Columbus, I.; Fridkin, G.; Chen, R. Active and strippable PVA/Borax/NaBO3 hydrogel for effective containment and decontamination of chemical warfare agents. ACS Omega 2021, 6, 5359–5367. [Google Scholar] [CrossRef]
- Varshney, N.; Sahi, A.K.; Poddar, S.; Vishwakarma, N.K.; Kavimandan, G.; Prakash, A.; Mahto, S.K. Freeze–Thaw-Induced Physically Cross-linked Superabsorbent Polyvinyl Alcohol/Soy Protein Isolate Hydrogels for Skin Wound Dressing: In Vitro and In Vivo Characterization. ACS Appl. Mater. Interfaces 2022, 14, 14033–14048. [Google Scholar] [CrossRef]
- Kenawy, E.-R.; Kamoun, E.A.; Eldin, M.S.M.; El-Meligy, M.A. Physically crosslinked poly (vinyl alcohol)-hydroxyethyl starch blend hydrogel membranes: Synthesis and characterization for biomedical applications. Arab. J. Chem. 2014, 7, 372–380. [Google Scholar] [CrossRef]
- Ou, K.; Dong, X.; Qin, C.; Ji, X.; He, J. Properties and toughening mechanisms of PVA/PAM double-network hydrogels prepared by freeze-thawing and anneal-swelling. Mater. Sci. Eng. C 2017, 77, 1017–1026. [Google Scholar] [CrossRef]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-based controlled release systems for therapeutics delivery and tissue engineering: From bench to bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef]
- Ng, J.Y.; Obuobi, S.; Chua, M.L.; Zhang, C.; Hong, S.; Kumar, Y.; Gokhale, R.; Ee, P.L.R. Biomimicry of microbial polysaccharide hydrogels for tissue engineering and regenerative medicine–A review. Carbohydr. Polym. 2020, 241, 116345. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.; Chen, F. Bioprocessing for Value-Added Products from Renewable Resources; Elsevier: Amsterdam, The Netherlands, 2006; pp. 131–161. [Google Scholar]
- Ramalingam, C.; Priya, J.; Mundra, S. Applications of microbial polysaccharides in food industry. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 322–324. [Google Scholar]
- Song, Y.; Li, S.; Gong, H.; Yip, R.C.S.; Chen, H. Biopharmaceutical applications of microbial polysaccharides as materials: A review. Int. J. Biol. Macromol. 2023, 239, 124259. [Google Scholar] [CrossRef]
- Halder, U.; Banerjee, A.; Bandopadhyay, R. Structural and functional properties, biosynthesis, and patenting trends of bacterial succinoglycan: A review. Indian J. Microbiol. 2017, 57, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Stredansky, M.; Conti, E.; Bertocchi, C.; Matulova, M.; Zanetti, F. Succinoglycan production by Agrobacterium tumefaciens. J. Ferment. Bioeng. 1998, 85, 398–403. [Google Scholar] [CrossRef]
- Bakhtiyari, M.; Moosavi-Nasab, M.; Askari, H. Optimization of succinoglycan hydrocolloid production by Agrobacterium radiobacter grown in sugar beet molasses and investigation of its physicochemical characteristics. Food Hydrocoll. 2015, 45, 18–29. [Google Scholar] [CrossRef]
- Ding, Z.; Zhao, Y.; Liu, J.; Ge, W.; Xu, X.; Wang, S.; Zhang, J. Dietary Succinoglycan Riclin Improves Glycemia Control in Mice with Type 2 Diabetes. J. Agric. Food Chem. 2022, 70, 1819–1829. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, X.; Zhao, Y.; Ge, W.; Ding, Z.; Liu, J.; Wang, L.; Xu, X.; Zhang, J. Anti-tumor activity and immunogenicity of a succinoglycan riclin. Carbohydr. Polym. 2021, 255, 117370. [Google Scholar] [CrossRef]
- Wu, Z.; Cheng, Y.; Yang, Y.; Gao, Y.; Sun, X.; Wang, L.; Sun, Q.; Zhang, J.; Xu, X. In vitro and in vivo anti-Listeria effect of Succinoglycan Riclin through regulating MAPK/IL-6 axis and metabolic profiling. Int. J. Biol. Macromol. 2020, 150, 802–813. [Google Scholar] [CrossRef]
- Kim, S.; Jeong, D.; Lee, H.; Kim, D.; Jung, S. Succinoglycan dialdehyde-reinforced gelatin hydrogels with toughness and thermal stability. Int. J. Biol. Macromol. 2020, 149, 281–289. [Google Scholar] [CrossRef]
- Kim, Y.; Hu, Y.; Jeong, J.-p.; Jung, S. Injectable, self-healable and adhesive hydrogels using oxidized Succinoglycan/chitosan for pH-responsive drug delivery. Carbohydr. Polym. 2022, 284, 119195. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-X.; Wang, Y.; Pellock, B.; Walker, G.C. Structural characterization of the symbiotically important low-molecular-weight succinoglycan of Sinorhizobium meliloti. J. Bacteriol. 1999, 181, 6788–6796. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-H.; Ko, S.-C.; Oh, G.-W.; Jang, Y.-M.; Kim, Y.-M.; Park, W.S.; Choi, I.-W.; Jung, W.-K. Characterization and biological activity of PVA hydrogel containing chitooligosaccharides conjugated with gallic acid. Carbohydr. Polym. 2018, 198, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, M.; Miyazono, Y.; Sasamoto, K.; Ohkura, Y.; Ueno, K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta 1997, 44, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Sarafian, A.; López, D.; Mijangos, C. Viscoelastic properties of poly (vinyl alcohol) hydrogels and ferrogels obtained through freezing–thawing cycles. Polymer 2004, 45, 5543–5549. [Google Scholar] [CrossRef]
- Bercea, M.; Morariu, S.; Teodorescu, M. Rheological investigation of poly (vinyl alcohol)/poly (N-vinyl pyrrolidone) mixtures in aqueous solution and hydrogel state. J. Polym. Res. 2016, 23, 142. [Google Scholar] [CrossRef]
- Ricciardi, R.; Mangiapia, G.; Lo Celso, F.; Paduano, L.; Triolo, R.; Auriemma, F.; De Rosa, C.; Lauprêtre, F. Structural organization of poly (vinyl alcohol) hydrogels obtained by freezing and thawing techniques: A SANS study. Chem. Mater. 2005, 17, 1183–1189. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, F.; Wu, J. Physically crosslinked hydrogels from polysaccharides prepared by freeze–thaw technique. React. Funct. Polym. 2013, 73, 923–928. [Google Scholar] [CrossRef]
- Qing, X.; He, G.; Liu, Z.; Yin, Y.; Cai, W.; Fan, L.; Fardim, P. Preparation and properties of polyvinyl alcohol/N–succinyl chitosan/lincomycin composite antibacterial hydrogels for wound dressing. Carbohydr. Polym. 2021, 261, 117875. [Google Scholar] [CrossRef]
- Baniasadi, H.; Madani, Z.; Ajdary, R.; Rojas, O.J.; Seppälä, J. Ascorbic acid-loaded polyvinyl alcohol/cellulose nanofibril hydrogels as precursors for 3D printed materials. Mater. Sci. Eng. C 2021, 130, 112424. [Google Scholar] [CrossRef]
- Hu, Z.; Cheng, J.; Xu, S.; Cheng, X.; Zhao, J.; Low, Z.W.K.; Chee, P.L.; Lu, Z.; Zheng, L.; Kai, D. PVA/pectin composite hydrogels inducing osteogenesis for bone regeneration. Mater. Today Bio 2022, 16, 100431. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, J.; Zhang, W.; Pu, Y.; Yang, R.; Wang, P.; Liu, S.; Tan, X.; Chi, B. 3D-printed antioxidant antibacterial carboxymethyl cellulose/ε-polylysine hydrogel promoted skin wound repair. Int. J. Biol. Macromol. 2021, 187, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Pacelli, S.; Di Muzio, L.; Paolicelli, P.; Fortunati, V.; Petralito, S.; Trilli, J.; Casadei, M.A. Dextran-polyethylene glycol cryogels as spongy scaffolds for drug delivery. Int. J. Biol. Macromol. 2021, 166, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, L.; Chen, G.; Zhou, Z.; Li, Q. A high water-content and high elastic dual-responsive polyurethane hydrogel for drug delivery. J. Mater. Chem. B 2015, 3, 8401–8409. [Google Scholar] [CrossRef]
- Guan, Y.; Qi, X.-M.; Zhang, B.; Chen, G.-G.; Peng, F.; Sun, R.-C. Physically crosslinked composite hydrogels of hemicelluloses with poly (vinyl alcohol phosphate) and chitin nanowhiskers. BioResources 2015, 10, 1378–1393. [Google Scholar] [CrossRef]
- Jayaramudu, T.; Ko, H.-U.; Kim, H.C.; Kim, J.W.; Muthoka, R.M.; Kim, J. Electroactive hydrogels made with polyvinyl alcohol/cellulose nanocrystals. Materials 2018, 11, 1615. [Google Scholar] [CrossRef]
- Hendrawan, H.; Khoerunnisa, F.; Sonjaya, Y.; Putri, A.D. Poly (vinyl alcohol)/glutaraldehyde/Premna oblongifolia merr extract hydrogel for controlled-release and water absorption application. In Proceedings of IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2019; p. 012048. [Google Scholar]
- Jipa, I.M.; Stoica, A.; Stroescu, M.; Dobre, L.-M.; Dobre, T.; Jinga, S.; Tardei, C. Potassium sorbate release from poly (vinyl alcohol)-bacterial cellulose films. Chem. Pap. 2012, 66, 138–143. [Google Scholar] [CrossRef]
- Tamer, T.M.; Sabet, M.M.; Alhalili, Z.A.; Ismail, A.M.; Mohy-Eldin, M.S.; Hassan, M.A. Influence of cedar essential oil on physical and biological properties of hemostatic, antibacterial, and antioxidant polyvinyl alcohol/cedar oil/kaolin composite hydrogels. Pharmaceutics 2022, 14, 2649. [Google Scholar] [CrossRef]
- Hu, Y.; Kim, Y.; Hong, I.; Kim, M.; Jung, S. Fabrication of flexible pH-responsive agarose/succinoglycan hydrogels for controlled drug release. Polymers 2021, 13, 2049. [Google Scholar] [CrossRef]
- Tadokoro, H.; Seki, S.; Nitta, I. Some information on the infrared absorption spectrum of polyvinyl alcohol from deuteration and pleochroism. J. Polym. Sci. 1956, 22, 563–566. [Google Scholar] [CrossRef]
- Kim, G.-M.; Asran, A.S.; Michler, G.H.; Simon, P.; Kim, J.-S. Electrospun PVA/HAp nanocomposite nanofibers: Biomimetics of mineralized hard tissues at a lower level of complexity. Bioinspir. Biomim. 2008, 3, 046003. [Google Scholar] [CrossRef] [PubMed]
- Bunn, C. Crystal structure of polyvinyl alcohol. Nature 1948, 161, 929–930. [Google Scholar] [CrossRef]
- Xiang, A.; Lv, C.; Zhou, H. Changes in crystallization behaviors of poly (vinyl alcohol) induced by water content. J. Vinyl Addit. Technol. 2020, 26, 613–622. [Google Scholar] [CrossRef]
- Chen, Y.-N.; Jiao, C.; Zhao, Y.; Zhang, J.; Wang, H. Self-assembled polyvinyl alcohol–tannic acid hydrogels with diverse microstructures and good mechanical properties. ACS Omega 2018, 3, 11788–11795. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Yang, L.; Tian, J.; Huang, L.; Huang, D.; Zhang, W.; Xie, F.; Niu, Y.; Jin, M.; Jia, C. Characterization and rheological properties analysis of the succinoglycan produced by a high-yield mutant of Rhizobium radiobacter ATCC 19358. Int. J. Biol. Macromol. 2021, 166, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Wang, H.; Xia, L.; Chen, M.; Li, L.; Cheng, J.; Li, X.; Jiang, S. Colorimetric film based on polyvinyl alcohol/okra mucilage polysaccharide incorporated with rose anthocyanins for shrimp freshness monitoring. Carbohydr. Polym. 2020, 229, 115402. [Google Scholar] [CrossRef]
- Liu, X.; Song, R.; Zhang, W.; Qi, C.; Zhang, S.; Li, J. Development of eco-friendly soy protein isolate films with high mechanical properties through HNTs, PVA, and PTGE synergism effect. Sci. Rep. 2017, 7, 44289. [Google Scholar] [CrossRef]
- Hu, Y.; Shin, Y.; Park, S.; Jeong, J.-p.; Kim, Y.; Jung, S. Multifunctional Oxidized Succinoglycan/Poly (N-isopropylacrylamide-co-acrylamide) Hydrogels for Drug Delivery. Polymers 2023, 15, 122. [Google Scholar] [CrossRef]
- Sedlařík, V.; Saha, N.; Kuřitka, I.; Saha, P. Preparation and characterization of poly (vinyl alcohol)/lactic acid compounded polymeric films. Int. J. Polym. Anal. Charact. 2006, 11, 253–270. [Google Scholar] [CrossRef]
- Lee, J.; Bhattacharyya, D.; Easteal, A.; Metson, J. Properties of nano-ZnO/poly (vinyl alcohol)/poly (ethylene oxide) composite thin films. Curr. Appl. Phys. 2008, 8, 42–47. [Google Scholar] [CrossRef]
- Kim, G.-M. Fabrication of bio-nanocomposite nanofibers mimicking the mineralized hard tissues via electrospinning process. In Nanofibers; IntechOpen: London, UK, 2010; pp. 69–88. [Google Scholar]
- Gupta, B.; Agarwal, R.; Sarwar Alam, M. Preparation and characterization of polyvinyl alcohol-polyethylene oxide-carboxymethyl cellulose blend membranes. J. Appl. Polym. Sci. 2013, 127, 1301–1308. [Google Scholar] [CrossRef]
- Zhou, T.; Chen, S.; Ding, X.; Hu, Z.; Cen, L.; Zhang, X. Fabrication and characterization of collagen/PVA dual-layer membranes for periodontal bone regeneration. Front. Bioeng. Biotechnol. 2021, 9, 630977. [Google Scholar] [CrossRef]
- Zhang, Y.; Ye, L.; Cui, M.; Yang, B.; Li, J.; Sun, H.; Yao, F. Physically crosslinked poly (vinyl alcohol)–carrageenan composite hydrogels: Pore structure stability and cell adhesive ability. Rsc Adv. 2015, 5, 78180–78191. [Google Scholar] [CrossRef]
- Hassan, C.M.; Peppas, N.A. Structure and morphology of freeze/thawed PVA hydrogels. Macromolecules 2000, 33, 2472–2479. [Google Scholar] [CrossRef]
- Patil, N.S.; Dordick, J.S.; Rethwisch, D.G. Macroporous poly (sucrose acrylate) hydrogel for controlled release of macromolecules. Biomaterials 1996, 17, 2343–2350. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Roy, A.; Pal, S. A Polysaccharide-Based pH-Sensitive Hybrid Hydrogel as a Sustained Release Matrix for Antimicrobial Drugs. ACS Appl. Polym. Mater. 2023, 5, 3348–3358. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Naeem, S.; Ramesh, S.; Ramesh, K. pH responsive N-succinyl chitosan/Poly (acrylamide-co-acrylic acid) hydrogels and in vitro release of 5-fluorouracil. PLoS ONE 2017, 12, e0179250. [Google Scholar]
- Yiğitoğlu, M.; Aydın, G.; Işıklan, N. Microwave-assisted synthesis of alginate-g-polyvinylpyrrolidone copolymer and its application in controlled drug release. Polym. Bull. 2014, 71, 385–414. [Google Scholar] [CrossRef]
- Qi, X.; Wei, W.; Shen, J.; Dong, W. Salecan polysaccharide-based hydrogels and their applications: A review. J. Mater. Chem. B 2019, 7, 2577–2587. [Google Scholar] [CrossRef]
- DeMerlis, C.; Schoneker, D. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, L.; Li, J.; Fu, R.; Wang, S.; Zhang, J. In vitro and in vivo anti-inflammatory activity of a succinoglycan Riclin from Agrobacterium sp. ZCC3656. J. Appl. Microbiol. 2019, 127, 1716–1726. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, R.; Sun, X.; Zhao, Y.; Yang, Y.; Gao, Y.; Ding, Z.; Ge, W.; Liu, J.; Wang, S. Safety assessment of functional oligooctasaccharide riclinoctaose: A pilot study of genotoxicity, acute toxicity, and subchronic toxicity. J. Food Sci. 2022, 87, 1306–1318. [Google Scholar] [CrossRef] [PubMed]





| PVA/SG Hydrogel | 6 wt% PVA (mL) | 1 wt% SG (mL) |
|---|---|---|
| PVA/SG 100/0 | 1 | 0 |
| PVA/SG 90/10 | 0.9 | 0.1 |
| PVA/SG 80/20 | 0.8 | 0.2 |
| PVA/SG 70/30 | 0.7 | 0.3 |
| PVA/SG 60/40 | 0.6 | 0.4 |
| PVA/SG 50/50 | 0.5 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.-p.; Kim, K.; Kim, J.; Kim, Y.; Jung, S. New Polyvinyl Alcohol/Succinoglycan-Based Hydrogels for pH-Responsive Drug Delivery. Polymers 2023, 15, 3009. https://doi.org/10.3390/polym15143009
Jeong J-p, Kim K, Kim J, Kim Y, Jung S. New Polyvinyl Alcohol/Succinoglycan-Based Hydrogels for pH-Responsive Drug Delivery. Polymers. 2023; 15(14):3009. https://doi.org/10.3390/polym15143009
Chicago/Turabian StyleJeong, Jae-pil, Kyungho Kim, Jaeyul Kim, Yohan Kim, and Seunho Jung. 2023. "New Polyvinyl Alcohol/Succinoglycan-Based Hydrogels for pH-Responsive Drug Delivery" Polymers 15, no. 14: 3009. https://doi.org/10.3390/polym15143009
APA StyleJeong, J.-p., Kim, K., Kim, J., Kim, Y., & Jung, S. (2023). New Polyvinyl Alcohol/Succinoglycan-Based Hydrogels for pH-Responsive Drug Delivery. Polymers, 15(14), 3009. https://doi.org/10.3390/polym15143009








