Novel PEG6000–Silica-MWCNTs Shape-Stabilized Composite Phase-Change Materials (ssCPCMs) for Thermal-Energy Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
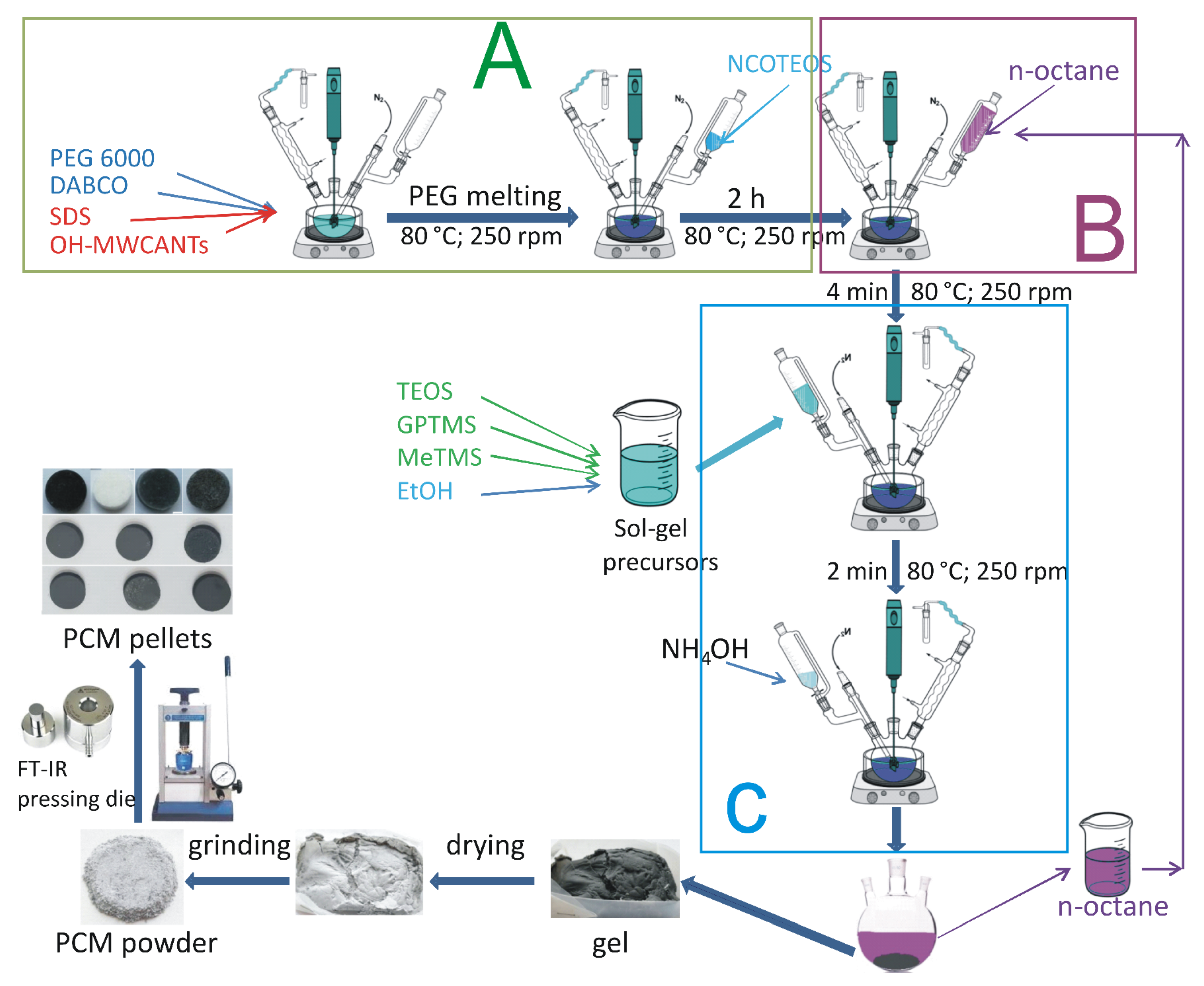
2.2. Preparation of PEG6000–Silica-MWCNTs ssCPCMs
2.3. Methods
3. Results and Discussions
3.1. Leakage Test
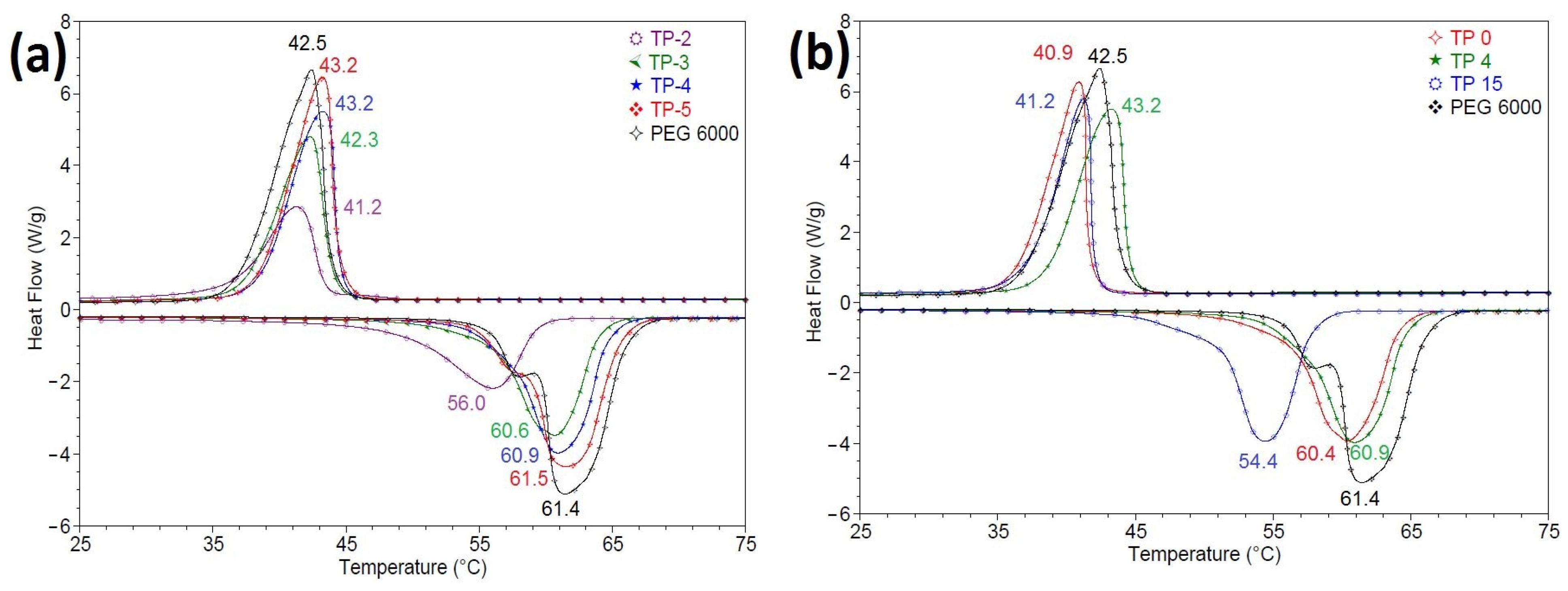
3.2. Evaluation of the Thermal Energy Storage Capacity for the Obtained Materials
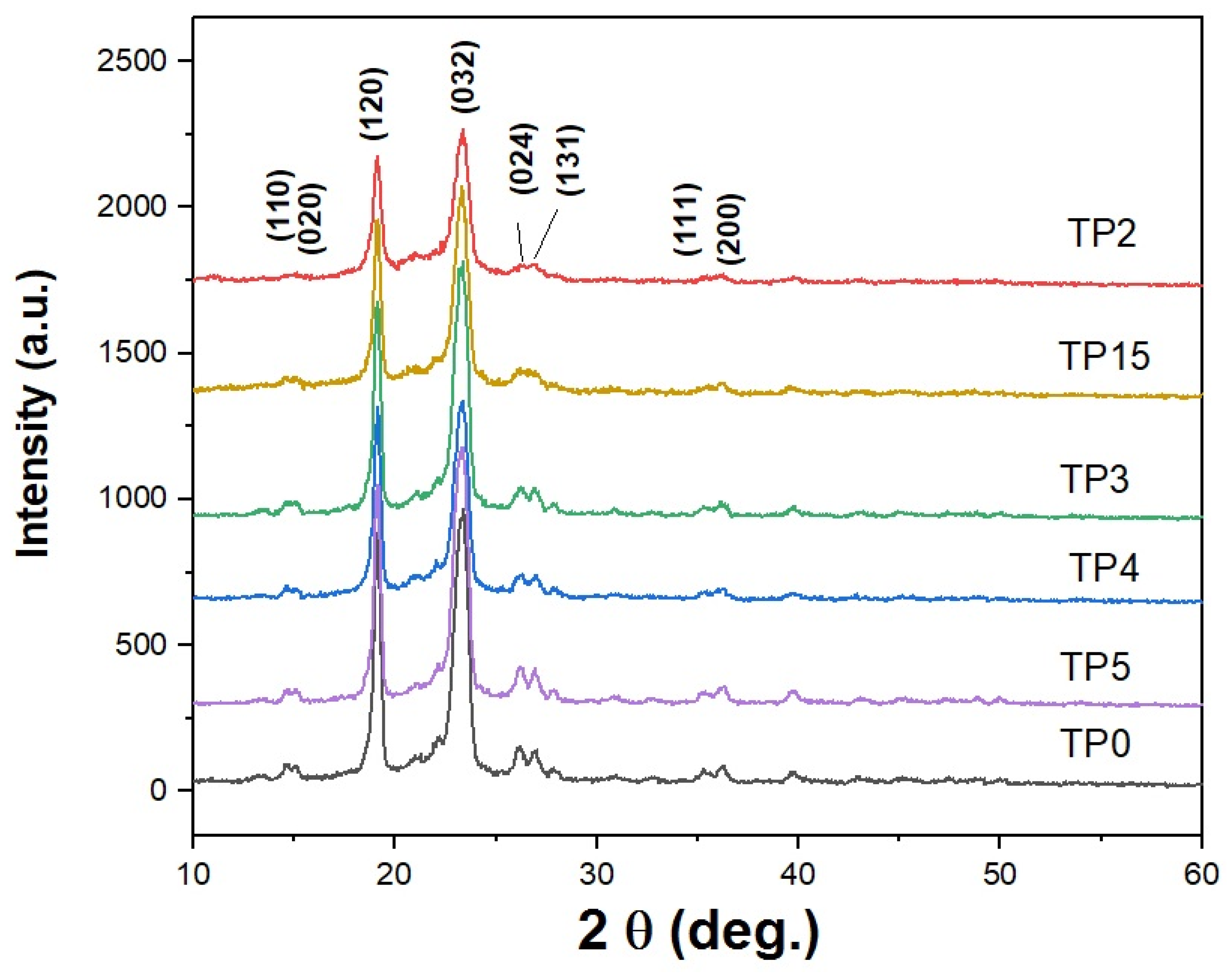
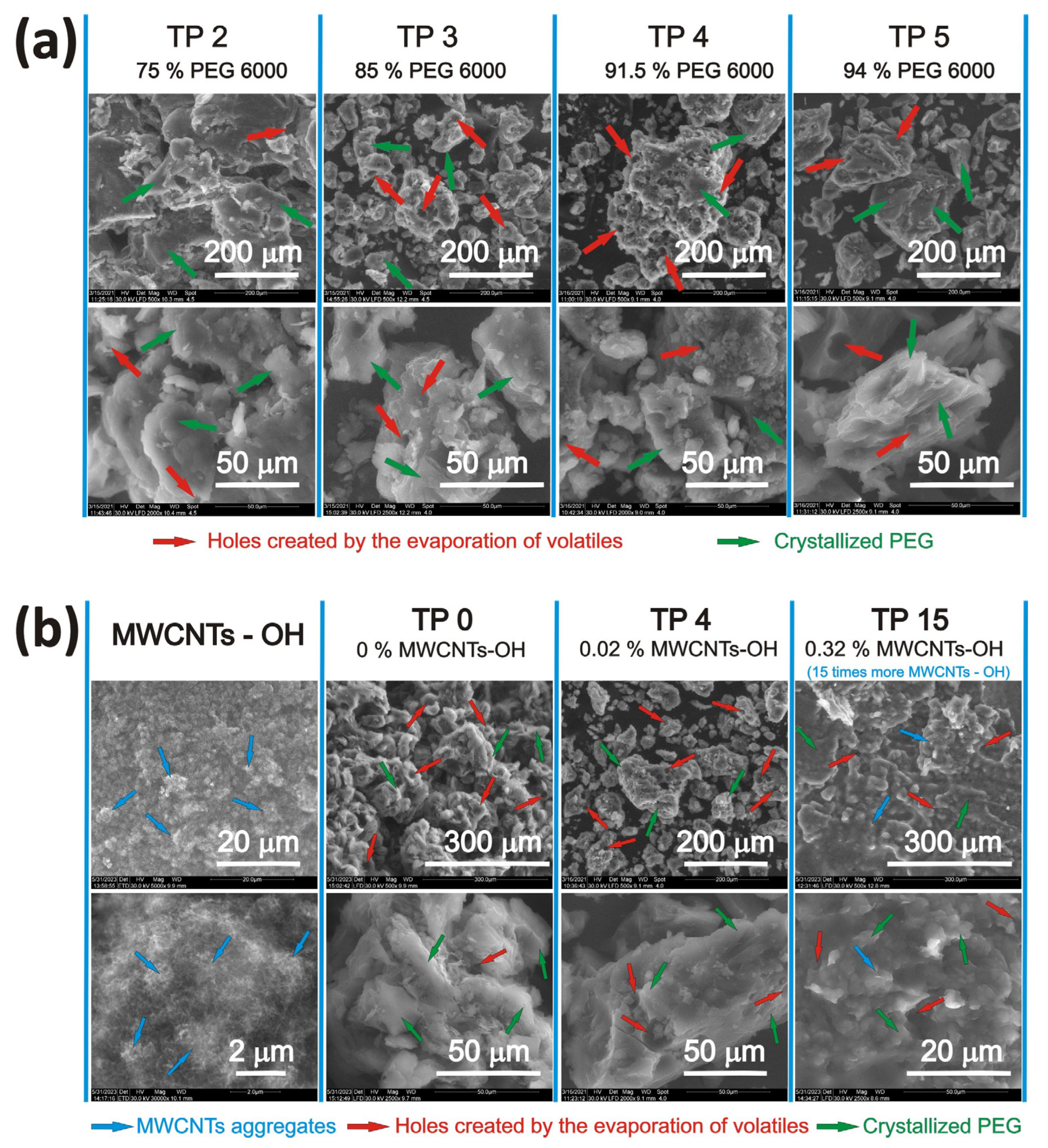
3.3. Structure and Morphology of the Semicrystalline PEG6000-Based Composites
3.4. FT-IR and UV-Raman Analysis
3.5. Monitoring the Thermal Stability of the ssCPCMs
3.6. Evaluation of Thermal Diffusivity, Specific Heat, Thermal Conductivity, and Effusivity for PEG6000–Silica-MWCNTs PCM Composites
3.7. Evaluation of the Mechanical Properties of Materials with Stabilized-Shape and Thermal-Energy Storage Properties (Dynamic Mechanical Analysis (DMA))
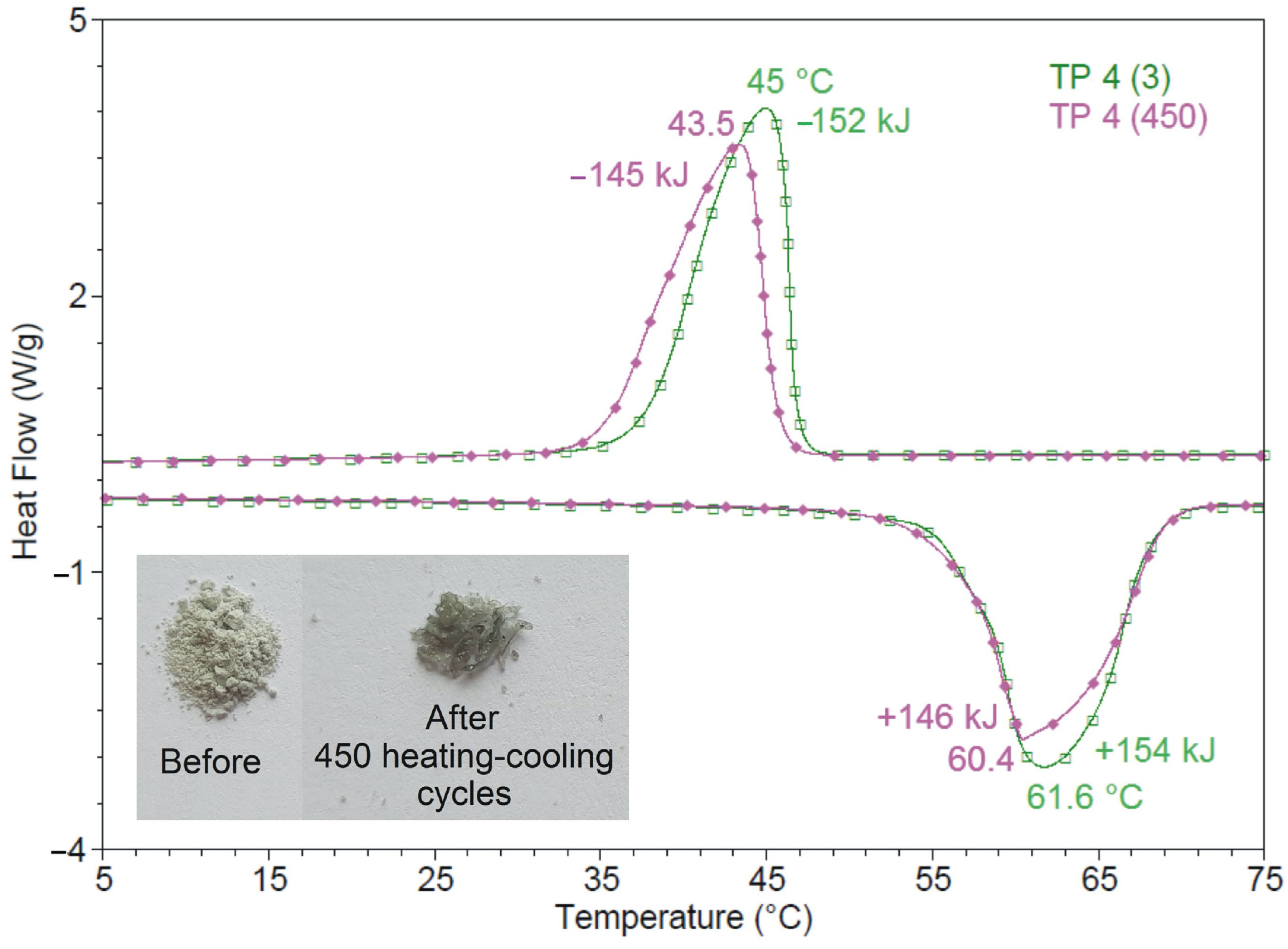
3.8. Thermal Reliability and Reusability
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| ssCPCMs = shape-stabilized composite phase change materials; |
| MWCNTs-OH = multi-wall carbon nanotubes functionalized with hydroxyl groups; |
| NCOTEOS = 3-(Triethoxysilyl)propyl isocyanate; |
| PEG6000-Si = PEG6000 coupled with NCOTEOS (newly created building block for the synthesis of the 3D network); |
| MWCNTs-Si = MWCNTs-OH coupled with NCOTEOS; |
| TEOS = tetraethylorthosilicate; |
| GPTMS = (3-Glycidyloxypropyl)trimethoxysilane; |
| MeTMS = methyltrimethoxysilane; |
| wPEG6000 = percentage of PEG6000 in the final material; |
| λm = phase-transition capacity for the heating stage; |
| ΔHm = experimental melting enthalpy (latent heat) of ssPCM; |
| ΔHmPEG6000 = melting enthalpy of pure PEG6000; |
| λf = phase-transition capacity for the cooling stage; |
| ΔHf = experimental crystallization enthalpy (latent heat) of ssPCM; |
| ΔHsPEG6000 = solidification enthalpy of pure PEG6000; |
| Tm = temperature at which the melting rate is maximum; |
| Tf = temperature at which the rate of crystallization is maximum; |
| α = thermal diffusivity; |
| Cp = specific heat capacity; |
| ρ = density of ssPCM composite; |
| λ = thermal conductivity; |
| e = thermal effusivity; |
| Tmo = onset melting temperature for cycling tests; |
| Tmp = temperature at which the melting rate is maximum for cycling tests; |
| ηm = melting efficiency = (ΔHm(cycle 450) − ΔHm(cycle 3))/ΔHm(cycle 3) × 100; |
| Tfo = onset crystallization temperature for cycling tests; |
| Tfp = temperature at which the crystallization rate is maximum for cycling tests; |
| ηf = crystallization efficiency = (ΔHf(cycle 450) − ΔHf(cycle 3))/ΔHf(cycle 3) × 100. |
References
- Diaconu, B.M.; Cruceru, M.; Anghelescu, L. Phase Change Materials—Applications and Systems Designs: A Literature Review. Designs 2022, 6, 117. [Google Scholar] [CrossRef]
- Salgado-Pizarro, R.; Padilla, J.A.; Xuriguera, E.; Barreneche, C.; Fernández, A.I. Novel Shape-Stabilized Phase Change Material with Cascade Character: Synthesis, Performance and Shaping Evaluation. Energies 2021, 14, 2621. [Google Scholar] [CrossRef]
- Li, Z.; Shahsavar, A.; Al-Rashed, A.A.; Talebizadehsardari, P. Effect of Porous Medium and Nanoparticles Presences in a Counter-Current Triple-Tube Composite Porous/Nano-PCM System. Appl. Therm. Eng. 2020, 167, 114777. [Google Scholar] [CrossRef]
- Yang, H.; Bai, Y.; Ge, C.; He, L.; Liang, W.; Zhang, X. Polyethylene Glycol-Based Phase Change Materials with High Photothermal Conversion Efficiency and Shape Stability in an Aqueous Environment for Solar Water Heater. Compos. Part A Appl. Sci. Manuf. 2022, 154, 106778. [Google Scholar] [CrossRef]
- Zhang, X.; Xu, M.; Liu, L.; Liu, L.; Wang, M.; Ji, H.; Song, K.-I. The Concept, Technical System and Heat Transfer Analysis on Phase-Change Heat Storage Backfill for Exploitation of Geothermal Energy. Energies 2020, 13, 4755. [Google Scholar] [CrossRef]
- Sun, K.; Kou, Y.; Zheng, H.; Liu, X.; Tan, Z.; Shi, Q. Using Silicagel Industrial Wastes to Synthesize Polyethylene Glycol/Silica-Hydroxyl Form-Stable Phase Change Materials for Thermal Energy Storage Applications. Sol. Energy Mater. Sol. Cells 2018, 178, 139–145. [Google Scholar] [CrossRef]
- Ferfera, R.S.; Madani, B. Thermal Characterization of a Heat Exchanger Equipped with a Combined Material of Phase Change Material and Metallic Foams. Int. J. Heat Mass Transf. 2020, 148, 119162. [Google Scholar] [CrossRef]
- Weiss, L.; Jha, R. Small-Scale Phase Change Materials in Low-Temperature Applications: A Review. Energies 2023, 16, 2841. [Google Scholar] [CrossRef]
- Gürel, B. A Numerical Investigation of the Melting Heat Transfer Characteristics of Phase Change Materials in Different Plate Heat Exchanger (Latent Heat Thermal Energy Storage) Systems. Int. J. Heat Mass Transf. 2020, 148, 119117. [Google Scholar] [CrossRef]
- Koytsoumpa, E.I.; Bergins, C.; Kakaras, E. The CO2 Economy: Review of CO2 Capture and Reuse Technologies. J. Supercrit. Fluids 2018, 132, 3–16. [Google Scholar] [CrossRef]
- Ali, H.M. Applications of Combined/Hybrid Use of Heat Pipe and Phase Change Materials in Energy Storage and Cooling Systems: A Recent Review. J. Energy Storage 2019, 26, 100986. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Y.; He, F.; Li, Y.; Zhou, Y.; Yang, Z.; He, R.; Zhang, K.; Yang, W. Experimental and Numerical Simulation of Phase Change Process for Paraffin in Three-Dimensional Graphene Aerogel. Appl. Therm. Eng. 2020, 167, 114773. [Google Scholar] [CrossRef]
- Ji, R.; Wei, S.; Xia, Y.; Huang, C.; Huang, Y.; Zhang, H.; Xu, F.; Sun, L.; Lin, X. Enhanced Thermal Performance of Form-Stable Composite Phase-Change Materials Supported by Novel Porous Carbon Spheres for Thermal Energy Storage. J. Energy Storage 2020, 27, 101134. [Google Scholar] [CrossRef]
- Wang, J.; Wu, Z.; Xie, H.; Wang, T.; Wang, Y.; Huang, Y.; Dong, L. Effect of Different Soft Segment Contents on the Energy Storage Capacity and Photo–Thermal Performance of Polyurethane-Based/Graphene Oxide Composite Solid–Solid Phase Change Materials. Polymers 2022, 14, 5161. [Google Scholar] [CrossRef]
- Zhou, T.; Ma, X.; Yuan, J.; Zheng, N.; Sun, Z. Moving Solid-Liquid Interface-Based Measurement Method for Thermal Conductivity Determination of Phase Change Materials in Liquid Phase. Exp. Therm. Fluid Sci. 2020, 113, 110042. [Google Scholar] [CrossRef]
- Deng, Y.; He, M.; Li, J.; Yang, Z. Polyethylene Glycol-Carbon Nanotubes/Expanded Vermiculite Form-Stable Composite Phase Change Materials: Simultaneously Enhanced Latent Heat and Heat Transfer. Polymers 2018, 10, 889. [Google Scholar] [CrossRef] [PubMed]
- Sundararajan, S.; Samui, A.B.; Kulkarni, P.S. Shape-Stabilized Poly(Ethylene Glycol) (PEG)-Cellulose Acetate Blend Preparation with Superior PEG Loading via Microwave-Assisted Blending. Sol. Energy 2017, 144, 32–39. [Google Scholar] [CrossRef]
- Kumar, A.; Kulkarni, P.S.; Samui, A.B. Polyethylene Glycol Grafted Cotton as Phase Change Polymer. Cellulose 2014, 21, 685–696. [Google Scholar] [CrossRef]
- Vélez, J.F.; Aparicio, M.; Mosa, J. Covalent Silica-PEO-LiTFSI Hybrid Solid Electrolytes via Sol-Gel for Li-Ion Battery Applications. Electrochim. Acta 2016, 213, 831–841. [Google Scholar] [CrossRef]
- Lin, W.; Wang, F.; Wang, H.; Li, H.; Fan, Y.; Chan, D.; Chen, S.; Tang, Y.; Zhang, Y. Thermal-Stable Separators: Design Principles and Strategies Towards Safe Lithium-Ion Battery Operations. ChemSusChem 2022, 15, e202201464. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, K.; Ali, M.; Gingrich, K.; Porter, D.L.; Chong, S.; Riley, B.J.; Peak, C.W.; Naleway, S.E.; Zharov, I.; Carlson, K. Sol-Gel Derived Silica: A Review of Polymer-Tailored Properties for Energy and Environmental Applications. Microp. Mesopor. Mater. 2022, 336, 111874. [Google Scholar] [CrossRef]
- Ghalambaz, M.; Shahabadi, M.; Mehryan, S.A.M.; Sheremet, M.; Younis, O.; Talebizadehsardari, P.; Yaici, W. Latent Heat Thermal Storage of Nano-Enhanced Phase Change Material Filled by Copper Foam with Linear Porosity Variation in Vertical Direction. Energies 2021, 14, 1508. [Google Scholar] [CrossRef]
- Zahir, M.H.; Rahman, M.M.; Irshad, K.; Shaikh, M.N.; Helal, A.; Aziz, M.A.; Ali, A.; Khan, F. Energy Conversion Efficiency Enhancement of Polyethylene Glycol and a SiO2 Composite Doped with Ni, Co, Zn, and Sc Oxides. ACS Omega 2022, 7, 22657–22670. [Google Scholar] [CrossRef]
- Serrano, A.; Borreguero, A.M.; Iglesias, I.; Acosta, A.; Rodríguez, J.F.; Carmona, M. Diffusion of Shape Stabilized PEG-SiO2 as a Driver for Producing Thermoregulating Facing Bricks. Materials 2021, 14, 1395. [Google Scholar] [CrossRef]
- Atinafu, D.G.; Wang, C.; Dong, W.; Chen, X.; Du, M.; Gao, H.; Wang, G. In-Situ Derived Graphene from Solid Sodium Acetate for Enhanced Photothermal Conversion, Thermal Conductivity, and Energy Storage Capacity of Phase Change Materials. Sol. Energy Mater. Sol. Cells 2020, 205, 110269. [Google Scholar] [CrossRef]
- Liu, L.; Su, D.; Tang, Y.; Fang, G. Thermal Conductivity Enhancement of Phase Change Materials for Thermal Energy Storage: A Review. Renew. Sust. Energy Rev. 2016, 62, 305–317. [Google Scholar] [CrossRef]
- Cabaleiro, D.; Hamze, S.; Fal, J.; Marcos, M.A.; Estellé, P.; Żyła, G. Thermal and Physical Characterization of PEG Phase Change Materials Enhanced by Carbon-Based Nanoparticles. Nanomaterials 2020, 10, 1168. [Google Scholar] [CrossRef] [PubMed]
- Marcos, M.A.; Podolsky, N.E.; Cabaleiro, D.; Lugo, L.; Zakharov, A.O.; Postnov, V.N.; Charykov, N.A.; Ageev, S.V.; Semenov, K.N. MWCNT in PEG-400 Nanofluids for Thermal Applications: A Chemical, Physical and Thermal Approach. J. Mol. Liq. 2019, 294, 111616. [Google Scholar] [CrossRef]
- Tang, B.; Wang, Y.; Qiu, M.; Zhang, S. A full-band sunlight-driven carbon nanotube/PEG/SiO2 composites for solar energy storage. Sol. Energy Mater. Sol. Cells 2014, 123, 7. [Google Scholar] [CrossRef]
- Chen, X.; Cheng, P.; Tang, Z.; Xu, X.; Gao, H.; Wang, G. Carbon-Based Composite Phase Change Materials for Thermal Energy Storage, Transfer, and Conversion. Adv. Sci. 2021, 8, 2001274. [Google Scholar] [CrossRef] [PubMed]
- Donchak, V.; Stetsyshyn, Y.; Bratychak, M.; Broza, G.; Harhay, K.; Stepina, N.; Kostenko, M.; Voronov, S. Nanoarchitectonics at surfaces using multifunctional initiators of surface-initiated radical polymerization for fabrication of the nanocomposites. Appl. Surf. Sci. Adv. 2021, 5, 100104. [Google Scholar] [CrossRef]
- Yang, Y.; Pang, Y.; Liu, Y.; Guo, H. Preparation and Thermal Properties of Polyethylene Glycol/Expanded Graphite as Novel Form-Stable Phase Change Material for Indoor Energy Saving. Mater. Lett. 2018, 216, 220–223. [Google Scholar] [CrossRef]
- Gao, J.; Tao, W.; Chen, D.; Guo, X.; Chen, Y.; Jiang, Y. High Performance Shape-Stabilized Phase Change Material with Nanoflower-Like Wrinkled Mesoporous Silica Encapsulating Polyethylene Glycol: Preparation and Thermal Properties. Nanomaterials 2018, 8, 385. [Google Scholar] [CrossRef]
- Min, X.; Fang, M.; Huang, Z.; Liu, Y.; Huang, Y.; Wen, R.; Qian, T.; Wu, X. Enhanced Thermal Properties of Novel Shape-Stabilized PEG Composite Phase Change Materials with Radial Mesoporous Silica Sphere for Thermal Energy Storage. Sci. Rep. 2015, 5, 12964. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, G.T. Polyethylene Glycol/Fumed Silica Composites as Shape-Stabilized Phase Change Materials with Effective Thermal Energy Storage. RSC Adv. 2023, 13, 7621–7631. [Google Scholar] [CrossRef]
- Mokhtari, S.; Madhkhan, M. The Performance Effect of PEG-Silica Fume as Shape-Stabilized Phase Change Materials on Mechanical and Thermal Properties of Lightweight Concrete Panels. Case Stud. Constr. Mater. 2022, 17, e01298. [Google Scholar] [CrossRef]
- Mohaisen, K.O.; Zahir, H.; Maslehuddin, M. Shape-stabilized phase change material for thermal energy storage: Sr+2 doped BaCO3 matrix incorporating polyethylene glycol. J. Energy Storage 2023, 58, 106369. [Google Scholar] [CrossRef]
- He, L.; Li, J.; Zhou, C.; Zhu, H.; Cao, X.; Tang, B. Phase Change Characteristics of Shape-Stabilized PEG/SiO2 Composites Using Calcium Chloride-Assisted and Temperature-Assisted Sol Gel Methods. Sol. Energy 2014, 103, 448–455. [Google Scholar] [CrossRef]
- Serrano, A.; Martín Del Campo, J.; Peco, N.; Rodriguez, J.F.; Carmona, M. Influence of Gelation Step for Preparing PEG–SiO2 Shape-Stabilized Phase Change Materials by Sol–Gel Method. J. Sol-Gel Sci. Technol. 2019, 89, 731–742. [Google Scholar] [CrossRef]
- Yang, H.; Feng, L.; Wang, C.; Zhao, W.; Li, X. Confinement Effect of SiO2 Framework on Phase Change of PEG in Shape-Stabilized PEG/SiO2 Composites. Eur. Polym. J. 2012, 48, 803–810. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J.; Yan, J. Enhanced Thermal Conductivity and Thermal Performance of Form-Stable Composite Phase Change Materials by Using β-Aluminum Nitride. Appl. Energy 2009, 86, 1196–1200. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J. Preparation and Performance of Form-Stable Polyethylene Glycol/Silicon Dioxide Composites as Solid–Liquid Phase Change Materials. Appl. Energy 2009, 86, 170–174. [Google Scholar] [CrossRef]
- Weng, Z.; Wu, K.; Luo, F.; Xiao, F.; Zhang, Q.; Wang, S.; Lu, M. Fabrication of High Thermal Conductive Shape-Stabilized Polyethylene Glycol/Silica Phase Change Composite by Two-Step Sol Gel Method. Compos. Part A Appl. Sci. Manuf. 2018, 110, 106–112. [Google Scholar] [CrossRef]
- Mishra, D.K.; Bhowmik, S.; Pandey, K.M. Polyethylene Glycol Based Form Stable Composite Phase Change Material: A Review. J. Phys. Conf. Ser. 2020, 1455, 012025. [Google Scholar] [CrossRef]
- Tao, Y.B.; Lin, C.H.; He, Y.L. Effect of Surface Active Agent on Thermal Properties of Carbonate Salt/Carbon Nanomaterial Composite Phase Change Material. Appl. Energy 2015, 156, 478–489. [Google Scholar] [CrossRef]
- Anghel, E.M.; Pavel, P.M.; Constantinescu, M.; Petrescu, S.; Atkinson, I.; Buixaderas, E. Thermal transfer performance of a spherical encapsulated PEG 6000-based composite for thermal energy storage. Appl. Energy 2017, 208, 1222–1231. [Google Scholar] [CrossRef]
- ASTM E 1461:2007; Standard Test Method for Thermal Diffusivity by the Flash Method. ASTM International: West Conshohocken, PA, USA, 2017.
- Zhang, L.; Wang, Y.-Z.; Yang, K.-K.; Wang, X.-L.; Chen, S.-C.; Li, J. Effect of PEG on the crystallization of PPDO/PEG blends. Eur. Polym. J. 2005, 41, 1243–1250. [Google Scholar] [CrossRef]
- Li, J.; He, L.; Liu, T.; Cao, X.; Zhu, X. Preparation and characterization of PEG/SiO2 composites as shape-stabilized phase change materials for thermal energy storage. Sol. Energy Mater. Sol. Cells 2013, 118, 48–53. [Google Scholar] [CrossRef]
- Yan, D.; Ming, W.; Liu, S.; Yin, G.; Zhang, Y.; Tang, B.; Zhang, S. Polyethylene glycol (PEG)/silicon dioxide grafted aminopropyl group and carboxylic multi-walled carbon nanotubes (SAM) composite as phase change material for light-to-heat energy conversion and storage. J. Energy Storage 2021, 36, 102428. [Google Scholar] [CrossRef]
- Yu, C.; Xie, Q.; Bao, Y.; Shan, G.; Pan, P. Crystalline and Spherulitic Morphology of Polymers Crystallized in Confined Systems. Crystals 2017, 7, 147. [Google Scholar] [CrossRef]
- Li, Y.; Ma, Q.; Huang, C.; Liu, G. Crystallization of Poly (Ethylene Glycol) in Poly (Methyl Methacrylate) Networks. Mater. Sci. 2013, 19, 147–151. [Google Scholar] [CrossRef]
- Wang, K.; Wen, R. Scaphium scaphigerum/graphene hybrid aerogel for composite phase change material with high phase change enthalpy and high thermal conductivity for energy storage. J. Energy Storage 2023, 58, 106302. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.N. The enthalpy of fusion and degree of crystallinity of polymers as measured by DSC. Eur. Polym. J. 2001, 39, 1721–1727. [Google Scholar] [CrossRef]
- Ficher, D.; Pospiech, D.; Scheler, U.; Navarro, R.; Messori, M.; Fabbri, P. Monitoring of the sol-gel synthesis of Organic-Inorganic Hybrids by FTIR Transmission, FTIR/ATR, NIR and Raman Spectroscopy. Macromol. Symp. 2008, 265, 134–143. [Google Scholar] [CrossRef]
- Luo, X.; Yu, Z.; Cai, Y.; Wu, Q.; Zeng, J. Facile Fabrication of Environmentally-Friendly Hydroxyl-Functionalized Multiwalled Carbon Nanotubes/Soy Oil-Based Polyurethane Nanocomposite Bioplastics with Enhanced Mechanical, Thermal, and Electrical Conductivity Properties. Polymers 2019, 11, 763. [Google Scholar] [CrossRef]
- Raj, C.R.; Suresh, S.; Vasudevan, S.; Chandrasekar, M.; Singh, V.K.; Bhavsar, R.R. Thermal performance of nano-enriched form-stable PCM implanted in a pin finned wall-less heat sink for thermal management application. Energy Conver. Manag. 2020, 226, 113466. [Google Scholar] [CrossRef]
- Salam, M.A.; Burk, R. Synthesis and characterization of multi-walled carbon nanotubes modified with octadecylamine and polyethylene glycol. Arab. Chem. J. 2017, 10, S921–S927. [Google Scholar] [CrossRef]
- Samuel, A.Z.; Umapathy, S. Energy funneling and macromolecular conformational dynamics: A 2D Raman correlation study of PEG melting. Polym. J. 2014, 46, 330–336. [Google Scholar] [CrossRef]
- Ravindran, T.R.; Jackson, B.R.; Badding, J.V. UV Raman Spectroscopy of Single-Walled Carbon Nanotubes. Chem. Mater. 2001, 13, 4187–4191. [Google Scholar] [CrossRef]
- Zhang, H.-B.; Lin, G.-D.; Zhou, Z.-H.; Dong, X.; Chen, T. Raman spectra of MWCNTs and MWCNT-based H2-adsorbing system. Carbon 2002, 40, 2429–2436. [Google Scholar] [CrossRef]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural study of sol–gel silicate glasses by IR and Raman spectroscopies. J. Non-Cryst. Solid 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Pielichowska, K.; Glowinkowski, S.; Lekki, J.; Binias, D.; Pielichowski, K.; Jenczyk, J. PEO/fatty acid blends for thermal energy storage materials. Structural/morphological features and hydrogen interactions. Eur. Polym. J. 2008, 44, 3344–3360. [Google Scholar] [CrossRef]
- Hernández-Escolano, M.; Ramis, X.; Jiménez-Morales, A.; Juan-Díaz, M.; Suay, J. Study of the Thermal Degradation of Bioactive Sol–Gel Coatings for the Optimization of Its Curing Process. J. Therm. Anal. Calorim. 2012, 107, 499–508. [Google Scholar] [CrossRef]
- Jiao, L.; Xiao, H.; Wang, Q.; Sun, J. Thermal Degradation Characteristics of Rigid Polyurethane Foam and the Volatile Products Analysis with TG-FTIR-MS. Polym. Degrad. Stab. 2013, 98, 2687–2696. [Google Scholar] [CrossRef]
- Pielichowski, K.; Flejtuch, K. Non-oxidative thermal degradation of poly(ethylene oxide): Kinetic and thermoanalytical study. J. Anal. Appl. Pyrolysis 2005, 73, 131–138. [Google Scholar] [CrossRef]
- Manasrah, A.D.; Laoui, T.; Zaidi, S.J.; Atieh, M.A. Effect of PEG functionalized carbon nanotubes on the enhancement of thermal and physical properties of nanofluids. Exp. Therm. Fluid Sci. 2017, 84, 231–241. [Google Scholar] [CrossRef]
- Lu, X.; Fang, C.; Sheng, X.; Zhang, L.; Qu, J. One-Step and Solvent-Free Synthesis of Polyethylene Glycol-Based Polyurethane As Solid–Solid Phase Change Materials for Solar Thermal Energy Storage. Ind. Eng. Chem. Res. 2019, 58, 3024–3032. [Google Scholar] [CrossRef]

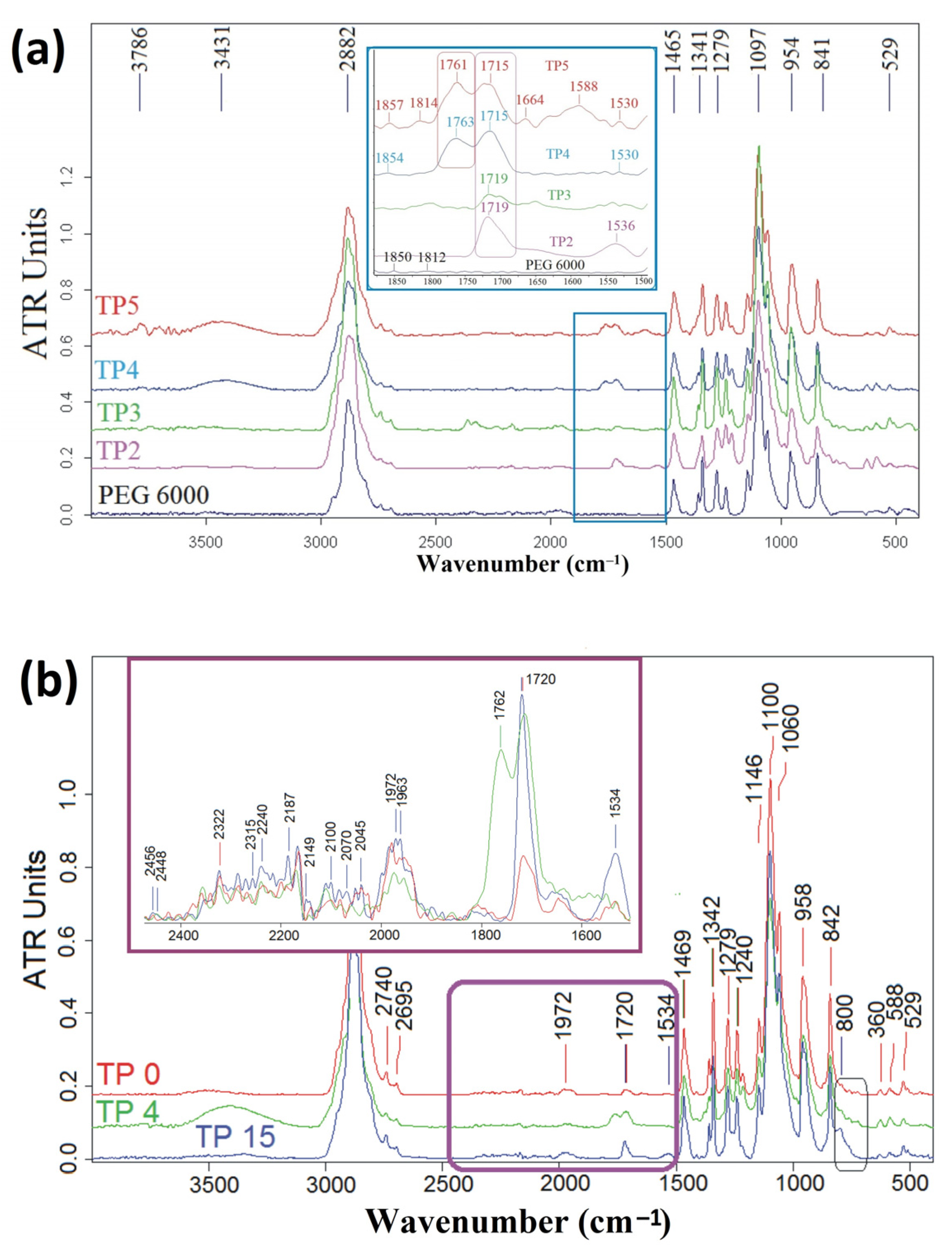




| Sample Code | PEG6000/NCOTEOS Molar Ratio | PEG6000 % wt. | Silica % wt. | MWCNTs-OH % wt. |
|---|---|---|---|---|
| TP2 | 1/2 | 75 | 10.58 | 0.07 |
| TP3 | 1/1 | 85 | 6.11 | 0.04 |
| TP15 | 2/1 | 91.23 | 3.30 | 0.32 |
| TP4 | 2/1 | 91.51 | 3.30 | 0.02 |
| TP0 | 2/1 | 91.53 | 3.30 | 0 |
| TP5 | 3/1 | 94 | 2.25 | 0.01 |
| Sample Code | A (PEG Mixture) | B (Solvent) | C (Sol–Gel Precursors Mixture) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PEG6000 (g) | DABCO (g) | MWCNTs (g) | NCOTES (g) | SDS (g) | n-octan (mL) | TEOS (g) | GPTMS (g) | MeTMS (g) | EtOH (mL) | NH4OH (mL) | |
| TP2 | 22 | 0.2 | 0.02 | 2 | 4 | 80 | 5 | 0.567 | 0.327 | 20 | 1.5 |
| TP3 | 43.2 | 0.43 | 0.02 | ||||||||
| TP15 | 86 | 0.86 | 0.306 | ||||||||
| TP4 | 86 | 0.86 | 0.02 | ||||||||
| TP0 | 86 | 0.86 | 0 | ||||||||
| TP5 | 130 | 1.3 | 0.02 | ||||||||
| Sample Code | wPEG | Tmo | Tm | ΔHm | 1 χc (DSC) | 2 XRD | Tfo | Tf | ΔHf | λm | λf | 3 ΔT |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (°C) | (°C) | (J/g) | % | % | (°C) | (°C) | (J/g) | (%) | (%) | (°C) | |
| TP2 | 75 | 48.9 | 56.0 | 92.64 | 67.17 | 69.44 | 43.5 | 41.2 | −91.37 | 65.83 | 66.77 | 14.8 |
| TP3 | 85 | 55.9 | 60.6 | 134.65 | 80.41 | 82.84 | 44.0 | 42.3 | −131.32 | 84.43 | 84.68 | 18.3 |
| TP15 | 91.24 | 50.8 | 54.4 | 141.76 | 78.87 | 75.92 | 41.9 | 41.2 | −137.16 | 82.81 | 82.40 | 13.3 |
| TP4 | 91.51 | 56.7 | 60.9 | 150.10 | 83.27 | 82.35 | 44.5 | 43.2 | −146.54 | 87.42 | 87.77 | 17.7 |
| TP0 | 91.53 | 56.2 | 60.4 | 153.04 | 84.87 | 87.86 | 41.5 | 40.9 | −149.31 | 89.12 | 89.41 | 19.5 |
| TP5 | 94 | 58.5 | 61.5 | 169.81 | 91.69 | 92.24 | 44.4 | 43.2 | −165.98 | 96.28 | 96.78 | 18.3 |
| PEG6000 | 100 | 59.4 | 61.4 | 187.63 | 94.77 | 43.4 | 42.5 | −182.45 | - | - | 18.9 |
| Sample Code | 0–215 °C | 215–260 °C | 260–450 °C | 450–700 °C | 1 Rezidue (exp) 700 °C | 2 Rezidue (theo) 700 °C | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Wt loss | Tmax | Wt loss | Tmax | Wt loss | Tmax | Wt loss | Tmax | |||
| (%) | (°C) | (%) | (°C) | (%) | (°C) | (%) | (°C) | (%) | (%) | |
| TP2 | 16.97 | 178.7 | 12.53 | 238.4 | 58.28 | 307.7 | 4.79 | 527.7 | 7.43 | 10.65 |
| TP3 | 17.84 | 194.3 | 14.04 | 242.1 | 59.24 | 303.9 | 3.42 | 518.9 | 5.46 | 6.15 |
| TP15 | 1.17 | - | 5.39 | 234.9 | 85.25 | 387.4 | 3.70 | - | 4.49 | 3.62 |
| TP4 | 0.93 | - | 4.52 | 233.2 | 89.07 | 383.7 | 1.15 | - | 4.33 | 3.32 |
| TP0 | 0.84 | - | 5.31 | 240.3 | 89.00 | 388.6 | 1.54 | - | 3.31 | 3.30 |
| TP5 | 0.42 | - | 1.51 | 250.8 | 94.54 | 387.4 | 0.66 | - | 2.87 | 2.26 |
| PEG6000 | 0.68 | - | 0.96 | - | 96.67 | 402.4 | 1.69 | - | 0 | 0 |
| Sample Code | PEG6000 % | MWCNT% | ρ ± U (g/cm3) | α ± U (mm2/s) | Cp ± U (J/g∙K) | λ ± U (W/m∙K) | e ± U (W∙s1/2/m2∙K) |
|---|---|---|---|---|---|---|---|
| MWCNT | 0 | 100 | 0.495 ± 0.001 | 0.708 ± 0.091 | 1.650 ± 0.169 | 0.343 ±0.004 | 0.522 ± 0.026 |
| TP2 | 75 | 0.07 | 0.973 ± 0.002 | 0.136 ± 0.001 | 3.153 ± 0.039 | 0.132 ± 0.001 | 0.637 ± 0.006 |
| TP3 | 85 | 0.04 | 1.198 ± 0.002 | 0.156 ± 0.001 | 2.611 ± 0.060 | 0.186 ± 0.001 | 0.764 ± 0.009 |
| TP15 | 91.23 | 0.32 | 1.131 ± 0.004 | 0.176 ± 0.001 | 3.702 ± 0.080 | 0.195 ± 0.001 | 0.819 ± 0.021 |
| TP4 | 91.51 | 0.02 | 1.187 ± 0.008 | 0.193 ± 0.001 | 2.568 ± 0.043 | 0.229 ± 0.001 | 0.836 ± 0.008 |
| TP0 | 91.53 | 0 | 1.182 ± 0.008 | 0.194 ± 0.002 | 3.707 ± 0.093 | 0.228 ± 0.002 | 1.003 ± 0.012 |
| TP5 | 94 | 0.01 | 1.199 ± 0.002 | 0.193 ± 0.002 | 2.425 ± 0.034 | 0.232 ± 0.002 | 0.822 ± 0.010 |
| PEG6000 | 100 | 0 | 1.200 ± 0.002 | 0.225 ± 0.009 | 1.780 ± 0.034 | 0.270 ± 0.011 | 0.759 ± 0.022 |
| Pyroceram 9606 | - | - | 2.545 | 2.4158 ± 0.004 | 0.800 | 4.1 | 2.889 |
| Sample TP4 | Tmo | Tmp | ΔHm | 1 ƞm | Tfo | Tfp | ΔHf | 2 ƞf |
|---|---|---|---|---|---|---|---|---|
| (°C) | (°C) | (J/g) | (%) | (°C) | (°C) | (J/g) | (%) | |
| Cycle 3 | 57.3 | 61.6 | 154.1 | 46.7 | 45.0 | −152.4 | ||
| Cycle 450 | 56.6 | 60.4 | 146.1 | 5.2 | 45.7 | 43.3 | −145.1 | 4.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, C.L.; Gifu, I.C.; Anghel, E.M.; Ianchis, R.; Cirstea, C.-D.; Nicolae, C.A.; Gabor, A.R.; Atkinson, I.; Petcu, C. Novel PEG6000–Silica-MWCNTs Shape-Stabilized Composite Phase-Change Materials (ssCPCMs) for Thermal-Energy Storage. Polymers 2023, 15, 3022. https://doi.org/10.3390/polym15143022
Nistor CL, Gifu IC, Anghel EM, Ianchis R, Cirstea C-D, Nicolae CA, Gabor AR, Atkinson I, Petcu C. Novel PEG6000–Silica-MWCNTs Shape-Stabilized Composite Phase-Change Materials (ssCPCMs) for Thermal-Energy Storage. Polymers. 2023; 15(14):3022. https://doi.org/10.3390/polym15143022
Chicago/Turabian StyleNistor, Cristina Lavinia, Ioana Catalina Gifu, Elena Maria Anghel, Raluca Ianchis, Cristiana-Diana Cirstea, Cristian Andi Nicolae, Augusta Raluca Gabor, Irina Atkinson, and Cristian Petcu. 2023. "Novel PEG6000–Silica-MWCNTs Shape-Stabilized Composite Phase-Change Materials (ssCPCMs) for Thermal-Energy Storage" Polymers 15, no. 14: 3022. https://doi.org/10.3390/polym15143022







