Effect of Chemical and Steam Explosion Pulping on the Physical and Mechanical Properties of Sugarcane Straw Pulp Trays
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Sugarcane Straw
2.3. Extraction of Sugarcane Straw Fiber Using Chemical and Steam Explosion Pulping Methods
2.4. Physicochemical Properties of Sugarcane Straw Fiber (SSF)
2.4.1. Soxhlet Extraction of Extractives from SSF
2.4.2. Holocellulose Content of SSF
2.4.3. Lignin Content of SSF
2.5. Formation of Molded Pulp Trays from the SSF
2.6. Physical, Mechanical, and Microstructural Properties of SSF Trays
2.6.1. Grammage of Molded Pulp Trays from the SSF
2.6.2. Water Wetting Time and Thickness Swelling of Molded Pulp Trays from the SSF
2.6.3. Color (L* a* b*) Values of Molded Pulp Trays from the SSF
2.6.4. Crystallinity Index of Molded Pulp Tray
2.6.5. Tensile and Compression Tests of Molded Pulp Trays from the SSF
2.6.6. Attenuated Total Reflectance Fourier-Transform Infrared (ATR–FTIR) Spectroscopy of SSF and Molded Pulp Tray
2.6.7. Scanning Electron and Two-Photon Microscopic Analysis of the Molded Pulp Tray
2.7. Statistical Analysis
3. Results and Discussion
3.1. Effect of Chemical and Steam Explosion Pulping Methods on the Characteristic Properties of Sugarcane Straw Fiber
Percent Yield and Component Composition of Sugarcane Straw Fiber
3.2. Effects of Treatments on the Physical and Mechanical Properties of SSF Trays
3.2.1. Physical Properties of SSF Trays
3.2.2. Mechanical Properties of Fabricated Trays
3.3. Fourier-Transform Infrared Spectroscopy (ATR-FTIR) and Crystallinity Index (CI) of SSF Trays
3.4. Impact of Chemical and Steam Explosion Pulping on the SSF Microstructure of Trays Evidenced by Scanning Electron and Two-Photon Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Negi, Y.S.; Choudhary, V.; Bhardwaj, N.K. Characterization of cellulose nanocrystals produced by acid-hydrolysis from sugarcane bagasse as agro-waste. J. Mater. Phys. Chem. 2014, 2, 1–8. [Google Scholar] [CrossRef]
- Vasconcelos, P.S.; Carpio, L.G.T. Bagasse, straws, tips and vinasse: From sugarcane waste to a clean and renewable bioenergy source. Int. J. Adv. Soc. Sci. Humanit. 2018, 5, 27–37. [Google Scholar]
- Seabra, J.E.; Tao, L.; Chum, H.L.; Macedo, I.C. A techno-economic evaluation of the effects of centralized cellulosic ethanol and co-products refinery options with sugarcane mill clustering. Biomass Bioenergy 2010, 34, 1065–1078. [Google Scholar] [CrossRef]
- Camargo, M.M.; Adefrs Taye, E.; Roether, J.A.; Tilahun Redda, D.; Boccaccini, A.R. A review on natural fiber-reinforced geopolymer and cement-based composites. Materials 2020, 13, 4603. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Lange, H.; Crestini, C.; Henn, A.; Österberg, M. Lignin for nano-and microscaled carrier systems: Applications, trends, and challenges. ChemSusChem 2019, 12, 2039–2054. [Google Scholar] [CrossRef]
- Qian, H.; Wang, J.; Yan, L. Synthesis of lignin-poly (N-methylaniline)-reduced graphene oxide hydrogel for organic dye and lead ions removal. J. Bioresour. Bioprod. 2020, 5, 204–210. [Google Scholar] [CrossRef]
- Lu, J.; Cheng, M.; Zhao, C.; Li, B.; Peng, H.; Zhang, Y.; Shao, Q.; Hassan, M. Application of lignin in preparation of slow-release fertilizer: Current status and future perspectives. Ind. Crops Prod. 2022, 176, 114267. [Google Scholar] [CrossRef]
- Tian, X.; Fang, Z.; Smith, R.L.; Wu, Z.; Liu, M. Properties, Chemical Characteristics and Application of Lignin and Its Derivatives. In Production of Biofuels and Chemicals from Lignin; Fang, Z., Smith, J.R.L., Eds.; Springer: Singapore, 2016; pp. 3–33. [Google Scholar]
- Yoo, C.G.; Meng, X.; Pu, Y.; Ragauskas, A.J. The critical role of lignin in lignocellulosic biomass conversion and recent pretreatment strategies: A comprehensive review. Bioresour. Technol. 2020, 301, 122784. [Google Scholar] [CrossRef]
- Worku, L.A.; Bachheti, A.; Bachheti, R.K.; Rodrigues Reis, C.E.; Chandel, A.K. Agricultural Residues as Raw Materials for Pulp and Paper Production: Overview and Applications on Membrane Fabrication. Membranes 2023, 13, 228. [Google Scholar] [CrossRef]
- Khristova, P.; Kordaschia, O.; Patt, R.; Karar, I. Comparative alkaline pulping of two bamboo species from Sudan. Cellul. Chem. Technol. 2006, 40, 325–334. [Google Scholar]
- Rullifank, K.; Roefinal, M.; Kostanti, M.; Sartika, L.; Evelyn, E. Pulp and paper industry: An overview on pulping technologies, factors, and challenges. IOP Conf. Ser. Mater. Sci. Eng. 2020, 845, 012005. [Google Scholar] [CrossRef]
- Iglesias, M.C.; Gomez-Maldonado, D.; Via, B.K.; Jiang, Z.; Peresin, M.S. Pulping processes and their effects on cellulose fibers and nanofibrillated cellulose properties: A review. For. Prod. J. 2020, 70, 10–21. [Google Scholar] [CrossRef]
- Ziegler-Devin, I.; Chrusciel, L.; Brosse, N. Steam Explosion Pretreatment of Lignocellulosic Biomass: A Mini-Review of Theorical and Experimental Approaches. Front. Chem. 2021, 9, 705358. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Ni, L.; Guo, Z.; Zeng, H.; Wu, M.; Zhang, M.; Zheng, B. Principle and Application of Steam Explosion Technology in Modification of Food Fiber. Foods 2022, 11, 3370. [Google Scholar] [CrossRef] [PubMed]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef]
- Frías, M.; Villar-Cociña, E.; Valencia-Morales, E. Characterisation of sugar cane straw waste as pozzolanic material for construction: Calcining temperature and kinetic parameters. Waste Manag. 2007, 27, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Robledo-Ortíz, J.R.; Martín Del Campo, A.S.; Blackaller, J.A.; González-López, M.E.; Pérez Fonseca, A.A. Valorization of Sugarcane Straw for the Development of Sustainable Biopolymer-Based Composites. Polymers 2021, 13, 3335. [Google Scholar] [CrossRef]
- Costa, S.M.; Mazzola, P.G.; Silva, J.C.A.R.; Pahl, R.; Pessoa, A.; Costa, S.A. Use of sugar cane straw as a source of cellulose for textile fiber production. Ind. Crops Prod. 2013, 42, 189–194. [Google Scholar] [CrossRef]
- Wang, K.; Chen, J.; Sun, S.-N.; Sun, R.-C. Chapter 6—Steam Explosion. In Pretreatment of Biomass; Pandey, A., Negi, S., Binod, P., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 75–104. [Google Scholar]
- Razak, S.I.A.; Sharif, N.; Hasraf, N.; Muhamad, I.; Yahya, M. Impregnation of Poly(lactic acid) on Biologically Pulped Pineapple Leaf Fiber for Packaging Materials. BioResources 2015, 10, 4350–4359. [Google Scholar] [CrossRef]
- Malucelli, L.; Ozeri, I.; Matos, M.; Magalhães, W.; Filho, C.M.; Eisen, M. High-flux, porous and homogeneous PVDF/cellulose microfiltration membranes. Cellulose 2022, 29, 1943–1953. [Google Scholar] [CrossRef]
- Shiekh, K.A.; Liangpanth, M.; Luesuwan, S.; Kraisitthisirintr, R.; Ngiwngam, K.; Rawdkuen, S.; Rachtanapun, P.; Karbowiak, T.; Tongdeesoontorn, W. Preparation and Characterization of Bioactive Chitosan Film Loaded with Cashew (Anacardium occidentale) Leaf Extract. Polymers 2022, 14, 540. [Google Scholar] [CrossRef] [PubMed]
- Inseemeesak, B.; Areeprasert, C. Fiber extraction and energy recovery from Cocos nucifera Linn mesocarp residues employing steam explosion and anaerobic digestion. Ind. Crops Prod. 2020, 147, 112180. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef] [PubMed]
- Areeprasert, C.; Zhao, P.; Ma, D.; Shen, Y.; Yoshikawa, K. Alternative solid fuel production from paper sludge employing hydrothermal treatment. J. Energy Fuels 2014, 28, 1198–1206. [Google Scholar] [CrossRef]
- Iroba, K.L.; Tabil, L.G.; Sokhansanj, S.; Dumonceaux, T. Pretreatment and fractionation of barley straw using steam explosion at low severity factor. Biomass Bioenergy 2014, 66, 286–300. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, M.; Li, C.; Sheng, X.; Yang, Q.; Li, N.; Niu, M. Surface sediments formation during auto-hydrolysis and its effects on the benzene-alcohol extractive, absorbability and chemical pulping properties of hydrolyzed acacia wood chips. Bioresour. Technol. 2019, 289, 121604. [Google Scholar] [CrossRef]
- Boonterm, M.; Sunyadeth, S.; Dedpakdee, S.; Athichalinthorn, P.; Patcharaphun, S.; Mungkung, R.; Techapiesancharoenkij, R. Characterization and comparison of cellulose fiber extraction from rice straw by chemical treatment and thermal steam explosion. J. Clean. Prod. 2016, 134, 592–599. [Google Scholar] [CrossRef]
- Jiang, W.; Han, G.; Zhou, C.; Gao, S.; Zhang, Y.; Li, M.; Gong, Y.; Via, B. The degradation of lignin, cellulose, and hemicellulose in kenaf bast under different pressures using steam explosion treatment. J. Wood Chem. Technol. 2017, 37, 359–368. [Google Scholar] [CrossRef]
- Simangunsong, E.; Ziegler-Devin, I.; Chrusciel, L.; Girods, P.; Wistara, N.; Brosse, N. Steam explosion of beech wood: Effect of the particle size on the xylans recovery. Waste Biomass Valorization J. 2020, 11, 625–633. [Google Scholar] [CrossRef]
- Ewunie, G.A.; Yigezu, Z.D.; Morken, J. Biochemical methane potential of Jatropha curcas fruit shell: Comparative effect of mechanical, steam explosion and alkaline pretreatments. Biomass Convers. Biorefin. 2021, 12, 4081–4094. [Google Scholar] [CrossRef]
- Verkasalo, E.; Roitto, M.; Möttönen, V.; Tanner, J.; Kumar, A.; Kilpeläinen, P.; Sikanen, L.; Ilvesniemi, H. Extractives of Tree Biomass of Scots Pine (Pinus sylvestris L.) for Biorefining in Four Climatic Regions in Finland—Lipophilic Compounds, Stilbenes, and Lignans. Forests 2022, 13, 779. [Google Scholar] [CrossRef]
- Wang, Z.; Li, J.; Barford, J.P.; Hellgradt, K.; McKay, G. A comparison of chemical treatment methods for the preparation of rice husk cellulosic fibers. Int. J. Environ. Agric. Res. 2016, 2, 67–77. [Google Scholar]
- Han, G.; Cheng, W.; Deng, J.; Dai, C.; Zhang, S.; Wu, Q. Effect of pressurized steam treatment on selected properties of wheat straws. Ind. Crops Prod. 2009, 30, 48–53. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Convers. Manag. 2019, 186, 82–92. [Google Scholar] [CrossRef]
- Uner, B.; Kombeci, K.; Akgul, M. The utilization of tomato stalk in fiber production: NaOH and CaO pulping process. Wood Res. 2016, 61, 927–936. [Google Scholar]
- Chen, X.; Yu, J.; Zhang, Z.; Lu, C. Study on structure and thermal stability properties of cellulose fibers from rice straw. Carbohydr. Polym. 2011, 85, 245–250. [Google Scholar] [CrossRef]
- Sabiha-Hanim, S.; Noor, M.A.M.; Rosma, A. Fractionation of oil palm frond hemicelluloses by water or alkaline impregnation and steam explosion. Carbohydr. Polym. 2015, 115, 533–539. [Google Scholar] [CrossRef]
- Maniet, G.; Schmetz, Q.; Jacquet, N.; Temmerman, M.; Gofflot, S.; Richel, A. Effect of steam explosion treatment on chemical composition and characteristic of organosolv fescue lignin. Ind. Crops Prod. 2017, 99, 79–85. [Google Scholar] [CrossRef]
- Tanpichai, S.; Witayakran, S.; Boonmahitthisud, A. Study on structural and thermal properties of cellulose microfibers isolated from pineapple leaves using steam explosion. J. Environ. Chem. Eng. 2019, 7, 102836. [Google Scholar] [CrossRef]
- Li, J.; Gellerstedt, G.; Toven, K. Steam explosion lignins; their extraction, structure and potential as feedstock for biodiesel and chemicals. Bioresour. Technol. 2009, 100, 2556–2561. [Google Scholar] [CrossRef]
- Bian, H.; Gao, Y.; Wang, R.; Liu, Z.; Wu, W.; Dai, H. Contribution of lignin to the surface structure and physical performance of cellulose nanofibrils film. Cellulose 2018, 25, 1309–1318. [Google Scholar] [CrossRef]
- Meléndez-Hernández, P.A.; Hernández-Beltrán, J.U.; Hernández-Guzmán, A.; Morales-Rodríguez, R.; Torres-Guzmán, J.C.; Hernández-Escoto, H. Comparative of alkaline hydrogen peroxide pretreatment using NaOH and Ca (OH) 2 and their effects on enzymatic hydrolysis and fermentation steps. Biomass Convers. Biorefin. 2021, 11, 1897–1907. [Google Scholar] [CrossRef]
- Gu, H. Tensile behaviours of the coir fibre and related composites after NaOH treatment. Mater. Des. 2009, 30, 3931–3934. [Google Scholar] [CrossRef]
- Dahham, O.S.; Noriman, N.; Hamzah, R.; Al-Samarrai, M.N.; Idrus, S.S.; Shayfull, Z.; Adam, T.; Mohammed, M. The influences naoh treatment on polypropylene/cyperus odoratus (pp/cy) composites: Tensile and morphology. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2018; p. 012057. [Google Scholar]
- Jiang, B.; Chen, C.; Liang, Z.; He, S.; Kuang, Y.; Song, J.; Mi, R.; Chen, G.; Jiao, M.; Hu, L. Lignin as a wood-inspired binder enabled strong, water stable, and biodegradable paper for plastic replacement. Adv. Funct. Mater. 2020, 30, 1906307. [Google Scholar] [CrossRef]
- Vänskä, E.; Vihelä, T.; Peresin, M.S.; Vartiainen, J.; Hummel, M.; Vuorinen, T. Residual lignin inhibits thermal degradation of cellulosic fiber sheets. Cellulose 2016, 23, 199–212. [Google Scholar] [CrossRef]
- Jaaskelainen, A.; Nuopponen, M.; Axelsson, P.; Tenhunen, M.; Loija, M.; Vuorinen, T. Determination of lignin distribution in pulps by FTIR ATR spectroscopy. J. Pulp Pap. Sci. 2003, 29, 328–331. [Google Scholar]
- Ling, Z.; Chen, S.; Zhang, X.; Xu, F. Exploring crystalline-structural variations of cellulose during alkaline pretreatment for enhanced enzymatic hydrolysis. Bioresour. Technol. 2017, 224, 611–617. [Google Scholar] [CrossRef]
- Romruen, O.; Karbowiak, T.; Tongdeesoontorn, W.; Shiekh, K.A.; Rawdkuen, S. Extraction and Characterization of Cellulose from Agricultural By-Products of Chiang Rai Province, Thailand. Polymers 2022, 14, 1830. [Google Scholar] [CrossRef]
- Pelissari, F.M.; Sobral, P.J.d.A.; Menegalli, F.C. Isolation and characterization of cellulose nanofibers from banana peels. Cellulose 2014, 21, 417–432. [Google Scholar] [CrossRef]
- Sari, N.H.; Wardana, I.; Irawan, Y.S.; Siswanto, E. The effect of sodium hydroxide on chemical and mechanical properties of corn husk fiber. Orient. J. Chem. 2017, 33, 3037–3042. [Google Scholar] [CrossRef]
- Chimenez, T.; Gehlen, M.; Marabezi, K.; Curvelo, A. Characterization of sugarcane bagasse by autofluorescence microscopy. Cellulose 2013, 21, 653–664. [Google Scholar] [CrossRef]
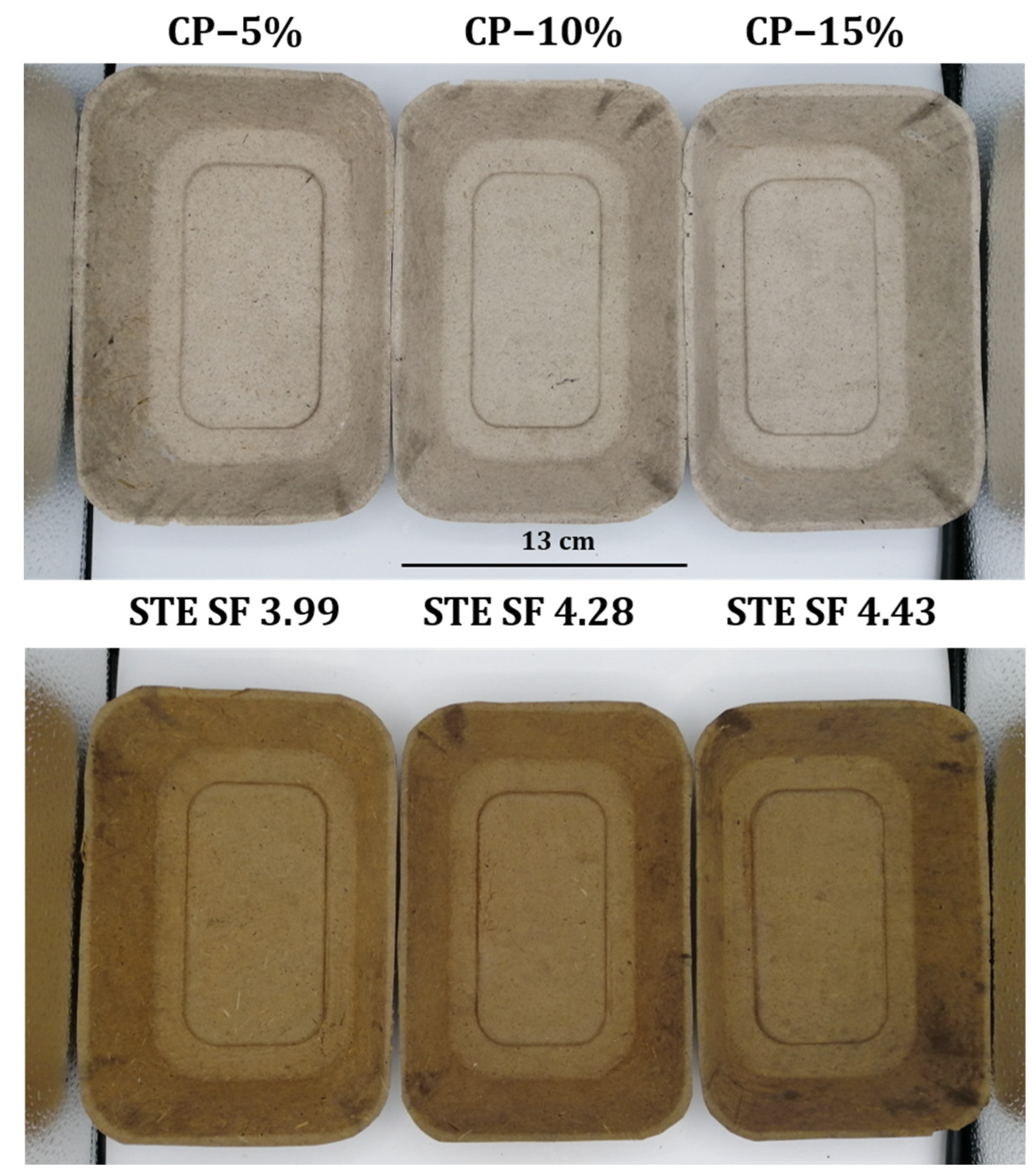
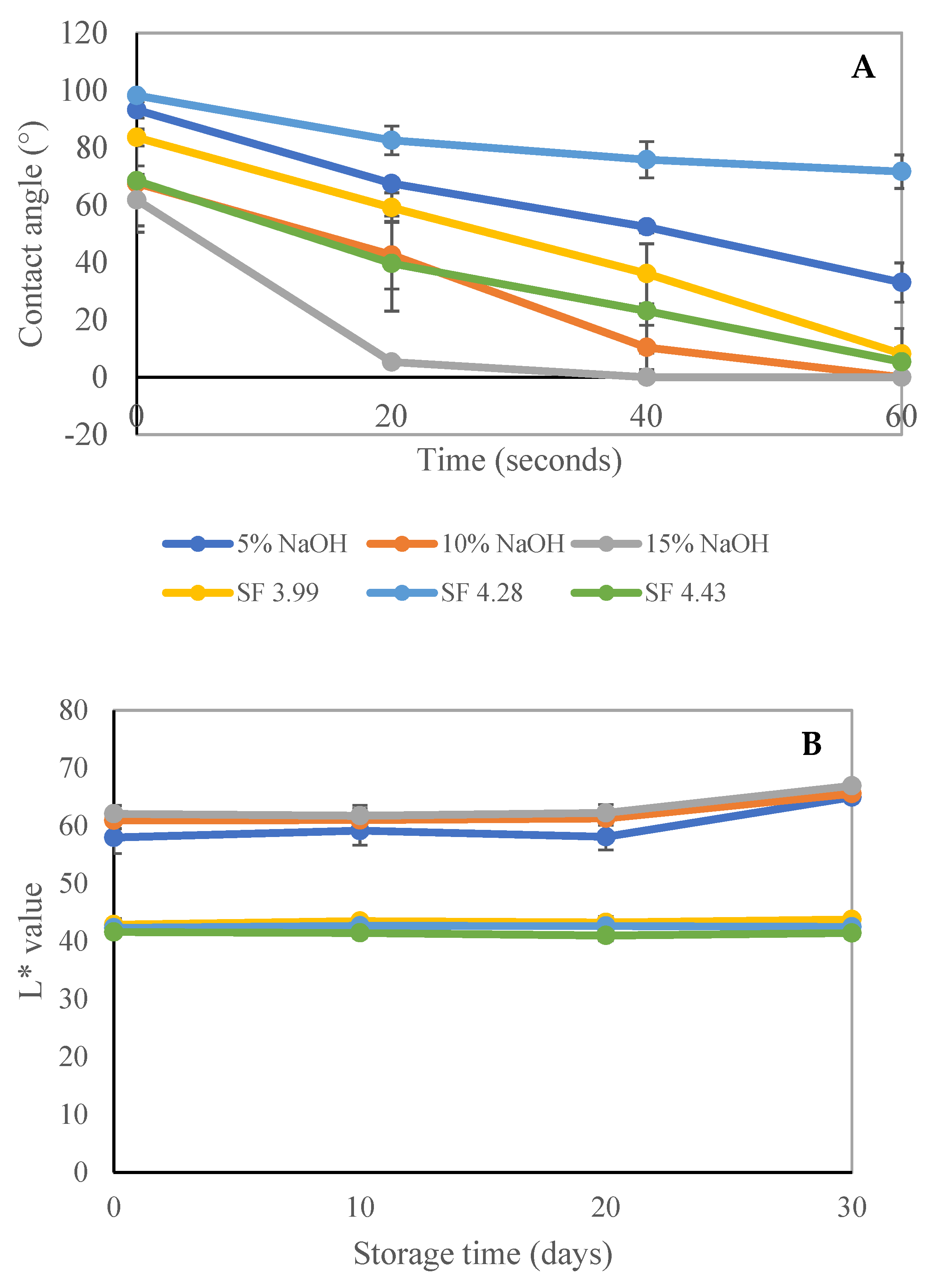
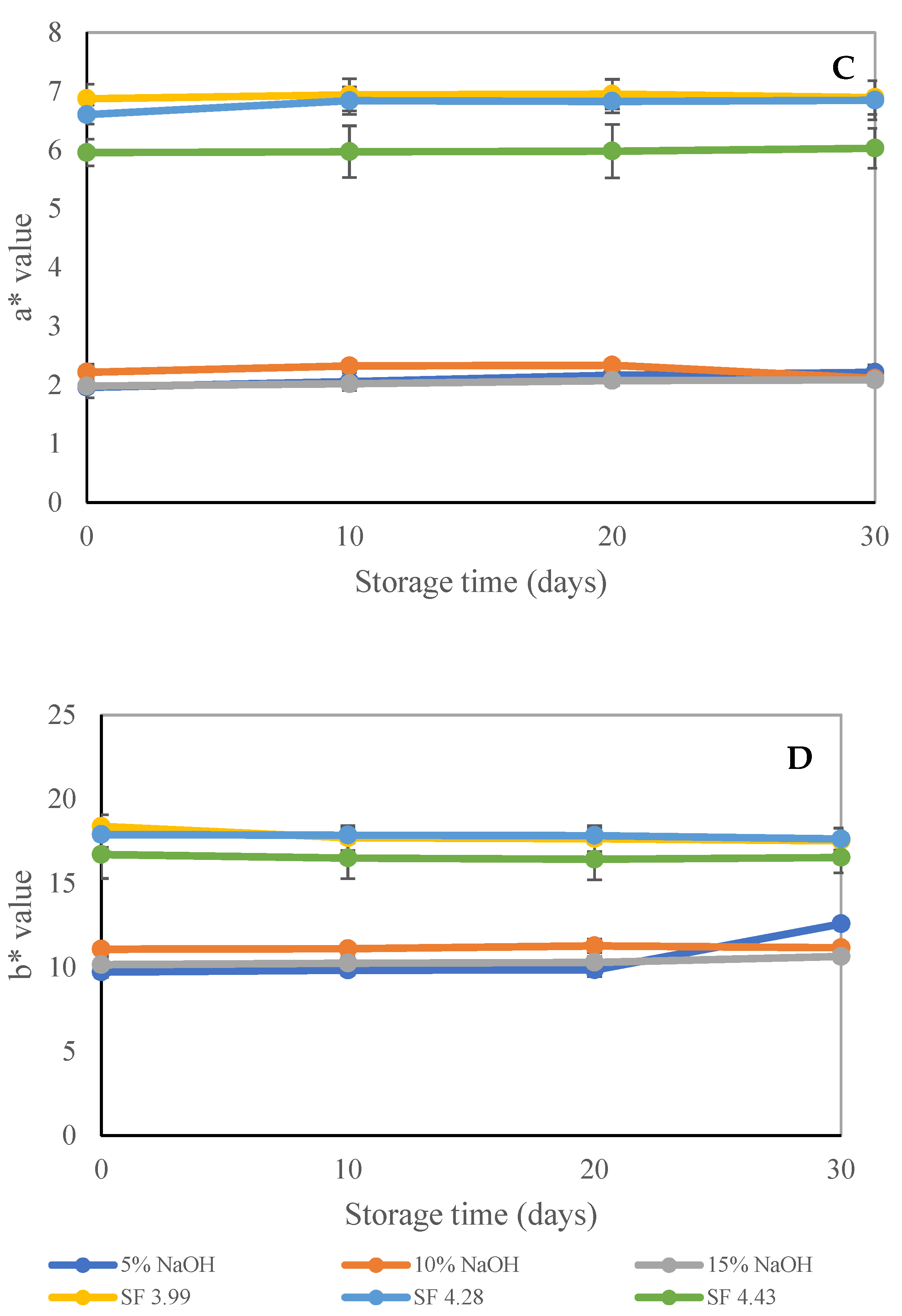

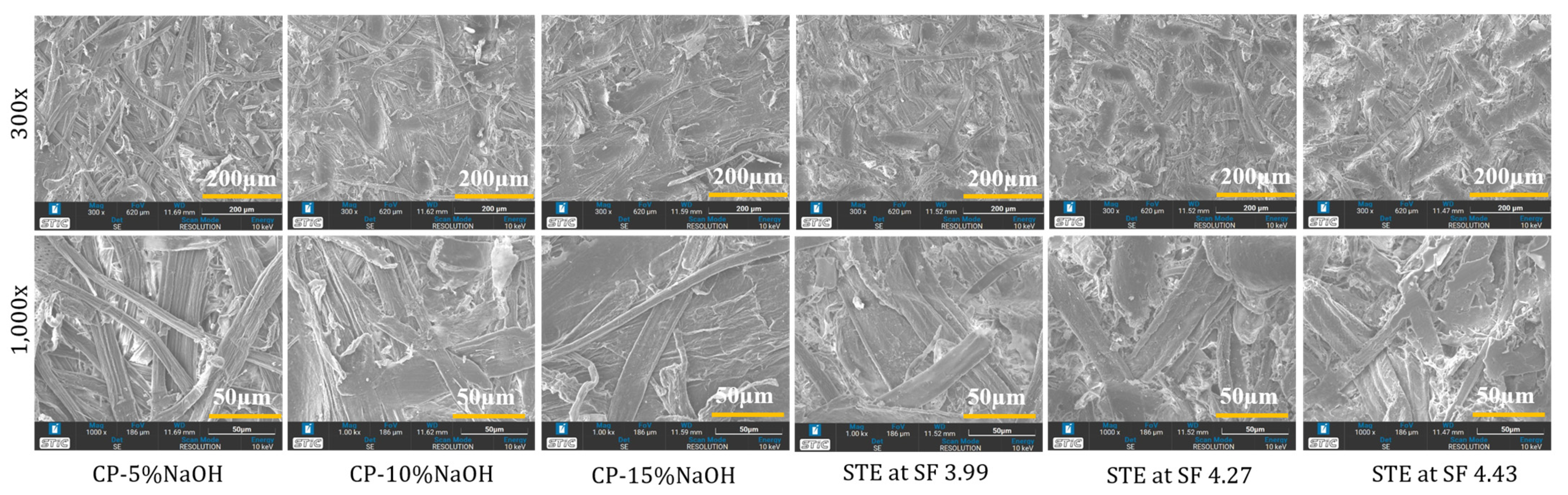

| Sample Treatments | % Yield (db.) | % Extractive | % Holocellulose | % Lignin |
|---|---|---|---|---|
| Sugarcane straw | – | 19.48 ± 0.58 | 80.52 ± 0.12 | 22.48 ± 0.14 |
| CP-5% NaOH | 47.93 ± 1.19 c | 10.03 ± 1.71 a | 62.71 ± 0.4 b | 6.52 ± 0.75 b |
| CP-10% NaOH | 40.25 ± 1.93 b | 8.55 ± 0.79 a | 65.99 ± 0.71 c | 3.30 ± 0.23 a,b |
| CP-15% NaOH | 31.95 ± 0.96 a | 8.36 ± 1.08 a | 66.69 ± 1.19 c | 2.93 ± 0.08 a |
| STE at SF 3.99 | 58.21 ± 0.71 e | 24.65 ± 0.73 b | 62.46 ± 1.83 b | 22.27 ± 2.61 c |
| STE at SF 4.28 | 59.62 ± 2.89 e | 22.45 ± 2.25 b | 65.00 ± 0.81 c | 20.65 ± 1.31 c |
| STE at SF 4.43 | 54.33 ± 1.12 d | 25.23 ± 1.36 b | 58.01 ± 0.15 a | 21.00 ± 3.37 c |
| SS Treatments | Weight/Tray (g) | Grammage (g/m2) | Thickness (mm) | Water Wetting Time (min) | Thickness Swelling (mm) |
|---|---|---|---|---|---|
| CP-5% NaOH | 17.11 ± 0.21 a | 636.47 ± 13.80 b,c | 0.73 ± 0.04 a | 4.94 ± 0.11 d | 16.57 ± 3.48 b |
| CP-10% NaOH | 16.91 ± 0.40 a | 611.24 ± 19.55 b,c | 0.78 ± 0.08 a,b | 3.31 ± 0.20 c | 22.94 ± 2.46 c |
| CP-15% NaOH | 16.30 ± 0.32 a | 531.21 ± 20.25 a | 0.75 ± 0.07 a,b | 0.99 ± 0.13 a | 36.62 ± 4.05 d |
| STE at SF 3.99 | 20.37 ± 1.12 b | 761.36 ± 3.68 d | 0.93 ± 0.04 d | 2.83 ± 0.34 bc | 12.12 ± 2.29 b |
| STE at SF 4.28 | 20.30 ± 1.47 b | 650.80 ± 30.67 c | 0.86 ± 0.04 c | 6.72 ± 0.70 e | 3.24 ± 1.13 a |
| STE at SF 4.43 | 19.80 ± 0.52 b | 607.09 ± 31.06 b | 0.83 ± 0.10 b,c | 2.30 ± 0.16 b | 21.85 ± 1.48 c |
| Sample Treatments | Tensile Strength (kN/m) | Tensile Index (Nm/g) | Elongation (mm) | Compression Strength (N/m2) | Compression Index (N/g) |
|---|---|---|---|---|---|
| CP-5% NaOH | 4.03 ± 0.33 b | 6.33 ± 0.53 b | 0.75 ± 0.06 d | 17579.1 ± 491.13 b | 27.62 ± 0.77 d |
| CP-10% NaOH | 1.89 ± 0.60 a | 3.08 ± 0.99 a | 0.45 ± 0.03 b | 12,887.66 ± 1062.58 c | 21.08 ± 1.74 c |
| CP-15% NaOH | 1.27 ± 0.06 a | 2.38 ± 0.11 a | 0.40 ± 0.02 b b,c | 10,055.92 ± 998.6 a | 18.93 ± 1.88 c |
| STE at SF 3.99 | 4.60 ± 0.61 b | 5.84 ± 0.77 b | 0.43 ± 0.08 b,c | 9855.04 ± 500.26 a | 12.51 ± 0.63 a |
| STE at SF 4.28 | 7.32 ± 1.69 c | 11.24 ± 2.60 c | 0.49 ± 0.03 c | 10,449.6 ± 1685.32 a | 16.06 ± 2.59 b |
| STE at SF 4.43 | 4.03 ± 0.43 b | 6.94 ± 0.74 b | 0.29 ± 0.04 a | 9150.85 ± 608.17 a | 15.77 ± 1.05 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngiwngam, K.; Chinvorarat, S.; Rachtanapun, P.; Auras, R.; Wittaya, T.; Tongdeesoontorn, W. Effect of Chemical and Steam Explosion Pulping on the Physical and Mechanical Properties of Sugarcane Straw Pulp Trays. Polymers 2023, 15, 3132. https://doi.org/10.3390/polym15143132
Ngiwngam K, Chinvorarat S, Rachtanapun P, Auras R, Wittaya T, Tongdeesoontorn W. Effect of Chemical and Steam Explosion Pulping on the Physical and Mechanical Properties of Sugarcane Straw Pulp Trays. Polymers. 2023; 15(14):3132. https://doi.org/10.3390/polym15143132
Chicago/Turabian StyleNgiwngam, Kittaporn, Sinchai Chinvorarat, Pornchai Rachtanapun, Rafael Auras, Thawien Wittaya, and Wirongrong Tongdeesoontorn. 2023. "Effect of Chemical and Steam Explosion Pulping on the Physical and Mechanical Properties of Sugarcane Straw Pulp Trays" Polymers 15, no. 14: 3132. https://doi.org/10.3390/polym15143132
APA StyleNgiwngam, K., Chinvorarat, S., Rachtanapun, P., Auras, R., Wittaya, T., & Tongdeesoontorn, W. (2023). Effect of Chemical and Steam Explosion Pulping on the Physical and Mechanical Properties of Sugarcane Straw Pulp Trays. Polymers, 15(14), 3132. https://doi.org/10.3390/polym15143132










