Fibrillar Hydrogel Based on Cellulose Nanocrystals Crosslinked via Diels–Alder Reaction: Preparation and pH-Sensitive Release of Benzocaine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthetic Procedures
2.2.1. Synthesis of Mal-CNC
2.2.2. Synthesis of Fur-aCNC
2.2.3. Investigation Gelation Conditions
2.2.4. Preparation of Benzocaine-Loaded Hydrogels
2.3. Characterization
3. Results and Discussion
3.1. Synthesis of Furan and Maleimide Modified Cellulose Nanocrystals
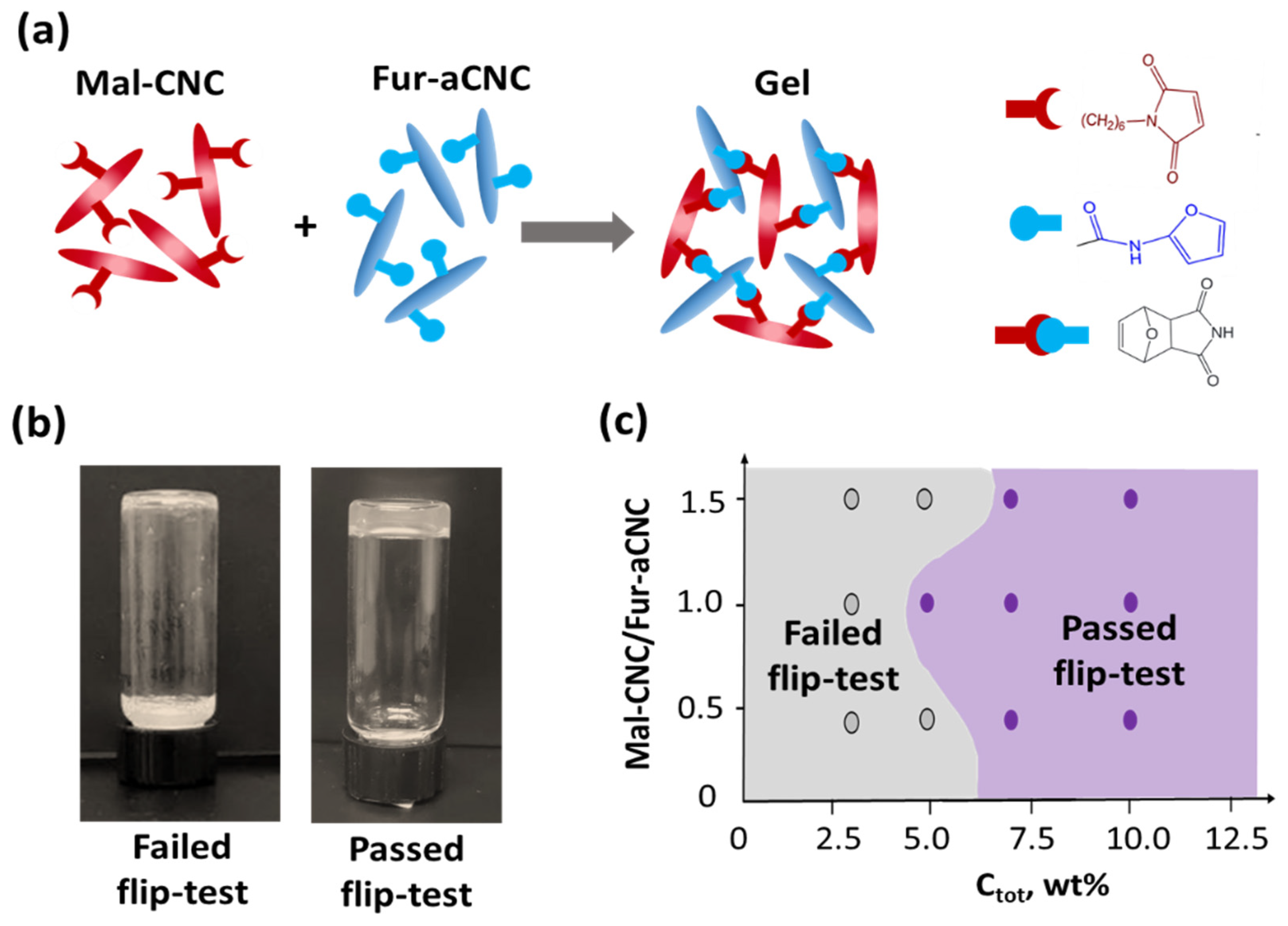
3.2. Gelation
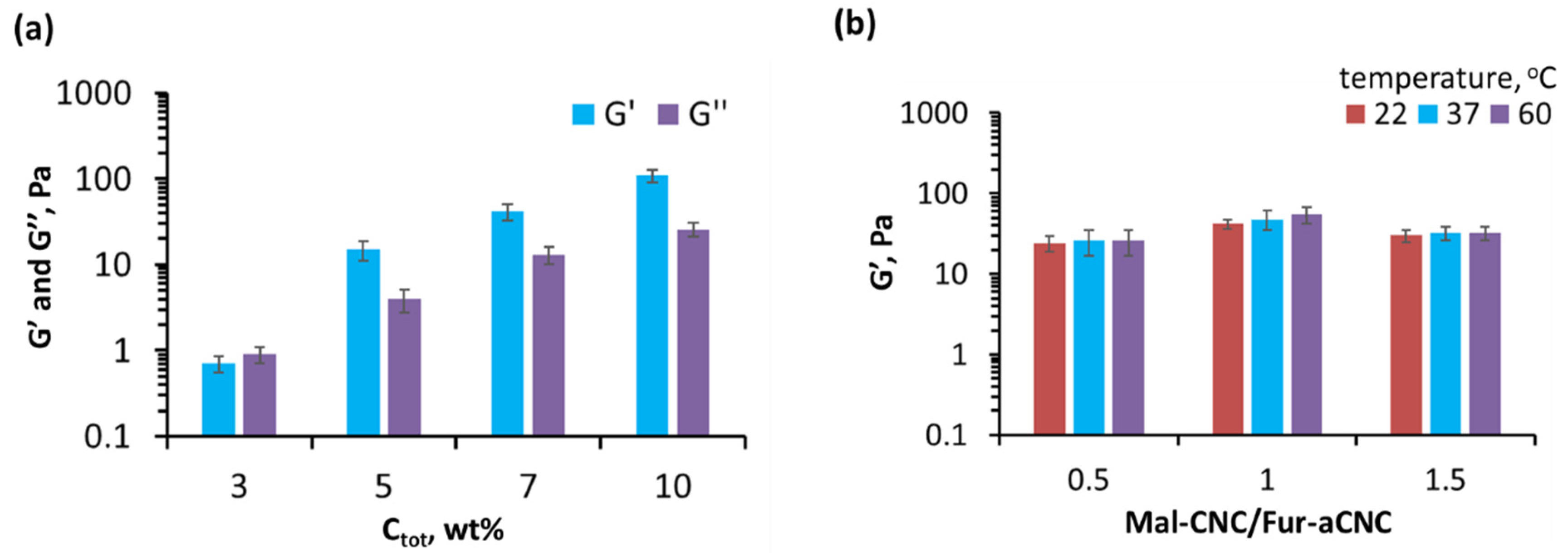
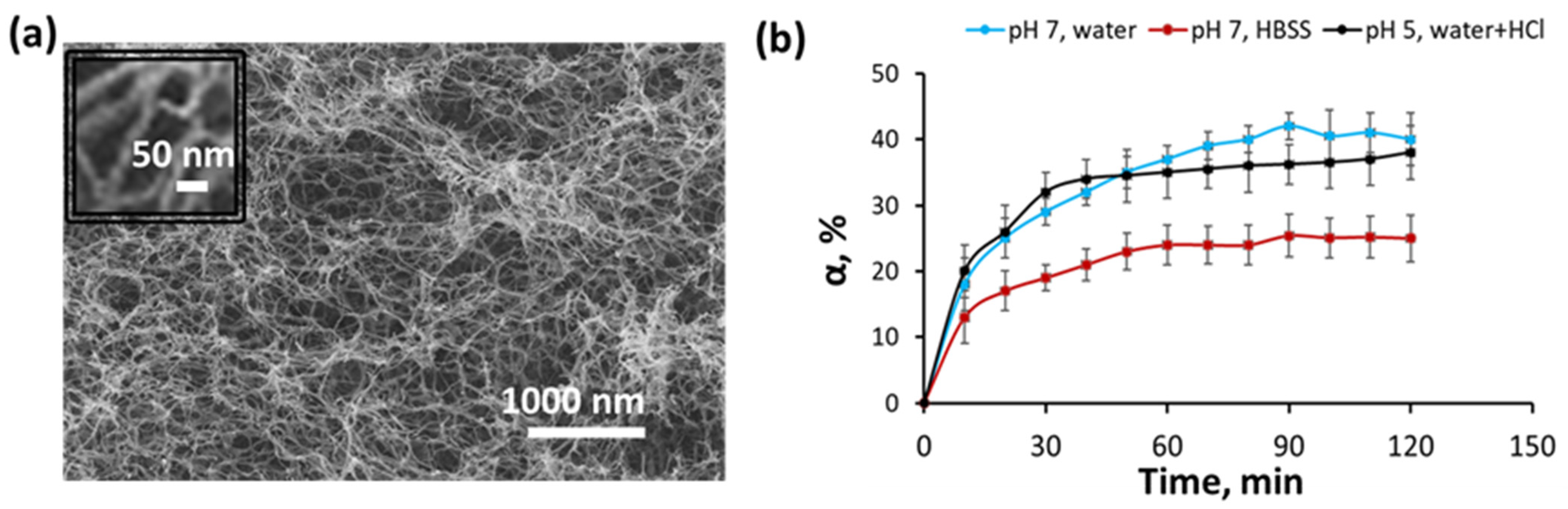
3.3. Rheological Properties and Structure of Hydrogel
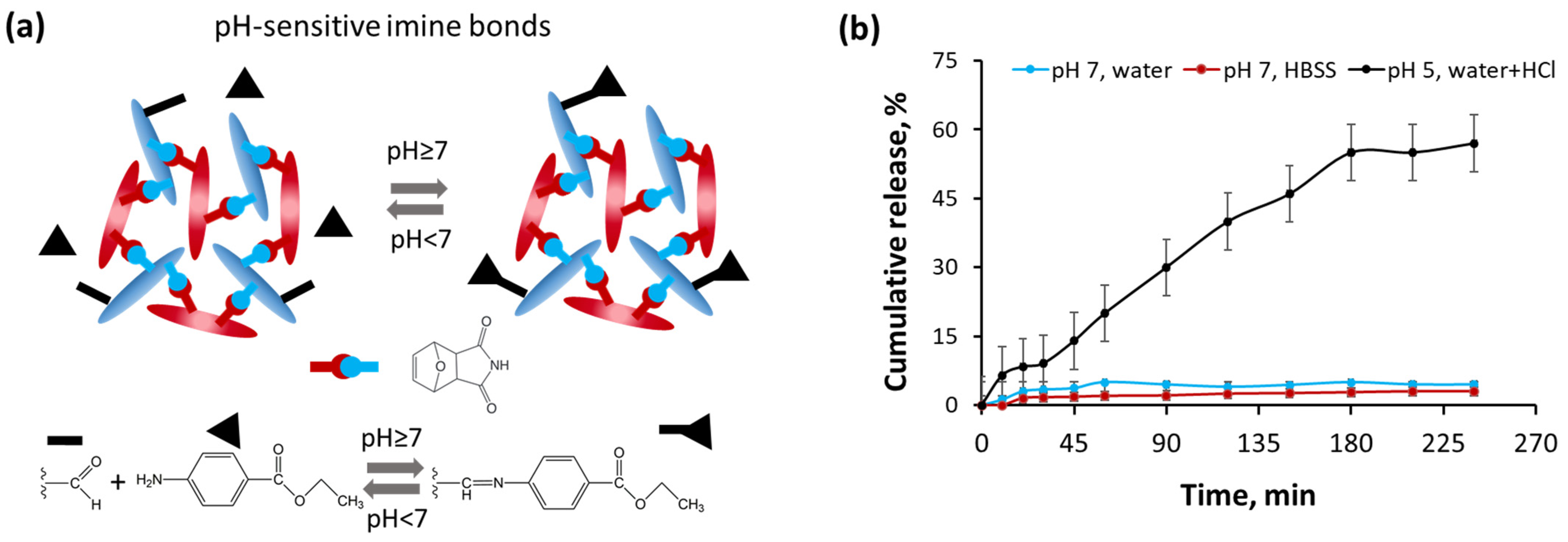
3.4. Release of Benzocaine from Hydrogel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Du, H.; Liu, W.; Zhang, M.; Si, C.; Zhang, X.; Li, B. Cellulose nanocrystals and cellulose nanofibrils based hydrogels for biomedical applications. Carbohydr. Polym. 2019, 209, 130–144. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Chau, M.; Sriskandha, S.E.; Pichugin, D.; Nykypanchuk, D.; Bouchard, J.; Gang, O.; Kumacheva, E. Ion-Mediated Gelation of Aqueous Suspensions of Cellulose Nanocrystals. Biomacromolecules 2015, 16, 2455–2462. [Google Scholar] [CrossRef] [PubMed]
- Belyaeva, A.A.; Tretyakov, I.V.; Kireynov, A.V.; Nashchekina, Y.A.; Solodilov, V.I.; Korzhikova-Vlakh, E.G.; Morozova, S.M. Fibrillar biocompatible colloidal gels based on cellulose nanocrystals and poly (N-isopropylacrylamide) for direct ink writing. J. Colloid Interface Sci. 2023, 635, 348–357. [Google Scholar] [CrossRef] [PubMed]
- Thérien-Aubin, H.; Wang, Y.; Nothdurft, K.; Prince, E.; Cho, S.; Kumacheva, E. Temperature-responsive nanofibrillar hydrogels for cell encapsulation. Biomacromolecules 2016, 17, 3244–3251. [Google Scholar] [CrossRef]
- Prince, E.; Chen, Z.; Khuu, N.; Kumacheva, E. Nano fi brillar Hydrogel Recapitulates Changes Occurring in the Fibrotic Extracellular Matrix. Biomacromolecules 2021, 22, 2352–2362. [Google Scholar] [CrossRef]
- Zhou, L.; He, H.; Li, M.C.; Huang, S.; Mei, C.; Wu, Q. Grafting polycaprolactone diol onto cellulose nanocrystals via click chemistry: Enhancing thermal stability and hydrophobic property. Carbohydr. Polym. 2018, 189, 331–341. [Google Scholar] [CrossRef]
- Shao, C.; Wang, M.; Chang, H.; Xu, F.; Yang, J. A Self-Healing Cellulose Nanocrystal-Poly(ethylene glycol) Nanocomposite Hydrogel via Diels–Alder Click Reaction. ACS Sustain. Chem. Eng. 2017, 5, 6167–6174. [Google Scholar] [CrossRef]
- González, K.; Gurrea, T.; Guaresti, O.; Algar, I.; Eceiza, A.; Gabilondo, N. Maleimide-grafted cellulose nanocrystals as cross-linkers for bionanocomposite hydrogels. Carbohydr. Polym. 2016, 149, 94–101. [Google Scholar]
- De Jong, S.J.; Van Eerdenbrugh, B.; van Nostrum, C.V.; Kettenes-Van Den Bosch, J.J.; Hennink, W.E. Physically crosslinked dextran hydrogels by stereocomplex formation of lactic acid oligomers: Degradation and protein release behavior. J. Control. Release 2001, 71, 261–275. [Google Scholar] [CrossRef]
- Mayr, J.; Saldías, C.; Díaz, D.D. Release of small bioactive molecules from physical gels. Chem. Soc. Rev. 2018, 47, 1484–1515. [Google Scholar] [CrossRef] [PubMed]
- Morozova, S.M.; Gevorkian, A.; Kumacheva, E. Design, characterization and applications of nanocolloidal hydrogels. Chem. Soc. Rev. 2023, 52, 5317–5339. [Google Scholar] [CrossRef] [PubMed]
- Parhi, R. Cross-linked hydrogel for pharmaceutical applications: A review. Adv. Pharm. Bull. 2017, 7, 515–530. [Google Scholar] [CrossRef] [PubMed]
- Gundogan, N.; Okay, O.; Oppermann, W. Swelling, elasticity and spatial inhomogeneity of poly (N, N-dimethylacrylamide) hydrogels formed at various polymer concentrations. Macromol. Chem. Phys. 2004, 205, 814–823. [Google Scholar] [CrossRef]
- D’errico, G.; De Lellis, M.; Mangiapia, G.; Tedeschi, A.; Ortona, O.; Fusco, S.; Borzacchiello, A.; Ambrosio, L. Structural and mechanical properties of UV-photo-cross-linked poly (N-vinyl-2-pyrrolidone) hydrogels. Biomacromolecules 2008, 9, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Cadamuro, F.; Russo, L.; Nicotra, F. Biomedical Hydrogels Fabricated Using Diels–Alder Crosslinking. Biomaterials 2021, 3, 374–382. [Google Scholar] [CrossRef]
- Diels, O.; Alder, K. Synthesen in der hydroaromatischen Reihe. Justus Liebigs Ann. Chem. 1928, 460, 98–122. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels–Alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–16698. [Google Scholar] [CrossRef]
- Nihal, T.; Sanyal, A. Furan-containing polymeric Materials: Harnessing the Diels-Alder chemistry for biomedical applications. Eur. Polym. J. 2021, 153, 110514. [Google Scholar]
- Ratwani, C.R.; Abdelkader, A. Self-healing by Diels-Alder cycloaddition in advanced functional polymers: A review. Prog. Mater. Sci. 2022, 131, 101001. [Google Scholar] [CrossRef]
- Morozova, S.M. Recent advances in hydrogels via Diels–Alder crosslinking: Design and applications. Gels 2023, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhu, Z.; Gao, R.; Yuan, J.; Zhang, J.; Li, H. Controlled release of MSC-derived small extracellular vesicles by an injectable Diels-Alder crosslinked hyaluronic acid/PEG hydrogel for osteoarthritis improvement. Acta Biomater. 2021, 128, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Guaresti, O.; Astrain, C.G.; Aguirresarobe, R.H.; Eceiza, A.; Gabilondo, N. Synthesis of stimuli–responsive chitosan–based hydrogels by Diels–Alder cross–linking ‘click’ reaction as potential carriers for drug administration. Carbohydr. Polym. 2018, 183, 278–286. [Google Scholar] [CrossRef] [PubMed]
- García-Astrain, C.; Gandini, A.; Peña, C.; Algar, I.; Eceiza, A.; Corcuera, M.; Gabilondo, N. Diels–Alder “click” chemistry for the cross-linking of furfuryl-gelatin-polyetheramine hydrogels. RSC Adv. 2014, 4, 35578–35587. [Google Scholar] [CrossRef]
- Hu, X.; Gao, Z.; Tan, H.; Wang, H.; Mao, X. An Injectable Hyaluronic Acid-Based Composite Hydrogel by DA Click Chemistry With pH Sensitive Nanoparticle for Biomedical Application. Front. Chem. 2019, 7, 477. [Google Scholar] [CrossRef] [PubMed]
- Prince, E.; Kumacheva, E. Design and applications of man-made biomimetic fibrillar hydrogels. Nat. Rev. 2019, 4, 99–115. [Google Scholar] [CrossRef]
- Wang, S.; Liu, H.; Wu, D.; Wang, X. Temperature and pH dual-stimuli-responsive phase-change microcapsules for multipurpose applications in smart drug delivery. J. Colloid Interface Sci. 2021, 583, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Morozova, S.M.; Li, T.; Li, S.; Naguib, H.E.; Kumacheva, E. Stimulus-Responsive Transport Properties of Nanocolloidal Hydrogels. Biomacromolecules 2023, 24, 1173–1183. [Google Scholar] [CrossRef]
- Ma, Y.; Adibnia, V.; Mitrache, M.; Halimi, I.; Walker, G.C.; Kumacheva, E. Stimulus-responsive nanoconjugates derived from phytoglycogen nanoparticles. Biomacromolecules 2022, 23, 1928–1937. [Google Scholar] [CrossRef]
- Wallace, L.A.; Gwynne, L.; Jenkins, T. Challenges and opportunities of pH in chronic wounds. Ther. Deliv. 2019, 10, 719–735. [Google Scholar] [CrossRef]
- Abuhelwa, A.Y.; Williams, D.B.; Upton, R.N.; Foster, D.J. Food, gastrointestinal pH, and models of oral drug absorption. Eur. J. Pharm. Biopharm. 2017, 112, 234–248. [Google Scholar] [CrossRef]
- Kato, Y.; Ozawa, S.; Miyamoto, C.; Maehata, Y.; Suzuki, A.; Maeda, T.; Baba, Y. Acidic extracellular microenvironment and cancer. Cancer Cell Int. 2013, 13, 89. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Liu, M.; Yang, X.; Zhai, G. The design of pH-sensitive chitosan-based formulations for gastrointestinal delivery. Drug Discov. Today 2015, 20, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, S. Flow in porous media I: A theoretical derivation of Darcy’s law. Transp. Porous Media 1986, 1, 3–25. [Google Scholar] [CrossRef]
- De Oliveira, J.C.; Rigolet, S.; Marichal, C.; Roucoules, V.; Laborie, M.P. Grafting Diels-Alder moieties on cellulose nanocrystals through carbamation. Carbohydr. Polym. 2020, 250, 116966. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Heindel, N.D. Determination of degree of substitution of formyl groups in polyaldehyde dextran by the hydroxylamine hydrochloride method. Pharm. Res. 1991, 8, 400–402. [Google Scholar] [CrossRef] [PubMed]
- Sirvio, J.; Hyvakko, U.; Liimatainen, H.; Niinimaki, J.; Hormi, O. Periodate oxidation of cellulose at elevated temperatures using metal salts as cellulose activators. Carbohydr. Polym. 2011, 83, 1293–1297. [Google Scholar] [CrossRef]
- Almdal, K.; Dyre, J.; Hvidt, S. Towards a Phenomenological Definition of the Term ‘Gel’. Polym. Gels Netw. 1993, 1, 5–17. [Google Scholar] [CrossRef]
- Raza, M.A.; Gull, N.; Lee, S.W.; Seralathan, K.K.; Park, S.H. Development of stimuli-responsive chitosan based hydrogels with anticancer efficacy, enhanced antibacterial characteristics, and applications for controlled release of benzocaine. J. Ind. Eng. Chem. 2022, 109, 210–220. [Google Scholar] [CrossRef]
- Benachenhou, F.; Mimouni, N.; Mederbel, Y.; Slimane, R.K. Hydrolysis study: Synthesis of novel styrenic Schiff bases derived from benzothiazole. Arab. J. Chem. 2012, 5, 245–250. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, P.; Tiwari, S. Dynamic imine bond based chitosan smart hydrogel with magnified mechanical strength for controlled drug delivery. Int. J. Biol. Macromol. 2020, 160, 489–495. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.L. Benzocaine. Anal. Profiles Drug Subst. 1983, 12, 73–104. [Google Scholar]
- Mahapatra, R.D.; Jo, A.; Imani, K.B.C.; Chung, J.W.; Yoon, J. Effective pH-regulated release of covalently conjugated antibiotics from antibacterial hydrogels. Polym. Chem. 2022, 13, 5234–5242. [Google Scholar] [CrossRef]
- Khuu, N.; Alizadehgiashi, M.; Gevorkian, A.; Galati, E.; Yan, N.; Kumacheva, E. Temperature-Mediated Microfluidic Extrusion of Structurally Anisotropic Hydrogels. Adv. Mater. Technol. 2019, 4, 1–9. [Google Scholar] [CrossRef]
- Tian, X.; Jiang, X. Preparing water-soluble 2,3-dialdehyde cellulose as a bio- origin cross-linker of chitosan. Cellulose 2018, 25, 987–998. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morozova, S.M.; Korzhikova-Vlakh, E.G. Fibrillar Hydrogel Based on Cellulose Nanocrystals Crosslinked via Diels–Alder Reaction: Preparation and pH-Sensitive Release of Benzocaine. Polymers 2023, 15, 4689. https://doi.org/10.3390/polym15244689
Morozova SM, Korzhikova-Vlakh EG. Fibrillar Hydrogel Based on Cellulose Nanocrystals Crosslinked via Diels–Alder Reaction: Preparation and pH-Sensitive Release of Benzocaine. Polymers. 2023; 15(24):4689. https://doi.org/10.3390/polym15244689
Chicago/Turabian StyleMorozova, Sofia M., and Evgenia G. Korzhikova-Vlakh. 2023. "Fibrillar Hydrogel Based on Cellulose Nanocrystals Crosslinked via Diels–Alder Reaction: Preparation and pH-Sensitive Release of Benzocaine" Polymers 15, no. 24: 4689. https://doi.org/10.3390/polym15244689
APA StyleMorozova, S. M., & Korzhikova-Vlakh, E. G. (2023). Fibrillar Hydrogel Based on Cellulose Nanocrystals Crosslinked via Diels–Alder Reaction: Preparation and pH-Sensitive Release of Benzocaine. Polymers, 15(24), 4689. https://doi.org/10.3390/polym15244689






