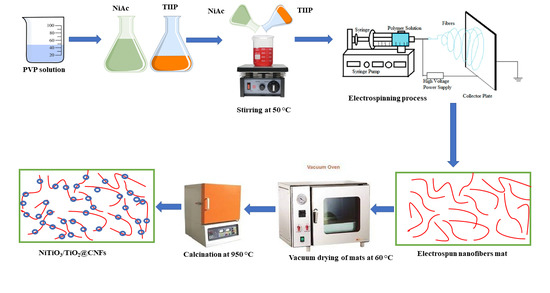
Synthesis of Ilmenite Nickel Titanite-Supported Carbon Nanofibers Derived from Polyvinylpyrrolidone as Photocatalyst for H2 Production from Ammonia Borane Photohydrolysis
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Composite NFs
2.2. Characterization
2.3. Photohydrolysis of AB
3. Results and Discussion
3.1. Photodehydrogenation of AB
3.2. Influence of Composite NF Loading
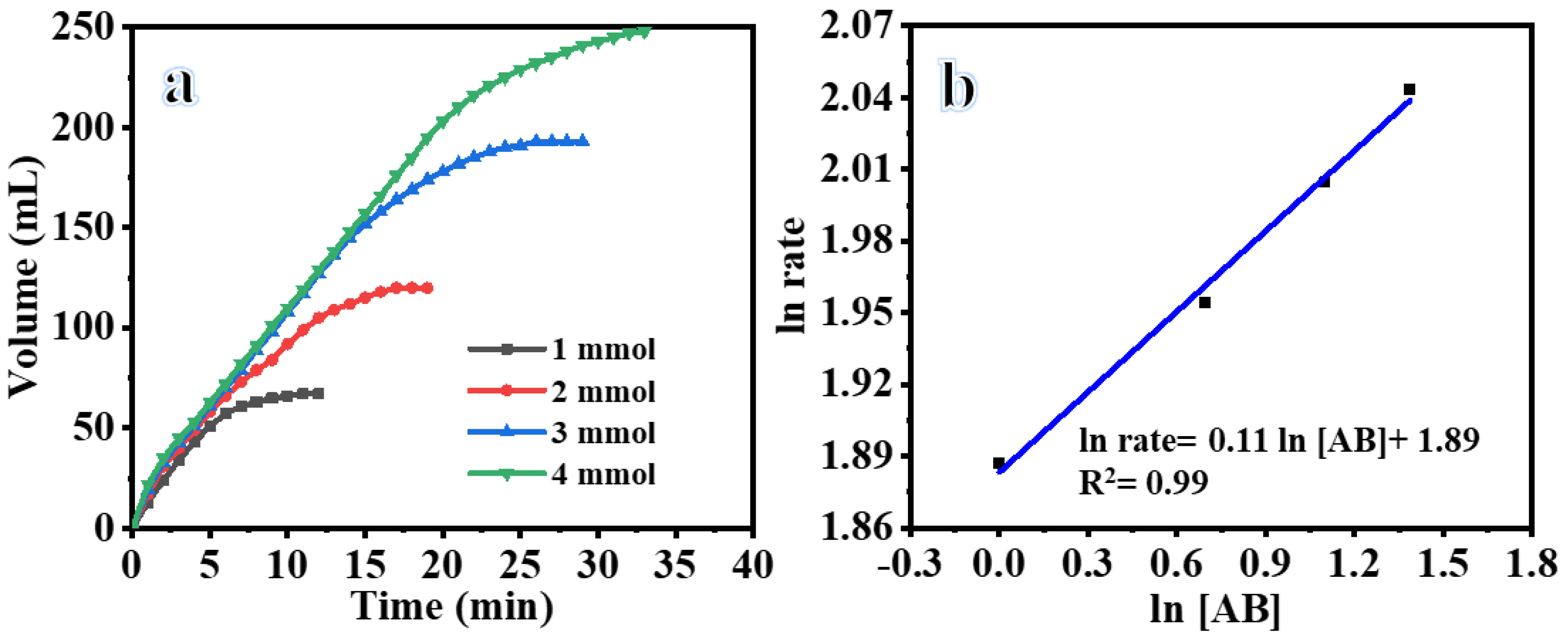
3.3. Influence of AB Concentration
3.4. Influence of Reaction Temperature
3.5. Catalyst Recyclability Data
3.6. Comparison of Our Results with the Literature
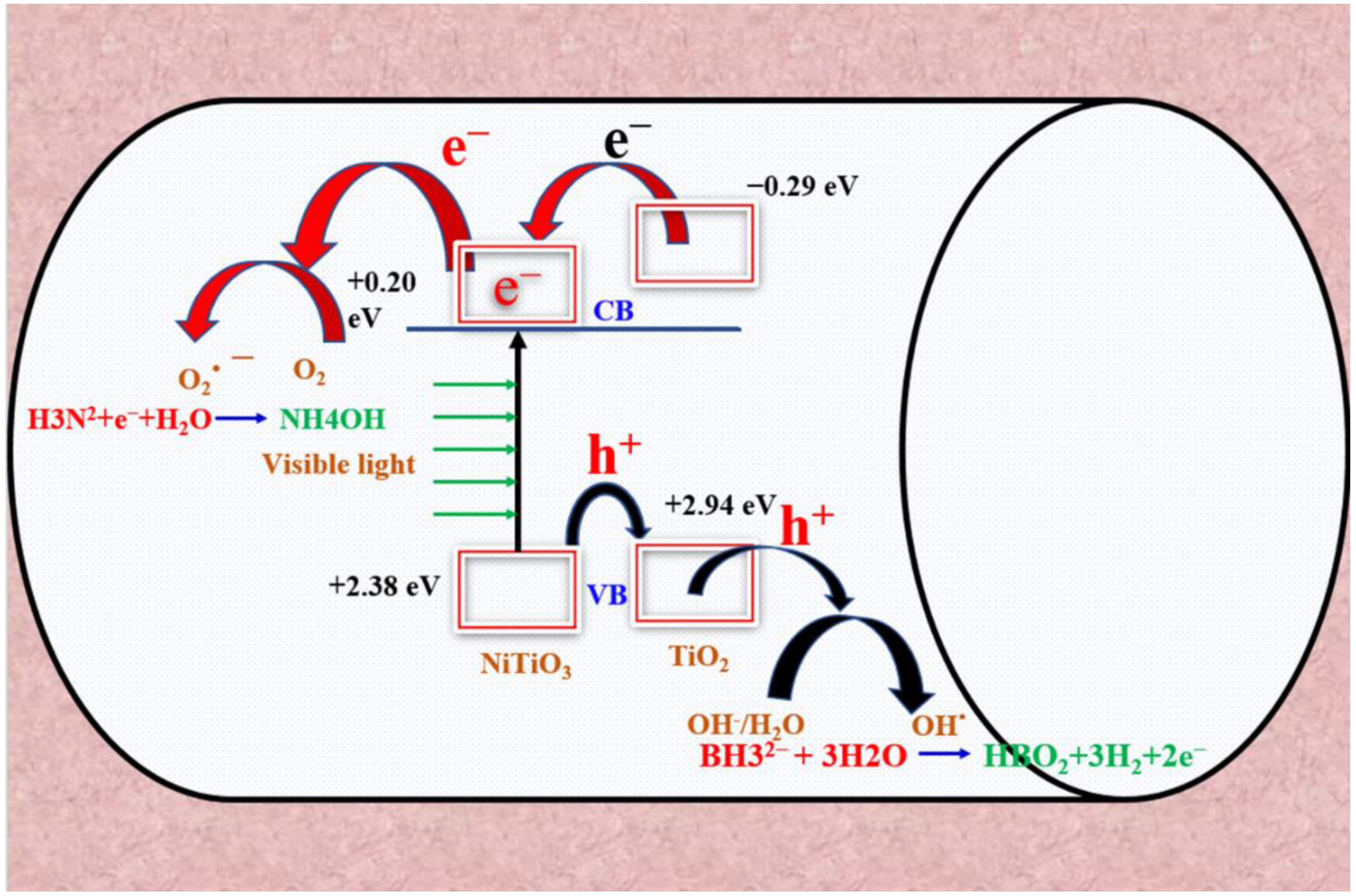
3.7. Photodehydrogenation Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, W.; Abbas, Q.; Alharthi, M.; Mohsin, M.; Hanif, I.; Vinh Vo, X.; Taghizadeh-Hesary, F. Integration of Renewable Hydrogen in Light-Duty Vehicle: Nexus between Energy Security and Low Carbon Emission Resources. Int. J. Hydrog. Energy 2020, 45, 27958–27968. [Google Scholar] [CrossRef]
- Omer, A.M. Green Energies and the Environment. Renew. Sustain. Energy Rev. 2008, 12, 1789–1821. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, J.; Cheng, H.; Zang, C.; Bian, F.; Sun, B.; Shen, Y.; Jiang, H. Photocatalytic H2 Evolution from Ammonia Borane: Improvement of Charge Separation and Directional Charge Transmission. ChemSusChem 2020, 13, 5264–5272. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the Use of Hydrogen for Maritime Applications. Energy Env. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Dematteis, E.M.; Barale, J.; Corno, M.; Sciullo, A.; Baricco, M.; Rizzi, P. Solid-State Hydrogen Storage Systems and the Relevance of a Gender Perspective. Energies 2021, 14, 6158. [Google Scholar] [CrossRef]
- Ahmad, M.A.N.; Sazelee, N.; Ali, N.A.; Ismail, M. An Overview of the Recent Advances of Additive-Improved Mg(BH4)2 for Solid-State Hydrogen Storage Material. Energies 2022, 15, 862. [Google Scholar] [CrossRef]
- Zhang, G.; Morrison, D.; Bao, G.; Yu, H.; Yoon, C.W.; Song, T.; Lee, J.; Ung, A.T.; Huang, Z. An Amine–Borane System Featuring Room-Temperature Dehydrogenation and Regeneration. Angew. Chem. Int. Ed. 2021, 60, 11725–11729. [Google Scholar] [CrossRef]
- Mboyi, C.D.; Poinsot, D.; Roger, J.; Fajerwerg, K.; Kahn, M.L.; Hierso, J. The Hydrogen-Storage Challenge: Nanoparticles for Metal-Catalyzed Ammonia Borane Dehydrogenation. Small 2021, 17, 2102759. [Google Scholar] [CrossRef]
- Giri, S.; Tripathi, A.K. Hydrogen Utilisation via Ammonia Borane Dehydrogenation and Regeneration: A Review. In Advances in Chemical, Bio and Environmental Engineering; Ratan, J.K., Sahu, D., Pandhare, N.N., Bhavanam, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2022; pp. 651–669. [Google Scholar]
- Liu, M.; Zhou, L.; Luo, X.; Wan, C.; Xu, L. Recent Advances in Noble Metal Catalysts for Hydrogen Production from Ammonia Borane. Catalysts 2020, 10, 788. [Google Scholar] [CrossRef]
- Li, H.; Yan, Y.; Feng, S.; Zhu, Y.; Chen, Y.; Fan, H.; Zhang, L.; Yang, Z. Transition Metal Tuned Semiconductor Photocatalyst CuCo/β-SiC Catalyze Hydrolysis of Ammonia Borane to Hydrogen Evolution. Int. J. Hydrog. Energy 2019, 44, 8307–8314. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.M.; Khalil, K.A.; Unnithan, A.R.; Panthi, G.; Pant, B.; Kim, H.Y. Photocatalytic Release of Hydrogen from Ammonia Borane-Complex Using Ni(0)-Doped TiO2/C Electrospun Nanofibers. Colloids Surf. A Physicochem. Eng. Asp. 2012, 410, 59–65. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, J.; Du, X.; Sun, S.; Yu, X.; Zhang, X.; Lu, Z.; Li, L.; Yang, X. Hydrogen Production from Ammonia Borane Hydrolysis Catalyzed by Non-Noble Metal-Based Materials: A Review. J. Mater. Sci. 2021, 56, 2856–2878. [Google Scholar] [CrossRef]
- Wei, L.; Yang, Y.; Yu, Y.-N.; Wang, X.; Liu, H.; Lu, Y.; Ma, M.; Chen, Y. Visible-Light-Enhanced Catalytic Hydrolysis of Ammonia Borane Using RuP2 Quantum Dots Supported by Graphitic Carbon Nitride. Int. J. Hydrog. Energy 2021, 46, 3811–3820. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, Y.; Liu, J.; Li, Y.; Lu, Y.; Liu, H. Visible-Light-Enhanced Hydrogen Evolution from Catalytic Hydrolysis of Ammonia Borane Using Ru Nanoparticles Supported on CdS-Modified Graphitic Carbon Nitride. New. J. Chem. 2022, 46, 19731–19739. [Google Scholar] [CrossRef]
- Simagina, V.I.; Komova, O.V.; Ozerova, A.M.; Netskina, O.V.; Odegova, G.V.; Kayl, N.L.; Filippov, T.N. TiO2-Based Photocatalysts for Controllable Hydrogen Evolution from Ammonia Borane. Catal. Today 2021, 379, 149–158. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, C.; Jia, T.; Li, H.; Shen, K. Efficient Hydrogen Production by an RGO/TiO 2 Composite Material from Ammonia Borane Hydrolysis in a Photocatalytic Reactor. Energy Fuels 2021, 35, 16065–16074. [Google Scholar] [CrossRef]
- Wang, C.; Yu, X.; Zhang, X.; Lu, Z.; Wang, X.; Han, X.; Zhao, J.; Li, L.; Yang, X. Enhanced Hydrogen Production from Ammonia Borane over CuNi Alloy Nanoparticles Supported on TiO2(B)/Anatase Mixed-Phase Nanofibers with High Specific Surface Area. J. Alloys Compd. 2020, 815, 152431. [Google Scholar] [CrossRef]
- Asim, M.; Zhang, S.; Ai, M.; Maryam, B.; Wang, Y.; Li, X.; Yang, J.; Zou, J.-J.; Pan, L. Photohydrolysis of Ammonia Borane for Effective H2 Evolution via Hot Electron-Assisted Energy Cascade of Au-WO2.72 /TiO2. Ind. Eng. Chem. Res. 2022, 61, 11429–11435. [Google Scholar] [CrossRef]
- Yan, Y.; Li, J.; Jia, T.; Li, H.; Shen, K.; He, Z. Preparation of TiO2 -Based Photocatalysts Synergistically Modified with Fe 3+–Graphene and Their Visible-Light-Catalyzed Hydrogen Production from Ammonia Borane. Energy Fuels 2021, 35, 16035–16045. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, G.; Zhang, Y.; Pan, L.; Zhang, X.; Zou, J.-J. Visible-Light-Induced Unbalanced Charge on NiCoP/TiO2 Sensitized System for Rapid H2 Generation from Hydrolysis of Ammonia Borane. Appl. Catal. B 2020, 260, 118183. [Google Scholar] [CrossRef]
- Trang, N.T.T.; Khang, D.M.; Dung, D.D.; Trung, N.N.; Phuong, N.T.; Bac, L.H. Synthesis of Ilmenite NiTiO3 Rods and Effect of PH on Rhodamine B Textile Dye Degradation under LED Visible-Light Irradiation. J. Electron. Mater. 2021, 50, 7188–7197. [Google Scholar] [CrossRef]
- Van, K.H.; Ramacharyulu, P.V.R.K.; Youn, D.H.; Kim, C.W. Determining Anisotropic Reactive Facet of Ilmenite Photocatalyst with Hubbard U Assisted Density Functional Theory. Comput. Mater. Sci. 2023, 219, 112024. [Google Scholar] [CrossRef]
- Pham, T.-T.; Shin, E.W. Inhibition of Charge Recombination of NiTiO3 Photocatalyst by the Combination of Mo-Doped Impurity State and Z-Scheme Charge Transfer. Appl. Surf. Sci. 2020, 501, 143992. [Google Scholar] [CrossRef]
- Jiang, K.; Jung, H.; Pham, T.-T.; Dao, D.Q.; Nguyen, T.K.A.; Yu, H.; Men, Y.; Shin, E.W. Modification of NiTiO3 Visible Light-Driven Photocatalysts by Nb Doping and NbOx Heterojunction: Oxygen Vacancy in the Nb-Doped NiTiO3 Structure. J. Alloys Compd. 2021, 861, 158636. [Google Scholar] [CrossRef]
- Li, S.; Hu, S.; Jiang, W.; Liu, Y.; Zhou, Y.; Liu, Y.; Mo, L. Hierarchical Architectures of Bismuth Molybdate Nanosheets onto Nickel Titanate Nanofibers: Facile Synthesis and Efficient Photocatalytic Removal of Tetracycline Hydrochloride. J. Colloid Interface Sci. 2018, 521, 42–49. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, Y.; Li, G.; Xue, C.; Guo, W. Hetero-Structural NiTiO3/TiO2 Nanotubes for Efficient Photocatalytic Hydrogen Generation. Renew. Energy 2017, 111, 410–415. [Google Scholar] [CrossRef]
- Komaraiah, D.; Radha, E.; Sivakumar, J.; Ramana Reddy, M.V.; Sayanna, R. Photoluminescence and Photocatalytic Activity of Spin Coated Ag+ Doped Anatase TiO2 Thin Films. Opt. Mater. 2020, 108, 110401. [Google Scholar] [CrossRef]
- Panthi, G.; Barakat, N.A.M.; Abdelrazek Khalil, K.; Yousef, A.; Jeon, K.-S.; Kim, H.Y. Encapsulation of CoS Nanoparticles in PAN Electrospun Nanofibers: Effective and Reusable Catalyst for Ammonia Borane Hydrolysis and Dyes Photodegradation. Ceram. Int. 2013, 39, 1469–1476. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.M.; Amna, T.; Al-Deyab, S.S.; Hassan, M.S.; Abdel-hay, A.; Kim, H.Y. Inactivation of Pathogenic Klebsiella Pneumoniae by CuO/TiO2 Nanofibers: A Multifunctional Nanomaterial via One-Step Electrospinning. Ceram. Int. 2012, 38, 4525–4532. [Google Scholar] [CrossRef]
- Yousef, A.; Barakat, N.A.M.; Kim, H.Y. Electrospun Cu-Doped Titania Nanofibers for Photocatalytic Hydrolysis of Ammonia Borane. Appl. Catal. A Gen. 2013, 467, 98–106. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.M.; EL-Newehy, M.H.; Al-Deyab, S.S.; Barakat, N.A.M. Cu 0/S-Doped TiO2 Nanoparticles-Decorated Carbon Nanofibers as Novel and Efficient Photocatalyst for Hydrogen Generation from Ammonia Borane. Ceram. Int. 2016, 42, 1507–1512. [Google Scholar] [CrossRef]
- Yousef, A.; Brooks, R.M.; El-Halwany, M.M.; Obaid, M.; El-Newehy, M.H.; Al-Deyab, S.S.; Barakat, N.A.M. A Novel and Chemical Stable Co–B Nanoflakes-like Structure Supported over Titanium Dioxide Nanofibers Used as Catalyst for Hydrogen Generation from Ammonia Borane Complex. Int. J. Hydrog. Energy 2016, 41, 285–293. [Google Scholar] [CrossRef]
- Yousef, A.; El-Halwany, M.M.; Barakat, N.A.M.; Al-Maghrabi, M.N.; Kim, H.Y. Cu0- Doped TiO2 Nanofibers as Potential Photocatalyst and Antimicrobial Agent. J. Ind. Eng. Chem. 2015, 26, 251–258. [Google Scholar] [CrossRef]
- Maafa, I.M.; Ali, M.A. Enhanced Organic Pollutant Removal Efficiency of Electrospun NiTiO3/TiO2-Decorated Carbon Nanofibers. Polymers 2023, 15, 109–125. [Google Scholar] [CrossRef]
- Gao, M.; Yu, Y.; Yang, W.; Li, J.; Xu, S.; Feng, M.; Li, H. Ni Nanoparticles Supported on Graphitic Carbon Nitride as Visible Light Catalysts for Hydrolytic Dehydrogenation of Ammonia Borane. Nanoscale 2019, 11, 3506–3513. [Google Scholar] [CrossRef]
- Metin, Ö.; Mazumder, V.; Özkar, S.; Sun, S. Monodisperse Nickel Nanoparticles and Their Catalysis in Hydrolytic Dehydrogenation of Ammonia Borane. J. Am. Chem. Soc. 2010, 132, 1468–1469. [Google Scholar] [CrossRef]
- Moradi, M.; Vasseghian, Y.; Khataee, A.; Harati, M.; Arfaeinia, H. Ultrasound-assisted Synthesis of FeTiO3/GO Nanocomposite for Photocatalytic Degradation of Phenol under Visible Light Irradiation. Sep. Purif. Technol. 2021, 261, 118274. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, C.; Mu, J.; Huang, X.; Zhang, Z.; Guo, Z.; Zhang, P.; Liu, Y. Hierarchical Heterostructures of Bi2MoO6 on Carbon Nanofibers: Controllable Solvothermal Fabrication and Enhanced Visible Photocatalytic Properties. J. Mater. Chem. 2012, 22, 577–584. [Google Scholar] [CrossRef]
- Song, J.; Wu, F.; Lu, Y.; Zhang, X.; Li, Z. CeVO4/CeO2 Heterostructure-Supported Co Nanoparticles for Photocatalytic H2 Production from Ammonia Borane under Visible Light. ACS Appl. Nano Mater. 2021, 4, 4800–4809. [Google Scholar] [CrossRef]
- Chandra, M.; Xu, Q. Room temperature hydrogen generation from aqueous ammonia-borane using noble metal nano-clusters as highly active catalysts. J. Power Sources 2007, 168, 135–142. [Google Scholar] [CrossRef]
- Zhu, J.; Ma, L.; Feng, J.; Geng, T.; Wei, W.; Xie, J. Facile synthesis of Cu nanoparticles on different morphology ZrO2 supports for catalytic hydrogen generation from ammonia borane. J. Mater. Sci. Mater. Electron. 2018, 29, 14971–14980. [Google Scholar] [CrossRef]
- Chen, M.; Xiong, R.; Cui, X.; Wang, Q.; Liu, X. SiO2-Encompassed Co@N-Doped Porous Carbon Assemblies as Recyclable Catalysts for Efficient Hydrolysis of Ammonia Borane. Langmuir 2019, 35, 671–677. [Google Scholar] [CrossRef]
- Wang, C.; Sun, D.; Yu, X.; Zhang, X.; Lu, Z.; Wang, X.; Zhao, J.; Li, L.; Yang, X. Cu/Ni nanoparticles supported on TiO2(B)nanotubes as hydrogen generation photocatalysts via hydrolysis of ammonia borane. Inorg. Chem. Front. 2018, 5, 2038–2044. [Google Scholar] [CrossRef]
- Cheng, H.; Kamegawa, T.; Mori, K.; Yamashita, H. Surfactant-free nonaqueous synthesis of plasmonic molybdenum oxide nanosheets with enhanced catalytic activity for hydrogen generation from ammonia borane under visible light. Angew Chem. Int. 2014, 53, 2910–2914. [Google Scholar] [CrossRef]
- Lu, Z.-H.; Li, J.; Feng, G.; Yao, Q.; Zhang, F.; Zhou, R.; Tao, D.; Chen, X.; Yu, Z. Synergistic catalysis of MCM-41 immobilized Cu-Ni nanoparticles in hydrolytic dehydrogeneration of ammonia borane. Int. J. Hydrogen Energy 2014, 39, 13389–13395. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The Absolute Energy Positions of Conduction and Valence Bands of Selected Semiconducting Minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Wen, M.; Kuwahara, Y.; Mori, K.; Zhang, D.; Li, H.; Yamashita, H. Synthesis of Ce Ions Doped Metal–Organic Framework for Promoting Catalytic H2 Production from Ammonia Borane under Visible Light Irradiation. J. Mater. Chem. A Mater. 2015, 3, 14134–14141. [Google Scholar] [CrossRef]
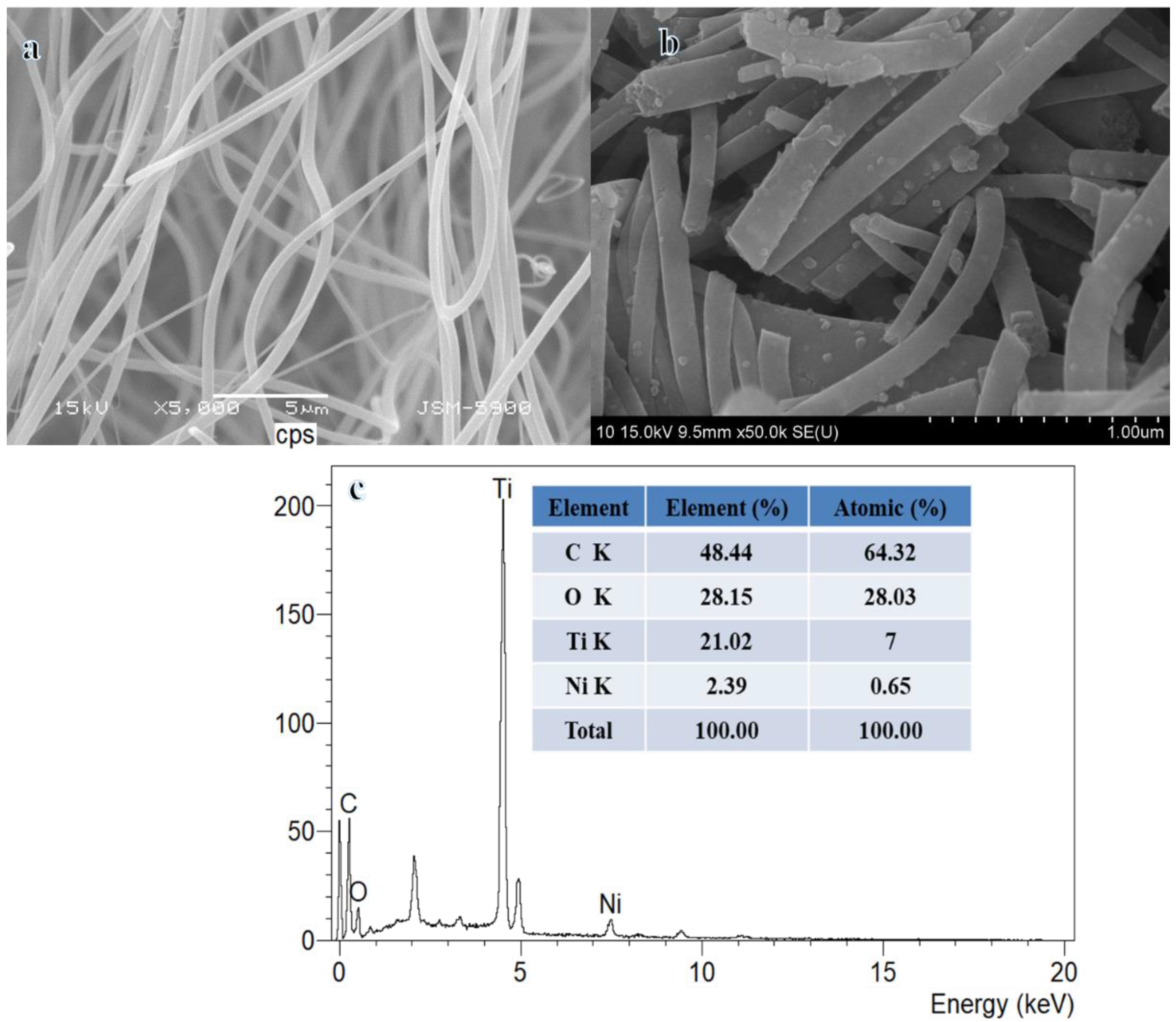
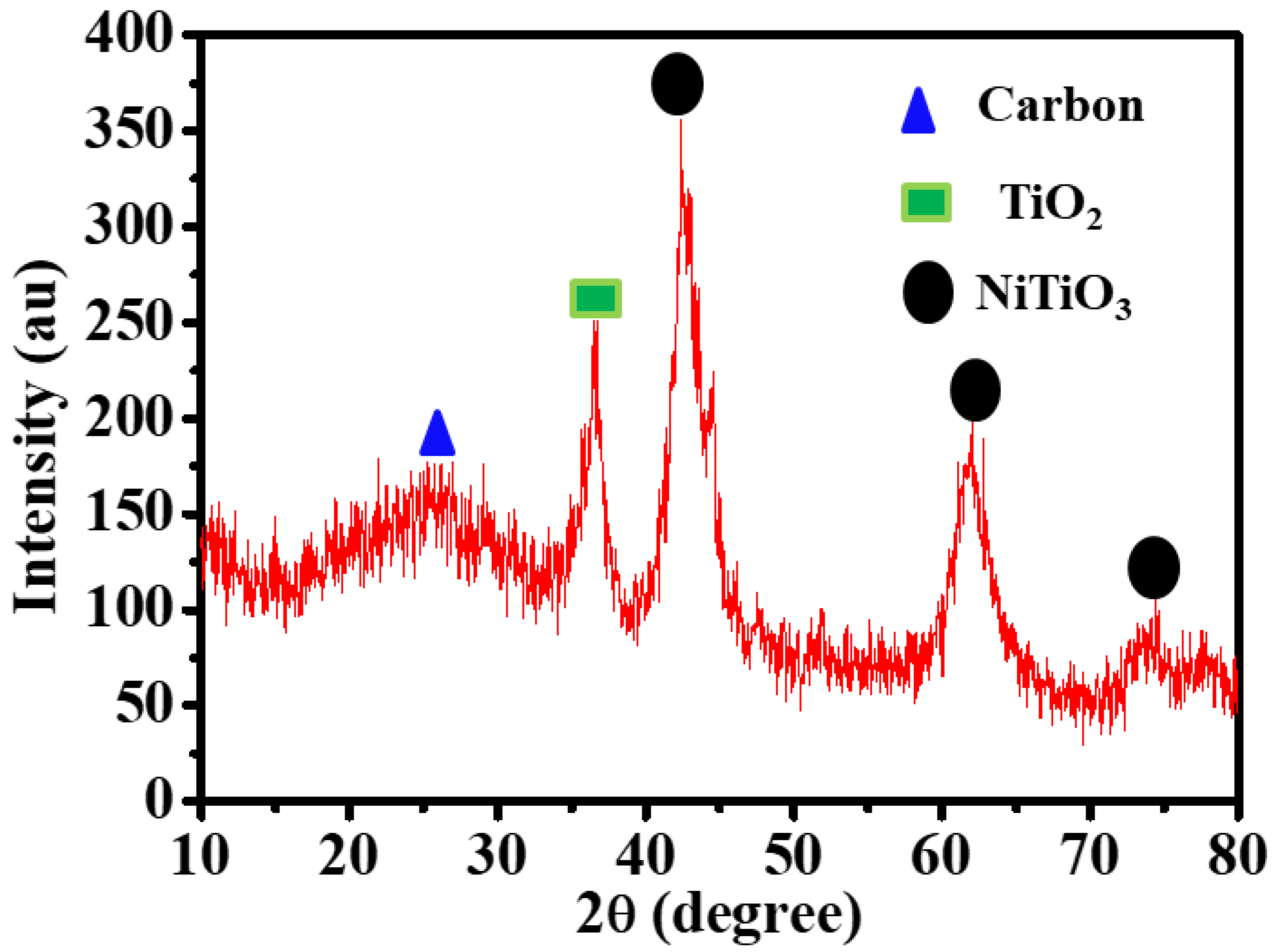




| Catalyst | TOF, mol(H2) mol(cat)−1 min−1 | Ea, kJ mol−1 | Ref. |
|---|---|---|---|
| Rh/g-Al2O3 | - | 21 | [42] |
| CuZrO2 | 0.384 | 22.34 | [43] |
| Co@CeN@SiO2 | 8.4 | 36.1 | [44] |
| Ni/g-C3N4 | 18.7 | 36 | [37] |
| Cu0.64Ni0.36-TiO2(B) NTs | 15.9 | 36.14 | [45] |
| Cu0.36Ni0.64-T700 | 21.87 | 27.40 | [19] |
| MoO3-x | 5.74 | -- | [46] |
| Cu0.2Ni0.8/MCM-41 | 10.7 | 38 | [47] |
| NiTiO3/TiO2@CNFs | 22.02 | 35.19 | This Work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maafa, I.M.; Zouli, N.; Abutaleb, A.; Yousef, A.; Qudsieh, I.Y.; Matar, S.M.; Adam, A.S.M.; El-Halwany, M.M. Synthesis of Ilmenite Nickel Titanite-Supported Carbon Nanofibers Derived from Polyvinylpyrrolidone as Photocatalyst for H2 Production from Ammonia Borane Photohydrolysis. Polymers 2023, 15, 3262. https://doi.org/10.3390/polym15153262
Maafa IM, Zouli N, Abutaleb A, Yousef A, Qudsieh IY, Matar SM, Adam ASM, El-Halwany MM. Synthesis of Ilmenite Nickel Titanite-Supported Carbon Nanofibers Derived from Polyvinylpyrrolidone as Photocatalyst for H2 Production from Ammonia Borane Photohydrolysis. Polymers. 2023; 15(15):3262. https://doi.org/10.3390/polym15153262
Chicago/Turabian StyleMaafa, Ibrahim M., Nasser Zouli, Ahmed Abutaleb, Ayman Yousef, Isam Y. Qudsieh, Saleh M. Matar, Abdel Samed M. Adam, and M. M. El-Halwany. 2023. "Synthesis of Ilmenite Nickel Titanite-Supported Carbon Nanofibers Derived from Polyvinylpyrrolidone as Photocatalyst for H2 Production from Ammonia Borane Photohydrolysis" Polymers 15, no. 15: 3262. https://doi.org/10.3390/polym15153262
APA StyleMaafa, I. M., Zouli, N., Abutaleb, A., Yousef, A., Qudsieh, I. Y., Matar, S. M., Adam, A. S. M., & El-Halwany, M. M. (2023). Synthesis of Ilmenite Nickel Titanite-Supported Carbon Nanofibers Derived from Polyvinylpyrrolidone as Photocatalyst for H2 Production from Ammonia Borane Photohydrolysis. Polymers, 15(15), 3262. https://doi.org/10.3390/polym15153262