Oxidation and Ablation Behavior of Particle-Filled SiCN Precursor Coatings for Thin-Film Sensors
Abstract
:1. Introduction
2. Experimental Section
2.1. The Fabrication of Thin-Film Coatings
2.2. Protection Performance Tests of the Thin-Film Coatings
2.3. Characterization
3. Results and Discussion
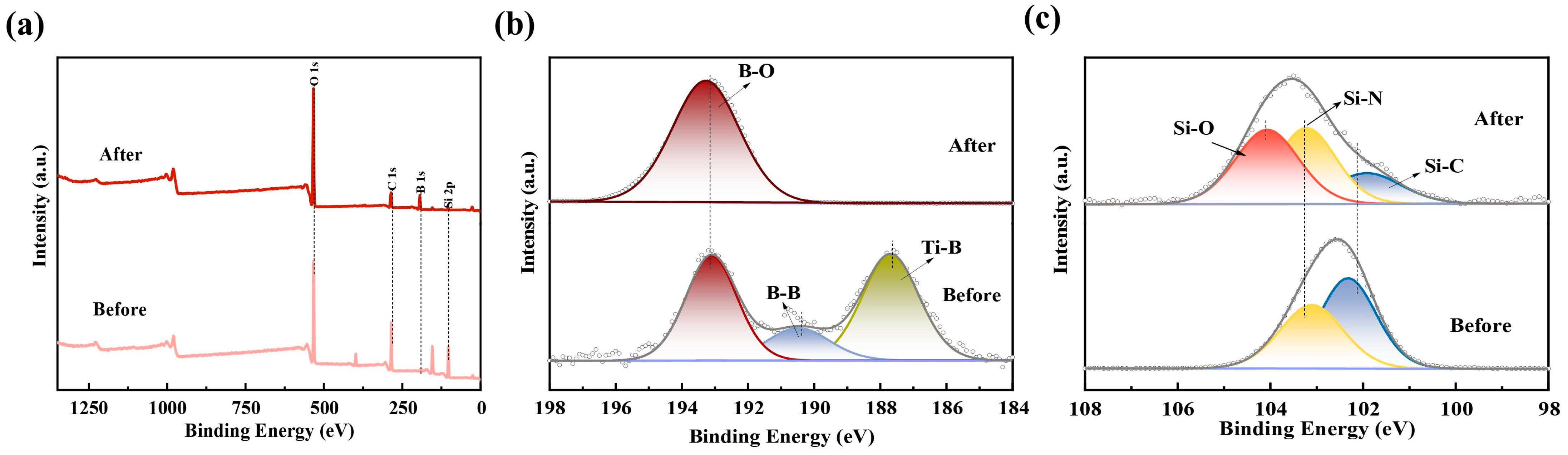
3.1. Phase Analysis and Microstructure of the Thin-Film Coatings
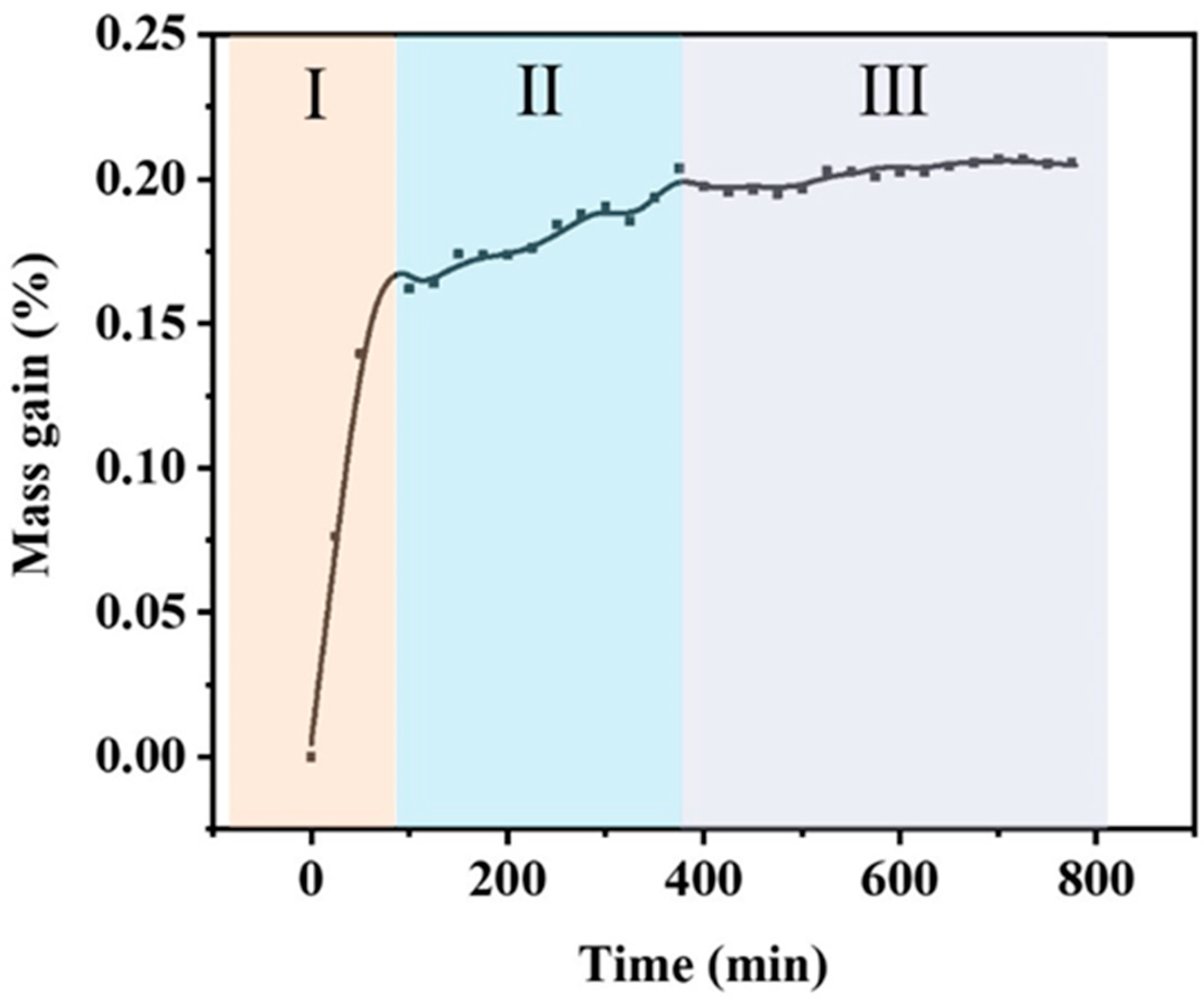
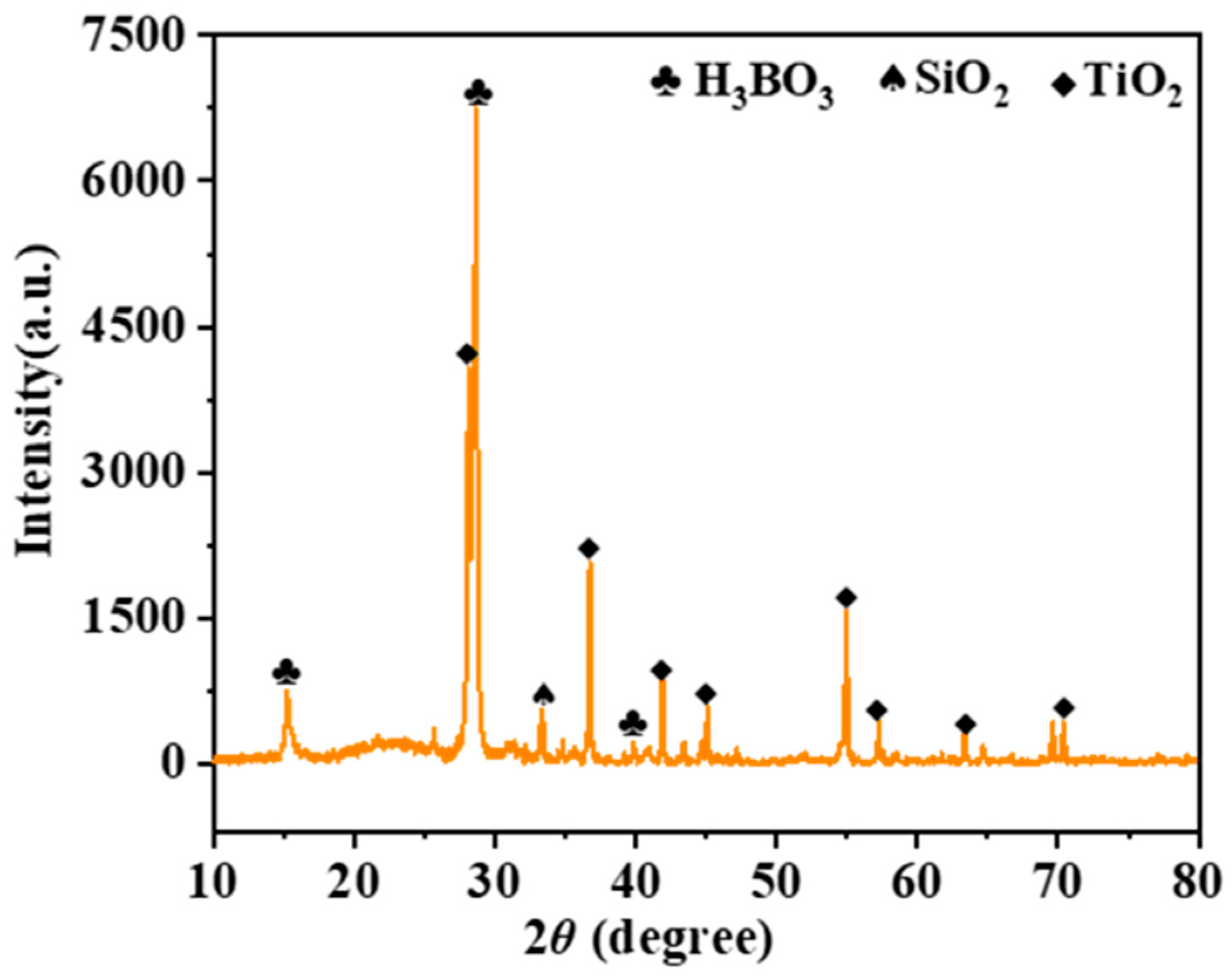
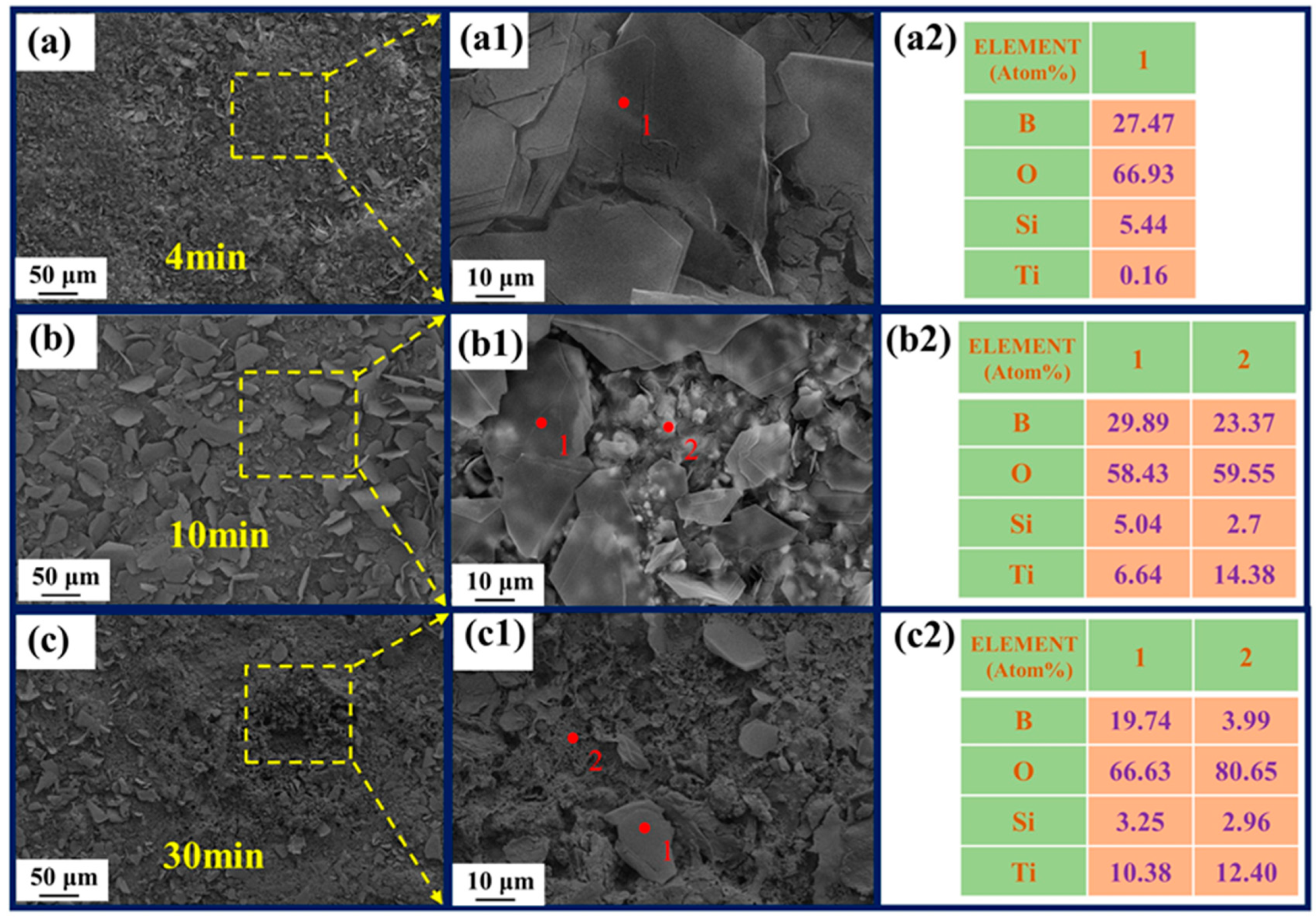
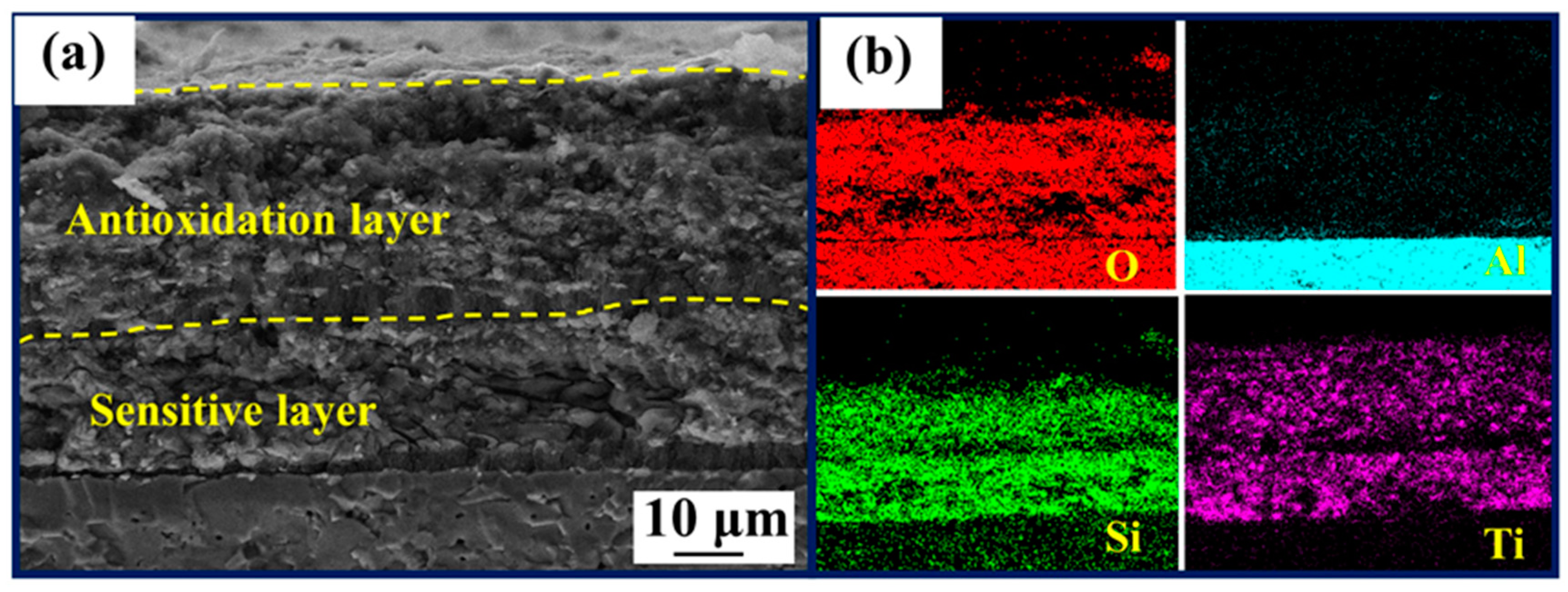
3.2. Oxidation Behavior of the Thin-Film Coatings
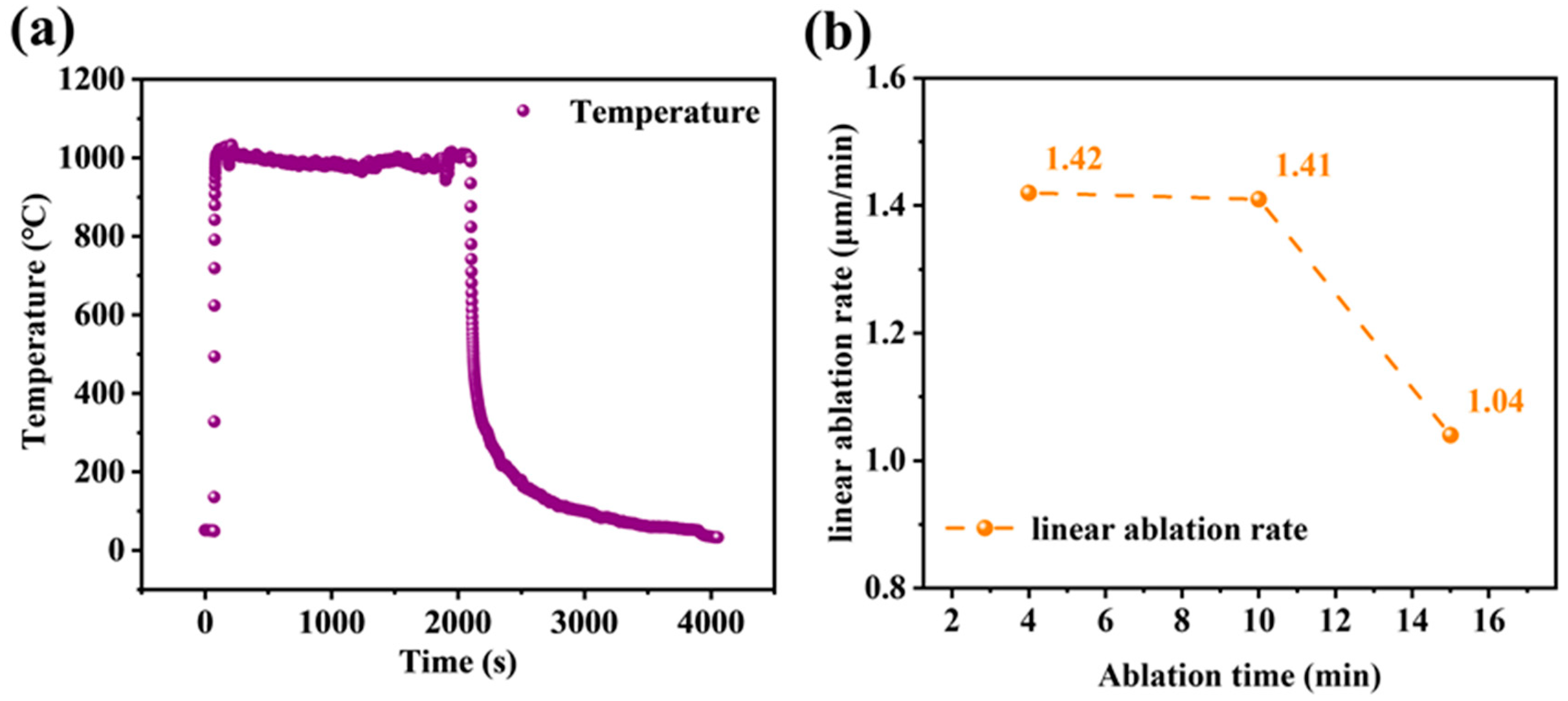
3.3. Ablation Behavior of the Coated Samples at Different Times
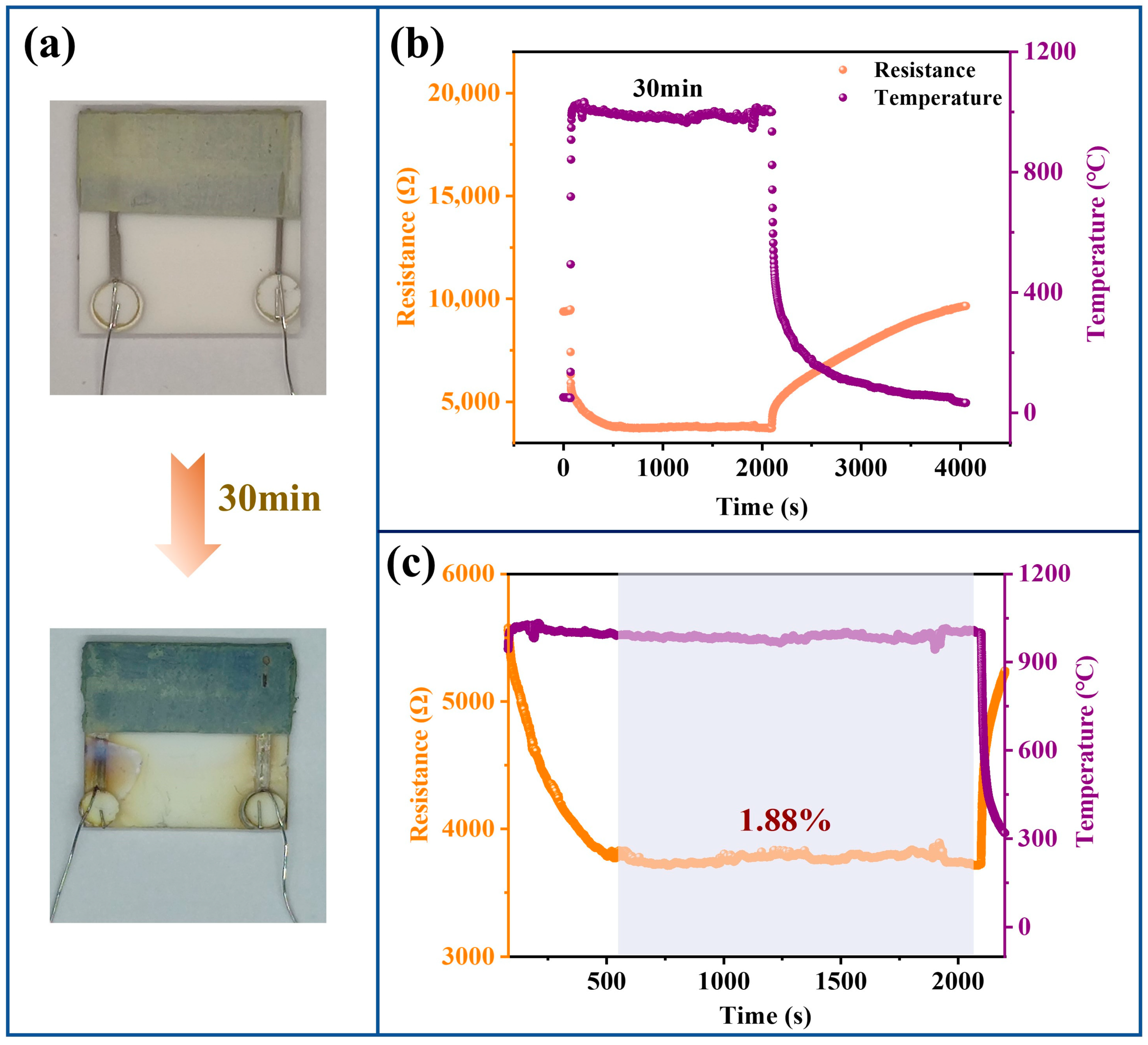
3.4. Thin-Film Temperature Sensor Oxidation Resistance Ablation Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.Y.; Li, H.F.; Lin, X.K.; Yao, J.Y.; Yang, Z.Q.; Zhang, C.C.; Wang, H.; Ding, G.F. Effect of Al2O3/Al bilayer protective coatings on the high-temperature stability of PdCr thin film strain gages. J. Alloys Compd. 2018, 759, 1–7. [Google Scholar] [CrossRef]
- Wrbanek, J.; Fralick, G.; Gonzalez, J. Developing multilayer thin film strain sensors with high thermal stability. In Proceedings of the 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Sacramento, CA, USA, 9–12 July 2006. [Google Scholar]
- Liu, H.; Mao, X.L.; Yang, Z.B.; Cui, J.T.; Jiang, S.W.; Zhang, W.L. High temperature static and dynamic strain response of PdCr thin film strain gauge prepared on Ni-based superalloy. Sens. Actuator A-Phys. 2019, 298, 9. [Google Scholar] [CrossRef]
- Wen, Q.B.; Yu, Z.J.; Riedel, R. The fate and role of in situ formed carbon in polymer-derived ceramics. Prog. Mater. Sci. 2020, 109, 63. [Google Scholar] [CrossRef]
- Cao, Y.J.; Yang, X.P.; Zhao, R.; Chen, Y.H.; Li, N.; An, L.N. Giant piezoresistivity in polymer-derived amorphous SiAlCO ceramics. J. Mater. Sci. 2016, 51, 5646–5650. [Google Scholar] [CrossRef]
- Shao, G.; Jiang, J.; Jiang, M.; Su, J.; Liu, W.; Wang, H.; Xu, H.; Lit, H.; Zhang, R. Polymer-derived SiBCN ceramic pressure sensor with excellent sensing performance. J. Adv. Ceram. 2020, 9, 374–379. [Google Scholar] [CrossRef]
- Ma, B.S.; Wang, Y.G. Fabrication of dense polymer-derived silicon carbonitride ceramic bulks by precursor infiltration and pyrolysis processes without losing piezoresistivity. J. Am. Ceram. Soc. 2018, 101, 2752–2759. [Google Scholar] [CrossRef]
- Wu, C.; Pan, X.C.; Lin, F.; Cui, Z.F.; He, Y.P.; Chen, G.C.; Zeng, Y.J.; Liu, X.L.; Chen, Q.N.; Sun, D.H.; et al. TiB2/SiCN Thin-Film Strain Gauges Fabricated by Direct Writing for High-Temperature Application. IEEE Sens. J. 2022, 22, 11517–11525. [Google Scholar] [CrossRef]
- Wu, C.; Pan, X.C.; Lin, F.; Cui, Z.F.; Li, X.; Chen, G.C.; Liu, X.L.; He, Y.P.; He, G.H.; Hai, Z.Y.; et al. High-temperature electrical properties of polymer-derived ceramic SiBCN thin films fabricated by direct writing. Ceram. Int. 2022, 48, 15293–15302. [Google Scholar] [CrossRef]
- Jung, S.; Seo, D.; Lombardo, S.J.; Feng, Z.C.; Chen, J.K.; Zhang, Y.W. Fabrication using filler controlled pyrolysis and characterization of polysilazane PDC RTD arrays on quartz wafers. Sens. Actuator A-Phys. 2012, 175, 53–59. [Google Scholar] [CrossRef]
- Seo, D.; Jung, S.H.; Lombardo, S.J.; Feng, Z.C.; Chen, J.K.; Zhang, Y.W. Fabrication and electrical properties of polymer-derived ceramic (PDC) thin films for high-temperature heat flux sensors. Sens. Actuator A-Phys. 2011, 165, 250–255. [Google Scholar] [CrossRef]
- Ma, B.S.; Cao, Y.J.; Gao, Y.; Wang, Y.G. Fabrication of a thin double-layer thermistor based on DVB-modified polymer-derived SiCN ceramics. J. Alloys Compd. 2018, 732, 491–497. [Google Scholar] [CrossRef]
- Li, L.; Xu, L.; He, Y.; Chen, G.; Zeng, Y.; Shao, C.; He, L.T.G.; Zhao, Y.; Sun, D.; Hai, Z.J.I.S.J. Fabrication of High-temperature Polymer-Derived Ceramic Thin Film Heat Flux Sensor by 3D Printing and Laser Pyrolysis. IEEE Sens. J. 2023, 23, 15391–15399. [Google Scholar] [CrossRef]
- Cui, Z.F.; Li, X.; Chen, G.C.; Wu, C.; He, G.H.; Hai, Z.Y.; Chen, Q.N.; Sun, D.H. Thin-film temperature sensor made from particle-filled polymer-derived ceramics pyrolyzed in vacuum. J. Eur. Ceram. Soc. 2022, 42, 2735–2742. [Google Scholar] [CrossRef]
- Cui, Z.F.; Chen, G.C.; Li, X.; Wu, C.; He, G.H.; Hai, Z.Y.; Chen, Q.N.; Sun, D.H. An anti-oxidative coating made from particle-filled SiCN precursor for applications up to 800 degrees C. J. Alloys Compd. 2022, 913, 7. [Google Scholar] [CrossRef]
- Xu, L.; Li, L.; Tang, L.; Zeng, Y.; Chen, G.; Shao, C.; Wu, C.; He, G.; Chen, Q.; Fang, G.; et al. Rapid Printing of High-Temperature Polymer-Derived Ceramic Composite Thin-Film Thermistor with Laser Pyrolysis. ACS Appl. Mater. Interfaces 2023, 15, 9996–10005. [Google Scholar] [CrossRef]
- Wu, C.; Pan, X.C.; Lin, F.; Chen, G.C.; Xu, L.D.; Zeng, Y.J.; He, Y.P.; Sun, D.H.; Hai, Z.Y. Al2O3-Modified Polymer-Derived Ceramic SiCN High-Temperature Anti-Oxidative Composite Coating Fabricated by Direct Writing. Polymers 2022, 14, 11. [Google Scholar] [CrossRef]
- Zhang, J.P.; Qu, J.L.; Fu, Q.G. Ablation behavior of nose-shaped HfB2-SiC modified carbon/carbon composites exposed to oxyacetylene torch. Corros. Sci. 2019, 151, 87–96. [Google Scholar] [CrossRef]
- Vinci, A.; Zoli, L.; Landi, E.; Sciti, D. Oxidation behaviour of a continuous carbon fibre reinforced ZrB2-SiC composite. Corros. Sci. 2017, 123, 129–138. [Google Scholar] [CrossRef]
- Jiang, Y.; Yin, S.; Li, M.F.; Zhang, Z.Q.; Tang, G.L.; Wang, N.; Ru, H.Q. Oxidation and ablation behaviour of multiphase ultra-high-temperature ceramic Ta0.5Zr0.5B2-Si-SiC protective coating for graphite. Ceram. Int. 2021, 47, 11358–11371. [Google Scholar] [CrossRef]
- Kong, J.A.; Zhang, Y.L.; Wang, H.H.; Chen, G.H.; Gai, W.H.; Zhang, P.F.; Li, H.J. Sublayer design and ablation resistance of CVD-TaC alternate coatings with different crystallite morphologies for C/C composites. J. Mater. Sci. Technol. 2023, 141, 1–10. [Google Scholar] [CrossRef]
- Liu, T.Y.; Fu, Q.G.; Zhang, J.P. Microstructure and mechanical performance evolution of C/C-ZrC composites exposed to oxyacetylene flame. Ceram. Int. 2021, 47, 22654–22661. [Google Scholar] [CrossRef]
- Liao, N.; Jia, D.C.; Yang, Z.H.; Niu, B.; Zhou, Y.; Li, Y.W. Enhanced mechanical properties, thermal shock resistance and ablation resistance of Si2BC3N ceramics with nano ZrB2 addition. J. Eur. Ceram. Soc. 2019, 39, 846–859. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.F.; Yu, S.J. Ablation behavior and mechanism analysis of C/SiC composites. J. Mater. Res. Technol-JMRT 2016, 5, 170–182. [Google Scholar] [CrossRef] [Green Version]
- Turcer, L.R.; Padture, N.P. Towards multifunctional thermal environmental barrier coatings (TEBCs) based on rare-earth pyrosilicate solid-solution ceramics. Scr. Mater. 2018, 154, 111–117. [Google Scholar] [CrossRef]
- Zhu, X.F.; Zhang, Y.L.; Li, H.G.; Zhang, J.; Fu, Y.Q.; Su, Y.Y. SiC/SiC-ZrSi2 coating with micro-pore to protect C/C composites against oxidation for long-life service at high temperatures. Corrosion Sci. 2021, 191, 11. [Google Scholar] [CrossRef]
- Hayashi, H.; Watanabe, M.; Inaba, H. Measurement of thermal expansion coefficient of LaCrO3. Thermochim. Acta 2000, 359, 77–85. [Google Scholar] [CrossRef]
- Munro, R.G. Material Properties of Titanium Diboride. J. Res. Natl. Inst. Stand. Technol. 2000, 105, 709–720. [Google Scholar] [CrossRef]
- Lundström, T.; Lönnberg, B.; Bauer, J. Thermal expansion of β rhombohedral boron. J. Alloys Compd. 1998, 267, 54–58. [Google Scholar] [CrossRef]
- Schultes, G.; Schmitt, M.; Goettel, D.; Freitag-Weber, O. Strain sensitivity of TiB2, TiSi2, TaSi2 and WSi2 thin films as possible candidates for high temperature strain gauges. Sens. Actuator A-Phys. 2006, 126, 287–291. [Google Scholar] [CrossRef]
- Kohn, J.A.; Nye, W.F.; Gaulé, G.K. Boron Synthesis, Structure, and Properties: Proceedings of the Conference on Boron; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Kulpa, A.; Troczynski, T. Oxidation of TiB2 powders below 900 degrees C. J. Am. Ceram. Soc. 1996, 79, 518–520. [Google Scholar] [CrossRef]
- Ivashchenko, V.I.; Kozak, A.O.; Porada, O.K.; Ivashchenko, L.A.; Sinelnichenko, O.K.; Lytvyn, O.S.; Tomila, T.V.; Malakhov, V.J. Characterization of SiCN thin films: Experimental and theoretical investigations. Thin Solid Film. 2014, 569, 57–63. [Google Scholar] [CrossRef]
- Senda, T. Oxidation behavior of titanium boride at elevated temperatures. J. Ceram. Soc. Jpn 1996, 104, 785–787. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Anthonysamy, S. Oxidation of boron carbide powder. J. Therm. Anal. Calorim. 2015, 122, 645–652. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Y.; Zhao, D.; Hu, H.; Zhang, Z. Mechanism of ablation of 3D C/ZrC–SiC composite under an oxyacetylene flame. Corros. Sci. 2013, 68, 168–175. [Google Scholar]
- Li, Z.; Li, H.; Zhang, S.; Wang, J.; Li, W.; Sun, F. Effect of reaction melt infiltration temperature on the ablation properties of 2D C/C–SiC–ZrC composites. Corros. Sci. 2012, 58, 12–19. [Google Scholar] [CrossRef]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; He, Y.; Xu, L.; Shao, C.; He, G.; Sun, D.; Hai, Z. Oxidation and Ablation Behavior of Particle-Filled SiCN Precursor Coatings for Thin-Film Sensors. Polymers 2023, 15, 3319. https://doi.org/10.3390/polym15153319
Li L, He Y, Xu L, Shao C, He G, Sun D, Hai Z. Oxidation and Ablation Behavior of Particle-Filled SiCN Precursor Coatings for Thin-Film Sensors. Polymers. 2023; 15(15):3319. https://doi.org/10.3390/polym15153319
Chicago/Turabian StyleLi, Lanlan, Yingping He, Lida Xu, Chenhe Shao, Gonghan He, Daoheng Sun, and Zhenyin Hai. 2023. "Oxidation and Ablation Behavior of Particle-Filled SiCN Precursor Coatings for Thin-Film Sensors" Polymers 15, no. 15: 3319. https://doi.org/10.3390/polym15153319



