Properties of Resorbable Conduits Based on Poly(L-Lactide) Nanofibers and Chitosan Fibers for Peripheral Nerve Regeneration
Abstract
:1. Introduction
2. Materials and Methods
Assessment of the Degree of Nerve Conduction Recovery
3. Results
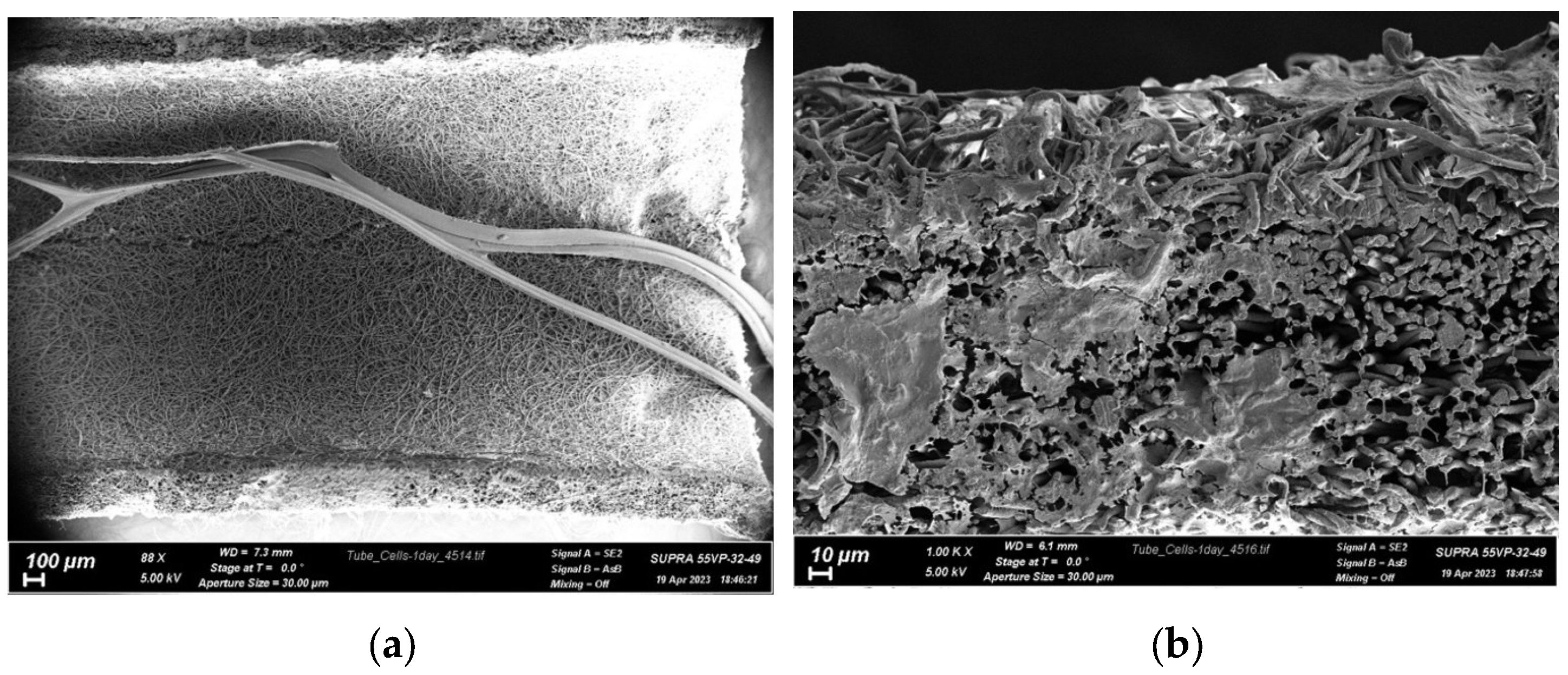
3.1. Physical and Mechanical Characteristics of Chitosan Fibers
3.2. Biocompatibility of Conduits
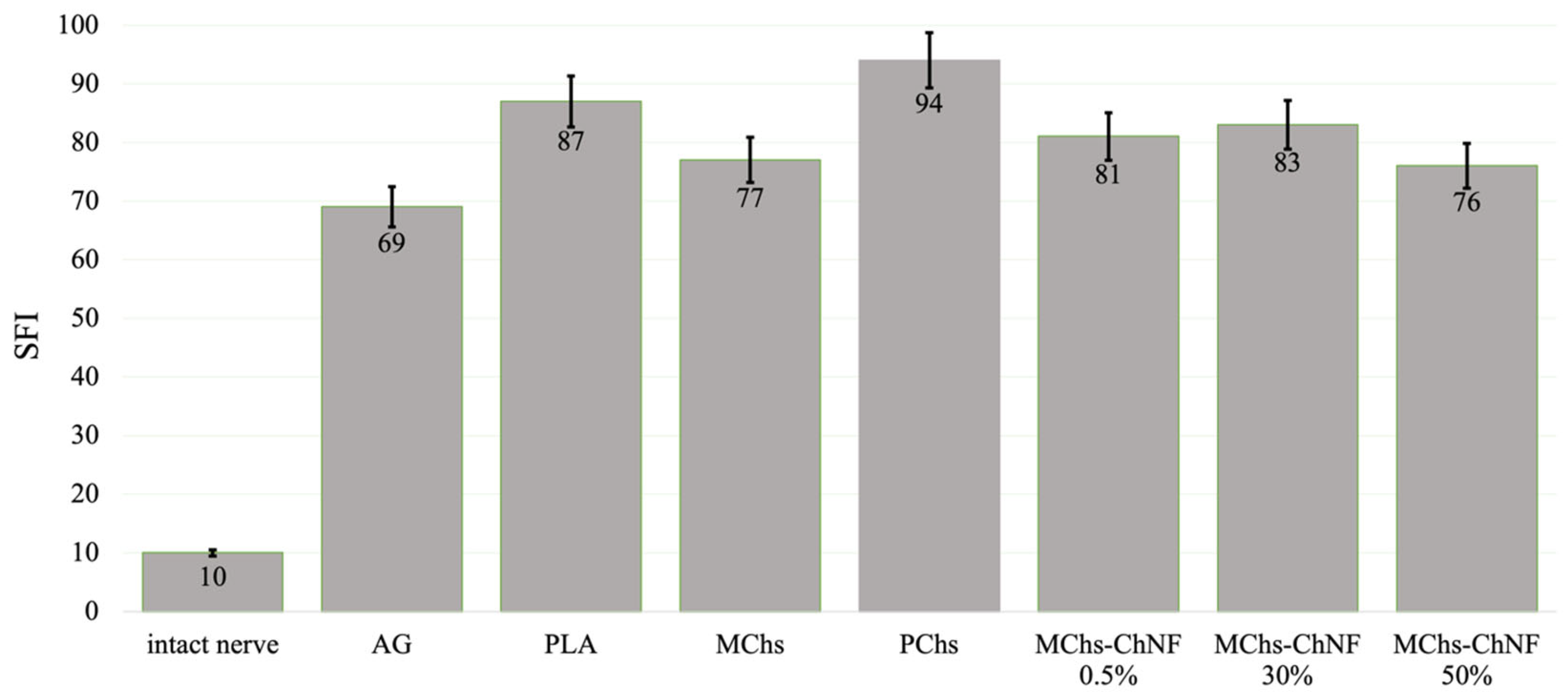
3.3. Nerve Conduction Recovery Assessment
3.4. Motor Recovery Assessment
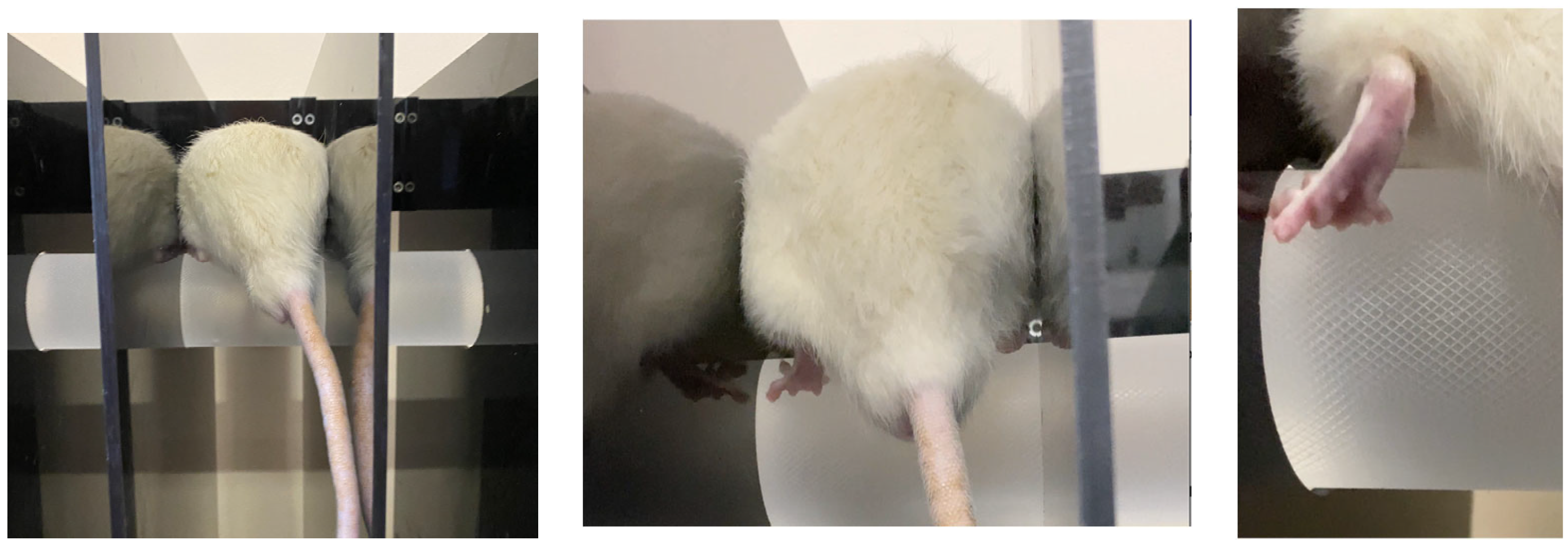
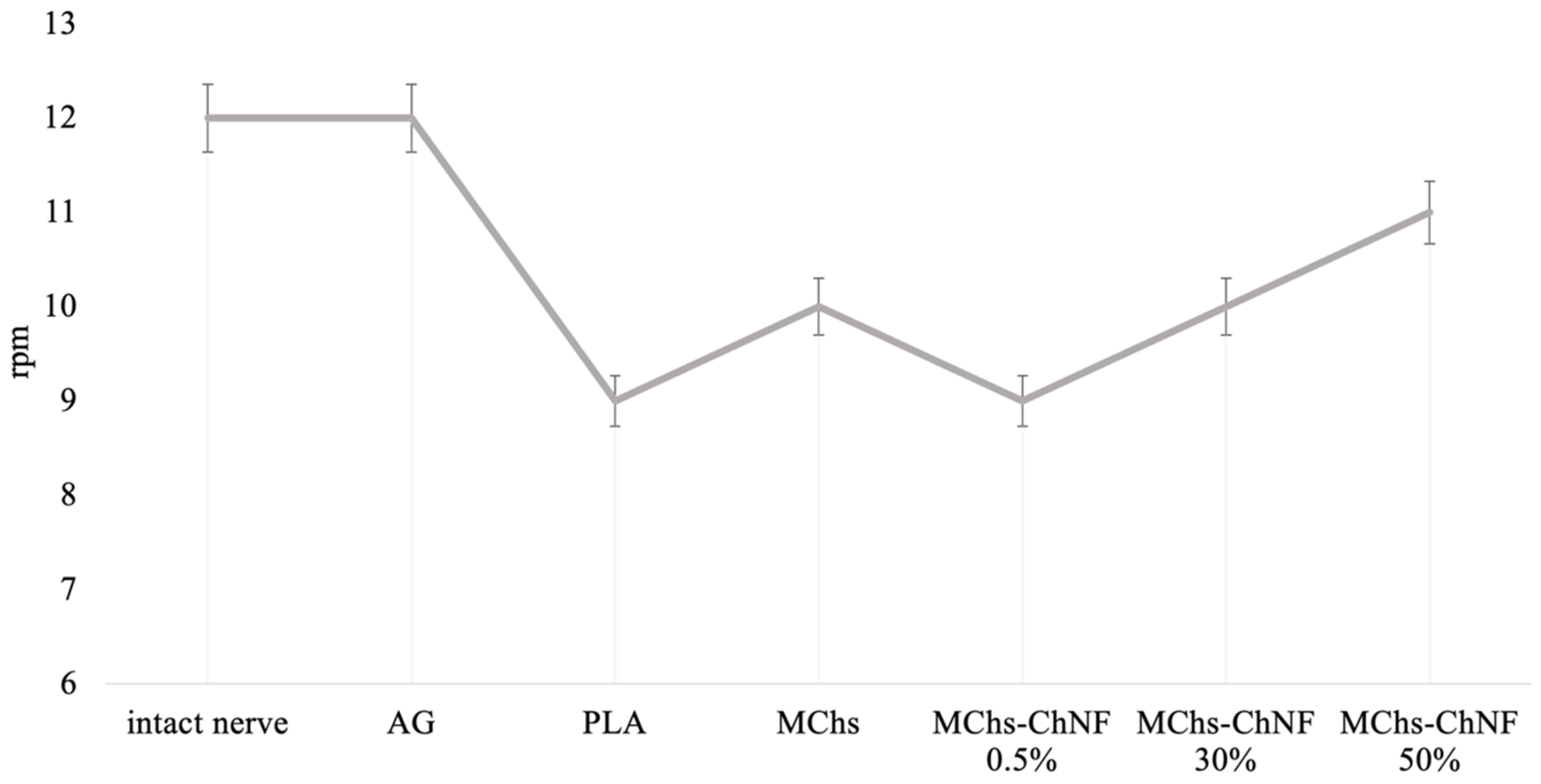
3.5. Investigation of Motor-Coordination Disorders
3.6. Neuromuscular Function Recovery Assessment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem. Neurosci. 2019, 10, 3349–3365. [Google Scholar] [CrossRef] [PubMed]
- Lundborg, G. A 25-Year Perspective of Peripheral Nerve Surgery: Evolving Neuroscientific Concepts and Clinical Significance. J. Hand Surg. Am. 2000, 25, 391–414. [Google Scholar] [CrossRef]
- Meek, M.F.; Coert, J.H. US Food and Drug Administration/Conformit Europe-Approved Absorbable Nerve Conduits for Clinical Repair of Peripheral and Cranial Nerves. Ann. Plast. Surg. 2008, 60, 110–116. [Google Scholar] [CrossRef]
- Hodgkin, A.L.; Huxley, A.F. Action Potentials Recorded from Inside a Nerve Fibre. Nature 1939, 144, 710–711. [Google Scholar] [CrossRef]
- Millesi, H. Bridging Defects: Autologous Nerve Grafts. Acta Neurochir. Suppl. 2007, 100, 37–38. [Google Scholar] [CrossRef]
- Babu, P.; Behl, A.; Chakravarty, B.; Bhandari, P.; Bhatti, T.; Maurya, S. Entubulation Techniques in Peripheral Nerve Repair. Indian J. Neurotrauma 2008, 5, 15–20. [Google Scholar] [CrossRef]
- de Ruiter, G.C.W.; Malessy, M.J.A.; Yaszemski, M.J.; Windebank, A.J.; Spinner, R.J. Designing Ideal Conduits for Peripheral Nerve Repair. Neurosurg. Focus 2009, 26, E5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlin, L.B.; Lundborg, G. Use of Tubes in Peripheral Nerve Repair. Neurosurg. Clin. N. Am. 2001, 12, 341–352. [Google Scholar] [CrossRef]
- Hudson, A.R.; Morris, J.; Weddell, G.; Drury, A. Peripheral Nerve Autografts. J. Surg. Res. 1972, 12, 267–274. [Google Scholar] [CrossRef]
- Martini, R. Expression and Functional Roles of Neural Cell Surface Molecules and Extracellular Matrix Components during Development and Regeneration of Peripheral Nerves. J. Neurocytol. 1994, 23, 1–28. [Google Scholar] [CrossRef]
- Huang, W.; Begum, R.; Barber, T.; Ibba, V.; Tee, N.C.H.; Hussain, M.; Arastoo, M.; Yang, Q.; Robson, L.G.; Lesage, S.; et al. Regenerative Potential of Silk Conduits in Repair of Peripheral Nerve Injury in Adult Rats. Biomaterials 2012, 33, 59–71. [Google Scholar] [CrossRef]
- Seddon, H.J. Three Types of Nerve Injury. Brain 1943, 66, 237–288. [Google Scholar] [CrossRef]
- Kehoe, S.; Zhang, X.F.; Boyd, D. FDA Approved Guidance Conduits and Wraps for Peripheral Nerve Injury: A Review of Materials and Efficacy. Injury 2012, 43, 553–572. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, W.; Cao, Y.; Yao, J.; Wu, J.; Gu, X. Dog Sciatic Nerve Regeneration across a 30-Mm Defect Bridged by a Chitosan/PGA Artificial Nerve Graft. Brain 2005, 128, 1897–1910. [Google Scholar] [CrossRef] [Green Version]
- Quigley, A.F.; Razal, J.M.; Thompson, B.C.; Moulton, S.E.; Kita, M.; Kennedy, E.L.; Clark, G.M.; Wallace, G.G.; Kapsa, R.M.I. A Conducting-Polymer Platform with Biodegradable Fibers for Stimulation and Guidance of Axonal Growth. Adv. Mater. 2009, 21, 4393–4397. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Ao, Q.; Wang, L.; Huang, Z.; Cai, Q.; Chen, G.; Yang, X.; Zhong, W. Constructing Conductive Conduit with Conductive Fibrous Infilling for Peripheral Nerve Regeneration. Chem. Eng. J. 2018, 345, 566–577. [Google Scholar] [CrossRef]
- Yoo, J.; Park, J.H.; Kwon, Y.W.; Chung, J.J.; Choi, I.C.; Nam, J.J.; Lee, H.S.; Jeon, E.Y.; Lee, K.; Kim, S.H.; et al. Augmented Peripheral Nerve Regeneration through Elastic Nerve Guidance Conduits Prepared Using a Porous PLCL Membrane with a 3D Printed Collagen Hydrogel. BioMater. Sci. 2020, 8, 6261–6271. [Google Scholar] [CrossRef]
- Chew, S.Y.; Mi, R.; Hoke, A.; Leong, K.W. Aligned Protein-Polymer Composite Fibers Enhance Nerve Regeneration: A Potential Tissue-Engineering Platform. Adv. Funct. Mater. 2007, 17, 1288–1296. [Google Scholar] [CrossRef] [Green Version]
- Newman, K.D.; McLaughlin, C.R.; Carlsson, D.; Li, F.; Liu, Y.; Griffith, M. Bioactive Hydrogel-Filament Scaffolds for Nerve Repair and Regeneration. Int. J. Artif. Organs. 2006, 29, 1082–1091. [Google Scholar] [CrossRef]
- Cai, J.; Peng, X.; Nelson, K.D.; Eberhart, R.; Smith, G.M. Permeable Guidance Channels Containing Microfilament Scaffolds Enhance Axon Growth and Maturation. J. Biomed. Mater. Res. A 2005, 75, 374–386. [Google Scholar] [CrossRef]
- Erturk, A.; Hellal, F.; Enes, J.; Bradke, F. Disorganized Microtubules Underlie the Formation of Retraction Bulbs and the Failure of Axonal Regeneration. J. Neurosci. 2007, 27, 9169–9180. [Google Scholar] [CrossRef] [Green Version]
- Menorca, R.M.G.; Fussell, T.S.; Elfar, J.C. Nerve Physiology: Mechanisms of Injury and Recovery. Hand Clin. 2013, 29, 317–330. [Google Scholar] [CrossRef] [Green Version]
- Evans, G.R. Peripheral Nerve Injury: A Review and Approach to Tissue Engineered Constructs. Anat. Rec. 2001, 263, 396–404. [Google Scholar] [CrossRef]
- Johnson, E.O.; Zoubos, A.B.; Soucacos, P.N. Regeneration and Repair of Peripheral Nerves. Injury 2005, 36 (Suppl. S4), S24–S29. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Lim, S.H.; Mao, H.-Q.; Chew, S.Y. Current Applications and Future Perspectives of Artificial Nerve Conduits. Exp. Neurol. 2010, 223, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Ngo, T.-T.B.; Waggoner, P.J.; Romero, A.A.; Nelson, K.D.; Eberhart, R.C.; Smith, G.M. Poly(L-Lactide) Microfilaments Enhance Peripheral Nerve Regeneration across Extended Nerve Lesions. J. Neurosci. Res. 2003, 72, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Saeki, M.; Tanaka, K.; Imatani, J.; Okamoto, H.; Watanabe, K.; Nakamura, T.; Gotani, H.; Ohi, H.; Nakamura, R.; Hirata, H. Efficacy and Safety of Novel Collagen Conduits Filled with Collagen Filaments to Treat Patients with Peripheral Nerve Injury: A Multicenter, Controlled, Open-Label Clinical Trial. Injury 2018, 49, 766–774. [Google Scholar] [CrossRef]
- Ceballos, D.; Navarro, X.; Dubey, N.; Wendelschafer-Crabb, G.; Kennedy, W.R.; Tranquillo, R.T. Magnetically Aligned Collagen Gel Filling a Collagen Nerve Guide Improves Peripheral Nerve Regeneration. Exp. Neurol. 1999, 158, 290–300. [Google Scholar] [CrossRef]
- Navissano, M.; Malan, F.; Carnino, R.; Battiston, B. Neurotube for Facial Nerve Repair. Microsurgery 2005, 25, 268–271. [Google Scholar] [CrossRef]
- Sundback, C.; Hadlock, T.; Cheney, M.; Vacanti, J. Manufacture of Porous Polymer Nerve Conduits by a Novel Low-Pressure Injection Molding Process. Biomaterials 2003, 24, 819–830. [Google Scholar] [CrossRef]
- Stang, F.; Fansa, H.; Wolf, G.; Reppin, M.; Keilhoff, G. Structural Parameters of Collagen Nerve Grafts Influence Peripheral Nerve Regeneration. Biomaterials 2005, 26, 3083–3091. [Google Scholar] [CrossRef] [PubMed]
- Allodi, I.; Udina, E.; Navarro, X. Specificity of Peripheral Nerve Regeneration: Interactions at the Axon Level. Prog. Neurobiol. 2012, 98, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Gámez, E.; Goto, Y.; Nagata, K.; Iwaki, T.; Sasaki, T.; Matsuda, T. Photofabricated Gelatin-Based Nerve Conduits: Nerve Tissue Regeneration Potentials. Cell Transpl. 2004, 13, 549–564. [Google Scholar] [CrossRef]
- Dobrovol’skaya, I.P.; Zavrazhnykh, N.A.; Popryadukhin, P.V.; Kasatkin, I.A.; Popova, E.N.; Ivankova, E.M.; Saprykina, N.N.; Yudin, V.E. Structure and thermomechanical properties of tubes based on poly(L-lactide) microfibers. High-Mol. Compd. A 2020, 62, 256–262. [Google Scholar] [CrossRef]
- Zavrazhnykh, N.A.; Yudin, V.E.; Dobrovolskaya, I.P.; Popryadukhin, P.V. SPbPU Science Week. In Proceedings of the Scientific Conference with International Participation, Saint-Petersburg, Russia, 19–24 of November 2018; Obtaining and studying the properties of porous materials based on polylactide nanofibers for vascular surgery; Vlasova, O.L., Malafeev, K.V., Pisarev, O.A., Yudin, V.E., Panarin, E.F., Zaitsev, A.V., Negulyaev, Y.A., Khodorkovsky, M.A., Eds.; POLYTECH-PRESS: St. Petersburg, Russia, 2018; pp. 65–68. [Google Scholar]
- Yuan, Y.; Zhang, P.; Yang, Y.; Wang, X.; Gu, X. The Interaction of SchwAnn. Cells with Chitosan Membranes and Fibers in Vitro. Biomaterials 2004, 25, 4273–4278. [Google Scholar] [CrossRef]
- Simões, M.J.; Gärtner, A.; Shirosaki, Y.; Gil da Costa, R.M.; Cortez, P.P.; Gartnër, F.; Santos, J.D.; Lopes, M.A.; Geuna, S.; Varejão, A.S.P.; et al. In Vitro and in Vivo Chitosan Membranes Testing for Peripheral Nerve Reconstruction. Acta Med. Port. 2011, 24, 43. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Stenberg, L.; Gonzalez-Perez, F.; Wrobel, S.; Ronchi, G.; Udina, E.; Suganuma, S.; Geuna, S.; Navarro, X.; Dahlin, L.B.; et al. Chitosan-Film Enhanced Chitosan Nerve Guides for Long-Distance Regeneration of Peripheral Nerves. Biomaterials 2016, 76, 33–51. [Google Scholar] [CrossRef] [Green Version]
- Stenberg, L.; Kodama, A.; Lindwall-Blom, C.; Dahlin, L.B. Nerve Regeneration in Chitosan Conduits and in Autologous Nerve Grafts in Healthy and in Type 2 Diabetic Goto-Kakizaki Rats. Eur. J. Neurosci. 2016, 43, 463–473. [Google Scholar] [CrossRef]
- Neubrech, F.; Sauerbier, M.; Moll, W.; Seegmüller, J.; Heider, S.; Harhaus, L.; Bickert, B.; Kneser, U.; Kremer, T. Enhancing the Outcome of Traumatic Sensory Nerve Lesions of the Hand by Additional Use of a Chitosan Nerve Tube in Primary Nerve Repair. Plast. Reconstr. Surg. 2018, 142, 415–424. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.; Sun, C.; Hu, W.; Zhao, J.; Li, G.; Zhang, L.; Liu, M.; Liu, Y.; Ding, F.; et al. Chitosan Degradation Products Promote Nerve Regeneration by Stimulating SchwAnn. Cell Proliferation via MiR-27a/FOXO1 Axis. Mol. Neurobiol. 2016, 53, 28–39. [Google Scholar] [CrossRef]
- Boecker, A.; Daeschler, S.C.; Kneser, U.; Harhaus, L. Relevance and Recent Developments of Chitosan in Peripheral Nerve Surgery. Front. Cell Neurosci. 2019, 13, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.-C.; Hong, L.; Chang, P.; Zhang, J.; Lu, S.-Y.; Zheng, B.-W.; Jiang, Z.-F. Chitooligosaccharides Attenuate Cu2+-Induced Cellular Oxidative Damage and Cell Apoptosis Involving Nrf2 Activation. Neurotox. Res. 2015, 27, 411–420. [Google Scholar] [CrossRef]
- Yudin, V.E.; Dobrovolskaya, I.P.; Neelov, I.M.; Dresvyanina, E.N.; Popryadukhin, P.V.; Ivan’kova, E.M.; Elokhovskii, V.Y.; Kasatkin, I.A.; Okrugin, B.M.; Morganti, P. Wet Spinning of Fibers Made of Chitosan and Chitin Nanofibrils. Carbohydr. Polym. 2014, 108, 176–182. [Google Scholar] [CrossRef]
- Dresvyanina, E.; Yudenko, A.; Yevlampieva, N.; Maevskaya, E.; Yudin, V.; Gubarev, A.; Slyusarenko, M.; Heppe, K. The molecular mass effect on mechanical properties of chitosan fibers. FibRes. Text. 2018, 25, 27–31. [Google Scholar]
- Dobrovol’skaya, I.P.; Kasatkin, I.A.; Yudin, V.E.; Ivan’kova, E.M.; Elokhovskii, V.Y. Supramolecular Structure of Chitin Nanofibrils. Polym. Sci. Ser. A 2015, 57, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Pavot, A.; Ignacio, D.; Tchou, S. Thermography in Peripheral Nerve Injury and Its Relationship to Electroneuromyography. Adv. Tech. Clin. Appl. Biomed. Thermol. 1994, 255, 255–280. [Google Scholar]
- Ribeiro-Resende, V.T.; Gomes, T.A.; De Lima, S.; Nascimento-Lima, M.; Bargas-Rega, M. Mice Lacking GD3 Synthase Display Morphological Abnormalities in the Sciatic Nerve and Neuronal Disturbances during Peripheral Nerve Regeneration. PLoS ONE 2014, 9, 108919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, N.D.; Jäger, R.; Purpura, M.; Wells, S.D.; Liao, K.; Godavarthi, A. Paraxanthine Supplementation Increases Muscle Mass. Strength Endur. Mice 2022, 14, 893. [Google Scholar] [CrossRef]
- Podgórski, R.; Wojasiński, M.; Ciach, T. Nanofibrous Materials Affect the Reaction of Cytotoxicity Assays. Sci. Rep. 2022, 12, 9047. [Google Scholar] [CrossRef]
- Sun, C.; Jin, X.; Holzwarth, J.M.; Liu, X.; Hu, J.; Gupte, M.J.; Zhao, Y.; Ma, P.X. Development of Channeled Nanofibrous Scaffolds for Oriented Tissue Engineering. Macromol. BioSci. 2012, 12, 761–769. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Tan, A.; Pastorin, G.; Ho, H.K. Nanomaterial Scaffolds for Stem Cell Proliferation and Differentiation in Tissue Engineering. Biotechnol. Adv. 2013, 31, 654–668. [Google Scholar] [CrossRef] [PubMed]
- Benatti, A.C.B.; Xavier, M.V.; Macedo, M.F.; Rodrigues, A.A.; Jardini, A.L.; Filho, R.M.; Kharmandayan, P. Comparative Analysis of Biocompatibility between Poly (Llactic Acid) (PLLA) and PLDL Purac® Nanofibers for Use in Tissue Engineering. Chem. Eng. Trans. 2016, 49, 199–204. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, F.; Li, C.; An, H.; Wan, T.; Zhang, P. Application of Chitosan and Its Derivative Polymers in Clinical Medicine and Agriculture. Polymers 2022, 14, 958. [Google Scholar] [CrossRef] [PubMed]




| Fibers | Tensile Strength, σ, MPa | Young Modulus, GPa | Elongation at Break, ε, % |
|---|---|---|---|
| MChs | 240.96 ± 7.98 | 13.83 ± 0.54 | 6.3 ± 0.72 |
| PChs | 186.58 ± 19.91 | 12.09 ± 1.9 | 3.36 ± 0.32 |
| PLA/Chs-ChNF 0.5% | 259.31 ± 10.45 | 14.41 ± 0.73 | 5.35 ± 0.34 |
| PLA/Chs-ChNF 30% | 174.37 ± 7.31 | 14.01 ± 0.81 | 3.87 ± 0.43 |
| PLA/Chs-ChNF 50% | 163.86 ± 9.26 | 13.87 ± 0.19 | 2.57 ± 0.55 |
| Fibers | Diameter, µm | ||||||
|---|---|---|---|---|---|---|---|
| Dry | 3 Days | 7 Days | 14 Days | 30 Days | 60 Days | 90 Days | |
| PChs | 24.17 ± 4.19 | 35.52 ± 3.91 | 37.55 ± 2.90 | 42.16 ± 3.99 | 65.37 ± 8.15 | 68.92 ± 5.21 | 70.34 ± 8.13 |
| MChs | 43.67 ± 3.79 | 52.67 ± 5.59 | 60.38 ± 5.21 | 78.23 ± 7.89 | 101.37 ± 7.73 | 102.72 ± 7.01 | 103.44 ± 6.93 |
| Chs-ChNF 0.5% | 42.75 ± 2.24 | 47.16 ± 3.67 | 59.74 ± 4.02 | 69.10 ± 3.72 | 95.48 ± 2.31 | 96.63 ± 6.29 | 98.04 ± 5.90 |
| Chs-ChNF 30% | 40.88 ± 1.98 | 60.01 ± 4.97 | 70.16 ± 3.18 | 83.87 ± 4.71 | 91.92 ± 5.01 | 93.51 ± 4.61 | 93.34 ± 4.01 |
| Chs-ChNF 50% | 43.10 ± 2.03 | 55.82 ± 3.72 | 75.20 ± 2.94 | 80.42 ± 4.71 | 90.15 ± 2.59 | 89.89 ± 4.75 | 91.56 ± 3.09 |
| Conduit | M-Response Amplitude (mV) | ||
|---|---|---|---|
| Intact Nerve | 4 Weeks | 16 Weeks | |
| AG | 28.9 ± 3.3 | 6.8 ± 2.4 | 15.1 ± 5.2 |
| PLA | 27.3 ± 3.8 | 5.1 ± 1.8 | 8.8 ± 3.9 |
| PLA/MChs | 27.2 ± 3.6 | 5.5 ± 2.2 | 13.8 ± 3.2 |
| PLA/PChs | 27.8 ± 2.1 | 3.9 ± 1.5 | 6.9 ± 4.7 |
| PLA/Chs-ChNF 0.5% | 30.1 ± 3.1 | 4.3 ± 1.7 | 12.5 ± 5.8 |
| PLA/Chs-ChNF 30% | 28.8 ± 3.7 | 4.9 ± 1.4 | 12.7 ± 3.7 |
| PLA/Chs-ChNF 50% | 28.2 ± 2.5 | 5.3 ± 0.9 | 14.1 ± 4.6 |
| Type if Conduit | Grip Force, N | ||
|---|---|---|---|
| Intact Nerve | Conduit | Recovery Degree, % | |
| AG | 2.77 ± 0.41 (14%) | 1.54 ± 0.21 (13%) | 55 |
| PLA | 1.80 ± 0.17 (10%) | 0.59 ± 0.1 (17%) | 33 |
| MChs | 2.54 ± 0.31 (12%) | 1.14 ± 0.41 (36%) | 45 |
| PChs | - | - | - |
| Chs-ChNF 0.5% | 1.95 ± 0.49 (25%) | 0.82 ± 0.17 (21%) | 42 |
| Chs-ChNF 30% | 2.06 ± 0.31 (15%) | 0.96 ± 0.25 (26%) | 46 |
| Chs-ChNF 50% | 2.73 ± 0.51 (19%) | 1.20 ± 0.48 (40%) | 44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tagandurdyyeva, N.A.; Trube, M.A.; Shemyakin, I.O.; Solomitskiy, D.N.; Medvedev, G.V.; Dresvyanina, E.N.; Nashchekina, Y.A.; Ivan’kova, E.M.; Dobrovol’skaya, I.P.; Kamalov, A.M.; et al. Properties of Resorbable Conduits Based on Poly(L-Lactide) Nanofibers and Chitosan Fibers for Peripheral Nerve Regeneration. Polymers 2023, 15, 3323. https://doi.org/10.3390/polym15153323
Tagandurdyyeva NA, Trube MA, Shemyakin IO, Solomitskiy DN, Medvedev GV, Dresvyanina EN, Nashchekina YA, Ivan’kova EM, Dobrovol’skaya IP, Kamalov AM, et al. Properties of Resorbable Conduits Based on Poly(L-Lactide) Nanofibers and Chitosan Fibers for Peripheral Nerve Regeneration. Polymers. 2023; 15(15):3323. https://doi.org/10.3390/polym15153323
Chicago/Turabian StyleTagandurdyyeva, Nurjemal A., Maxim A. Trube, Igor’ O. Shemyakin, Denis N. Solomitskiy, German V. Medvedev, Elena N. Dresvyanina, Yulia A. Nashchekina, Elena M. Ivan’kova, Irina P. Dobrovol’skaya, Almaz M. Kamalov, and et al. 2023. "Properties of Resorbable Conduits Based on Poly(L-Lactide) Nanofibers and Chitosan Fibers for Peripheral Nerve Regeneration" Polymers 15, no. 15: 3323. https://doi.org/10.3390/polym15153323







