Recycling of Nanocellulose from Polyester–Cotton Textile Waste for Modification of Film Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
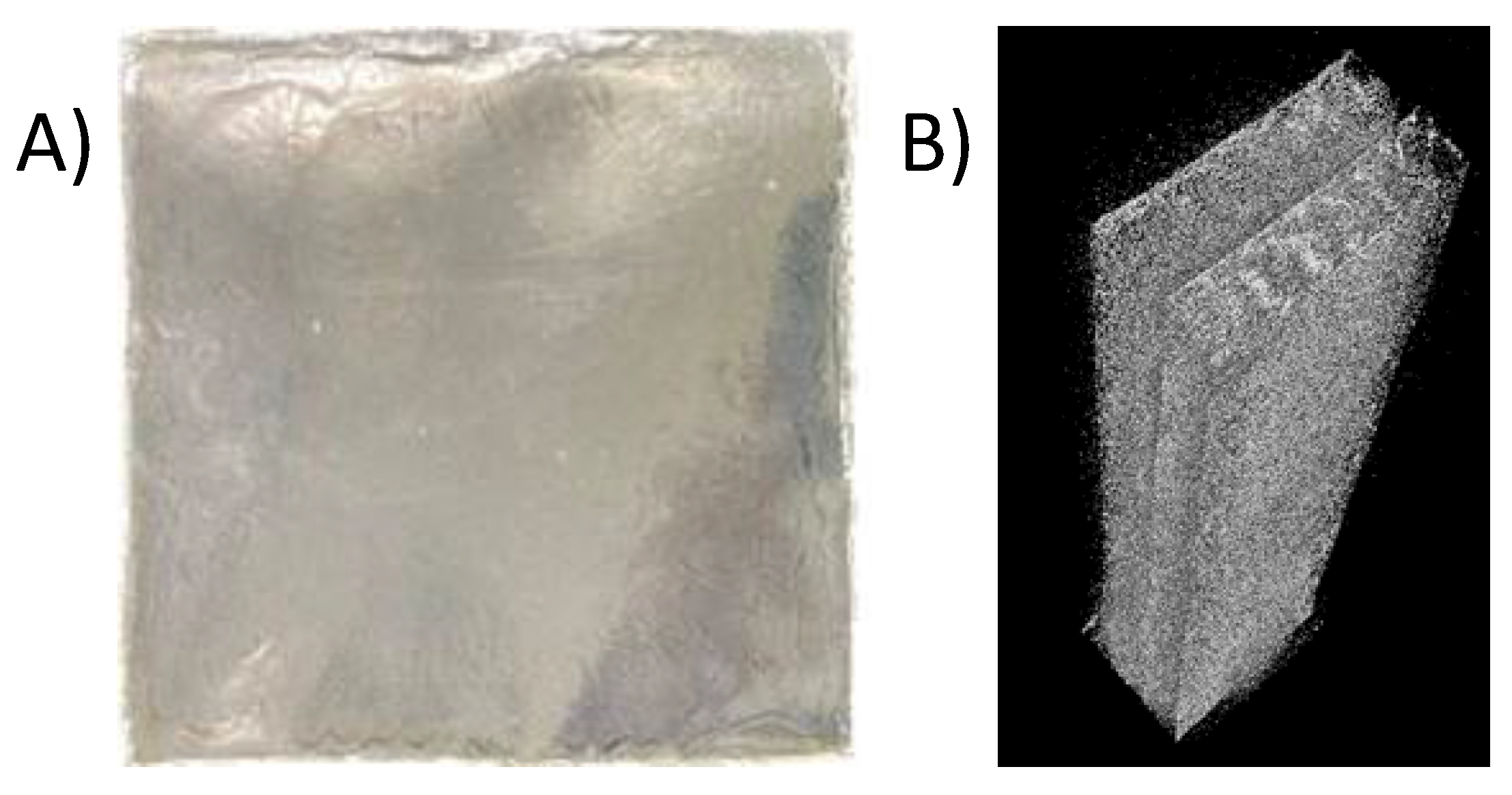
2.2. Preparation of PVA Film Incorporated with Nanocellulose
2.3. Characterizations
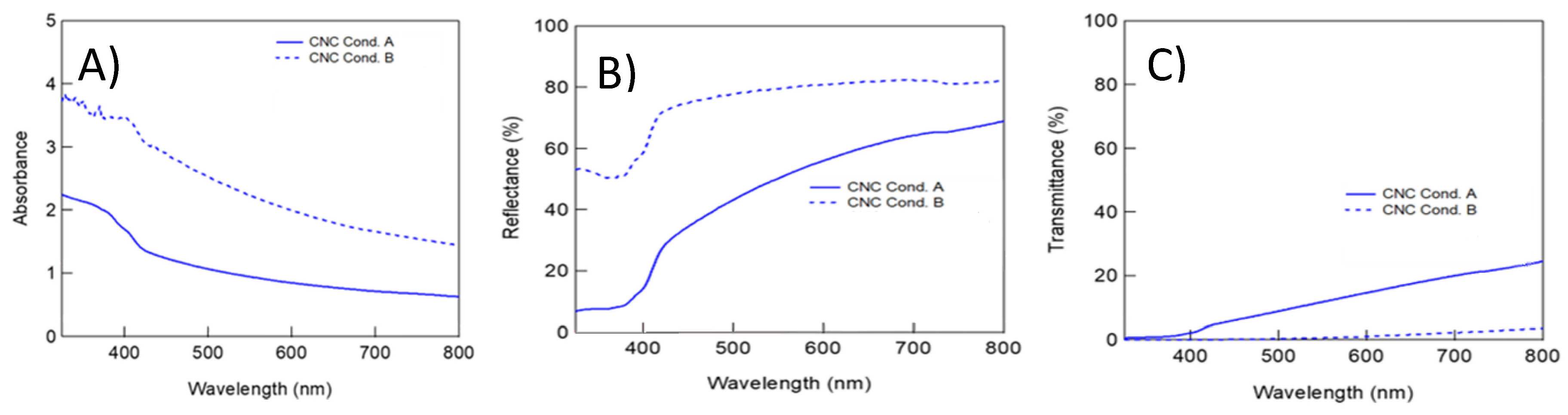
2.3.1. Photophysical Properties
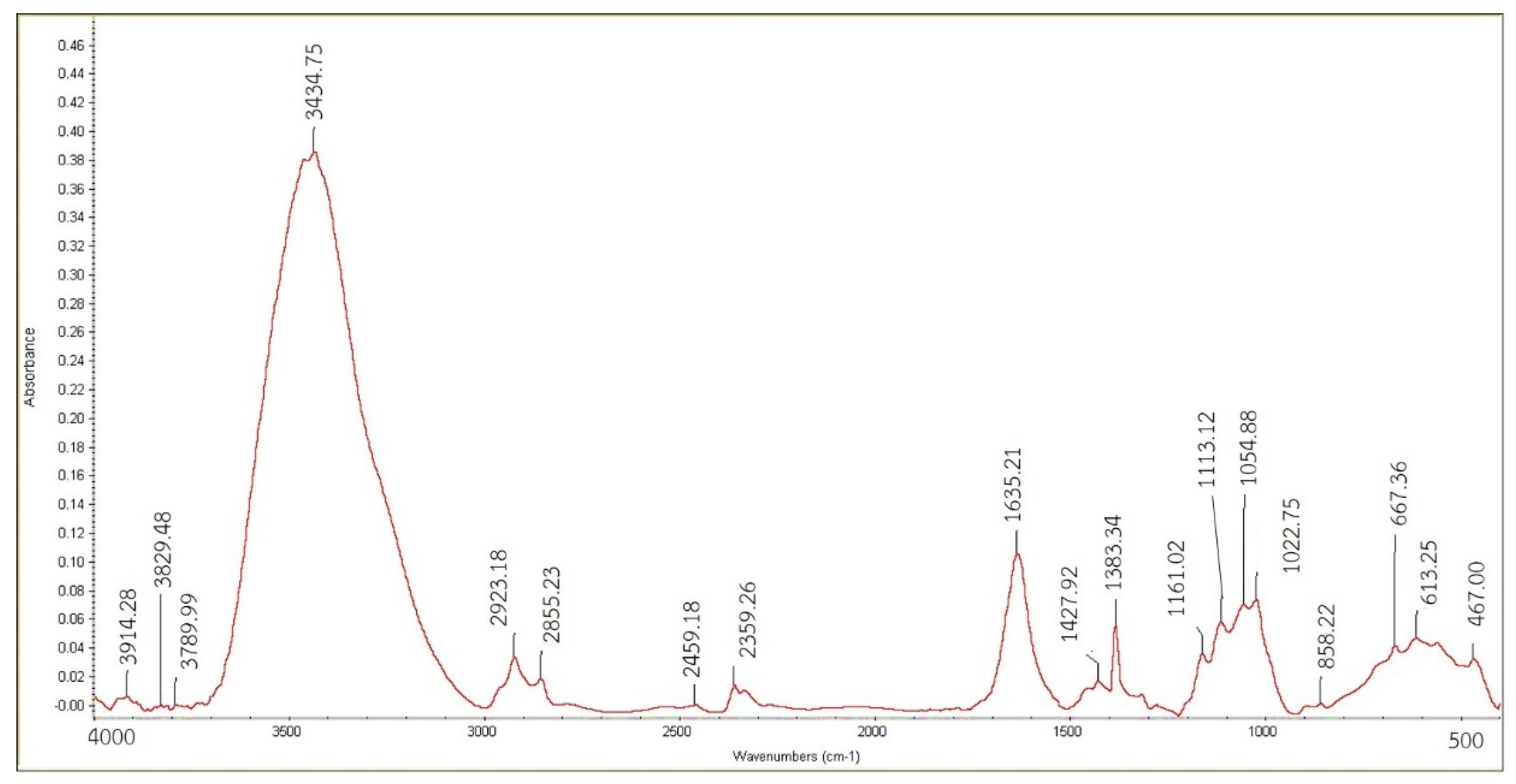
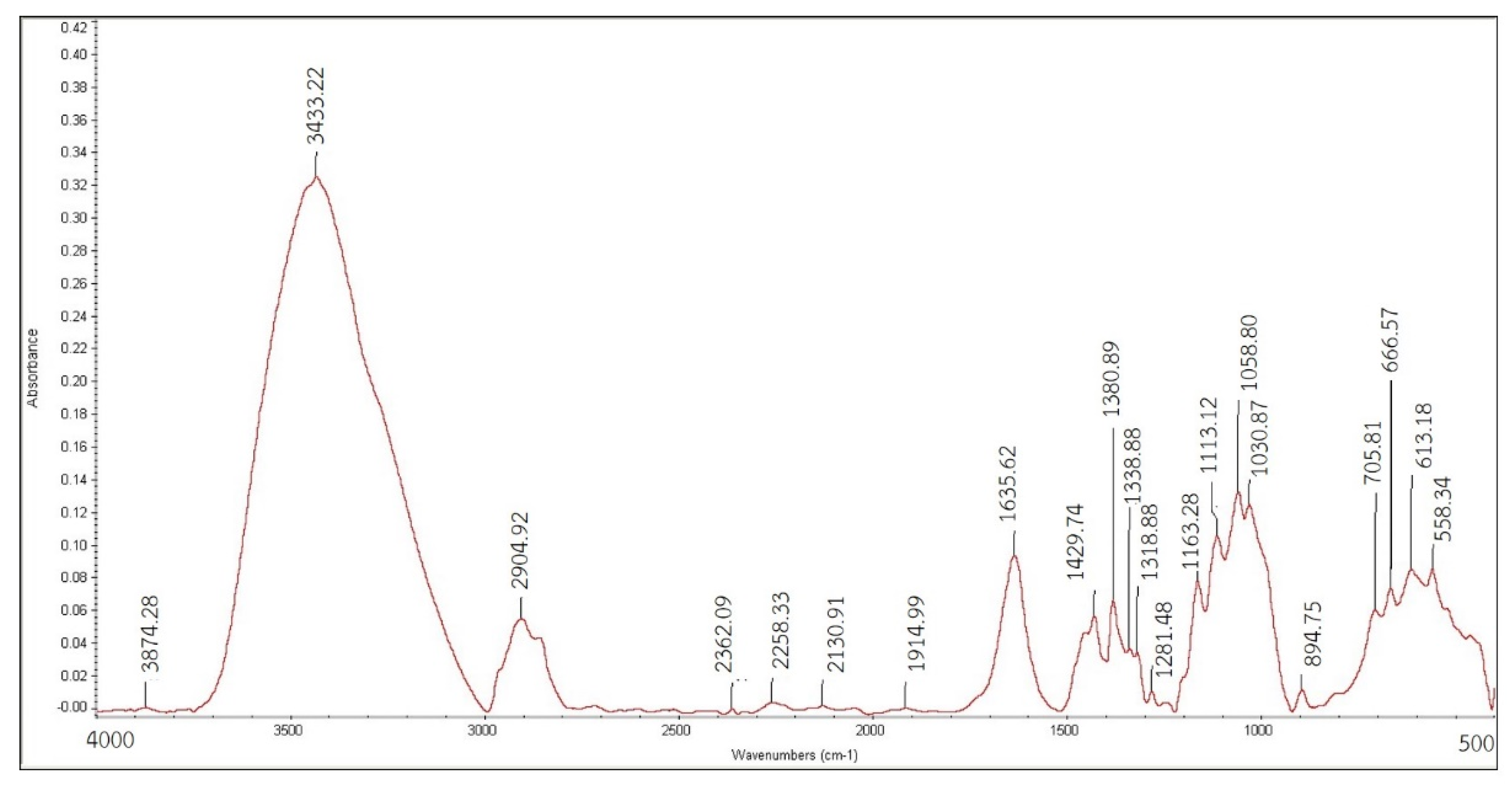
2.3.2. Infrared Radiation Absorption Property
| A | = | Absorbance |
| ε | = | Molar absorptivity unit dm3 cm−1 g−1 |
| c | = | Concentration unit g dm−3 or mol L−1 or molar |
| I0 | = | Light intensity |
| I | = | Light intensity when passing the sample |
| b | = | Thickness of the sample |
2.3.3. Basis Weight
| = | Basis weight (g/m2) | |
| m | = | Mass (g) |
| A | = | Area (m2) |
2.3.4. X-ray Computed Tomography
2.3.5. Mechanical Properties
3. Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shirvanimoghaddam, K.; Motamed, B.; Ramakrishna, S.; Naebe, M. Death by waste: Fashion and textile circular economy case. Sci. Total Environ. 2020, 718, 137317. [Google Scholar] [CrossRef] [PubMed]
- Papamichael, I.; Chatziparaskeva, G.; Pedreño, J.N.; Voukkali, I.; Candel, M.B.A.; Zorpas, A.A. Building a new mind set in tomorrow fashion development through circular strategy models in the framework of waste management. Curr. Opin. Green Sustain. Chem. 2022, 36, 100638. [Google Scholar] [CrossRef]
- Ikram, M. Transition toward green economy: Technological Innovation’s role in the fashion industry. Curr. Opin. Green Sustain. Chem. 2022, 37, 100657. [Google Scholar] [CrossRef]
- McCauley, E.; Jestratijevic, I. Exploring the Business Case for Textile-to-Textile Recycling Using Post-Consumer Waste in the US: Challenges and Opportunities. Sustainability 2023, 15, 1473. [Google Scholar] [CrossRef]
- Woodside, A.G.; Fine, M.B. Sustainable fashion themes in luxury brand storytelling: The sustainability fashion research grid. J. Glob. Fash. Mark. 2019, 10, 111–128. [Google Scholar] [CrossRef]
- Kozlowski, A.; Searcy, C.; Bardecki, M. The reDesign canvas: Fashion design as a tool for sustainability. J. Clean. Prod. 2018, 183, 194–207. [Google Scholar] [CrossRef]
- Patti, A.; Acierno, D. Towards the Sustainability of the Plastic Industry through Biopolymers: Properties and Potential Applications to the Textiles World. Polymers 2022, 14, 692. [Google Scholar] [CrossRef]
- Palme, A.; Peterson, A.; de la Motte, H.; Theliander, H.; Brelid, H. Development of an efficient route for combined recycling of PET and cotton from mixed fabrics. Text. Cloth. Sustain. 2017, 3, 4. [Google Scholar] [CrossRef] [Green Version]
- Hatch, K.L. Fiber Properties and Identification. In Textile Science; West Publishing: Eagan, MN, USA, 1993; pp. 108–127. [Google Scholar]
- Šajn, N. Environmental Impact of the Textile and Clothing Industry; European Parliamentary Research Service: Brussels, Belgium, 2019. [Google Scholar]
- Centobelli, P.; Abbate, S.; Nadeem, S.P.; Garza-Reyes, J.A. Slowing the fast fashion industry: An all-round perspective. Curr. Opin. Green Sustain. Chem. 2022, 38, 100684. [Google Scholar] [CrossRef]
- Nimkar, U. Sustainable chemistry: A solution to the textile industry in a developing world. Curr. Opin. Green Sustain. Chem. 2018, 9, 13–17. [Google Scholar] [CrossRef]
- Ouchi, A.; Toida, T.; Kumaresan, S.; Ando, W.; Kato, J. A new methodology to recycle polyester from fabric blends with cellulose. Cellulose 2010, 17, 215–222. [Google Scholar] [CrossRef]
- De Silva, R.; Wang, X.; Byrne, N. Recycling textiles: The use of ionic liquids in the separation of cotton polyester blends. RSC Adv. 2014, 4, 29094–29098. [Google Scholar] [CrossRef]
- Morley, N.J. Maximising Reuse and Recycling of UK Clothing and Textiles: A Report to the Department for Environment; Food and Rural Affairs, Oakdene Hollins Ltd.: Aylesbury, UK, 2009. [Google Scholar]
- Kaabel, S.; Arciszewski, J.; Borchers, T.H.; Therien, J.P.D.; Friščić, T.; Auclair, K. Solid-State Enzymatic Hydrolysis of Mixed PET/Cotton Textiles. ChemSusChem 2023, 16, e202201613. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Jung, S.; Lin, K.-Y.A.; Kwon, E.E.; Lee, J. Upcycling textile waste using pyrolysis process. Sci. Total Environ. 2023, 859, 160393. [Google Scholar] [CrossRef]
- Quartinello, F.; Vecchiato, S.; Weinberger, S.; Kremenser, K.; Skopek, L.; Pellis, A.; Guebitz, G.M. Highly Selective Enzymatic Recovery of Building Blocks from Wool-Cotton-Polyester Textile Waste Blends. Polymers 2018, 10, 1107. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.; Viana, A.; Silva, C.; Marques, E.F.; Azoia, N.G. Recycling of textile wastes, by acid hydrolysis, into new cellulosic raw materials. Waste Manag. 2022, 153, 99–109. [Google Scholar] [CrossRef]
- Jeihanipour, A.; Karimi, K.; Niklasson, C.; Taherzadeh, M.J. A novel process for ethanol or biogas production from cellulose in blended-fibers waste textiles. Waste Manag. 2010, 30, 2504–2509. [Google Scholar] [CrossRef]
- Wang, S.; Salmon, S. Progress toward Circularity of Polyester and Cotton Textiles. Sustain. Chem. 2022, 3, 376–403. [Google Scholar] [CrossRef]
- Wojnowska-Baryła, I.; Bernat, K.; Zaborowska, M. Strategies of Recovery and Organic Recycling Used in Textile Waste Management. Int. J. Environ. Res. Public Health 2022, 19, 5859. [Google Scholar] [CrossRef]
- Gholamzad, E.; Karimi, K.; Masoomi, M. Effective conversion of waste polyester–cotton textile to ethanol and recovery of polyester by alkaline pretreatment. Chem. Eng. J. 2014, 253, 40–45. [Google Scholar] [CrossRef]
- Bengtsson, J.; Peterson, A.; Idström, A.; de la Motte, H.; Jedvert, K. Chemical Recycling of a Textile Blend from Polyester and Viscose, Part II: Mechanism and Reactivity during Alkaline Hydrolysis of Textile Polyester. Sustainability 2022, 14, 6911. [Google Scholar] [CrossRef]
- Sanchis-Sebastiá, M.; Ruuth, E.; Stigsson, L.; Galbe, M.; Wallberg, O. Novel sustainable alternatives for the fashion industry: A method of chemically recycling waste textiles via acid hydrolysis. Waste Manag. 2021, 121, 248–254. [Google Scholar] [CrossRef]
- Ruuth, E.; Sanchis-Sebastiá, M.; Larsson, P.T.; Teleman, A.; Jiménez-Quero, A.; Delestig, S.; Sahlberg, V.; Salén, P.; Ortiz, M.S.; Vadher, S.; et al. Reclaiming the Value of Cotton Waste Textiles: A New Improved Method to Recycle Cotton Waste Textiles via Acid Hydrolysis. Recycling 2022, 7, 57. [Google Scholar] [CrossRef]
- Basak, P.; Das, P.; Biswas, S.; Biswas, N.C.; Mahapatra, G.K.D. Green Synthesis and Characterization of Gelatin-PVA Silver Nanocomposite Films for Improved Antimicrobial Activity. IOP Conf. Ser. Mater. Sci. Eng. 2018, 410, 012019. [Google Scholar] [CrossRef]
- Marambio-Jones, C.; Hoek, E.M.V. A review of the antibacterial effects of silver nanomaterials and potential implications for human health and the environment. J. Nanoparticle Res. 2010, 12, 1531–1551. [Google Scholar] [CrossRef]
- Ram, F.; Radhakrishnan, S.; Ambone, T.; Shanmuganathan, K. Highly Flexible Mechanical Energy Harvester Based on Nylon 11 Ferroelectric Nanocomposites. ACS Appl. Polym. Mater. 2019, 1, 1998–2005. [Google Scholar] [CrossRef]
- Joshi, K.M.; Shinde, D.R.; Nikam, L.K.; Panmand, R.; Sethi, Y.A.; Kale, B.B.; Chaskar, M.G. Fragmented lignin-assisted synthesis of a hierarchical ZnO nanostructure for ammonia gas sensing. RSC Adv. 2019, 9, 2484–2492. [Google Scholar] [CrossRef] [PubMed]
- Kalita, E.; Nath, B.K.; Agan, F.; More, V.; Deb, P. Isolation and characterization of crystalline, autofluorescent, cellulose nanocrystals from saw dust wastes. Ind. Crops Prod. 2015, 65, 550–555. [Google Scholar] [CrossRef]
- Onkarappa, S.B.; Bhat, N.S.; Parashuram, D.; Dutta, S. Catalytic Conversion of Biomass-Derived Carbohydrates into Levulinic Acid Assisted by a Cationic Surface Active Agent. ChemistrySelect 2019, 4, 13021–13024. [Google Scholar] [CrossRef]
- Bianchi, S.; Bartoli, F.; Bruni, C.; Fernandez-Avila, C.; Rodriguez-Turienzo, L.; Mellado-Carretero, J.; Spinelli, D.; Coltelli, M.-B. Opportunities and Limitations in Recycling Fossil Polymers from Textiles. Macromol 2023, 3, 120–148. [Google Scholar] [CrossRef]
- Daukantienė, V. Analysis of the sustainability aspects of fashion: A literature review. Text. Res. J. 2022, 93, 991–1002. [Google Scholar] [CrossRef]
- Bozaci, E.; Tağaç, A.A. Extraction and Characterization of New Cellulosic Fiber from Catalpa bignonioides Fruits for Potential Use in Sustainable Products. Polymers 2023, 15, 201. [Google Scholar] [CrossRef] [PubMed]
- Eyupoglu, S.; Merdan, N. Physicochemical Properties of New Plant Based Fiber from Lavender Stem. J. Nat. Fibers 2022, 19, 9248–9258. [Google Scholar] [CrossRef]
- Baskaran, P.G.; Kathiresan, M.; Senthamaraikannan, P.; Saravanakumar, S.S. Characterization of New Natural Cellulosic Fiber from the Bark of Dichrostachys Cinerea. J. Nat. Fibers 2018, 15, 62–68. [Google Scholar] [CrossRef]
- Thomas, S.K.; Parameswaranpillai, J.; Krishnasamy, S.; Begum, P.M.S.; Nandi, D.; Siengchin, S.; George, J.J.; Hameed, N.; Salim, N.V.; Sienkiewicz, N. A comprehensive review on cellulose, chitin, and starch as fillers in natural rubber biocomposites. Carbohydr. Polym. Technol. Appl. 2021, 2, 100095. [Google Scholar] [CrossRef]
- Żelaziński, T. Properties of Biocomposites from Rapeseed Meal, Fruit Pomace and Microcrystalline Cellulose Made by Press Pressing: Mechanical and Physicochemical Characteristics. Materials 2021, 14, 890. [Google Scholar] [CrossRef]
- Damayanti, H.-S. Strategic Possibility Routes of Recycled PET. Polymers 2021, 13, 1475. [Google Scholar] [CrossRef]
- Benyathiar, P.; Kumar, P.; Carpenter, G.; Brace, J.; Mishra, D.K. Polyethylene Terephthalate (PET) Bottle-to-Bottle Recycling for the Beverage Industry: A Review. Polymers 2022, 14, 2366. [Google Scholar] [CrossRef]
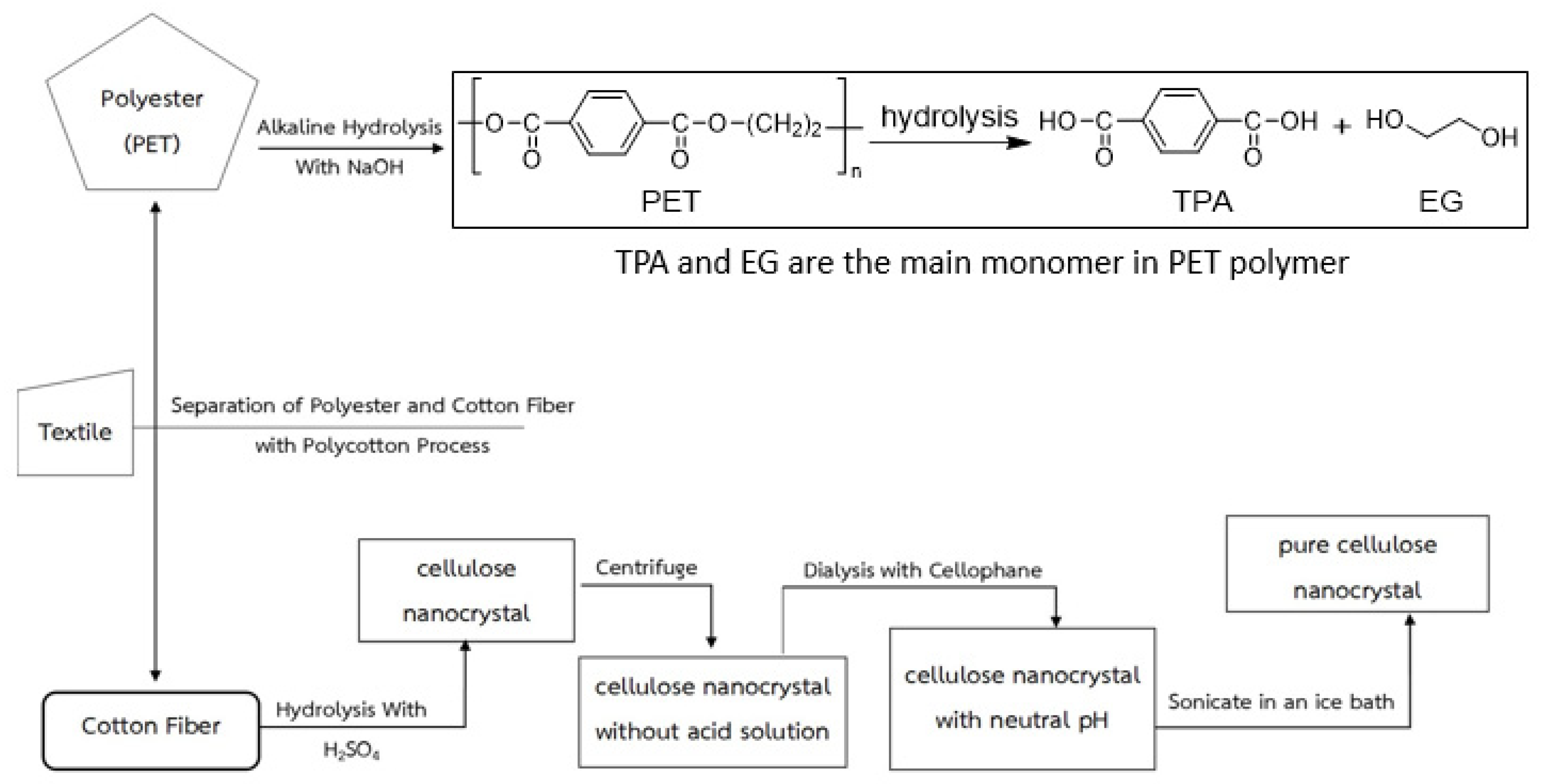


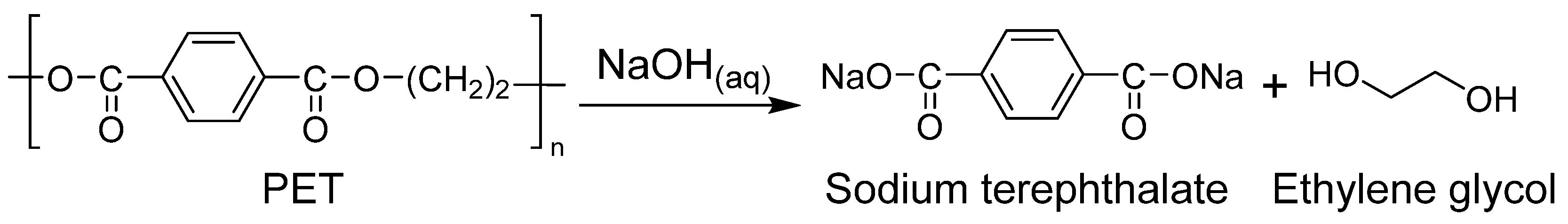
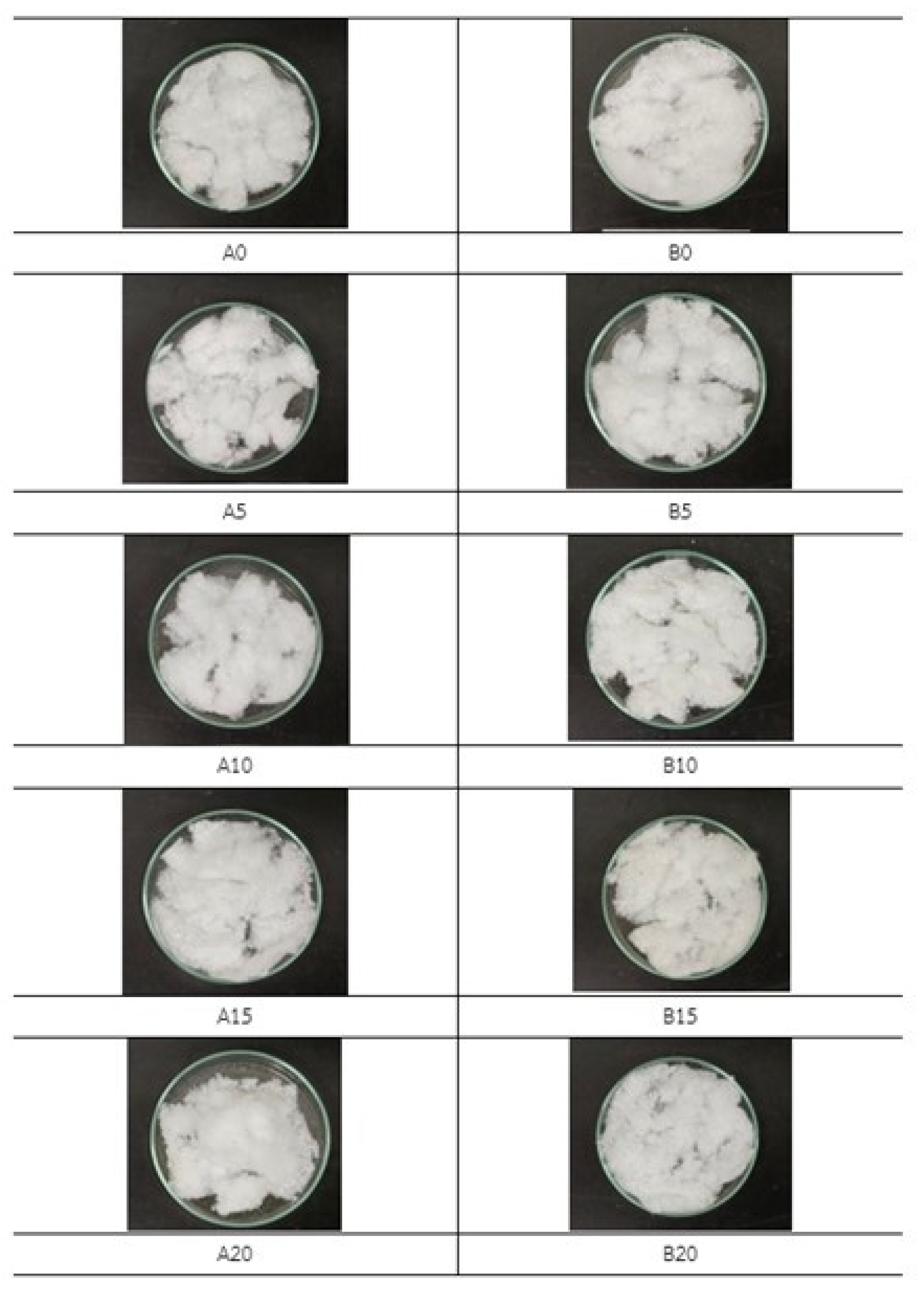


| No. of Samples | NaOH (%) | Temp. (°C) | Time (h) | PET Mixed Cellulose Yield (%) |
|---|---|---|---|---|
| A0 | 0 | 85 | 2 | 62.14 |
| A5 | 5 | 85 | 2 | 69.88 |
| A10 | 10 | 85 | 2 | 71.21 |
| A15 | 15 | 85 | 2 | 69.36 |
| A20 | 20 | 85 | 2 | 71.76 |
| B0 | 0 | 85 | 4 | 67.76 |
| B5 | 5 | 85 | 4 | 68.73 |
| B10 | 10 | 85 | 4 | 69.21 |
| B15 | 15 | 85 | 4 | 68.32 |
| B20 | 20 | 85 | 4 | 70.83 |
| Samples | Average Tensile (MPa) | Average Elogation (%) |
|---|---|---|
| PVA film incorporated with 5 wt% cellulose and 0.3 wt% AgNPs | 1.71 ± 0.38 | 43.90 ± 20.20 |
| PVA film incorporated with 5 wt% nanocellulose and 0.3 wt% AgNPs | 2.37 ± 0.32 | 214.26 ± 44.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srichola, P.; Witthayolankowit, K.; Sukyai, P.; Sampoompuang, C.; Lobyam, K.; Kampakun, P.; Toomtong, R. Recycling of Nanocellulose from Polyester–Cotton Textile Waste for Modification of Film Composites. Polymers 2023, 15, 3324. https://doi.org/10.3390/polym15153324
Srichola P, Witthayolankowit K, Sukyai P, Sampoompuang C, Lobyam K, Kampakun P, Toomtong R. Recycling of Nanocellulose from Polyester–Cotton Textile Waste for Modification of Film Composites. Polymers. 2023; 15(15):3324. https://doi.org/10.3390/polym15153324
Chicago/Turabian StyleSrichola, Preeyanuch, Kuntawit Witthayolankowit, Prakit Sukyai, Chaiyaporn Sampoompuang, Keowpatch Lobyam, Prapakorn Kampakun, and Raveewan Toomtong. 2023. "Recycling of Nanocellulose from Polyester–Cotton Textile Waste for Modification of Film Composites" Polymers 15, no. 15: 3324. https://doi.org/10.3390/polym15153324






