Interpolymer Complexes Based on Cellulose Ethers: Application
Abstract
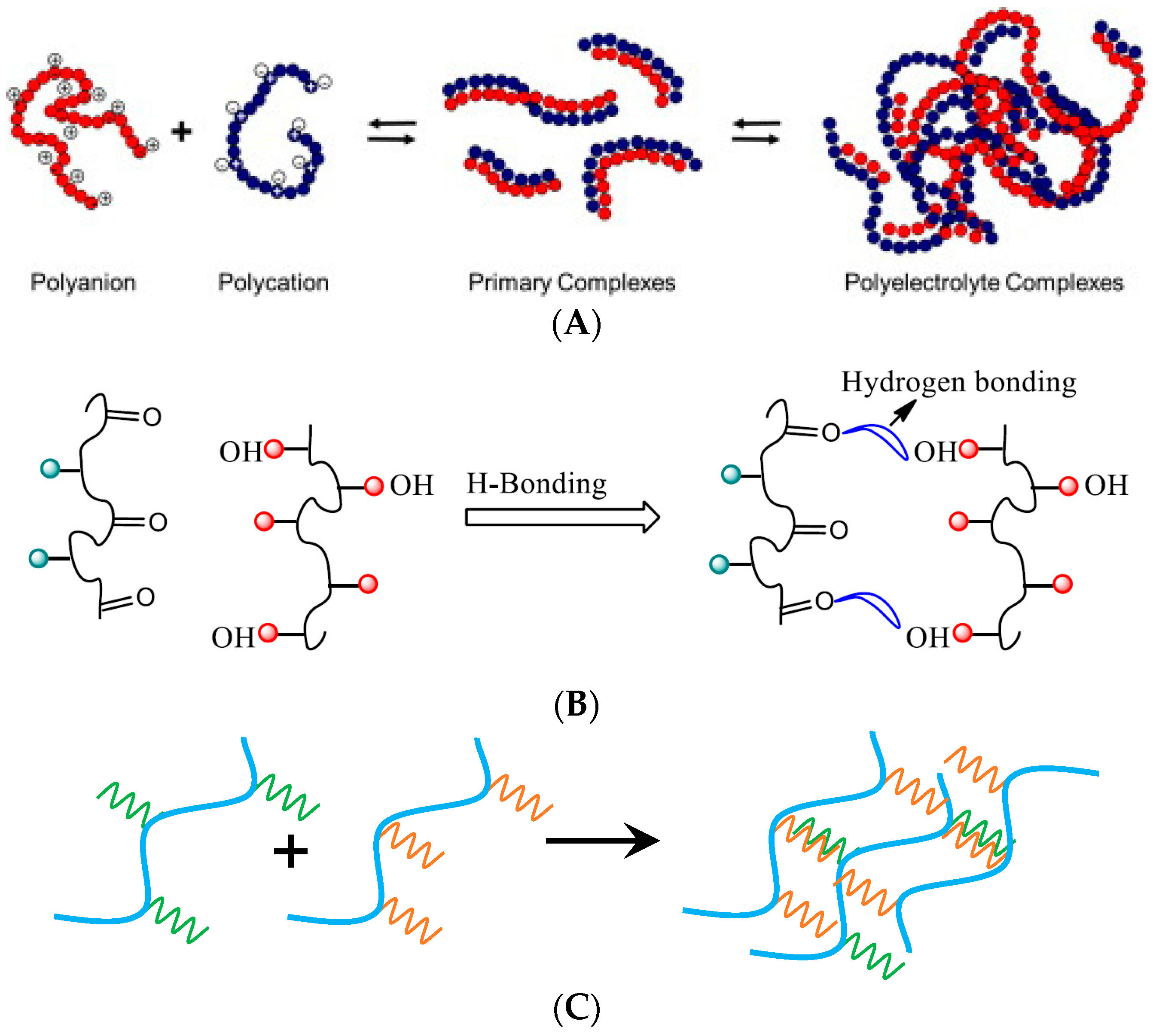
:1. Introduction
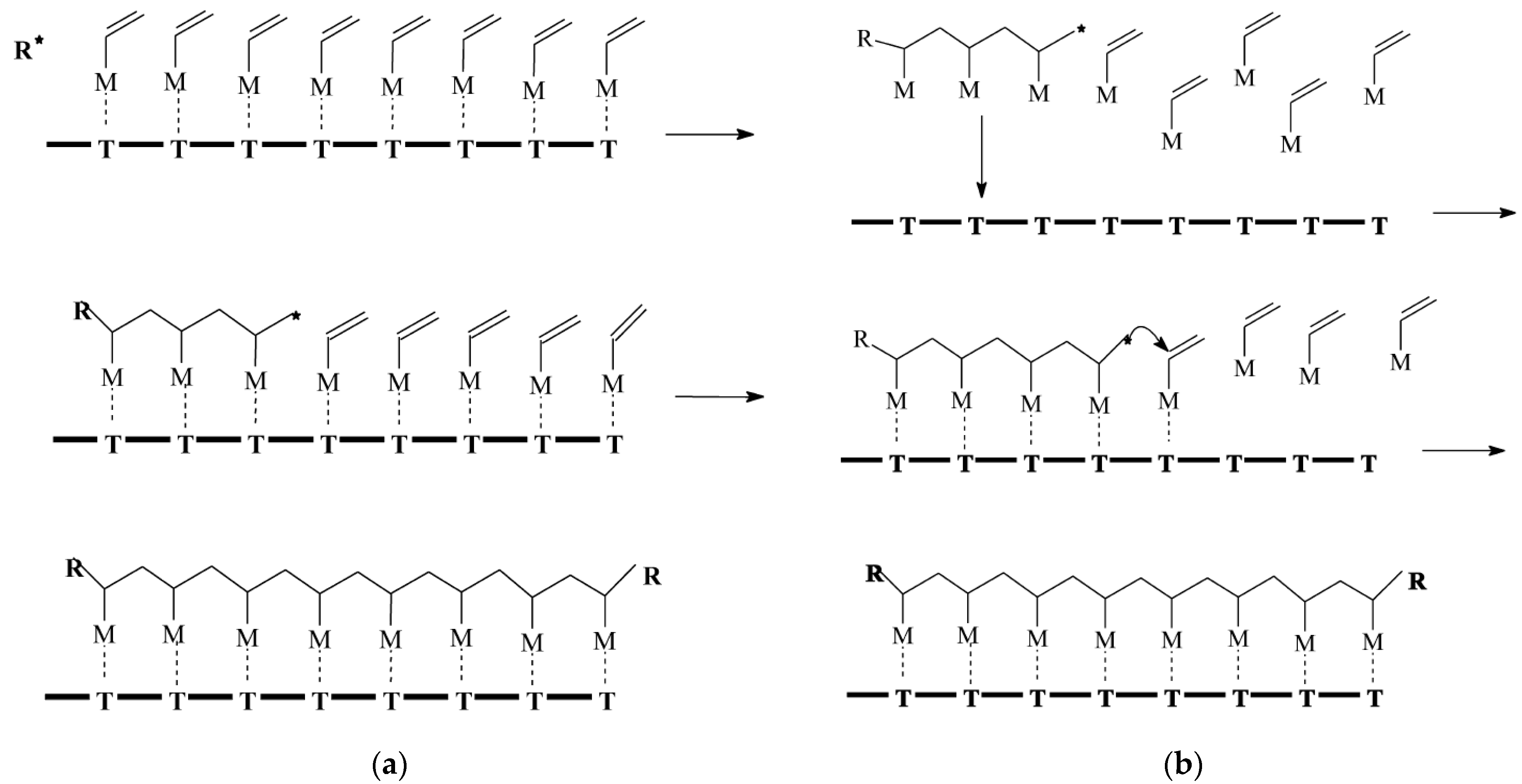
2. Methods of Obtaining
3. Methods of Studying IPCs
4. IPCs Based on Cellulose Ethers
5. Application of IPCs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kossel, A. Ueber Die Peptone Und Ihr Verhältniss Zu Den Eiweisskörpern. Pflüger Arch. Für Die Gesammte Physiol. Des Menschen Und Der Thiere 1880, 21, 179–184. [Google Scholar] [CrossRef]
- de Jong, H.G.B.; Winkler, K.C. Entmischung in Kristalloiden Neutralsalzlösungen, Analog Der Komplexkoazervation von Biokolloidsolen. Z. Für Anorg. Und Allg. Chem. 1937, 232, 119–132. [Google Scholar] [CrossRef]
- Holleman, L.W.J.; de Jong, H.G.B.; Modderman, R.S.T. Zur Kenntnis Der Lyophilen Kolloide-XXI. Mitteilung. Über Koazervation I: Einfache Koazervation von Gelatinesolen. Kolloid-Beihefte 1934, 39, 334–420. [Google Scholar] [CrossRef]
- Bungenberg de Jong, H.G.; Dekker, W.A.L. Zur Kenntnis Der Lyophilen Kolloide-XXVI. Mitteilung. Über Koazervation III: Komplexkoazervation Des Systems Gummiarabikum-Gelatine. Zweiter Teil. Kolloid-Beihefte 1936, 43, 213–271. [Google Scholar] [CrossRef]
- de Jong, H.G.B.; Dekker, W.A.L. Zur Kenntnis Der Lyophilen Kolloide-XXV. Mitteilung. Über Koazervation II: Komplexkoazervation Des Systems Gummiarabikum-Gelatine. Kolloid-Beihefte 1935, 43, 143–212. [Google Scholar] [CrossRef]
- Liquori, A.M.; Anzuino, G.; Coiro, V.M.; D’Alagni, M.; De Santis, P.; Savino, M. Complementary Stereospecific Interaction between Isotactic and Syndiotactic Polymer Molecules. Nature 1965, 206, 358–362. [Google Scholar] [CrossRef]
- Barone, G.; Crescenzi, V.; Liquori, A.M.; Quadrifoglio, F. Solubilization of Polycyclic Aromatic Hydrocarbons in Poly(Methacrylic Acid) Aqueous Solutions. Phys. Chem. 1967, 71, 2341–2345. [Google Scholar] [CrossRef]
- Bekturov, E.A.; Bimendina, L.A. Interpolymer Complexes. In Speciality Polymers; Springer: Berlin/Heidelberg, Germany, 1981; pp. 99–147. ISBN 978-3-540-38525-7. [Google Scholar]
- Kabanov, V.A.; Zezin, A.B. Soluble Interpolymeric Complexes as a New Class of Synthetic Polyelectrolytes. Pure Appl. Chem. 1984, 56, 343–354. [Google Scholar] [CrossRef] [Green Version]
- Baranovsky, V.Y.; Litmanovich, A.A.; Papisov, I.M.; Kabanov, V.A. Quantitative Studies of Interaction between Complementary Polymers and Oligomers in Solutions. Eur. Polym. J. 1981, 17, 969–979. [Google Scholar] [CrossRef]
- Kabanov, A.V.; Bronich, T.K.; Kabanov, V.A.; Yu, K.; Eisenberg, A. Soluble Stoichiometric Complexes from Poly(N-Ethyl-4-Vinylpyridinium) Cations and Poly(Ethylene Oxide)-Block-Polymethacrylate Anions. Macromolecules 1996, 29, 6797–6802. [Google Scholar] [CrossRef] [Green Version]
- Tsuchida, E.; Abe, K. Interactions Between Macromolecules in Solution and Intermacromolecular Complexes; Tsuchida, E., Abe, K., Eds.; Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 1982; Volume 45, ISBN 3-540-11624-9. [Google Scholar]
- Khutoryanskiy, V.V.; Smyslov, R.Y.; Yakimansky, A.V. Modern Methods for Studying Polymer Complexes in Aqueous and Organic Solutions. Polym. Sci. Ser. A 2018, 60, 553–576. [Google Scholar] [CrossRef]
- Pavlyuchenko, V.N.; Ivanchev, S.S. Composite Polymer Hydrogels. Polym. Sci. Ser. A 2009, 51, 743–760. [Google Scholar] [CrossRef]
- Bizley, S.C.; Williams, A.C.; Khutoryanskiy, V.V. Thermodynamic and Kinetic Properties of Interpolymer Complexes Assessed by Isothermal Titration Calorimetry and Surface Plasmon Resonance. Soft Matter 2014, 10, 8254–8260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumrudov, V.A. Self-Assembly and Molecular “Recognition” Phenomena in Solutions of (Bio)Polyelectrolyte Complexes. Russ. Chem. Rev. 2008, 77, 401–415. [Google Scholar] [CrossRef]
- Du, J.; Dai, J.; Liu, J.-L.; Dankovich, T. Novel PH-Sensitive Polyelectrolyte Carboxymethyl Konjac Glucomannan-Chitosan Beads as Drug Carriers. React. Funct. Polym. 2006, 66, 1055–1061. [Google Scholar] [CrossRef]
- Kabanov, V.A. Polyelectrolyte Complexes in Solution and in Bulk. Russ. Chem. Rev. 2005, 74, 3–20. [Google Scholar] [CrossRef]
- Putro, J.N.; Lunardi, V.B.; Soetaredjo, F.E.; Yuliana, M.; Santoso, S.P.; Wenten, I.G.; Ismadji, S. A Review of Gum Hydrocolloid Polyelectrolyte Complexes (PEC) for Biomedical Applications: Their Properties and Drug Delivery Studies. Processes 2021, 9, 1796. [Google Scholar] [CrossRef]
- Bourganis, V.; Karamanidou, T.; Kammona, O.; Kiparissides, C. Polyelectrolyte Complexes as Prospective Carriers for the Oral Delivery of Protein Therapeutics. Eur. J. Pharm. Biopharm. 2017, 111, 44–60. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V. Hydrogen-Bonded Interpolymer Complexes as Materials for Pharmaceutical Applications. Int. J. Pharm. 2007, 334, 15–26. [Google Scholar] [CrossRef]
- Yan, S.; Yao, L.; Kang, B.; Lee, J.Y. Solvent Effect on Hydrogen Bonded Tyr⋯Asp⋯Arg Triads: Enzymatic Catalyzed Model System. Comput. Biol. Chem. 2016, 65, 140–147. [Google Scholar] [CrossRef]
- Gadwal, I. A Brief Overview on Preparation of Self-Healing Polymers and Coatings via Hydrogen Bonding Interactions. Macromol 2020, 1, 18–36. [Google Scholar] [CrossRef]
- Anufrieva, E.V.; Krakovyak, M.G.; Nekrasova, T.N.; Smyslov, R.Y. Effect of Solvent on the Formation of Stereocomplexes in Poly(Methyl Methacrylate) Solutions. Polym. Sci. Ser. A 1996, 38, 190–193. [Google Scholar]
- Munim, S.A.; Raza, Z.A. Poly(Lactic Acid) Based Hydrogels: Formation, Characteristics and Biomedical Applications. J. Porous Mater. 2019, 26, 881–901. [Google Scholar] [CrossRef]
- De Geest, B.G.; Sukhorukov, G.B.; Möhwald, H. The Pros and Cons of Polyelectrolyte Capsules in Drug Delivery. Expert Opin. Drug Deliv. 2009, 6, 613–624. [Google Scholar] [CrossRef] [PubMed]
- Mazzoldi, P.; Carturan, S.; Quaranta, A.; Sada, C.; Sglavo, V.M. Ion Exchange Process: History, Evolution and Applications. Riv. del Nuovo Cim. 2013, 36, 397–460. [Google Scholar] [CrossRef]
- Pergushov, D.V.; Borisov, O.V.; Zezin, A.B.; Müller, A.H.E. Interpolyelectrolyte Complexes Based on Polyionic Species of Branched Topology. Adv. Polym. Sci. 2011, 241, 131–161. [Google Scholar] [CrossRef]
- Yashin, V.V.; Ermakov, I.V.; Litmanovich, A.D.; Plate, N.A. Theory of Macromolecular Reaction in the Polymer Mixture Complicated by Diffusion. Vysokomol. Soedin. Ser. A Ser. B Ser. C Kratk. Soobshcheniya 1994, 36, 955–963. [Google Scholar]
- Izumrudov, V.A.; Zezin, A.B.; Kabanov, V.A. Equilibria in Interpolyelectrolyte Reactions and the Phenomenon of Molecular “Recognition” in Solutions of Interpolyelectrolyte Complexes. Russ. Chem. Rev. 1991, 60, 792–806. [Google Scholar] [CrossRef]
- Kabanov, V.A.; Papisov, I.M. Formation of Complexes between Complementary Synthetic Polymers and Oligomers in Dilute Solution Review. Polym. Sci. USSR 1979, 21, 261–307. [Google Scholar] [CrossRef]
- Shovsky, A.; Varga, I.; Makuška, R.; Claesson, P.M. Formation and Stability of Water-Soluble, Molecular Polyelectrolyte Complexes: Effects of Charge Density, Mixing Ratio, and Polyelectrolyte Concentration. Langmuir 2009, 25, 6113–6121. [Google Scholar] [CrossRef]
- Zhang, Y.; Jin, Q.; Zhao, J.; Wu, C.; Fan, Q.; Wu, Q. Facile Fabrication of PH-Sensitive Core-Shell Nanoparticles Based on HEC and PMAA via Template Polymerization. Eur. Polym. J. 2010, 46, 1425–1435. [Google Scholar] [CrossRef]
- Połowiński, S. Template Polymerisation and Co-Polymerisation. Prog. Polym. Sci. 2002, 27, 537–577. [Google Scholar] [CrossRef]
- Papisov, I.M. Matrix Polymerization and Other Matrix and Pseudomatrix Processes as a Method to Obtain Composite Materials. Polym. Sci. Ser. B 1997, 39, 122–133. [Google Scholar]
- Novakov, I.A.; Navrotskij, A.V.; Navrotskii, A.V. Polymerization of 1,2-Dimethyl-5-Vinylpyridinium Methyl Sulfate and the Properties of the Resultant Polyelectrolytes. Polym. Sci. Ser. C 2002, 44, 49–62. [Google Scholar]
- Salimi, H.; Aryanasab, F.; Banazadeh, A.R.; Shabanian, M.; Seidi, F. Designing Syntheses of Cellulose and Starch Derivatives with Basic or Cationic N -Functions: Part I-Cellulose Derivatives. Polym. Adv. Technol. 2016, 27, 5–32. [Google Scholar] [CrossRef]
- Bekturov, E.A.; Bimendina, L.A. Complexes of Water-Soluble Polymers. J. Macromol. Sci. Part C 1997, 37, 501–518. [Google Scholar] [CrossRef]
- Panarin, E.F.; Kopejkin, V.V. Biological Activity of Synthetic Polyelectrolyte Complexes of Ionogenic Surfactants. Vysokomol. Soedin. Ser. A Ser. B Ser. C Kratk. Soobshcheniya 2002, 44, 2340–2351. [Google Scholar]
- Gazizov, A.; Zhumadilova, G.; Bimendina, L.; Kudaibergenov, S. Interpolymer Complexes of Some Vinyl Copolymers in a Solution and on the Boundary of Two Liquid Phases. Polymer 2000, 41, 5793–5797. [Google Scholar] [CrossRef]
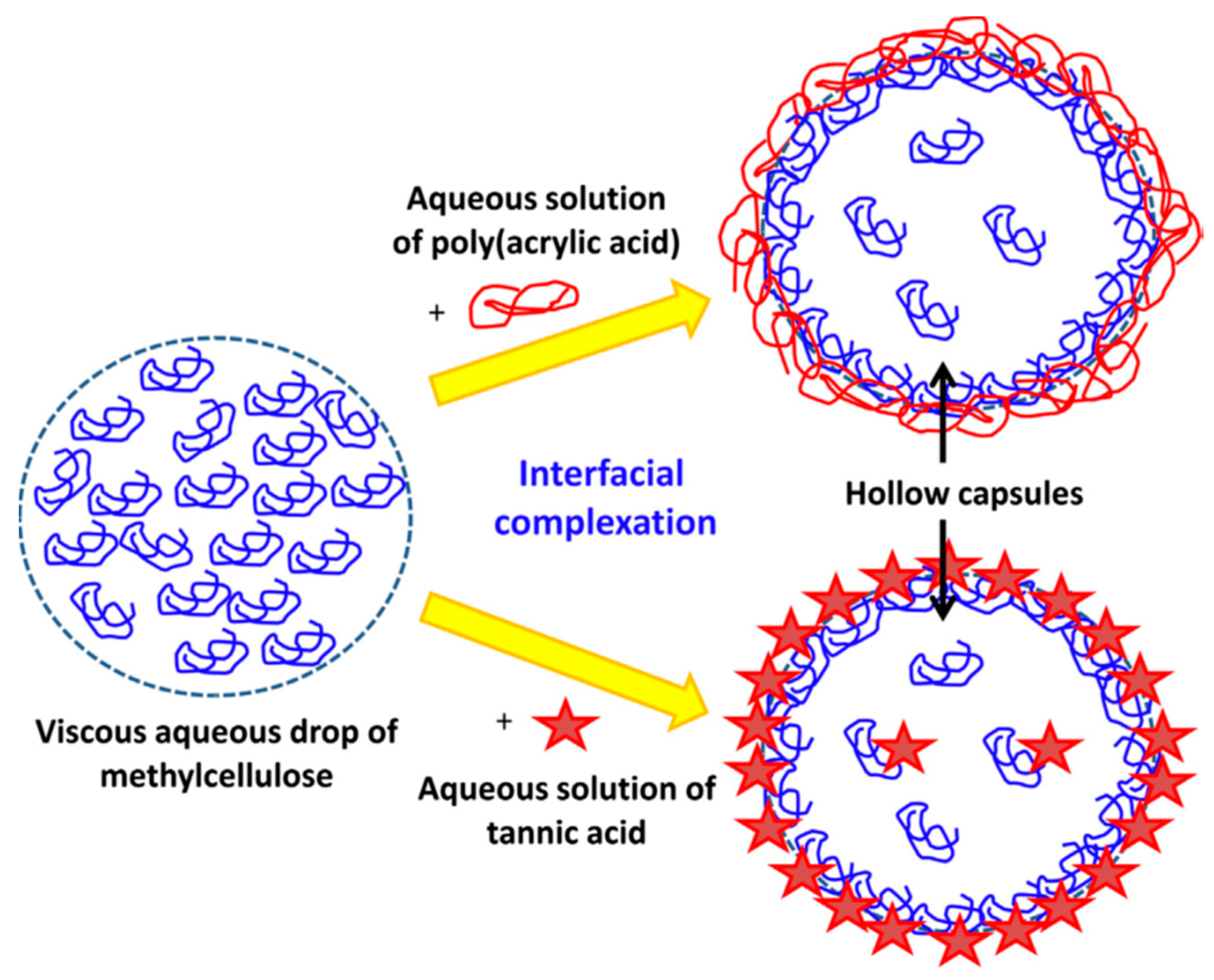
- Driver, K.; Baco, S.; Khutoryanskiy, V.V. Hollow Capsules Formed in a Single Stage via Interfacial Hydrogen-Bonded Complexation of Methylcellulose with Poly(Acrylic Acid) and Tannic Acid. Eur. Polym. J. 2013, 49, 4249–4256. [Google Scholar] [CrossRef]
- Piculell, L.; Thuresson, K.; Lindman, B. Mixed Solutions of Surfactant and Hydrophobically Modified Polymer. Polym. Adv. Technol. 2001, 12, 44–69. [Google Scholar] [CrossRef]
- De Geest, B.G.; De Koker, S.; Sukhorukov, G.B.; Kreft, O.; Parak, W.J.; Skirtach, A.G.; Demeester, J.; De Smedt, S.C.; Hennink, W.E. Polyelectrolyte Microcapsules for Biomedical Applications. Soft Matter 2009, 5, 282–291. [Google Scholar] [CrossRef]
- Khutoryanskaya, O.V.; Williams, A.C.; Khutoryanskiy, V.V. PH-Mediated Interactions between Poly(Acrylic Acid) and Methylcellulose in the Formation of Ultrathin Multilayered Hydrogels and Spherical Nanoparticles. Macromolecules 2007, 40, 7707–7713. [Google Scholar] [CrossRef]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Szwarc, M. Replica Polymerization. J. Polym. Sci. 1954, 13, 317–318. [Google Scholar] [CrossRef]
- Petrov, A.I.; Antipov, A.A.; Sukhorukov, G.B. Base−Acid Equilibria in Polyelectrolyte Systems: From Weak Polyelectrolytes to Interpolyelectrolyte Complexes and Multilayered Polyelectrolyte Shells. Macromolecules 2003, 36, 10079–10086. [Google Scholar] [CrossRef]
- Mun, G.A.; Khutoryanskiy, V.V.; Akhmetkalieva, G.T.; Shmakov, S.N.; Dubolazov, A.V.; Nurkeeva, Z.S.; Park, K. Interpolymer Complexes of Poly(Acrylic Acid) with Poly(2-Hydroxyethyl Acrylate) in Aqueous Solutions. Colloid Polym. Sci. 2004, 283, 174–181. [Google Scholar] [CrossRef]
- Bumbu, G.G.; Munteanu, B.S.; Eckelt, J.; Vasile, C. Influence of the Composition of Hydroxypropyl Cellulose/Maleic Acid-Alt-Styrene Copolymer Blends on Their Properties as Matrix for Drug Release. Express Polym. Lett. 2009, 3, 286–294. [Google Scholar] [CrossRef]
- Castro-Guerrero, C.F.; Morales-Cepeda, A.; Rivera-Armenta, J.; Mendoza-Martfnez, A.; Álvarez-Castillo, A. Gels from Acrylic Acid and Hydroxypropyl Cellulose via Free Radical Polymerization. E-Polymers 2008, 147, 1697–1704. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Shen, X.-Y.; Gong, G.-L.; Wang, D. Compatibility Studies with Blends Based on Hydroxypropylcellulose and Polyacrylonitrile. Carbohydr. Polym. 2008, 73, 191–200. [Google Scholar] [CrossRef]
- Liu, W.; Liu, Y.; Hao, X.; Zeng, G.; Wang, W.; Liu, R.; Huang, Y. Backbone-Collapsed Intra- and Inter-Molecular Self-Assembly of Cellulose-Based Dense Graft Copolymer. Carbohydr. Polym. 2012, 88, 290–298. [Google Scholar] [CrossRef]
- Zhang, R.; Chu, G.; Vasilyev, G.; Martin, P.; Camposeo, A.; Persano, L.; Pisignano, D.; Zussman, E. Hybrid Nanocomposites for 3D Optics: Using Interpolymer Complexes with Cellulose Nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 19324–19330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gärdlund, L.; Wågberg, L.; Norgren, M. New Insights into the Structure of Polyelectrolyte Complexes. J. Colloid Interface Sci. 2007, 312, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.M.F.; Sikdar, P.P.; Haque, B.; Bhuiyan, M.A.R.; Ali, A.; Islam, M.N. Cellulose-Based Hydrogel Materials: Chemistry, Properties and Their Prospective Applications. Prog. Biomater. 2018, 7, 153–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mun, G.A.; Khutoryanskiy, V.V.; Nurkeeva, Z.S.; Urkimbaeva, P.I.; Zhunuspaev, D. Stabilization of Water/n-Hexane Emulsions by Amphiphilic Copolymers Based on Vinyl Ethers and Their Polycomplexes with Poly(Acrylic Acid). J. Polym. Sci. Part B Polym. Phys. 2004, 42, 2625–2632. [Google Scholar] [CrossRef]
- Ambrogi, V.; Perioli, L.; Marmottini, F.; Moretti, M.; Lollini, E.; Rossi, C. Chlorhexidine MCM-41 Mucoadhesive Tablets for Topical Use. J. Pharm. Innov. 2009, 4, 156–164. [Google Scholar] [CrossRef]
- Saeedi, M.; Akbari, J.; Enayatifard, R.; Morteza-Semnani, K.; Tahernia, M.; Valizadeh, H. In Situ Cross-Linking of Polyanionic Polymers to Sustain the Drug Release from Theophylline Tablets. Iran. J. Pharm. Res. 2009, 8, 241–249. [Google Scholar] [CrossRef]
- Mizrahi, B.; Golenser, J.; Wolnerman, J.S.; Domb, A.J. Adhesive Tablet Effective for Treating Canker Sores in Humans. J. Pharm. Sci. 2004, 93, 2927–2935. [Google Scholar] [CrossRef]
- Mizrahi, B.; Domb, A.J. Mucoadhesive Tablet Releasing Iodine for Treating Oral Infections. J. Pharm. Sci. 2007, 96, 3144–3150. [Google Scholar] [CrossRef]
- Ameye, D.; Mus, D.; Foreman, P.; Remon, J.P. Spray-Dried Amioca® Starch/Carbopol® 974P Mixtures as Buccal Bioadhesive Carriers. Int. J. Pharm. 2005, 301, 170–180. [Google Scholar] [CrossRef]
- Ameye, D.; Pringels, E.; Foreman, P.; Remon, J.P.; Adriaensens, P.; Storme, L.; Gelan, J. Correlation between the Molecular Morphology and the Biocompatibility of Bioadhesive Carriers Prepared from Spray-Dried Starch/Carbopol® Blends. Polymer 2005, 46, 2338–2345. [Google Scholar] [CrossRef]
- Dou, H.; Jiang, M. Fabrication, Characterization and Drug Loading of PH-Dependent Multi-Morphological Nanoparticles Based on Cellulose. Polym. Int. 2007, 56, 1206–1212. [Google Scholar] [CrossRef]
- Ozeki, T.; Yuasa, H.; Okada, H. Controlled Release of Drug via Methylcellulose-Carboxyvinylpolymer Interpolymer Complex Solid Dispersion. AAPS PharmSciTech 2005, 6, E231–E236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perioli, L.; Ambrogi, V.; Rubini, D.; Giovagnoli, S.; Ricci, M.; Blasi, P.; Rossi, C. Novel Mucoadhesive Buccal Formulation Containing Metronidazole for the Treatment of Periodontal Disease. J. Control. Release 2004, 95, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.R.P.; Figueiredo, A.G.P.R.; Silva, N.H.C.S.; Barros-Timmons, A.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Antimicrobial Bacterial Cellulose Nanocomposites Prepared by in Situ Polymerization of 2-Aminoethyl Methacrylate. Carbohydr. Polym. 2015, 123, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Vuković, J.S.; Babić, M.M.; Antić, K.M.; Filipović, J.M.; Stojanović, S.T.; Najman, S.J.; Tomić, S.L. In Vitro Cytotoxicity Assessment of Intelligent Acrylate Based Hydrogels with Incorporated Copper in Wound Management. Mater. Chem. Phys. 2016, 175, 158–163. [Google Scholar] [CrossRef]
- Shim, J.-W.; Nho, Y.-C. Preparation of Poly(Acrylic Acid)-Chitosan Hydrogels by Gamma Irradiation and In Vitro Drug Release. J. Appl. Polym. Sci. 2003, 90, 3660–3667. [Google Scholar] [CrossRef]
- Nafee, N.A.; Boraie, N.A.; Ismail, F.A.; Mortada, L.M. Design and Characterization of Mucoadhesive Buccal Patches Containing Cetylpyridinium Chloride. Acta Pharm. 2003, 53, 199–212. [Google Scholar]
- Barzic, A.I.; Nechifor, C.D.; Stoica, I.; Dorohoi, D.O. On the Effects of UV Radiation on the Release Ability of Glucose Embedded in Hydroxypropyl Cellulose Films. J. Macromol. Sci. Part B Phys. 2016, 55, 575–590. [Google Scholar] [CrossRef]
- Musial, W. The Effect of Methylcellulose on Metronidazole Release from Polyacrylic Acid Hydrogels. Chem. Pharm. Bull. 2007, 55, 1141–1147. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Zhao, C.; Bai, Y.; Deng, M.; Shan, H.; Zhuang, X.; Chen, X.; Jing, X. Thermo- and PH-Responsive HPC-g-AA/AA Hydrogels for Controlled Drug Delivery Applications. Polymer 2011, 52, 676–682. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, D.; Mao, Z.; He, Y.; Hu, Y.; Wu, W.; Jiang, X. Synthesis of Hydroxypropylcellulose-Poly(Acrylic Acid) Particles with Semi-Interpenetrating Polymer Network Structure. Biomacromolecules 2008, 9, 2609–2614. [Google Scholar] [CrossRef] [PubMed]
- Khutoryanskiy, V.V.; Cascone, M.G.; Lazzeri, L.; Barbani, N.; Nurkeeva, Z.S.; Mun, G.A.; Bitekenova, A.B.; Dzhusupbekova, A.B. Hydrophilic Films Based on Blends of Poly(Acrylic Acid) and Poly(2-Hydroxyethyl Vinyl Ether): Thermal, Mechanical, and Morphological Characterization. Macromol. Biosci. 2003, 3, 117–122. [Google Scholar] [CrossRef]
- Khutoryanskaya, O.V.; Morrison, P.W.J.; Seilkhanov, S.K.; Mussin, M.N.; Ozhmukhametova, E.K.; Rakhypbekov, T.K.; Khutoryanskiy, V.V. Hydrogen-Bonded Complexes and Blends of Poly(Acrylic Acid) and Methylcellulose: Nanoparticles and Mucoadhesive Films for Ocular Delivery of Riboflavin. Macromol. Biosci. 2014, 14, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Adeleke, O.A. Premium Ethylcellulose Polymer Based Architectures at Work in Drug Delivery. Int. J. Pharm. X 2019, 1, 100023. [Google Scholar] [CrossRef]
- Vlaia, L.; Coneac, G.; Olariu, I.; Vlaia, V.; Lupuleasa, D. Cellulose-Derivatives-Based Hydrogels as Vehicles for Dermal and Transdermal Drug Delivery. In Emerging Concepts in Analysis and Applications of Hydrogels; InTech: Wien, Austria, 2016. [Google Scholar]
- Nurkeeva, Z.S.; Tyukova, I.S.; Suvorova, A.I.; Mun, G.A.; Dzhusupbekova, A.B.; Khutoryanskiy, V.V. Miscibility Studies in Poly(Methyl Vinyl Ether)/Hydroxypropylcellulose Binary System in Aqueous Solutions and Solid State. Carbohydr. Polym. 2005, 62, 80–86. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V.; Mun, G.A.; Nurkeeva, Z.S.; Dubolazov, A.V. PH and Salt Effects on Interpolymer Complexation via Hydrogen Bonding in Aqueous Solutions. Polym. Int. 2004, 53, 1382–1387. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V.; Dubolazov, A.V.; Mun, G.A. PH-and Ionic Strength Effects on Interpolymer Complexation via Hydrogen-Bonding. In Hydrogen-Bonded Interpolymer Complexes; World Scientific: Singapore, 2009; pp. 1–21. ISBN 978-981-270-785-7. [Google Scholar]
- Nurkeeva, Z.S.; Mun, G.A.; Khutoryanskiy, V.V. Interpolymer Complexes of Water-Soluble Nonionic Polysaccharides with Polycarboxylic Acids and Their Applications. Macromol. Biosci. 2003, 3, 283–295. [Google Scholar] [CrossRef]
- Khutoryanskaya, O.V.; Potgieter, M.; Khutoryanskiy, V.V. Multilayered Hydrogel Coatings Covalently-Linked to Glass Surfaces Showing a Potential to Mimic Mucosal Tissues. Soft Matter 2010, 6, 551–557. [Google Scholar] [CrossRef]
- Dubolazov, A.V.; Nurkeeva, Z.S.; Mun, G.A.; Khutoryanskiy, V.V. Design of Micoadhesive Polymeric Films Based on Blends of Poly(Acrylic Acid) and (Hydroxypropyl)Cellulose. Biomacromolecules 2006, 7, 1637–1643. [Google Scholar] [CrossRef]
- Vallet-Regi, M.; Rámila, A.; Del Real, R.P.; Pérez-Pariente, J. A New Property of MCM-41: Drug Delivery System. Chem. Mater. 2001, 13, 308–311. [Google Scholar] [CrossRef]
- Vincent, S.D.; Lilly, G.E. Clinical, Historic, and Therapeutic Features of Aphthous Stomatitis. Literature Review and Open Clinical Trial Employing Steroids. Oral Surg. Oral Med. Oral Pathol. 1992, 74, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Moles, M.A.; Morales-Garcia, P.; Rodrigues-Archilla, A. The Treatment of Oral Apthous Ulceration or Erosive Lichen Planus with Toppical Clobetasol Propionate in Three Preparations. A Clinical Study on 54 Patients (Lo Muzio et al.). J. Oral Pathol. Med. 2002, 31, 284–285. [Google Scholar] [CrossRef] [PubMed]
- Park, C.R.; Munday, D.L. Development and Evaluation of a Biphasic Buccal Adhesive Tablet for Nicotine Replacement Therapy. Int. J. Pharm. 2002, 237, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Christersson, L.A.; Zambon, J.J.; Genco, R.J. Dental Bacterial Plaques. Nature and Role in Periodontal Disease. J. Clin. Periodontol. 1991, 18, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Liu, H.; Yu, L.; Liu, X.; Chen, L.; Zhang, N. Developing Hydroxypropyl Methylcellulose/Hydroxypropyl Starch Blends for Use as Capsule Materials. Carbohydr. Polym. 2013, 98, 73–79. [Google Scholar] [CrossRef]
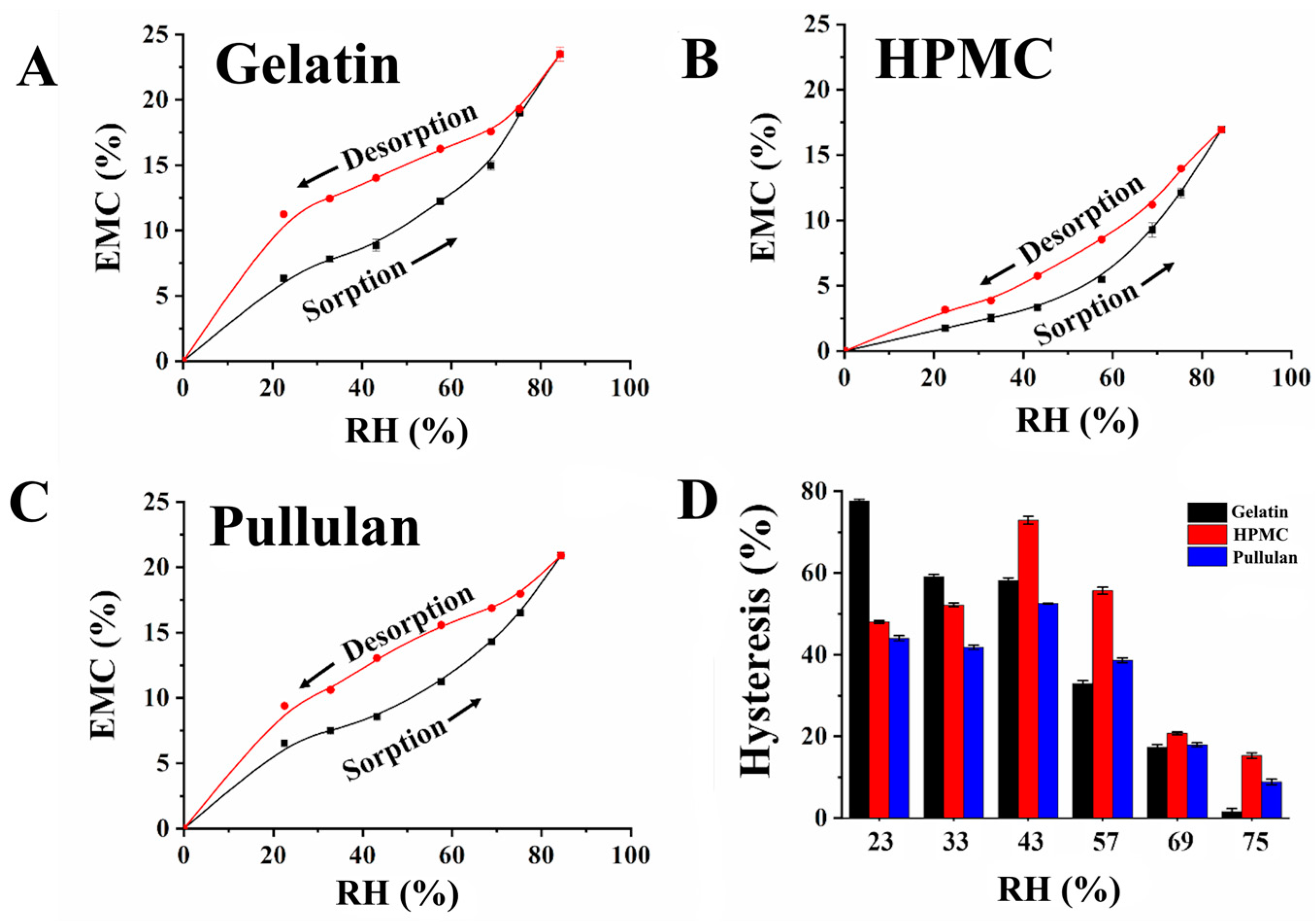
- Yang, N.; Chen, H.; Jin, Z.; Hou, J.; Zhang, Y.; Han, H.; Shen, Y.; Guo, S. Moisture Sorption and Desorption Properties of Gelatin, HPMC and Pullulan Hard Capsules. Int. J. Biol. Macromol. 2020, 159, 659–666. [Google Scholar] [CrossRef]
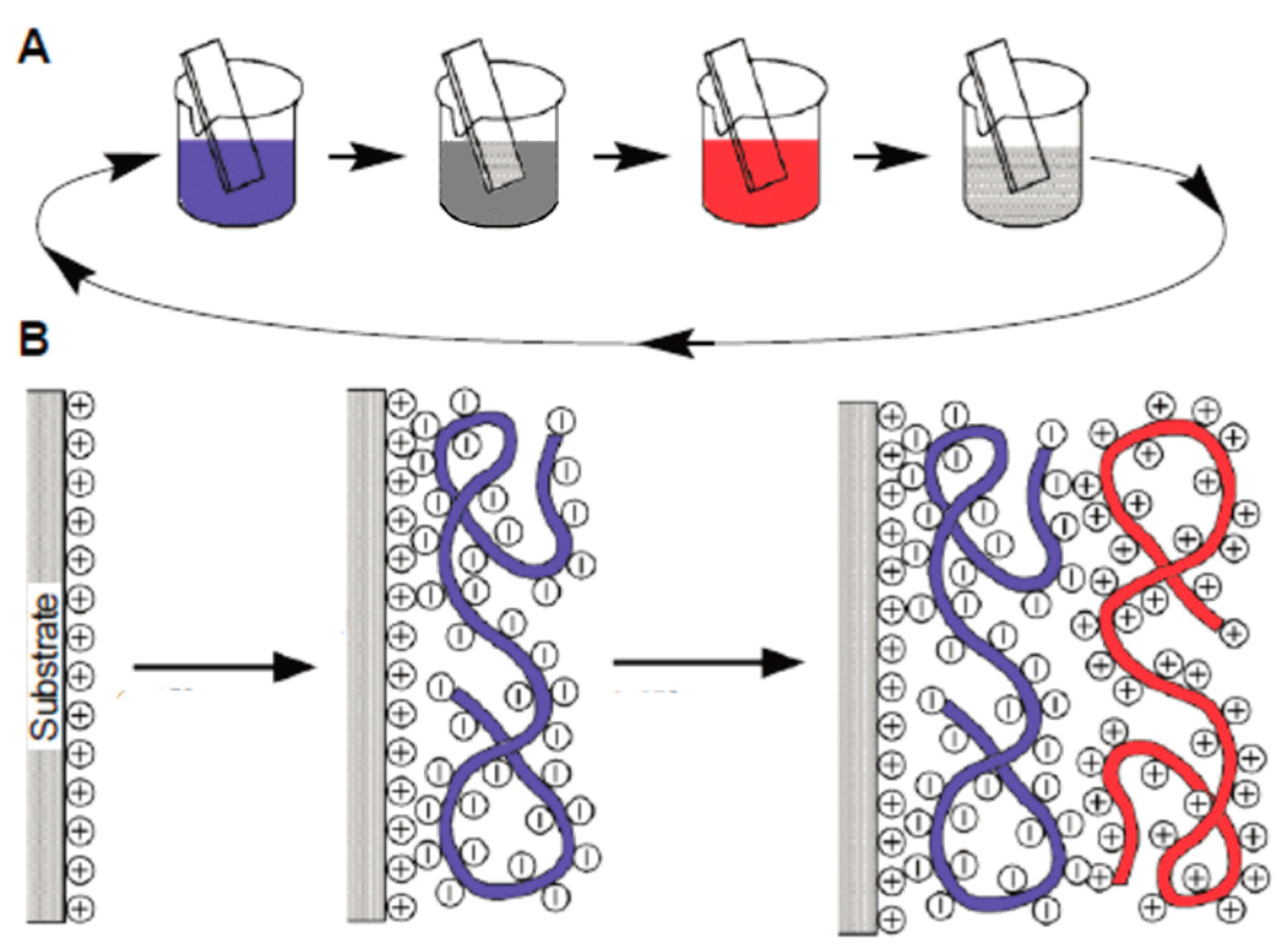
- Bizley, S.C.; Williams, A.C.; Kemp, F.; Khutoryanskiy, V.V. Optimizing Layer-by-Layer Deposition of Interpolymer Complexes on Solid Substrates Using Biacore. Soft Matter 2012, 8, 6782–6787. [Google Scholar] [CrossRef]
- Maleki, A.; Kjøniksen, A.L.; Nyström, B. Effect of Shear on Intramolecular and Intermolecular Association during Cross-Linking of Hydroxyethylcellulose in Dilute Aqueous Solutions. J. Phys. Chem. B 2005, 109, 12329–12336. [Google Scholar] [CrossRef] [Green Version]
- Maleki, A.; Beheshti, N.; Zhu, K.; Kjøniksen, A.L.; Nyström, B. Shrinking of Chemically Cross-Linked Polymer Networks in the Postgel Region. Polym. Bull. 2007, 58, 435–445. [Google Scholar] [CrossRef]
- Liao, J.; Zhu, C.; Gao, B.; Zhao, Z.; Liu, X.; Tian, L.; Zeng, Y.; Zhou, X.; Xie, Z.; Gu, Z. Multiresponsive Elastic Colloidal Crystals for Reversible Structural Color Patterns. Adv. Funct. Mater. 2019, 29, 1902954. [Google Scholar] [CrossRef]
- Guan, Y.; Zhang, Y.; Zhou, T.; Zhou, S. Stability of Hydrogen-Bonded Hydroxypropylcellulose/Poly(Acrylic Acid) Microcapsules in Aqueous Solutions. Soft Matter 2009, 5, 842–849. [Google Scholar] [CrossRef]
- Mun, G.A.; Nurkeeva, Z.S.; Khutoryanskij, V.V.; Dubolazov, A.V.; Khutoryanskiy, V.V.; Dubolazov, A.V. Effect of PH and Ionic Strength on Complex Formation between Poly(Acrylic Acid) and Hydroxyethyl Cellulose in Aqueous Solutions. Polym. Sci. Ser. B 2003, 45, 361–364. [Google Scholar]
- Yin, J.B.; Khutoryanskiy, V.V.; Mun, G.A.; Nurkeeva, Z.S. Polycomplexes and Film Compositions Based on Hydroxyethylcellulose and Poly(Acrylic Acid) as Systems for the Controlled Release of Levomycetin. Polym. Sci. Ser. A 2002, 44, 1094–1098. [Google Scholar]
- Zhang, Z.; Chen, L.; Deng, M.; Bai, Y.; Chen, X.J.X. Biodegradable Thermo- and PH-Responsive Hydrogels for Oral Drug Delivery. Wiley Period. Inc. J. Polym Sci. Part A Polym. Chem. 2011, 49, 2941–2951. [Google Scholar] [CrossRef]
- Nair, A.B.; Kumria, R.; Harsha, S.; Attimarad, M.; Al-Dhubiab, B.E.; Alhaider, I.A. In Vitro Techniques to Evaluate Buccal Films. J. Control. Release 2013, 166, 10–21. [Google Scholar] [CrossRef]
- Qian, H.; Yao, W.; Yu, S.; Chen, Y.; Wu, W.; Jiang, X. Spontaneous Formation of Giant Polymer Vesicles through a Nucleation and Growth Pathway. Chem. Asian J. 2012, 7, 1875–1880. [Google Scholar] [CrossRef]
- Khutoryanskaya, O.V.; Khutoryanskiy, V.V.; Pethrick, R.A. Characterisation of Blends Based on Hydroxyethylcellulose and Maleic Acid-Alt-Methyl Vinyl Ether. Macromol. Chem. Phys. 2005, 206, 1497–1510. [Google Scholar] [CrossRef]
- Ofori-Kwakye, K.; Fell, J.T. Leaching of Pectin from Mixed Films Containing Pectin, Chitosan and HPMC Intended for Biphasic Drug Delivery. Int. J. Pharm. 2003, 250, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Mangazbaeva, R.A.; Mun, G.A.; Nurkeeva, Z.S.; Khutoryanskiy, V.V. Interpolymer Complexes of Hydroxy-Propylmethylcellulose with Polycarboxylic Acids in Aqueous Solutions. Polym. Int. 2006, 55, 668–674. [Google Scholar] [CrossRef]
- Yao, R.; Xu, J.; Lu, X.; Deng, S. Phase Transition Behavior of HPMC-AA and Preparation of HPMC-PAA Nanogels. J. Nanomater. 2011, 2011, 507542. [Google Scholar] [CrossRef]
- Şakar-Deliormanli, A. Flow Behavior of Hydroxypropyl Methyl Cellulose/Polyacrylic Acid Interpolymer Complexes in Aqueous Media. Polym. Int. 2012, 61, 1751–1757. [Google Scholar] [CrossRef]
- Bumbu, G.G.; Vasile, C.; Chitanu, G.C.; Staikos, G. Interpolymer Complexes between Hydroxypropylcellulose and Copolymers of Maleic Acid: A Comparative Study. Macromol. Chem. Phys. 2005, 206, 540–546. [Google Scholar] [CrossRef]
- Bumbu, G.G.; Vasile, C.; Eckelt, J.; Wolf, B.A. Investigation of the Interpolymer Complex between Hydroxypropyl Cellulose and Maleic Acid-Styrene Copolymer, 1: Dilute Solutions Studies. Macromol. Chem. Phys. 2004, 205, 1869–1876. [Google Scholar] [CrossRef]
- Gong, G.-L.; Li, H.; Li, X. yan Studies on Swelling Kinetics for HPC/PAN Thermo-Sensitive Blending Films. Polym. Adv. Technol. 2011, 22, 1422–1426. [Google Scholar] [CrossRef]
- Yoo, D.; Shiratori, S.S.; Rubner, M.F. Controlling Bilayer Composition and Surface Wettability of Sequentially Adsorbed Multilayers of Weak Polyelectrolytes. Macromolecules 1998, 31, 4309–4318. [Google Scholar] [CrossRef]
- Schoeler, B.; Poptoshev, E.; Caruso, F. Growth of Multilayer Films of Fixed and Variable Charge Density Polyelectrolytes: Effect of Mutual Charge and Secondary Interactions. Macromolecules 2003, 36, 5258–5264. [Google Scholar] [CrossRef]
- Li-Bin, D.; Dong-Qing, Z.; Shou-Ping, L.; Yun-Xiang, Z. Effects of Ethylene Oxide Spacer Length on Solution Properties of Water-Soluble Fluorocarbon-Containing Hydrophobically Associat-Ing Poly (Acrylic Acid-Co-Rf-PEG Macromonomer). Chin. J. Chem. 2003, 2, 698–705. [Google Scholar]
- Zhang, X.; Lin, F.; Yuan, Q.; Zhu, L.; Wang, C.; Yang, S. Hydrogen-Bonded Thin Films of Cellulose Ethers and Poly(Acrylic Acid). Carbohydr. Polym. 2019, 215, 58–62. [Google Scholar] [CrossRef]
- Babiker, D.M.D.; Zhu, L.; Yagoub, H.; Xu, X.; Zhang, X.; Shibraen, M.H.M.A.; Yang, S. Hydrogen-Bonded Methylcellulose/Poly(Acrylic Acid) Complex Membrane for Oil-Water Separation. Surf. Coatings Technol. 2019, 367, 49–57. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, M.; Zhao, J.; Wang, Z.; Dou, H.; Chen, D. PH-Responsive Core-Shell Particles and Hollow Spheres Attained by Macromolecular Self-Assembly. Langmuir 2005, 21, 1531–1538. [Google Scholar] [CrossRef]
- Dou, H.J.; Yang, W.H.; Sun, K. A Facile Fabrication to Cellulose-Based Nanoparticles with Thermo-Responsivity and Carboxyl Functional Groups. Chem. Lett. 2006, 35, 1374–1375. [Google Scholar] [CrossRef]
- Liao, Q.; Shao, Q.; Wang, H.; Qiu, G.; Lu, X. Hydroxypropylcellulose Templated Synthesis of Surfactant-Free Poly(Acrylic Acid) Nanogels in Aqueous Media. Carbohydr. Polym. 2012, 87, 2648–2654. [Google Scholar] [CrossRef]
- Liao, Q.; Shao, Q.; Qiu, G.; Lu, X. Methacrylic Acid-Triggered Phase Transition Behavior of Thermosensitive Hydroxypropylcellulose. Carbohydr. Polym. 2012, 89, 1301–1304. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Sun, K.; Yang, W. The Self-Assembly of Hydroxypropylcellulose and Carboxyl-Ended Surfactants to Multi-Morphological Nanoparticles. Macromol. Chem. Phys. 2006, 207, 1899–1904. [Google Scholar] [CrossRef]
- Khamitova, T.O.; Burkeev, M.Z.; Havlicek, D. Synthesis and Study of the Properties of Polymer-Immobilized Silver and Nickel Nanoparticles. Bull. L.N. Gumilyov Eurasian Natl. Univ. Chem. Geogr. Ecol. Ser. 2022, 141, 7–18. [Google Scholar] [CrossRef]
- El-Hag Ali, A.; Abd El-Rehim, H.A.; Kamal, H.; Hegazy, D.E.-S.A. Synthesis of Carboxymethyl Cellulose Based Drug Carrier Hydrogel Using Ionizing Radiation for Possible Use as Site Specific Delivery System. J. Macromol. Sci. Part A Pure Appl. Chem. 2008, 45, 628–634. [Google Scholar] [CrossRef]
- Ohno, T.; Yoshizawa, S.; Miyashita, Y.; Nishio, Y. Interaction and Scale of Mixing in Cellulose Acetate/Poly(N-Vinyl Pyrrolidone-Co-Vinyl Acetate) Blends. Cellulose 2005, 12, 281–291. [Google Scholar] [CrossRef]
- Ishida, M.; Machida, Y.; Nambu, N.; Nagai, T. New Mucosal Dosage Form of Insulin. Chem. Pharm. Bull. 1981, 29, 810–816. [Google Scholar] [CrossRef] [Green Version]
- Ishida, M.; Nambu, N.; Nagai, T. Highly Viscous Gel Ointment Containing Carbopol for Application to the Oral Mucosa. Chem. Pharm. Bull. 1983, 31, 4561–4564. [Google Scholar] [CrossRef] [Green Version]
- Nikolaeva, O.; Budtova, T.; Alexeev, V.; Frenkel, S. Interpolymer Complexation between Polyacrylic Acid and Cellulose Ethers: Formation and Properties. J. Polym. Sci. Part B Polym. Phys. 2000, 38, 1323–1330. [Google Scholar] [CrossRef]
- Nikolaeva, O.; Budtova, T.; Brestkin, Y.; Zoolshoev, Z.; Frenkel, S. Rheological Properties of an Interpolymer Complex Formed between Poly(Acrylic Acid) and Methyl Cellulose. J. Appl. Polym. Sci. 1999, 72, 1523–1528. [Google Scholar] [CrossRef]
- Khutoryanskiy, V.V.; Cascone, M.G.; Lazzeri, L.; Nurkeeva, Z.S.; Mun, G.A.; Mangazbaeva, R.A. Phase Behaviour of Methylcellulose-Poly(Acrylic Acid) Blends and Preparation of Related Hydrophilic Films. Polym. Int. 2003, 52, 62–67. [Google Scholar] [CrossRef]
- Dang, F.; Hasegawa, T.; Biju, V.; Ishikawa, M.; Kaji, N.; Yasui, T.; Baba, Y. Spontaneous Adsorption on a Hydrophobic Surface Governed by Hydrogen Bonding. Langmuir 2009, 25, 9296–9301. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.L.; Xu, G.Y.; Cai, Z.T.; Jiang, Y.S. Molecular Simulation Studies on the Interaction between Different Polymers in Aqueous Solution. Colloid Polym. Sci. 2003, 281, 66–72. [Google Scholar] [CrossRef]
- Ma, S.; Qi, X.; Cao, Y.; Yang, S.; Xu, J. Hydrogen Bond Detachment in Polymer Complexes. Polymer 2013, 54, 5382–5390. [Google Scholar] [CrossRef]

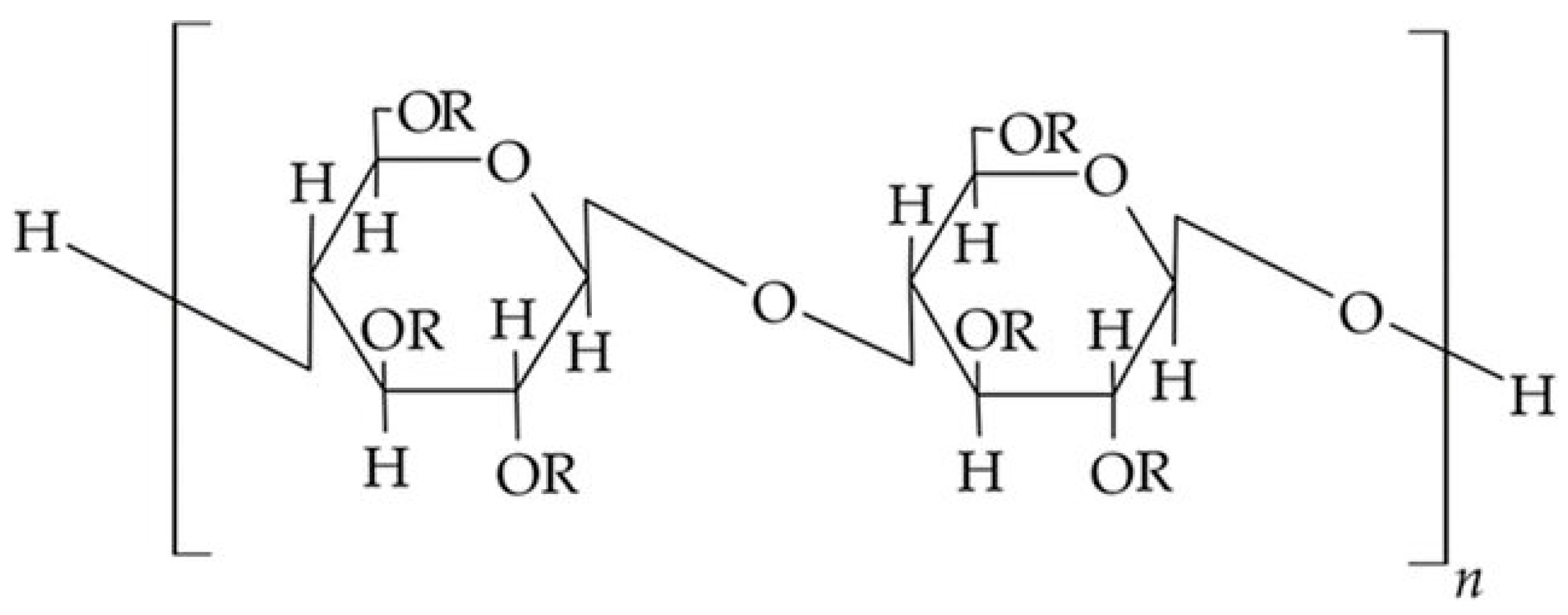
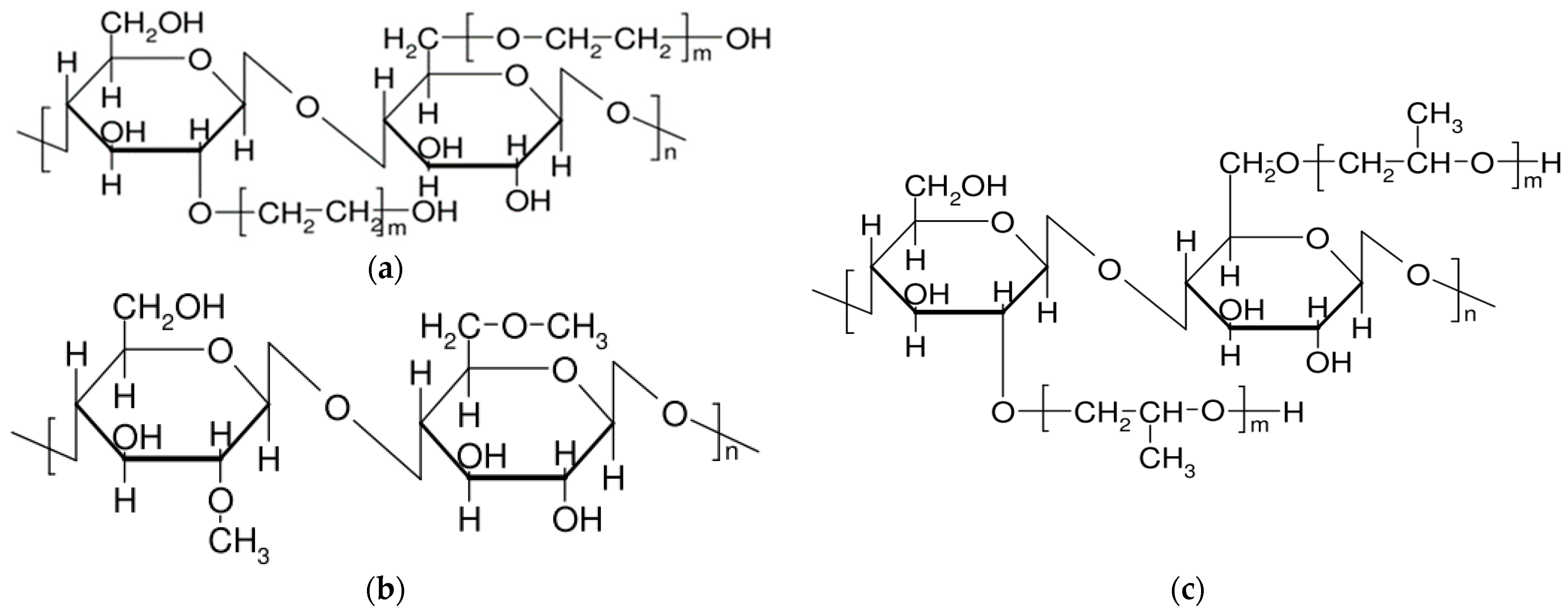
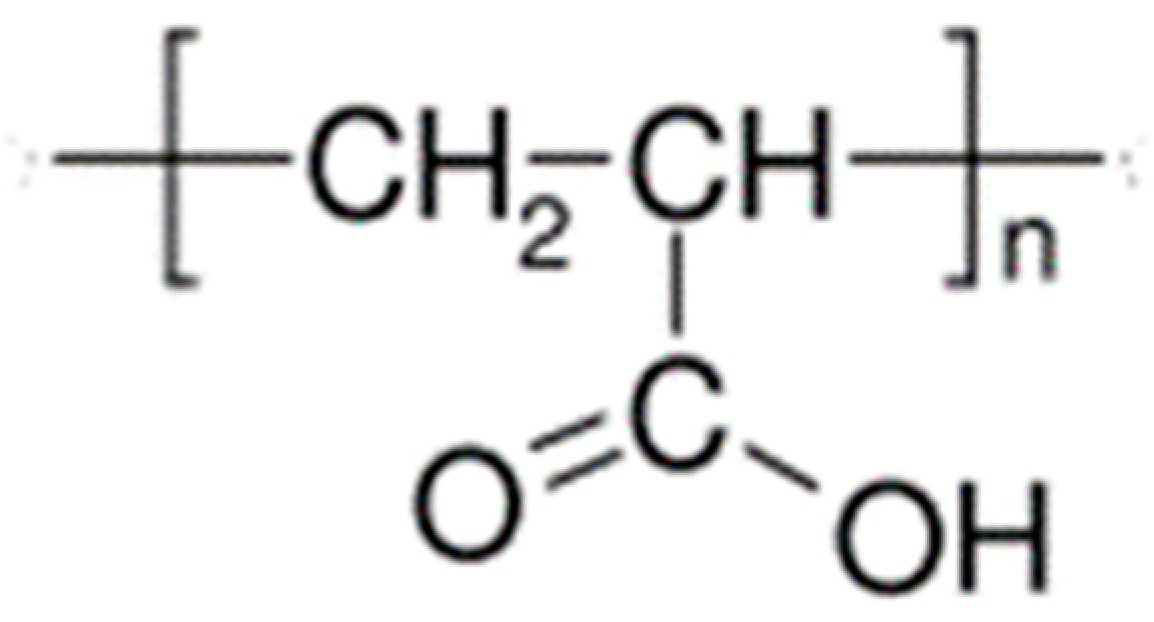
| Cellulose Esters | R Groups | Cellulose Ethers | R Groups |
|---|---|---|---|
| Acetate | H, I | Methylcellulose | H, CH3 |
| Acetate trimellitate | H, I, II | Ethyl cellulose | H, CH2CH3 |
| Acetate phthalate | I, III | Hydroxyethylmethyl cellulose | H, CH3, [CH2CH2O]nH |
| Hydroxypropyl methyl phthalate | H, CH3, CH2CH(OH)CH3, III, IV | Hydroxypropyl cellulose | H, [CH2CH(CH3)O]nH |
| Hydroxypropyl methyl phthalate acetate succinate | H, CH3, CH2CH(OH)CH3, I, V | Carboxymethylcellulose | H, CH2COONa |
| Dosage Forms | Drugs and Medicinal Products | Links |
|---|---|---|
| Mucoadhesive tablets | Metronidazole; chlorhexidine diacetate; theophylline; magnesium chloride; diminazene; phenacetin; iodine. | [56,57,58,59,60,61,62,63,64,65] |
| Medical patches | Antimicrobial biomaterials; 5-fluorouracil; cetylpyridinium chloride. | [66,67,68,69,70] |
| Hydrogels | Metronidazole; oxaliplatin, Voltaren Emulgel (carbomer and propylene glycol alginate) | [71,72,73] |
| Eye drops | Riboflavin | [74,75] |
| Topical creams and ointments: | Diclofenac sodium topical gel | [76] |
| Inhalation products | Beclomethasone dipropionate inhaler | [77] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keldibekova, R.; Suleimenova, S.; Nurgozhina, G.; Kopishev, E. Interpolymer Complexes Based on Cellulose Ethers: Application. Polymers 2023, 15, 3326. https://doi.org/10.3390/polym15153326
Keldibekova R, Suleimenova S, Nurgozhina G, Kopishev E. Interpolymer Complexes Based on Cellulose Ethers: Application. Polymers. 2023; 15(15):3326. https://doi.org/10.3390/polym15153326
Chicago/Turabian StyleKeldibekova, Raushan, Symbat Suleimenova, Gulden Nurgozhina, and Eldar Kopishev. 2023. "Interpolymer Complexes Based on Cellulose Ethers: Application" Polymers 15, no. 15: 3326. https://doi.org/10.3390/polym15153326