Synthesis, Characterization, and Potential Application of Cyclodextrin-Based Polyrotaxanes for Reinforced Atelocollagen Threads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
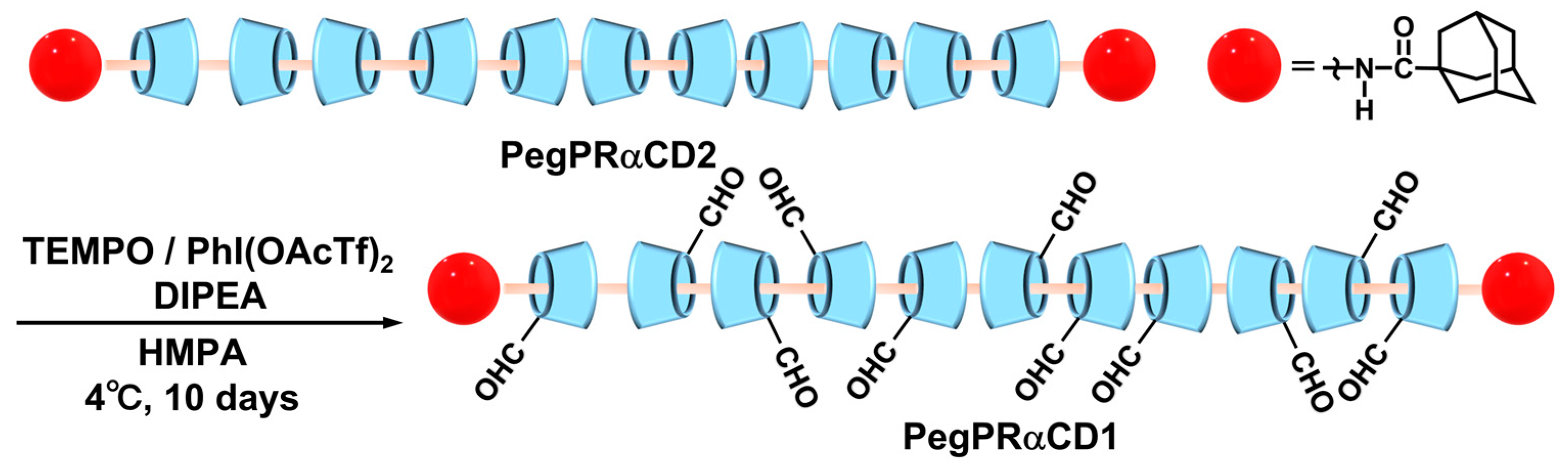
2.2. PegPRαCD1 General Synthesis Procedure
2.3. Synthesis of PluPRβCD1
2.3.1. PluPRβCD3 General Synthesis Procedure
2.3.2. PluPRβCD2 General Synthesis Procedure
2.3.3. PluPRβCD1 General Synthesis Procedure
2.4. Quantification of Aldehyde Groups Using DMHZ
2.5. Preparation of PRATs
2.6. Tensile Testing
2.7. Analytical Methods and Apparatus
3. Results and Discussion
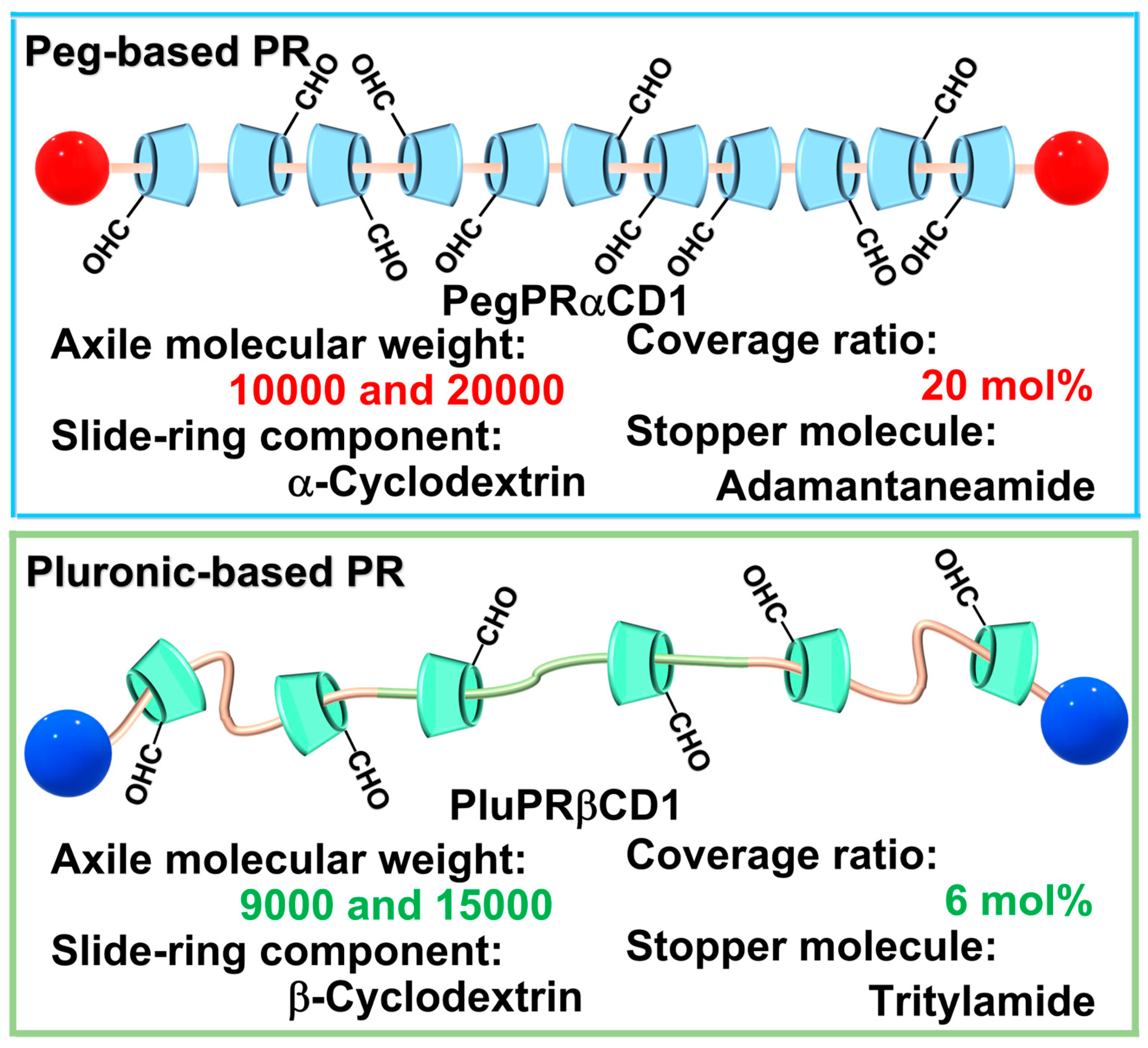
3.1. Synthesis and Characterization of PegPRαCD1
3.2. Synthesis and Characterization of PluPRβCD1
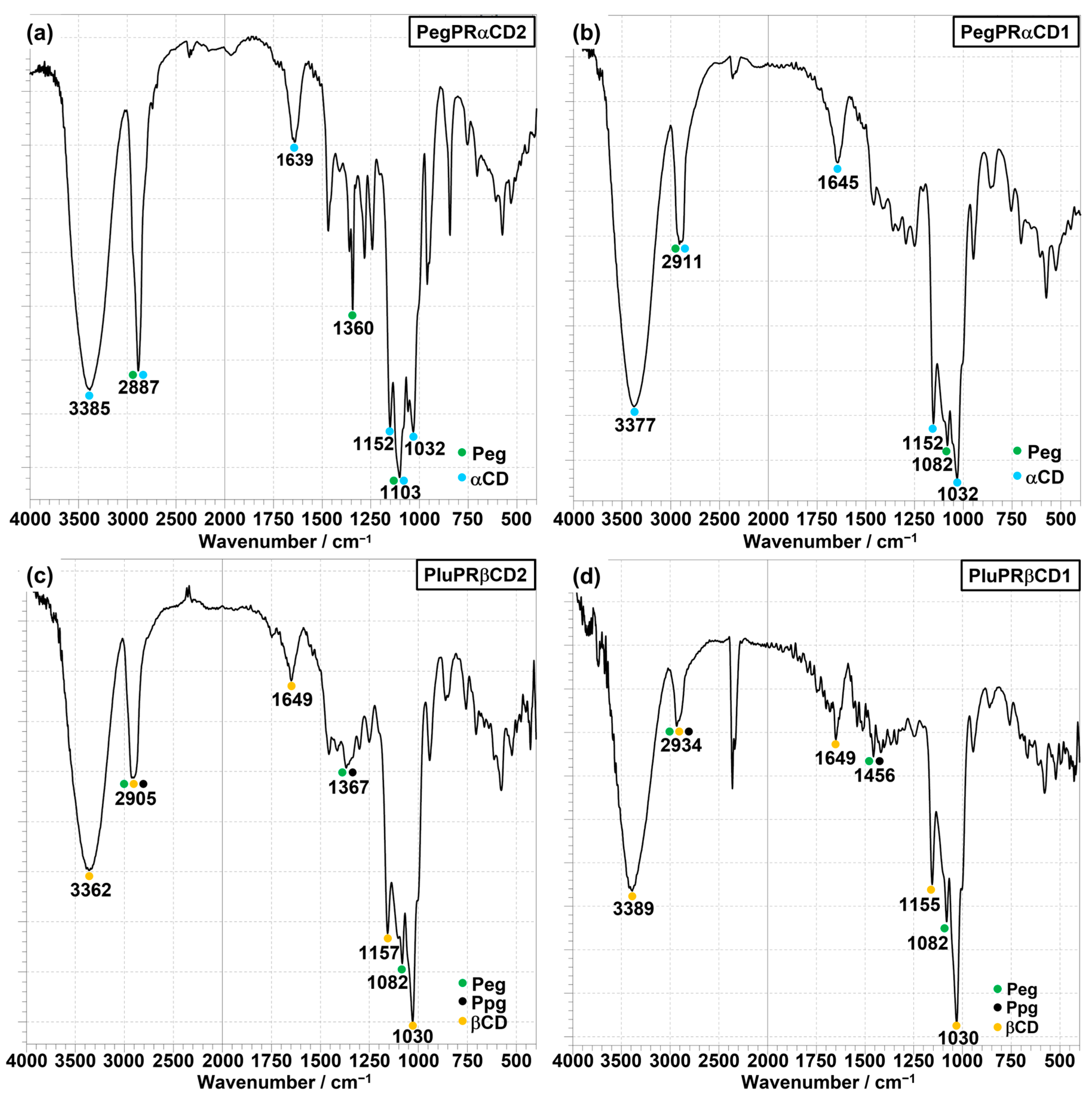
3.3. FT–IR Measurements
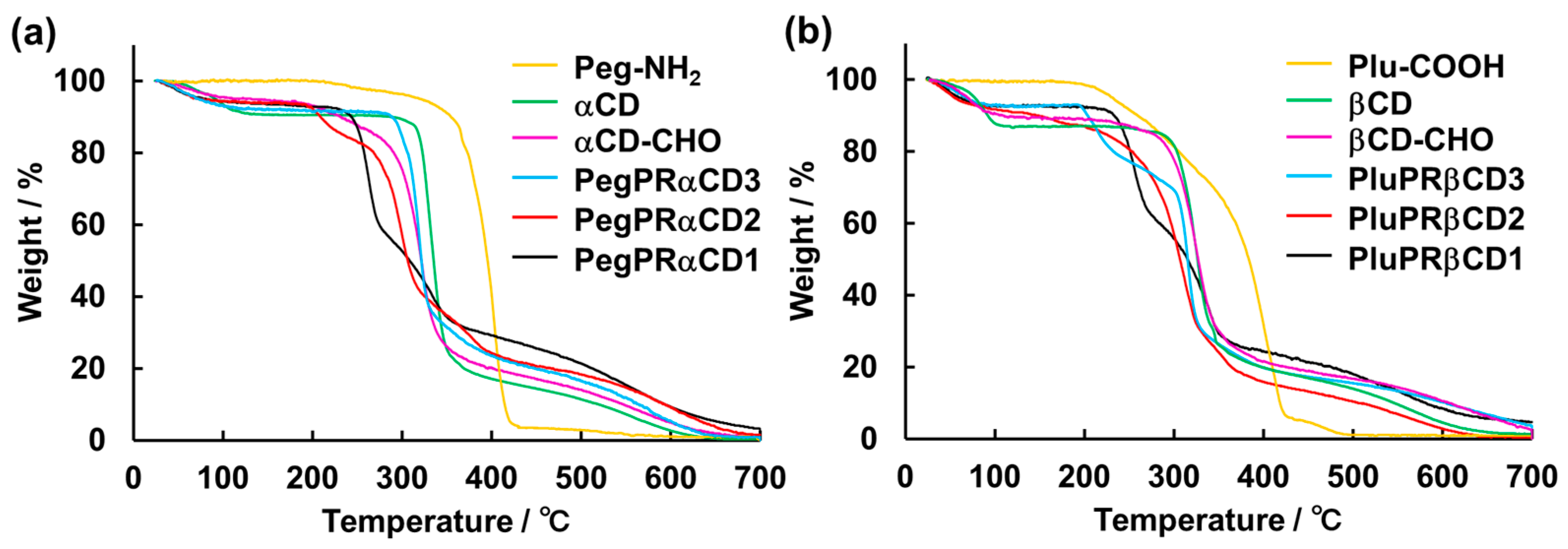
3.4. TGA Measurements
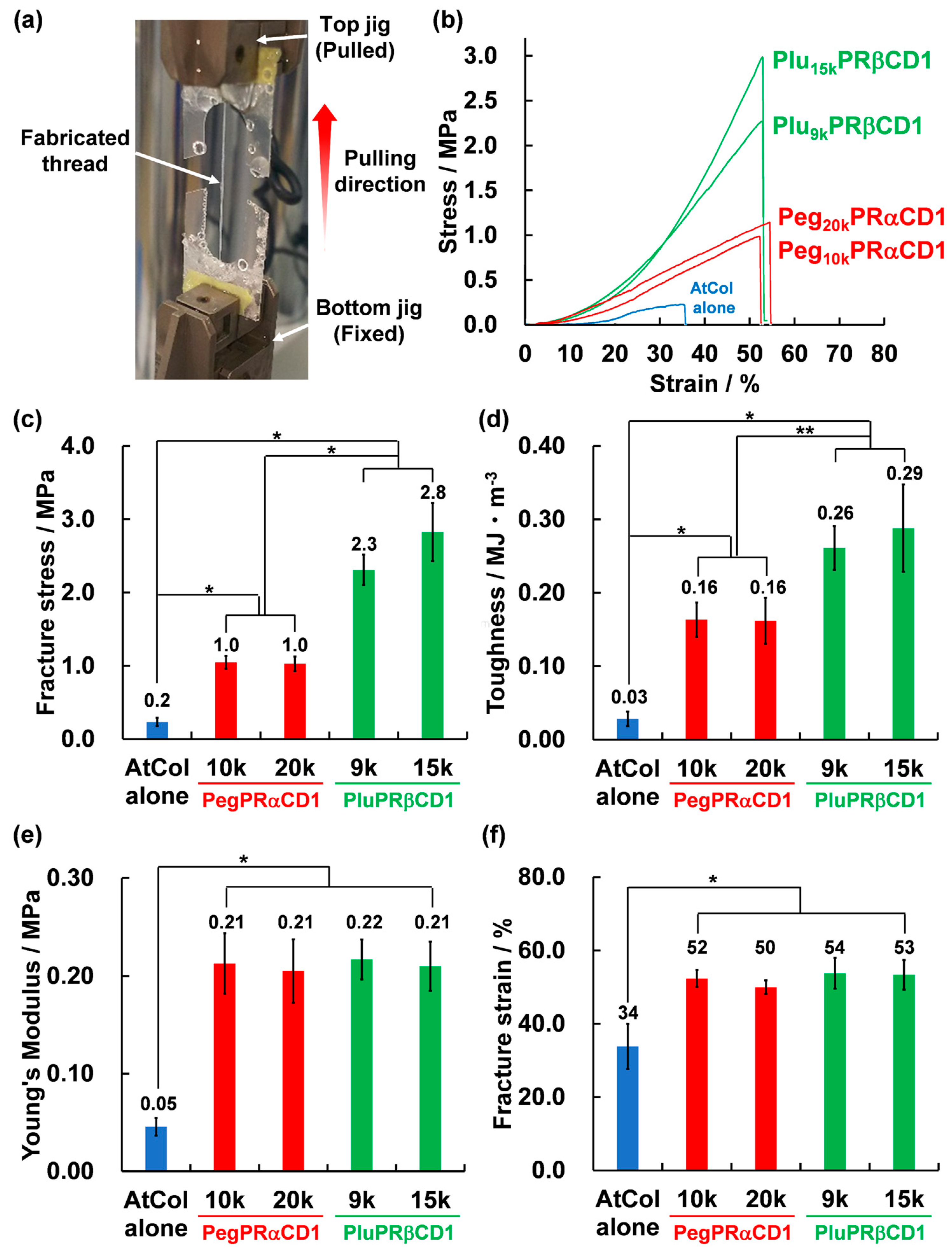
3.5. Tensile Testing of PRATs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harada, A. Preparation and Structures of Supramolecules Between Cyclodextrins and Polymers. Coord. Chem. Rev. 1996, 148, 115–133. [Google Scholar] [CrossRef]
- Harada, A.; Hashidzume, A.; Yamaguchi, H.; Takashima, Y. Polymeric Rotaxanes. Chem. Rev. 2009, 109, 5974–6023. [Google Scholar] [CrossRef]
- Stoddart, J.F. Mechanically Interlocked Molecules (MIMs)—Molecular Shuttles, Switches, and Machines (Nobel Lecture). Angew. Chem. Int. Ed. 2017, 56, 11094–11125. [Google Scholar] [CrossRef]
- Williams, G.T.; Haynes, C.J.E.; Fares, M.; Caltagirone, C.; Hiscock, J.R.; Gale, P.A. Advances in Applied Supramolecular Technologies. Chem. Soc. Rev. 2021, 50, 2737–2763. [Google Scholar] [CrossRef]
- Seale, J.S.W.; Feng, Y.; Feng, L.; Astumian, R.D.; Stoddart, J.F. Polyrotaxanes and the Pump Paradigm. Chem. Soc. Rev. 2022, 51, 8450–8475. [Google Scholar] [CrossRef]
- Harada, A.; Li, J.; Kamachi, M. The Molecular Necklace: A Rotaxane Containing Many Threaded α-Cyclodextrins. Nature 1992, 356, 325–327. [Google Scholar] [CrossRef]
- Araki, J.; Ito, K. Recent Advances in the Preparation of Cyclodextrin-Based Polyrotaxanes and Their Applications to Soft Materials. Soft Matter 2007, 3, 1456–1473. [Google Scholar] [CrossRef]
- Nakahata, M.; Mori, S.; Takashima, Y.; Yamaguchi, H.; Harada, A. Self-Healing Materials Formed by Cross-Linked Polyrotaxanes with Reversible Bonds. Chem 2016, 1, 766–775. [Google Scholar] [CrossRef] [Green Version]
- Resmerita, A.-M.; Assaf, K.I.; Lazar, A.I.; Nau, W.M.; Farcas, A. Polyrotaxanes based on PEG-amine with cucurbit[7]uril, α-cyclodextrin and its tris-O-methylated derivative. Eur. Polym. J. 2017, 93, 323–333. [Google Scholar] [CrossRef]
- Kwan, C.-S.; Leung, K.C.-F. Development and Advancement of Rotaxane Dendrimers as Switchable Macromolecular Machines. Mater. Chem. Front. 2020, 4, 2825–2844. [Google Scholar] [CrossRef]
- Hart, L.F.; Hertzog, J.E.; Rauscher, P.M.; Rawe, B.W.; Tranquilli, M.M.; Rowan, S.J. Material Properties and Applications of Mechanically Interlocked Polymers. Nat. Rev. Mater. 2021, 6, 508–530. [Google Scholar] [CrossRef]
- Brinkmann, J.; Cavatorta, E.; Sankaran, S.; Schmidt, B.; van Weerd, J.; Jonkheijm, P. About Supramolecular Systems for Dynamically Probing Cells. Chem. Soc. Rev. 2014, 43, 4449–4469. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Lorenzo, C.; García-González, C.A.; Concheiro, A. Cyclodextrins as Versatile Building Blocks for Regenerative Medicine. J. Control Release 2017, 268, 269–281. [Google Scholar] [CrossRef]
- Arisaka, Y.; Yui, N. Polyrotaxane-Based Biointerfaces with Dynamic Biomaterial Functions. J. Mater. Chem. B 2019, 7, 2123–2129. [Google Scholar] [CrossRef]
- Xu, C.; Wu, Y.-L.; Li, Z.; Loh, X.J. Cyclodextrin-Based Sustained Gene Release Systems: A Supramolecular Solution Towards Clinical Applications. Mater. Chem. Front. 2019, 3, 181–192. [Google Scholar] [CrossRef]
- Yasen, W.; Dong, R.; Aini, A.; Zhu, X. Recent Advances in Supramolecular Block Copolymers for Biomedical Applications. J. Mater. Chem. B 2020, 8, 8219–8231. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Qiu, N. Cyclodextrin-Based Polymeric Drug Delivery Systems for Cancer Therapy. Polymers 2023, 15, 1400. [Google Scholar] [CrossRef]
- Yu, S.; Zhang, Y.; Wang, X.; Zhen, X.; Zhang, Z.; Wu, W.; Jiang, X. Synthesis of Paclitaxel-Conjugated β-Cyclodextrin Polyrotaxane and Its Antitumor Activity. Angew. Chem. Int. Ed. 2013, 52, 7272–7277. [Google Scholar] [CrossRef]
- Ardeleanu, R.; Dascalu, A.I.; Neamtu, A.; Peptanariu, D.; Uritu, C.M.; Maier, S.S.; Nicolescu, A.; Simionescu, B.C.; Barboiu, M.; Pinteala, M. Multivalent Polyrotaxane Vectors as Adaptive Cargo Complexes for Gene Therapy. Polym. Chem. 2018, 9, 845–859. [Google Scholar] [CrossRef]
- Taneda, H.; Shundo, A.; Matsuno, H.; Tanaka, K. Design of a Well-Defined Polyrotaxane Structure on a Glassy Polymer Surface. Langmuir 2018, 34, 709–714. [Google Scholar] [CrossRef] [Green Version]
- Zu, G.; Cao, Y.; Dong, J.; Zhou, Q.; van Rijn, P.; Liu, M.; Pei, R. Development of an Aptamer-Conjugated Polyrotaxane-Based Biodegradable Magnetic Resonance Contrast Agent for Tumor-Targeted Imaging. ACS Appl. Bio Mater. 2019, 2, 406–416. [Google Scholar] [CrossRef]
- Masuda, H.; Arisaka, Y.; Sekiya-Aoyama, R.; Yoda, T.; Yui, N. Biological Effects of Polyrotaxane Surfaces on Cellular Responses of Fibroblast, Preosteoblast and Preadipocyte Cell Lines. Polymers 2020, 12, 924. [Google Scholar] [CrossRef] [Green Version]
- Kubota, R.; Naritomi, M.; Fujimoto, I. Synthesis of a Stretchable Polymer Crosslinker for Reinforced Atelocollagen Threads. React. Funct. Polym. 2023, 182, 105462. [Google Scholar] [CrossRef]
- Dess, D.B.; Martin, J.C. A Useful 12-I-5 Triacetoxyperiodinane (the Dess–Martin Periodinane) for the Selective Oxidation of Primary or Secondary Alcohols and a Variety of Related 12-I-5 Species. J. Am. Chem. Soc. 1991, 113, 7277–7287. [Google Scholar] [CrossRef]
- Arterburn, J.B. Selective Oxidation of Secondary Alcohols. Tetrahedron 2001, 57, 9765–9788. [Google Scholar] [CrossRef]
- Liu, S.; Cai, J.; Ren, L.; Wang, L.; Wang, Y. β-Cyclodextrin Polyrotaxane Monoaldehyde: A Novel Bio-Crosslinker with High Biocompatibility. RSC Adv. 2014, 4, 18608–18611. [Google Scholar] [CrossRef]
- Liu, S.; Xie, R.; Cai, J.; Wang, L.; Shi, X.; Ren, L.; Wang, Y. Crosslinking of Collagen Using a Controlled Molecular Weight Bio-crosslinker: β-Cyclodextrin Polyrotaxane Multi-Aldehydes. RSC Adv. 2015, 5, 46088–46094. [Google Scholar]
- Zhao, X.; Song, W.; Li, W.; Liu, S.; Wang, L.; Ren, L. Collagen Membranes Crosslinked by β-Cyclodextrin Polyrotaxane Monoaldehyde with Good Biocompatibilities and Repair Capabilities for Cornea Repair. RSC Adv. 2017, 7, 28865–28875. [Google Scholar]
- Lei, X.; Jia, Y.-G.; Song, W.; Qi, D.; Jin, J.; Liu, J.; Ren, L. Mechanical and Optical Properties of Reinforced Collagen Membranes for Corneal Regeneration through Polyrotaxane Cross-Linking. ACS Appl. Bio Mater. 2019, 2, 3861–3869. [Google Scholar] [CrossRef]
- Cho, I.S.; Ooya, T. Cell-Encapsulating Hydrogel Puzzle: Polyrotaxane-Based Self-Healing Hydrogels. Chem. Eur. J. 2020, 26, 913–920. [Google Scholar] [CrossRef]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [Green Version]
- Shoulders, M.D.; Raines, R.T. Collagen Structure and Stability. Annu. Rev. Biochem. 2009, 78, 929–958. [Google Scholar] [CrossRef] [Green Version]
- Yunoki, S.; Nagai, N.; Suzuki, T.; Munekata, M. Novel Biomaterial from Reinforced Salmon Collagen Gel Prepared by Fibril Formation and Cross-Linking. J. Biosci. Bioeng. 2004, 98, 40–47. [Google Scholar] [CrossRef]
- Heinemann, S.; Coradin, T.; Desimone, M.F. Bio-Inspired Silica–Collagen Materials: Applications and Perspectives in the Medical Field. Biomater. Sci. 2013, 1, 688–702. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, K.M.; Best, S.M.; Cameron, R.E. Collagen: A Network for Regenerative Medicine. J. Mater. Chem. B 2016, 4, 6484–6496. [Google Scholar] [CrossRef] [Green Version]
- An, B.; Lin, Y.-S.; Brodsky, B. Collagen Interactions: Drug Design and Delivery. Adv. Drug Deliv. Rev. 2016, 97, 69–84. [Google Scholar] [CrossRef]
- Miyata, T.; Taira, T.; Noishiki, Y. Collagen Engineering for Biomaterial Use. Clin. Mater. 1992, 9, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.K.; Yannas, I.V.; Bonfield, W. Antigenicity and Immunogenicity of Collagen. J. Biomed. Mater. Res. B Appl. Biomater. 2004, 71, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Sano, A.; Maeda, M.; Nagahara, S.; Ochiya, T.; Honma, K.; Itoh, H.; Miyata, T.; Fujioka, K. Atelocollagen for Protein and Gene Delivery. Adv. Drug Deliv. Rev. 2003, 55, 1651–1677. [Google Scholar] [CrossRef]
- Nimesh, S. Atelocollagen. In Gene Therapy; Nimesh, S., Ed.; Woodhead Publishing: Sawston, Cambridge, UK, 2013; pp. 225–235. [Google Scholar]
- Elliott, D.M.; Mauck, R.L.; Shah, R.P.; Schaer, T.P.; Maher, S.A. Biomaterials for Replacement and Repair of the Meniscus and Annulus Fibrosus. In Comprehensive Biomaterials II; Ducheyne, P., Ed.; Elsevier: Oxford, UK, 2017; pp. 174–193. [Google Scholar]
- Fujimoto, I.; Takei, Y. Atelocollagen-Mediated siRNA Delivery: Future Promise for Therapeutic Application. Therap. Deliv. 2014, 5, 369–371. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, R.; Nakamura, R.; Nakaegawa, Y.; Nomoto, Y.; Fujimoto, I.; Semura, K.; Hazama, A.; Omori, K. Optimal Bovine Collagen Concentration to Achieve Tracheal Epithelial Coverage of Collagen Sponges. Laryngoscope 2016, 126, E396–E403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Semura, K.; Fujimoto, I. Micro-Dimpled Surface Atelocollagen Maintains Primary Human Hepatocytes in Culture and May Promote Their Functionality Compared with Collagen Coat Culture. Int. J. Mol. Med. 2019, 44, 960–972. [Google Scholar] [CrossRef] [PubMed]
- Araki, J.; Zhao, C.; Ito, K. Efficient Production of Polyrotaxanes from α-Cyclodextrin and Poly(ethylene glycol). Macromolecules 2005, 38, 7524–7527. [Google Scholar]
- Uenuma, S.; Maeda, R.; Takahashi, S.; Kato, K.; Yokoyama, H.; Ito, K. Self-Assembled Structure of Polyrotaxane Consisting of β-Cyclodextrin and Poly(ethylene oxide)-block-poly(propylene oxide)-block-poly(ethylene oxide) Triblock Copolymer in Bulk System. Chem. Lett. 2016, 45, 991–993. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Ajiro, H. Preparation of Stereocomplex and Pseudo-Polyrotaxane with Various Cyclodextrins as Wheel Components Using Triblock Copolymer of Poly(ethylene glycol) and Polylactide. Soft Matter 2022, 18, 8885–8893. [Google Scholar]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular Inclusion Complex of Curcumin–β-Cyclodextrin Nanoparticle to Enhance Curcumin Skin Permeability from Hydrophilic Matrix Gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Chieng, B.W.; Ibrahim, N.A.; Wan Yunus, W.M.Z.; Hussein, M.Z. Poly(lactic acid)/Poly(ethylene glycol) Polymer Nanocomposites: Effects of Graphene Nanoplatelets. Polymers 2014, 6, 93–104. [Google Scholar]
- Yin, G.-Z.; Hobson, J.; Duan, Y.; Wang, D.-Y. Polyrotaxane: New Generation of Sustainable, Ultra-Flexible, Form-Stable and Smart Phase Change Materials. Energy Storage Mater. 2021, 40, 347–357. [Google Scholar] [CrossRef]
- Liu, C.; Yin, Q.; Li, X.; Hao, L.; Zhang, W.; Bao, Y.; Ma, J. A Waterborne Polyurethane–Based Leather Finishing Agent with Excellent Room Temperature Self-Healing Properties and Wear-Resistance. Adv. Compos. Hybrid Mater. 2021, 4, 138–149. [Google Scholar] [CrossRef]
- Qian, T.; Li, J.; Feng, W.; Nian, H. Enhanced Thermal Conductivity of Form-Stable Phase Change Composite with Single-Walled Carbon Nanotubes for Thermal Energy Storage. Sci. Rep. 2017, 7, 44710. [Google Scholar] [CrossRef] [Green Version]
- Shown, I.; Banerjee, S.; Ramchandran, A.V.; Geckeler, K.E.; Murthy, C.N. Synthesis of Cyclodextrin and Sugar-Based Oligomers for the Efavirenz Drug Delivery. Macromol. Symp. 2010, 287, 51–59. [Google Scholar] [CrossRef]
- Manohara, G.V.; Maroto-Valer, M.; Garcia, S. A Simple and Green Synthesis Method for Ca-adamantanecarboxylate: A Novel Precursor for High Temperature CO2 Capture Sorbent Materials. Sustain. Energy Fuels 2019, 3, 3318–3323. [Google Scholar] [CrossRef]
- Hodge, S.A.; Buckley, D.J.; Yau, H.C.; Skipper, N.T.; Howard, C.A.; Shaffer, M.S.P. Chemical Routes to Discharging Graphenides. Nanoscale 2017, 9, 3150–3158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K. Slide-Ring Materials Using Cyclodextrin. Chem. Pharm. Bull. 2017, 65, 326–329. [Google Scholar] [CrossRef] [Green Version]
- Ohmori, K.; Abu Bin, I.; Seki, T.; Liu, C.; Mayumi, K.; Ito, K.; Takeoka, Y. Molecular Weight Dependency of Polyrotaxane-Cross-Linked Polymer Gel Extensibility. Chem. Commun. 2016, 52, 13757–13759. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Lee, D.H.; Arisaka, Y.; Kang, T.W.; Yui, N. Post-Cross-Linking of Collagen Hydrogels by Carboxymethylated Polyrotaxanes for Simultaneously Improving Mechanical Strength and Cell Proliferation. ACS Biomater. Sci. Eng. 2022, 8, 588–597. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Morimoto, N.; Jiang, L.; Kawahara, S.; Noritomi, T.; Yokoyama, H.; Mayumi, K.; Ito, K. Tough Hydrogels with Rapid Self-Reinforcement. Science 2021, 372, 1078–1081. [Google Scholar] [CrossRef]
- Kato, K.; Karube, K.; Nakamura, N.; Ito, K. The Effect of Ring Size on the Mechanical Relaxation Dynamics of Polyrotaxane Gels. Polym. Chem. 2015, 6, 2241–2248. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kubota, R.; Fujimoto, I. Synthesis, Characterization, and Potential Application of Cyclodextrin-Based Polyrotaxanes for Reinforced Atelocollagen Threads. Polymers 2023, 15, 3325. https://doi.org/10.3390/polym15153325
Kubota R, Fujimoto I. Synthesis, Characterization, and Potential Application of Cyclodextrin-Based Polyrotaxanes for Reinforced Atelocollagen Threads. Polymers. 2023; 15(15):3325. https://doi.org/10.3390/polym15153325
Chicago/Turabian StyleKubota, Riku, and Ichiro Fujimoto. 2023. "Synthesis, Characterization, and Potential Application of Cyclodextrin-Based Polyrotaxanes for Reinforced Atelocollagen Threads" Polymers 15, no. 15: 3325. https://doi.org/10.3390/polym15153325






