Phase-Separated Structure of NBR/PVC Blends with Different Acrylonitrile Contents Investigated Using STEM–EDS Mapping Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. DMA Measurement
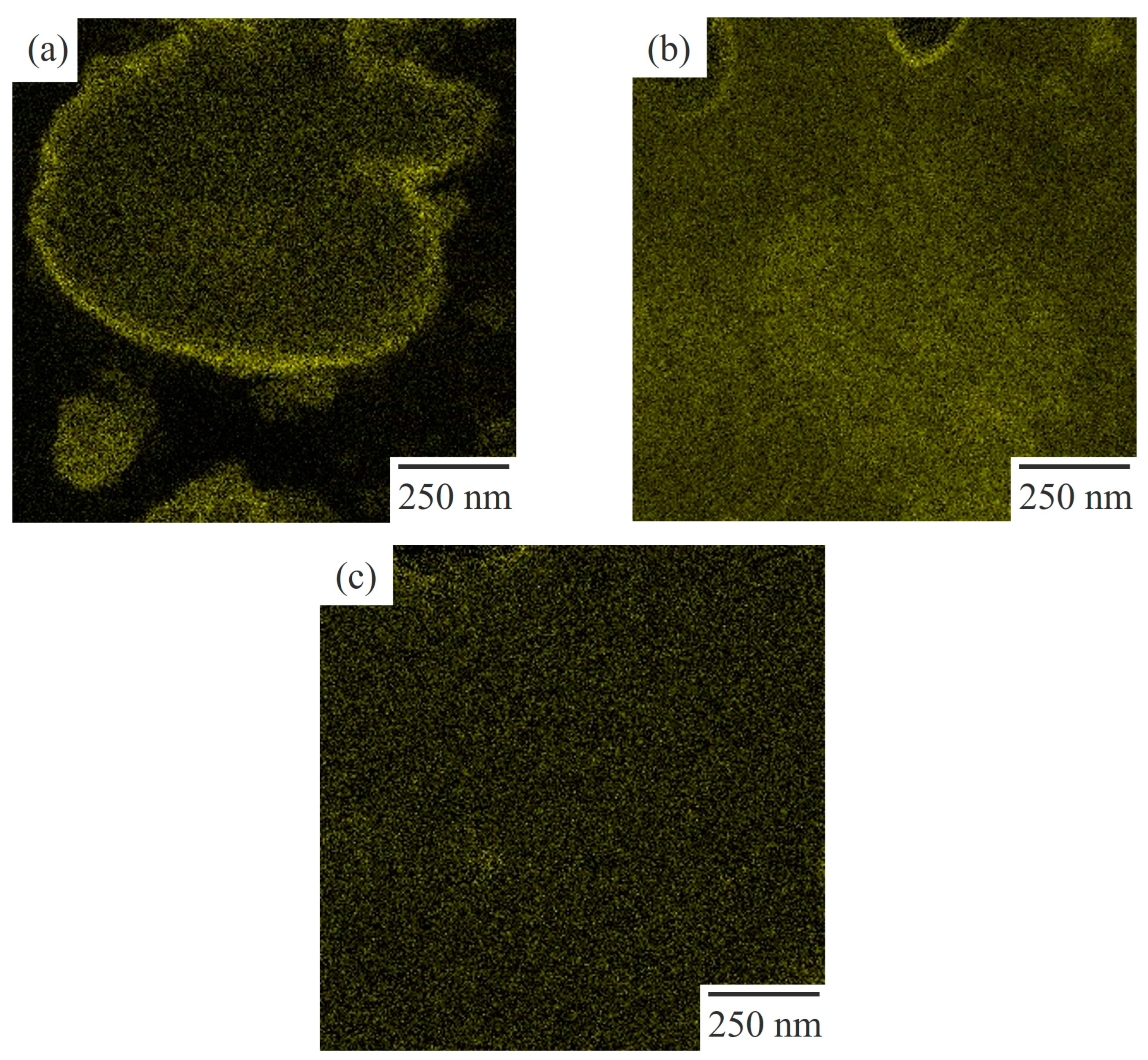
2.4. STEM Observation
3. Results and Discussion
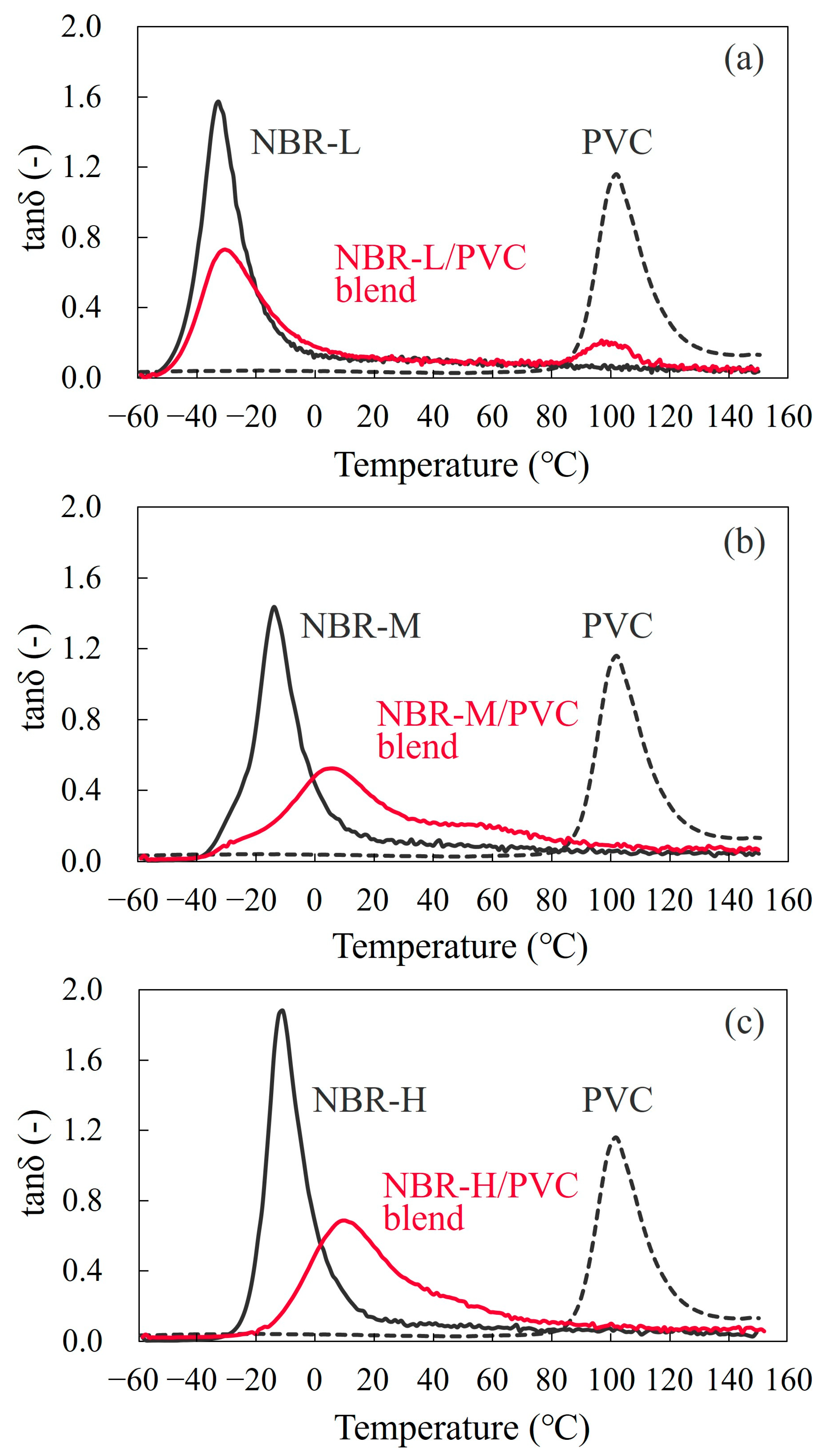
3.1. Glass Transition
3.2. Solubility Parameter
3.3. Two-Phase Morphology
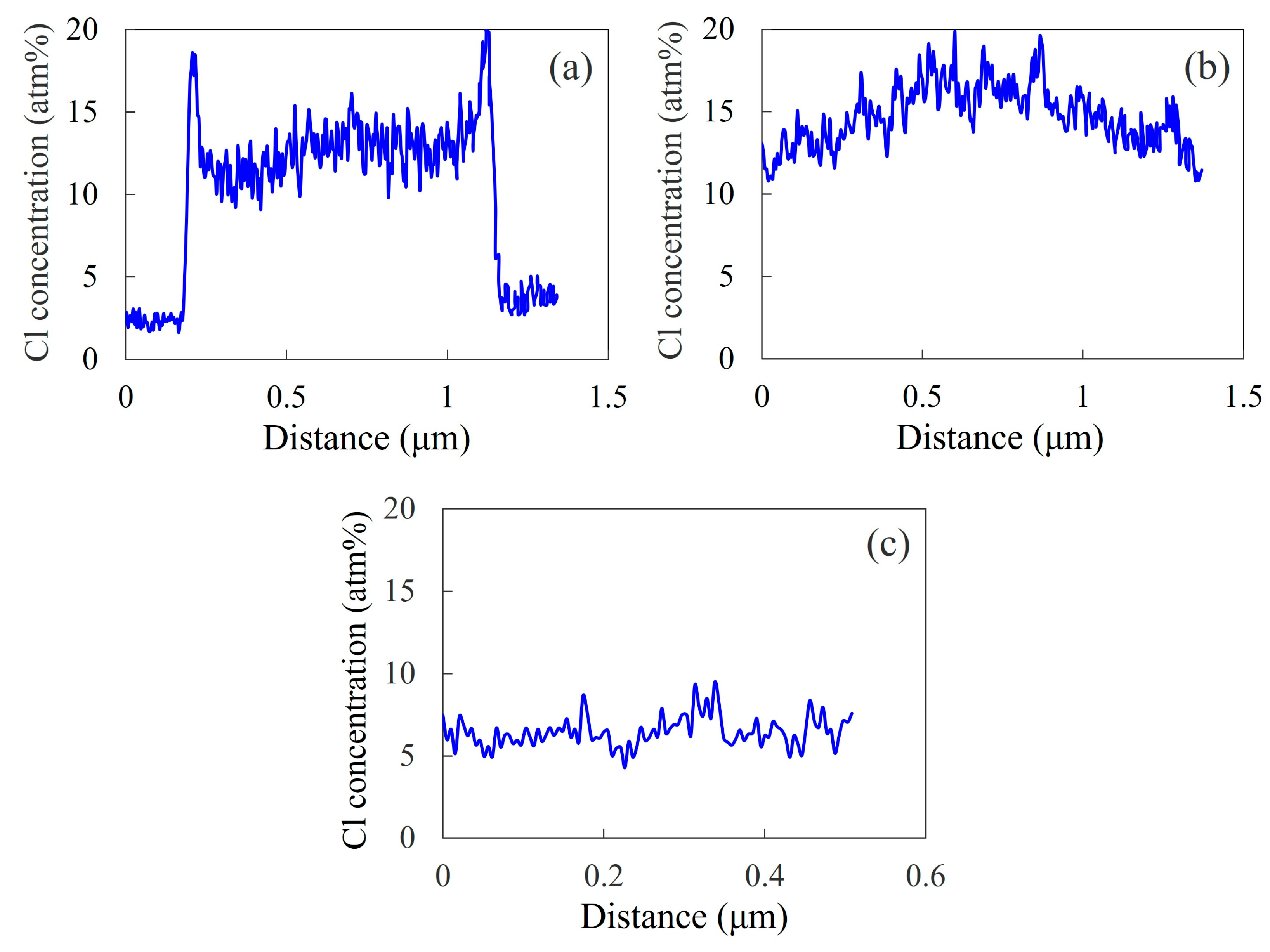
3.4. Concentration Distribution
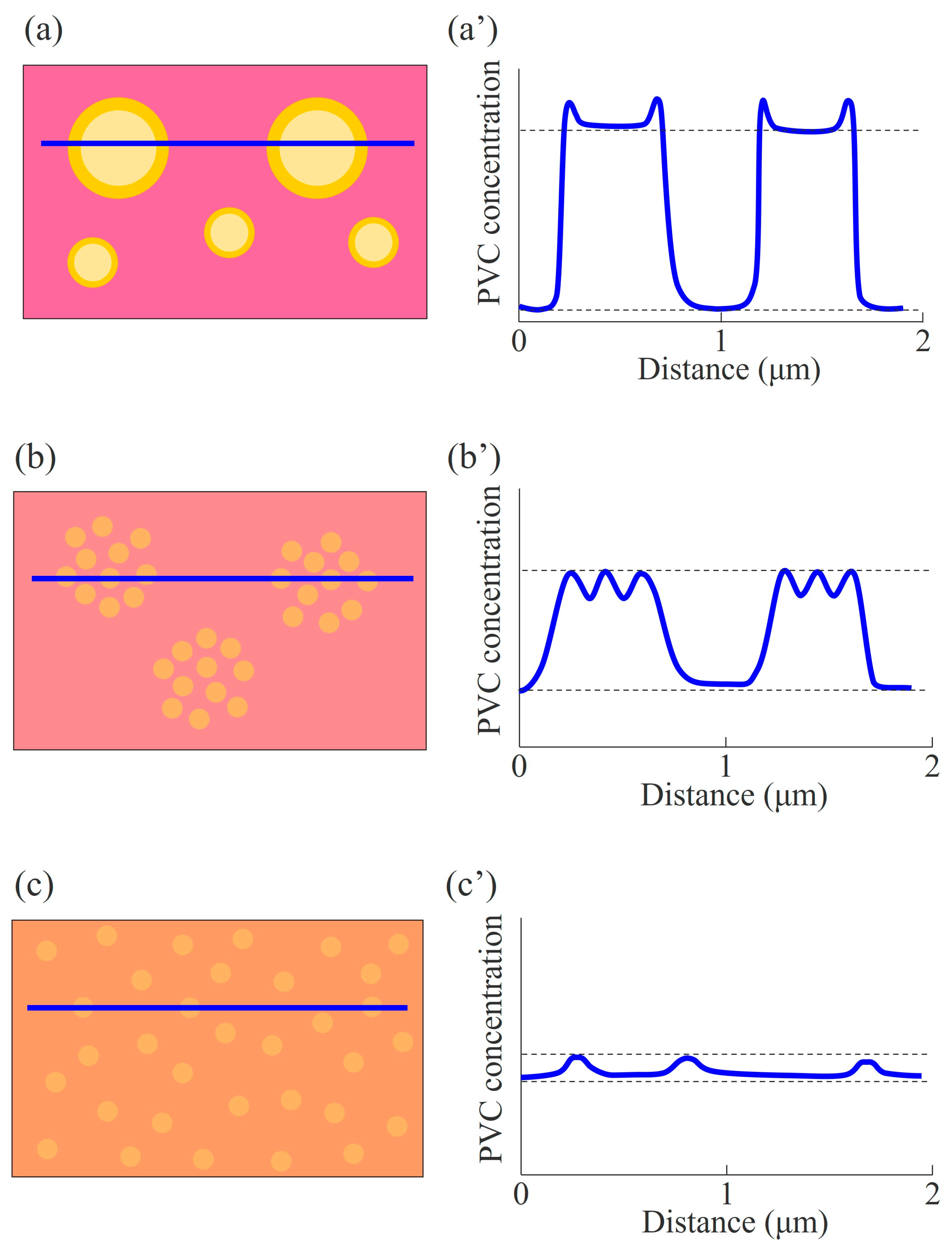
3.5. Phase-Separated Structure of NBR/PVC Blends
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olabis, O. Polymer-Polymer Miscibility; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Paul, D.R. Polymer Blends Volume 1; Elsevier: Amsterdam, The Netherlands, 2012; Volume 1. [Google Scholar]
- Utracki, L.A.; Wilkie, C.A. Polymer Blends Handbook; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2002; Volume 1. [Google Scholar]
- Thomas, S.; Grohens, Y.; Jyotishkumar, P. Characterization of Polymer Blends: Miscibility, Morphology and Interfaces; John Wiley & Sons: Hoboken, NJ, USA, 2014. [Google Scholar]
- Kader, M.; Kim, W.; Kaang, S.; Nah, C. Morphology and dynamic mechanical properties of natural rubber/nitrile rubber blends containing trans-polyoctylene rubber as a compatibilizer. Polym. Int. 2005, 54, 120–129. [Google Scholar] [CrossRef]
- Samsudin, S.A.; Kelly, C.A.; Kukureka, S.N.; Jenkins, M.J. Development of partial miscibility in polycarbonate/polypropylene blends via annealing. J. Polym. Eng. 2017, 37, 707–714. [Google Scholar] [CrossRef]
- Takamatsu, K.; Suzuki, S.; Nishimura, Y.; Saito, H. Reduction of birefringence by dynamic asymmetry in miscible blends of dissimilar polycarbonates. Polymer 2021, 222, 123632. [Google Scholar] [CrossRef]
- Nair, T.M.; Kumaran, M.; Unnikrishnan, G.; Pillai, V. Dynamic mechanical analysis of ethylene–propylene–diene monomer rubber and styrene–butadiene rubber blends. J. Appl. Polym. Sci. 2009, 112, 72–81. [Google Scholar] [CrossRef]
- Ziquinatti, F.; Hugen, R.G.; Oliveira, C.M.; Coelho, L.A.; Pezzin, S.H. Miscibility and mechanical behavior of SAN/NBR blends. Macromol. Symp. 2005, 229, 276–280. [Google Scholar] [CrossRef]
- Nara, S.; Sagawa, H.; Saito, H.; Oyama, H.T. Synergetic toughening of poly (phenylene sulfide) by poly (phenylsulfone) and poly (ethylene-ran-methacrylate-ran-glycidyl methacrylate). J. Appl. Polym. Sci. 2021, 138, 49994. [Google Scholar] [CrossRef]
- Sun, H.; Yu, B.; Sheng, J. Phase morphology and miscibility of iPP/PcBR blends. Polym.-Plast. Technol. Eng. 2010, 49, 414–417. [Google Scholar] [CrossRef]
- Wu, C.; Akiyama, S. Dynamic mechanical and adhesive properties of acrylate rubber/chlorinated polypropylene blends compatibilized with a hindered phenol compound. Polym. J. 2001, 33, 955–958. [Google Scholar] [CrossRef]
- Komori, Y.; Taniguchi, A.; Shibata, H.; Goto, S.; Saito, H. Partial miscibility and concentration distribution of two-phase blends of crosslinked NBR and PVC. Polymers 2023, 15, 1383. [Google Scholar] [CrossRef]
- Shi, X.; Li, Q.; Fu, G.; Jia, L. The effects of a polyol on the damping properties of EVM/PLA blends. Polym. Test. 2014, 33, 1–6. [Google Scholar] [CrossRef]
- Liu, B.; Gao, X.; Zhao, Y.; Dai, L.; Xie, Z.; Zhang, Z. 9, 10-Dihydro-9-oxa-10-phosphaphenanthrene 10-oxide-based oligosiloxane as a promising damping additive for methyl vinyl silicone rubber (VMQ). J. Mater. Sci. 2017, 52, 8603–8617. [Google Scholar] [CrossRef]
- Liu, C.; Fan, J.; Chen, Y. Design of regulable chlorobutyl rubber damping materials with high-damping value for a wide temperature range. Polym. Test. 2019, 79, 106003. [Google Scholar] [CrossRef]
- Li, Z.; Lu, X.; Tao, G.; Guo, J.; Jiang, H. Damping elastomer with broad temperature range based on irregular networks formed by end-linking of hydroxyl-terminated poly (dimethylsiloxane). Polym. Eng. Sci. 2016, 56, 97–102. [Google Scholar] [CrossRef]
- Lu, X.; Yang, Y.; Cheng, B.; Li, T. High damping epoxized natural rubber/diallyl adiallyl phthalate prepolymer (ENR/DAP-A) blends engineered by interphase crosslinking. J. Appl. Polym. Sci. 2018, 135, 46300. [Google Scholar] [CrossRef]
- Li, J.-X.; Chan, C.-M. Effect of the size of the dispersed NBR phase in PVC/NBR blends on the stability of PVC to electron irradiation. Polymer 2001, 42, 6833–6839. [Google Scholar] [CrossRef]
- Slater, T.J.; Janssen, A.; Camargo, P.H.; Burke, M.G.; Zaluzec, N.J.; Haigh, S.J. STEM-EDX tomography of bimetallic nanoparticles: A methodological investigation. Ultramicroscopy 2016, 162, 61–73. [Google Scholar] [CrossRef]
- Kraxner, J.; Schäfer, M.; Röschel, O.; Kothleitner, G.; Haberfehlner, G.; Paller, M.; Grogger, W. Quantitative EDXS: Influence of geometry on a four detector system. Ultramicroscopy 2017, 172, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Bender, H.; Richard, O.; Kundu, P.; Favia, P.; Zhong, Z.; Palenstijn, W.J.; Batenburg, K.J.; Wirix, M.; Kohr, H.; Schoenmakers, R. Combined STEM-EDS tomography of nanowire structures. Semicond. Sci. Technol. 2019, 34, 114002. [Google Scholar] [CrossRef] [Green Version]
- Stockdale, M.K. Thermoplastic elastomers from NBR and polyvinyl chloride. J. Vinyl Technol. 1990, 12, 235–244. [Google Scholar] [CrossRef]
- Mousa, A.; Ishiaku, U.; Mohd Ishak, Z. Oil resistance of dynamically vulcanized poly (vinyl chloride)/nitrile butadiene rubber thermoplastic elastomers. Polym. Bull. 2005, 53, 203–212. [Google Scholar] [CrossRef]
- Gheno, S.; Passador, F.; Pessan, L. Investigation of the phase morphology of dynamically vulcanized PVC/NBR blends using atomic force microscopy. J. Appl. Polym. Sci. 2010, 117, 3211–3219. [Google Scholar] [CrossRef]
- Sen, A.; Mukherjee, G. Studies on the thermodynamic compatibility of blends of poly (vinyl chloride) and nitrile rubber. Polymer 1993, 34, 2386–2391. [Google Scholar] [CrossRef]
- Liu, Z.; Zhu, X.; Wu, L.; Li, Y.; Qi, Z.; Choy, C.; Wang, F. Effects of interfacial adhesion on the rubber toughening of poly (vinyl chloride) Part 1. Impact tests. Polymer 2001, 42, 737–746. [Google Scholar] [CrossRef]
- George, K.; Joseph, R.; Francis, D.J. Studies on NBR/PVC blends. J. Appl. Polym. Sci. 1986, 32, 2867–2873. [Google Scholar] [CrossRef]
- Fukumori, K.; Sato, N.; Kurauchi, T. Pulsed NMR-study of motional heterogeneity in acrylonitrile butadiene poly(vinyl chloride) blends. Rubber Chem. Technol. 1991, 64, 522–533. [Google Scholar] [CrossRef]
- Zakrzewski, G. Investigation of the compatibility of butadiene—Acrylonitrile copolymers with poly (vinyl chloride). Polymer 1973, 14, 347–351. [Google Scholar] [CrossRef]
- Perera, M.S.; Ishiaku, U.; Ishak, Z.M. Characterisation of PVC/NBR and PVC/ENR50 binary blends and PVC/ENR50/NBR ternary blends by DMA and solid state NMR. Eur. Polym. J. 2001, 37, 167–178. [Google Scholar] [CrossRef]
- Pena, J.; Hidalgo, M.; Mijangos, C. Plastification of poly (vinyl chloride) by polymer blending. J. Appl. Polym. Sci. 2000, 75, 1303–1312. [Google Scholar] [CrossRef]
- Kwak, S.-Y.; Nakajima, N. Monitoring of homogenization and analysis of nanoscale structure in a butadiene−acrylonitrile copolymer/poly (vinyl chloride) blend. Macromolecules 1996, 29, 5446–5452. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, X.; Liu, S.; Gui, H.; Lai, J.; Liu, Y.; Gao, J.; Huang, F.; Song, Z.; Tan, B. Ultrafine full-vulcanized powdered rubbers/PVC compounds with higher toughness and higher heat resistance. Polymer 2005, 46, 10614–10617. [Google Scholar] [CrossRef]
- Cliff, G.; Lorimer, G.W. The quantitative analysis of thin specimens. J. Microsc. 1975, 103, 203–207. [Google Scholar] [CrossRef]
- Fedors, R.F. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Araki, Y.; Shimizu, D.; Hori, Y.; Nakatani, K.; Saito, H. Mechanical properties and microphase structure of hydrogenated S-SB-S triblock copolymers. Polym. J. 2013, 45, 1140–1145. [Google Scholar] [CrossRef] [Green Version]
- Esmizadeh, E.; Naderi, G.; Ghoreishy, M.H.R. Modification of theoretical models to predict mechanical behavior of PVC/NBR/organoclay nanocomposites. J. Appl. Polym. Sci. 2013, 130, 3229–3239. [Google Scholar] [CrossRef]
- Zhang, S.; Zhai, Y.; Zhang, Y. Microwave-absorbing performance and mechanical properties of poly (vinyl chloride)/acrylonitrile–butadiene rubber thermoplastic elastomers filled with multiwalled carbon nanotubes and silicon carbide. J. Appl. Polym. Sci. 2013, 130, 345–351. [Google Scholar] [CrossRef]
- Lai, S.; Cheng, C.; Liao, Y.; Su, X.; Tan, Q.; Yang, S.; Bai, S. The structure and properties of mechanochemically modified acrylonitrile butadiene rubber (NBR)/poly (vinyl chloride)(PVC) scraps and fresh NBR composites. Waste Manag. 2023, 159, 93–101. [Google Scholar] [CrossRef]



| Material | Grade | Supplier | Additive Amount (g) |
|---|---|---|---|
| stabilizer for PVC | ADK STAB | Adeka Corp., Tokyo, Japan | 0.01 |
| SC-308E | |||
| cross-linking agent | colloidal | Hosoi Chemical Industry Co., Ltd., Tokyo, Japan | 0.018 |
| sulfur | |||
| zinc oxide | JIS-certified | Hakusui Tech Co., Ltd., Osaka, Japan | 0.036 |
| (ZnO) | Class 2 *2 | ||
| stearic acid | stearic acid | NOF Corp., Tokyo, Japan | 0.012 |
| N-cyclohexyl-2- | Nocceler CZ | Ouchi Shinko Chemical Industrial Co., Ltd., Tokyo, Japan | 0.018 |
| benzothiazolyl | |||
| sulfenamide (CZ) | |||
| tetramethylthiuram disulfide (TT) | Nocceler TT | Ouchi Shinko Chemical Industrial Co., Ltd., Tokyo, Japan | 0.002 |
| Polymer | AN (%) | EC (J/mol) | V (cm3/mol) | δi ((MPa)1/2) | Δδ (=δPVC − δNBR) ((MPa)1/2) |
|---|---|---|---|---|---|
| NBR-L | 18.0 | 21,272 | 55.6 | 19.6 | 3.0 |
| NBR-M | 29.0 | 22,966 | 53.4 | 20.7 | 1.9 |
| NBR-H | 33.5 | 23,659 | 52.5 | 21.2 | 1.4 |
| PVC | - | 19,920 | 39.1 | 22.6 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komori, Y.; Taniguchi, A.; Shibata, H.; Goto, S.; Saito, H. Phase-Separated Structure of NBR/PVC Blends with Different Acrylonitrile Contents Investigated Using STEM–EDS Mapping Analysis. Polymers 2023, 15, 3343. https://doi.org/10.3390/polym15163343
Komori Y, Taniguchi A, Shibata H, Goto S, Saito H. Phase-Separated Structure of NBR/PVC Blends with Different Acrylonitrile Contents Investigated Using STEM–EDS Mapping Analysis. Polymers. 2023; 15(16):3343. https://doi.org/10.3390/polym15163343
Chicago/Turabian StyleKomori, Yuka, Aoi Taniguchi, Haruhisa Shibata, Shinya Goto, and Hiromu Saito. 2023. "Phase-Separated Structure of NBR/PVC Blends with Different Acrylonitrile Contents Investigated Using STEM–EDS Mapping Analysis" Polymers 15, no. 16: 3343. https://doi.org/10.3390/polym15163343
APA StyleKomori, Y., Taniguchi, A., Shibata, H., Goto, S., & Saito, H. (2023). Phase-Separated Structure of NBR/PVC Blends with Different Acrylonitrile Contents Investigated Using STEM–EDS Mapping Analysis. Polymers, 15(16), 3343. https://doi.org/10.3390/polym15163343






