Abstract
With the success of several clinical trials of products based on human serum albumin (HSA) and the rapid development of nanotechnology, HSA-based nanodrug delivery systems (HBNDSs) have received extensive attention in the field of nanomedicine. However, there is still a lack of comprehensive reviews exploring the broader scope of HBNDSs in biomedical applications beyond cancer therapy. To address this gap, this review takes a systematic approach. Firstly, it focuses on the crystal structure and the potential binding sites of HSA. Additionally, it provides a comprehensive summary of recent progresses in the field of HBNDSs for various biomedical applications over the past five years, categorized according to the type of therapeutic drugs loaded onto HSA. These categories include small-molecule drugs, inorganic materials and bioactive ingredients. Finally, the review summarizes the characteristics and current application status of HBNDSs in drug delivery, and also discusses the challenges that need to be addressed for the clinical transformation of HSA formulations and offers future perspectives in this field.
1. Introduction
Human serum albumin (HSA) is the most abundant protein in human plasma, with concentrations ranging from 35–50 g/L, accounting for approximately 50% of the total plasma protein []. Serving as a natural transporter in the bloodstream, HSA exhibits reversible binding capabilities with a range of endogenous and exogenous substances, such as fatty acids, hormones and metal ions []. It efficiently transports these substances to their targeted tissues. A variety of albumin-binding proteins (ABPs), including neonatal Fc receptor (FcRn), glycoprotein 60 kDa (gp60), and secreted protein acidic and rich in cysteine (SPARC), have been studied extensively []. They have also been demonstrated to be essential in the regulation of HSA circulation and distribution in the body. The intrinsic properties of HSA make it an attractive drug vehicle for delivering a variety of therapeutic agents. Over the past few decades, the extensive development of nanotechnology has also facilitated the development of drug delivery systems []. HSA, owing to its superior biodegradability and biocompatibility, non-toxicity, and non-immunogenicity, has been widely explored as a multifunctional nanodrug delivery system for biomedical applications. Various drugs can be combined to HSA through covalent conjugation, electrostatic adsorption, or hydrophobic interaction. As a result, HSA nanoparticles of different particle sizes can be easily fabricated using mild and facile strategies [,]. Furthermore, the presence of charged functional groups, including carboxyl and amino groups, provides possibilities for diverse surface modifications of HSA-based nanoparticles [].
Several HSA-based drug formulations have been approved for clinical trials and marketed. Among them is Abraxane, the first HSA-based product to be approved by the Food and Drug Administration (FDA) for human use in 2005 []. This formulation is manufactured by Nab™ technology and is clinically utilized in the treatment of breast cancer, non-small cell lung cancer, and pancreatic cancer. Furthermore, numerous other products are currently undergoing pre-clinical and clinical trials for a variety of biomedical applications, including cancers, diabetes, hemophilia, and rheumatoid arthritis (RA) []. These aforementioned facts have increased researchers’ interest in employing HSA as a nanocarrier for various biomedical applications. However, up until now, there has been a lack of specialized reviews specifically focusing on HSA-based nanocarrier for biomedical applications. In this review, our primary focus is on the application of HSA-based nanodrug delivery systems (HBNDSs) in the biomedical field (Figure 1), with particular emphasis on the recent progress made within the past five years. We hope that this review will provide a comprehensive understanding of HBNDSs, especially their applications in the biomedical field, and bring some possible inspiration to the clinical translation of HSA-based nanoformulations in the future.
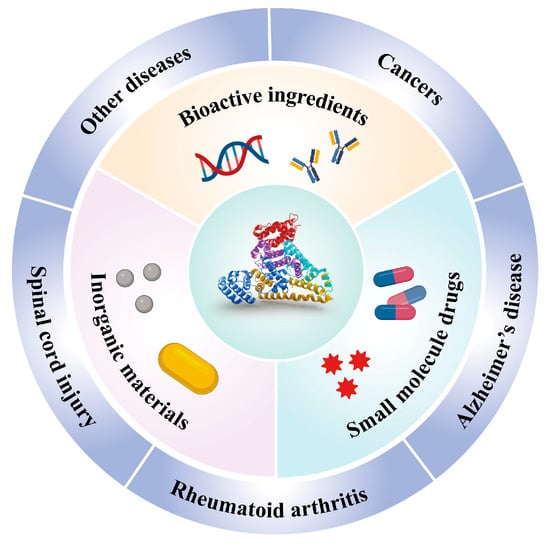
Figure 1.
Overview of HSA-based nanocarriers for the delivery of diverse therapeutic agents (including small-molecule drugs, inorganic materials, and bioactive ingredients), and the potential biomedical applications of HSA-based nanocarriers.
2. Structure and Properties of HSA
HSA, synthesized by liver parenchymal cells, is composed of 585 amino acid residues and has a molecular weight of 66,500 Da []. HSA is characterized by a low content of aromatic amino acids, with only one tryptophan present, but it possesses a significant number of amino acids containing carboxyl and amino groups [,]. These amino acid residues contribute to the exceptional solubility and stability of HSA. Notably, HSA demonstrates remarkable stability over a broad pH range (pH 4–9) and exhibits excellent tolerance to specific organic solvents, withstanding concentrations of up to 40% ethanol. Additionally, HSA can endure high temperatures without denaturation, maintaining its structural integrity for a duration of 10 h at 60 °C [,]. These exceptional properties enable HSA to retain its structural characteristics even under harsh processing conditions.
Figure 2 depicts the crystal structure of HSA, highlighting the specific binding sites for therapeutic drugs. In terms of structure, HSA consists of three homologous domains (domain I, II, and III), each of which is further divided into two subdomains, A and B. Each subdomain consists of six helical structures, and within each subdomain, hydrophobic and positively charged groups form a pocket-like structure. This arrangement creates an advantageous spatial environment for encapsulating hydrophobic nutrients, vitamins, hormones, and other molecules. As a result, HSA serves as a natural carrier for hydrophobic substances within the body. The unique spatial structural advantages of HSA facilitate its binding with a wide range of substances [,]. HSA possesses two major binding sites: Sudlow site I, located in subdomain IIA, and Sudlow site II, situated in subdomain IIIA (also known as the warfarin site and the benzodiazepine site). These sites exhibit high affinity for various molecules, including small-molecule drugs, peptides, nucleic acids, and so on []. Furthermore, HSA also has four metal binding sites, namely N-terminal binding site (NTS), cysteine 34 (cys34), and metal binding sites-A (MBS-A) and MBS-B, enabling the binding of different kinds of metal ions.
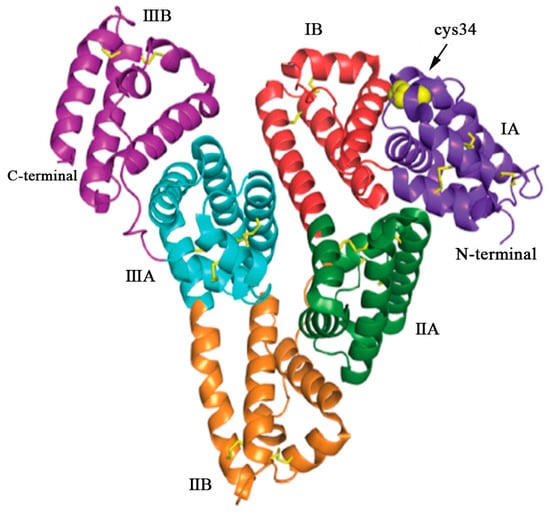
Figure 2.
The structure of HSA, a single polypeptide consisting of 585 amino acids. HSA adopts a heart-shaped configuration, assembled by three homologous domains: domain I (residues 1–197), domain II (residues 189–385), and domain III (residues 381–585). Each domain comprises two subdomains, denoted as A and B, which exhibit common structural motifs. The domains are color coded: IA (purple), IB (red), IIA (green), IIB (orange), IIIA (blue), and IIIB (violet). The yellow sticks represent the disulfide bridges, while the yellow spheres represent the free cysteine residue located at position 34 (cys34) in domain IA. Reproduced with permission from the work of Kudarha et al. (2017) [].
3. HSA-Based Multifunctional Nanocarrier
Due to its favorable attributes such as excellent biocompatibility, non-toxicity, non-immunogenicity, and prolonged circulation time, HBNDSs have garnered significant attention for a wide range of biomedical applications []. They have emerged as crucial carriers for delivering diverse therapeutic drugs, including small-molecule drugs, inorganic materials, and bioactive ingredients, thereby enhancing both imaging performance and therapeutic efficacy across various diseases []. In this section, we will systematically summarize the recent advancements in HSA-based multifunctional nanocarriers within the past five years.
3.1. Small-Molecule Drugs
The unique advantages of delivering small-molecule drugs using HSA as a carrier are as follows. (1) Good biocompatibility: HSA, being an endogenous substance, does not typically induce autoimmune reactions and adverse effects when used as a carrier. (2) High drug-loading ability. The unique spatial structure and abundant surface functional groups of HSA provide a convenient route for encapsulating diverse hydrophilic and hydrophobic small-molecule drugs in a high-affinity manner. (3) Prolonged half-life: When combined with drugs, HSA significantly extends the half-life time of drugs during the blood circulation. HSA itself exhibits a circulation half-life of approximately 19 days in the body. Additionally, HSA is negatively charged in the blood, making it less susceptible to clearance by macrophages. This feature provides the possibility of achieving prolonged blood circulation of drugs. (4) High stability: HSA can be utilized as a valuable carrier for exogenous drugs to improve their stabilities during systemic circulation and protect them from enzymatic degradation [], thereby reducing drug leakage. The binding strategy between HSA and drugs is generally classified into two categories: covalent binding and non-covalent binding, based on the different approaches used to establish the interaction between HSA and the drugs. Typical examples of HSA-based nanocarriers for the delivery of small-molecule drugs within the past five years are summarized in Table 1.

Table 1.
Typical examples of HSA NPs loaded with small-molecule drugs for various biomedical applications.
3.1.1. Covalent Binding
Theoretically, each HSA molecule contains one N-terminal carboxyl group, one cysteine (thiol group), and fifty-nine lysine residues (free amino group) []. Covalent binding refers to the preparation of HSA-drug nanoconjugate, by linking these abundant groups on the HSA with drugs through various chemical addition reactions, such as thiol-maleimide coupling, carbodiimide coupling, and Michael addition reactions []. In general, small-molecule drugs that can be combined with HSA through covalent binding include certain chemotherapeutic drugs, photosensitizers, and sonosensitizers. Examples of such drugs include methotrexate (MTX), doxorubicin (DOX), chlorine e6 (Ce6), and some prodrugs []. The nanoconjugates generated using this approach exhibit relatively high stability, reducing the potential side effects arising from drug leakage during blood circulation [,,]. However, achieving controlled release of covalently connected drugs upon reaching the target sites has become a significant research focus in recent years. For instance, tumor tissue microenvironments possess distinctive characteristics, such as slight acidity and enzyme overexpression, in comparison to normal tissue []. A pH-sensitive linker, azidomethyl-methylmaleic anhydride (AzMMMan), was first employed to modify catalase (CAT) and HSA-Pt (IV) prodrug conjugates, respectively. Subsequently, a protein–drug conjugate (HSAP-DC-CAT, where DC represents dibenzocyclooctyne/chlorin) was synthesized by linking the two components through the click chemistry reaction between azide groups of AzMMMan and dibenzocyclooctyne (DBCO) groups of DBCO coupled Ce6 (DBCO-Ce6) []. The formation of stable covalent bonds between the drugs and HSA contributes to the systemic delivery of the nanoparticle, subsequently triggering trace lysis and release of bioactive Pt (IV), Ce6 and CAT in the acidic tumor microenvironment (TME). In addition to utilizing the special acidic TME, controlled drug release can also be achieved by establishing specific associations with overexpressed enzymes at the target site. In one instance, a light-activated, reactive oxygen species (ROS)-responsive nanoplatform (M-IR820/αCD47@NP) was designed by Lu et al. []. Notably, the photosensitizer IR820 was covalently linked by using a matrix metalloproteinase (MMP)-sensitive peptide as a linker and an αCD47@HSA NP with ROS responsiveness (Figure 3A). The M-IR820/αCD47@NP system releases the conjugated IR820 at the tumor site through MMP activation, enabling photodynamic therapy and inducing immunogenic cell death upon near-infrared (NIR) light irradiation. Additionally, the combination of CD47 blockade and photo-immunotherapy mediated by M-IR820/αCD47@NP elicits a significant antitumor immune response to effectively suppress the growth of 4T1 tumors and avoid tumor recurrence (Figure 3B).
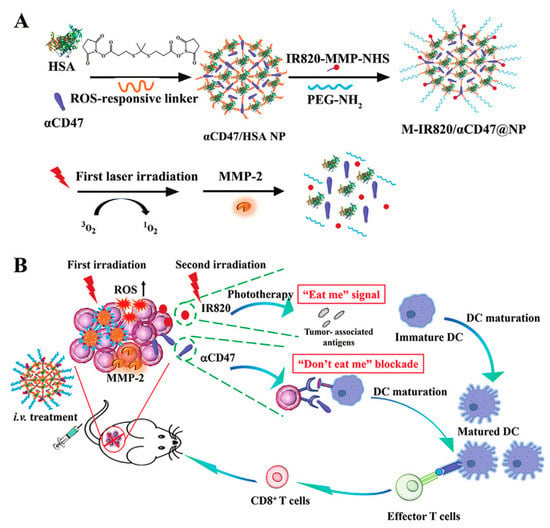
Figure 3.
(A) Preparation and (B) simplified mechanism of M-IR820/αCD47@NP combining immunogenic cell death induction and CD47 blockade to improve cancer immunotherapy. Reproduced with permission from Lu et al. (2022) [].
Furthermore, the rapid growth and high metabolic activity of tumor tissues leads to inadequate blood supply, resulting in a hypoxic environment surrounding the tumor [,]. Exploiting the contrast between the low oxygen levels in tumor tissues and the normoxic conditions in normal tissues, it is possible to design hypoxia-sensitive nanoparticles. Although large nanoparticles (~100 nm) demonstrate excellent serum stability and accumulate in tumor tissues, their limited ability to penetrate deep-seated tumor regions restricts their efficacy. Conversely, small-sized nanoparticles (<20 nm) greatly enhance tissue penetration but exhibit reduced tumor accumulation []. This size contradiction hinders nanoparticles from achieving both effective tumor accumulation and deep tissue penetration. To overcome this limitation, Yang et al. developed a hypoxia-sensitive dissociable HSA nanoparticle []. They utilized the properties that azobenzene derivatives can be reduced to aniline derivatives under hypoxic conditions and various reductases [,]. By crosslinking the azobenzene group between HSA conjugated with the photosensitizer Ce6 (HC) and HSA conjugated with the oxaliplatin prodrug (HO), a size-tunable nanoplatform known as an HSA-based nanosystem (HCHOA) was created. This nanoplatform demonstrated favorable stability in the bloodstream, with an approximate diameter of 130 nm. Leveraging the enhanced permeability and retention (EPR) effect, the nanosized particle exhibited effective targeting of tumor tissues. Upon reaching the tumor site, the specific hypoxic environment triggered the cleavage of the azobenzene groups within the nanoparticles, leading to the disintegration of HCHOA into smaller HC and HO nanoparticles with sizes below 10 nm. This facilitated deep tissue penetration. Before and after dissociation, the photodynamic activity of Ce6 within HC experienced a transition from quenching to activation, resulting in an increase in singlet oxygen production. Ultimately, the combination of photodynamic therapy (PDT) and chemotherapy achieved highly efficient treatment of breast tumors.
3.1.2. Non-Covalent Binding
As mentioned above, the crystal structure of HSA contains several specific binding sites that have the ability to bind various molecules with high affinity. One approach to non-covalent binding involves utilizing these inherent binding sites to form nanosized drug-HSA complexes. The available binding sites mainly include Sudlow sites I, Sudlow sites II, and cys34. Through reversible binding between HSA and drugs, the drug-HSA complexes can improve drug pharmacokinetics and enhance therapeutic efficacy by facilitating targeted accumulation and adequate drug release [].
Another method of production of drug-loaded HSA nanoparticles involves encapsulating small-molecule drugs into the interior of nanoparticles during the process of HSA nanocrystallization; this can be achieved through techniques such as desolvation, emulsification method and self-assembly method []. The binding between drugs and HSA in this approach relies on hydrogen bonding interaction, hydrophobic interaction, and electrostatic adsorption. Compared with the covalent binding method, the non-covalent binding method is widely applicable and suitable for combining small-molecule drugs []. A smart nanoplatform was fabricated using a desolvation approach []. This involved integrating IR780 iodide (IR780) and piceatannol (PIC) into the core of the HSA nanoparticle, resulting in the formation of HSA/IR780/PIC NPs. These nanoparticles (NPs)were designed for photoacoustic imaging-guided combined cancer therapy, specifically enhanced sonodynamic therapy, and chemotherapy. In order to improve the therapeutic efficacy of HSA-bound drugs, it is crucial to understand how nanoformulation influences their behavior in TME. In another report by Li et al., a potential therapeutic strategy was proposed to enhance the effectiveness of nanoparticulate HSA-bound drugs in cancer treatment by reprogramming nutrient signaling and enhancing macropinocytosis in cancer cells []. The researchers revealed that the level of macropinocytosis in cancer cells can be upregulated in the manner of nutrient deprivation, which increases the accumulation of HSA-bound drugs in tumors and enhances the therapeutic efficacy.
Furthermore, the abundant functional groups present on the HSA allowed for the surface modification of active ligands, enhancing the targeting ability of the drug-loaded nanoparticles to the desired site. Arg-Gly-Asp (RGD) peptide, MMP-2 reaction sequence, and polyhistidine (pHis) were introduced at the end of the HSA by gene fusion technology, and PTX was loaded into the pHis micelle core through hydrophobic interaction []. Drug-loaded HSA nanoparticles with a three-stage propulsive effect (namely 3RGD-HSA-MMP-18His nanoparticles, RHMH18 NPs) were successfully constructed. This system aimed to achieve active tumor targeting, MMP-2 digestion-mediated deep penetration, and pH-responsive drug release. In vitro and in vivo experimental results demonstrated that RHMH18 NPs exhibited superior tumor growth inhibition and lower toxic side effects compared to Abraxane.
Another approach to enhance targeting is the utilization of microenvironment-sensitive HBNDSs, which enhance the responsiveness of nanoparticles to disease microenvironments such as pH, glutathione (GSH), and enzymes, allowing for controlled and sustained drug release. This strategy enables the nanoparticles to maintain their structural integrity under normal physiological conditions while selectively releasing the drug at the site of the lesion, minimizing damage to normal tissues and reducing toxic side effects []. GSH, a bioreductant present in human cells, exhibits significantly higher concentrations within cells compared to body fluids and extracellular matrix, with tumor tissues showing GSH levels over four times higher than normal tissues []. This disparity can be exploited to achieve reduction-triggered drug release. For instance, Zhang et al. employed non-covalent binding to encapsulate a disulfide bond bridged paclitaxel-pentadecanoic acid conjugate (PTX-SS-C10-COOH) within HSA, resulting in the formation of oxidation reduction-responsive nanoparticles (HPTX NPs) []. In vitro experiments demonstrated the nanoparticles’ stability under physiological conditions, with only 13.2% cumulative release of PTX within 30 h. In contrast, the nanoparticles exhibited faster and more complete PTX release, reaching a cumulative release of 81.3%, in simulated tumor microenvironment conditions (10 mM GSH). Moreover, compared to the commercialized Abraxane, these nanoparticles demonstrated enhanced tumor growth inhibition and lower biotoxicity.
Compared with free drugs, albumin-binding prodrugs tends to be more effective in cancer therapy. However, no clear studies have clarified the tumor-targeting ability of these prodrugs. Um et al. verified the in vitro and in vivo targeting efficiency of three albumin-binding molecules []. They are albumin-binding peptide (PEP), palmitic acid (PA), and maleimide (MI), which are labeled with the NIR fluorescent dye cyanine 5.5 (Cy5.5), respectively. Among these three compounds, PA-Cy5.5 bound to albumin non-covalently and formed the most stable complex, due to its reversible and multivalent binding affinities. In addition, PA-Cy5.5 showed the longest half-life (395.3 ± 192.9 h·µg/mL) and the highest tumor-targeting efficiency after intravenous injection into the tumor-bearing mice compared to the other two molecules. This suggests that albumin-binding molecules with reversible and multivalent affinities to native albumin would greatly improve their in vivo pharmacokinetics and enhance tumor-targeting efficiency.
3.2. Inorganic Materials
In addition to small-molecule drug binding sites, the crystal structure of HSA also contains multiple metal binding sites. These binding sites allow for reversible binding of various metal ions (such as Ag+, Cu2+ and Mn2+), and they play an important role in the transport of metal ions during specific physiological or pathological processes in the living body []. The existence of these metal-binding sites has led to extensive exploration of HSA as a template for the synthesis of inorganic metal nanomaterials, including silver sulfide (Ag2S), gadolinium oxide (Gd2O3), manganese dioxide (MnO2), and copper sulfide (CuS) []. The process is similar to the biomineralization process occurring within the living organisms. Sun et al. constructed a NIR-II laser mediated photothermal Fenton nanocatalyst (PFN) by depositing MnO2 nanoparticles and CuS nanoparticles through a biomimetic biomineralization process using HSA as a stabilizer and template []. Due to the existence of CuS, PFN showed a good photothermal conversion efficiency under laser irradiation. This property not only enabled photothermal therapy (PTT), but also enhanced Cu+-mediated Fenton-like reaction. The combined effect of PFN-mediated PTT and chemodynamic therapy (CDT) exerted a synergistic ablation effect on xenograft tumors. However, the therapeutic efficacy of CDT is also critically dependent on the H2O2 level. Recently, a similar strategy was employed to develop a copper peroxide-based, tumor pH-responsive autocatalytic nanoreactor (CESAR), as reported by Liu et al. []. Upon exposure to the acidic TME, CESAR underwent collapse and instantly generated H2O2 and O2. The generated H2O2 facilitated the Cu+-catalyzed Fenton-like reaction, resulting in the production of a significant amount of ·OH for efficient CDT. Furthermore, the released O2 helped alleviate tumor hypoxia, thereby enhancing the efficacy of Ce6-mediated PDT (Figure 4). Overall, this strategy provides a promising paradigm to improve cancer therapies in cases of hyperoxide deficiency or oxygen limitation.
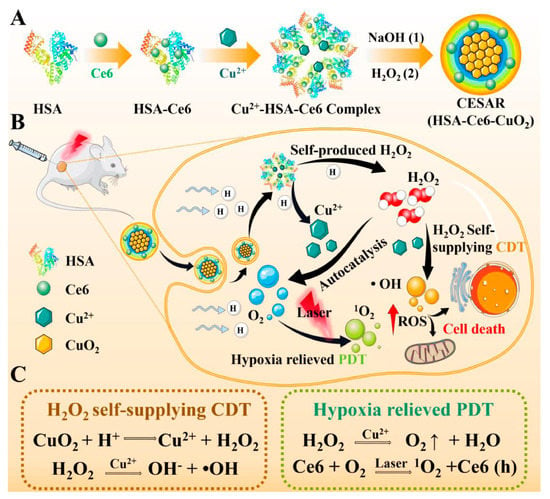
Figure 4.
(A) The synthesis route of HSA-based tumor pH-responsive nanoreactor (CESAR). (B,C) The scheme of therapeutic mechanism of CESAR nanoreactor, which integrates the capacity of locally triggered H2O2/O2 self-supply and Cu2+ release for realizing efficient PDT/CDT. Reproduced with permission from Liu et al. (2022) [].
In addition to the mineralization of inorganic metal nanoparticles on the HSA, the surface functional groups of HSA can also be covalently linked to specific inorganic non-metallic nanoparticles. Alzheimer’s disease (AD) is the most prevalent form of dementia, characterized by the accumulation of amyloid-beta (Aβ) proteins and elevated levels of ROS [,]. Currently, AD affects over 50 million individuals worldwide [], underscoring the urgent need for comprehensive diagnostic and therapeutic interventions. For instance, Wang et al. developed a multifunctional nanoparticle based on HSA (HSA-BFP) by introducing an Aβ fluorescent probe (F) and a cell-penetrating peptide (Penetratin, Pen) onto basified HSA (HSA-B) []. Further coupling of carbon dots (CDs) with HSA-BFP using N-succinimidyl-3-(2-pyridyldithio) propionate (SPDP) as a linker resulted in a targeted Aβ multifunctional protein-carbon dot conjugate (HSA-BFP@CDs). Upon interaction with Aβ aggregates, HSA-BFP@CDs exhibited a fluorescence signal at 700 nm, transitioning from an off state to an on state, showcasing the potential for early diagnosis of AD. Furthermore, in vitro and in vivo experiments utilizing Caenorhabditis elegans as a model demonstrated that HSA-BFP@CDs effectively suppressed Aβ aggregation and mitigated oxidative stress in vivo, underscoring the potential and prospects of protein-carbon dot conjugates in the multi-targeted treatment of AD.
3.3. Bioactive Ingredients
As a non-toxic, non-immunogenic, stable, and biocompatible material, HSA nanoparticles are also employed as useful vehicles for bioactive ingredients, including nucleic acids (DNA and RNA), antibodies, peptides, cytokines, and enzymes []. Recent studies of HSA-based nanoparticles for the delivery of bioactive ingredients and their biomedical applications are listed in Table 2. On the one hand, using HSA nanoparticles for the delivery of these bioactive ingredients can avoid unnecessary immunogenicity and potential safety issues. On the other hand, utilizing HSA nanoparticles as carriers can provide significant protection to these active components against enzymatic digestion, enhance their systemic stability during blood circulation, and effectively improve their pharmacokinetics.

Table 2.
Typical examples of HSA-based nanocarriers for the delivery of bioactive ingredients and their biomedical applications.
AML is the most common hematological malignancy, with a high recurrence rate and poor long-term survival, accounting for 42% of all leukemia deaths []. Some studies have shown that the polycomb protein Bmi1 is overexpressed in AML and plays a role in disease development through its downstream targets [], making it a potential epigenetic target. Kushwaha et al. proposed a delivery strategy using HSA nanoparticles as carriers (nanocarriers, NCs) for Bmi1 siRNA, which was stabilized by polyethylenimine (PEI) []. This innovative approach, known as PEI@HSANCs, aimed to enhance the effectiveness of Bmi1 siRNA delivery. The PEI-modified HSA nanocarrier demonstrated the ability to protect Bmi1 siRNA from degradation by ribonucleases, resulting in a significant increase in the transfection efficiency of Bmi1 siRNA through caveolae-mediated endocytosis (Figure 5). The siRNA transfection efficiency in the PEI@HSANCs group was nearly 2.4 times higher than that in the siRNA group. In another study, Kaundal et al. identified another member of the polycomb histone family, Enhancer of Zeste Homolog 2 (EZH2), which is highly expressed in AML []. The authors proposed an effective EZH2 siRNA delivery strategy based on HSA (HSANPs-PEI@EZH2siRNA). EZH2 siRNA was loaded in PEI-modified HSA nanoparticles, which displayed enhanced system stability and blood compatibility. These nanoparticles precisely targeted the EZH2 gene in AML cells, leading to enhanced EZH2 gene silencing.
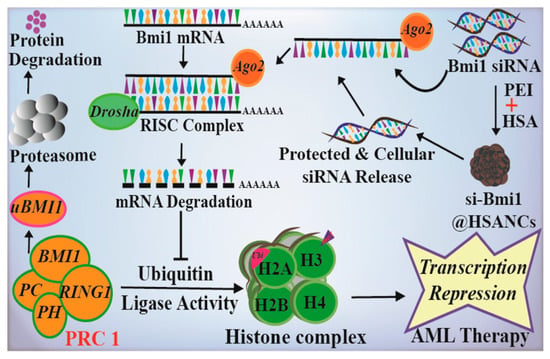
Figure 5.
Schematic representation of the construction of HSA NPs-PEI@EZH2 siRNA and the possible mechanism of AML therapy. Reproduced with permission from the work of Kushwaha et al. (2020) [].
RA is a prevalent chronic systemic autoimmune disease that poses a serious threat to human health and well-being []. In light of this, an HSA-based nanodrug co-loaded with the anti-RA medication MTX and the ROS scavenger superoxide dismutase (SOD) was designed, and is now known as HSA-SOD-MTX []. Owing to the overexpression of SPARC at arthritic inflammatory sites, HSA-SOD-MTX nanoparticles can specifically target these sites, thereby overcoming the challenges of insufficient affinity of SOD for the target and the lack of evident drug tropism. The SOD incorporated in the nanoparticles exhibited effective scavenging of O2•− and notably reduced the expression levels of various in vitro inflammation-associated cytokines (granulocyte colony-stimulating factor G-CSF, monocyte chemotactic protein-1 MCP-1 as well as regulated on activation, normal T cell expressed and secreted RANTES), which is beneficial to the treatment of RA. In vivo results showed that the combined action of SOD-mediated ROS scavenging and MTX-mediated anti-rheumatism was able to significantly inhibit the inflammatory response in the joints of CIA mice without inducing obvious systemic toxicity.
4. Conclusions and Future Perspectives
Owing to its advantages of good biocompatibility, non-toxicity, non-immunogenicity, and long half-life, HSA has received much attention in the study of drug delivery systems. Utilizing the intrinsic binding sites of HSA and the extensive developments in nanotechnology for HSA nanocrystallization, therapeutic drugs can be effectively conjugated with HSA or encapsulated within HSA nanoparticles. These drugs include small-molecule drugs, inorganic materials, as well as bioactive ingredients (such as nucleic acids, peptides, antibodies, cytokines, and enzymes). They help to enhance systemic stability, improve pharmacokinetics, and enhance the therapeutic efficacy of drugs while minimizing systemic side effects. The existence of functional groups on HSA allows for their modification with multiple targeted ligands, enhancing the targeting of drug delivery systems and reducing the accumulation of therapeutic drugs in non-targeted regions. Additionally, HSA-based stimuli-responsive nanodrug delivery systems can be designed for on-demand drug release according to various covalent or non-covalent approaches, on the basis of the characteristics of the lesion microenvironment and the structural characteristics of HSA. Stimulus-sensitive HBNDSs can decompose and release drugs, under specific endogenous conditions (e.g., pH, GSH, and multiple enzymes) or exogenous conditions (e.g., laser and ultrasound), effectively increasing the drug concentration at the desired region. For example, due to the rapid growth and vigorous metabolism of tumor tissue, it requires a lot of energy and protein. Albumin serves as the main source of energy and amino acids for tumor tissue. A large number of receptors and proteins will be over-expressed on tumor cells, which can bind with HSA in a high-affinity manner. HSA-based nanoparticles can preferentially accumulate in tumors through this mechanism. Moreover, due to the increased albumin metabolism in tumors, the targeted delivery of cytotoxic agents to tumor tissue can be realized. This may have beneficial implications for the design of clinical antitumor drugs, such as targeted therapy for pan-KRAS mutant cancers []. Similarly, the overexpression of SPARC is also confirmed in arthritic inflammatory sites [], compared to healthy tissues. These findings demonstrate the great potential of HBNDSs for use in the biomedical field.
Despite the unique advantages of this method, there are still some problems and mechanisms that need to be solved and explored. Firstly, in terms of cancer therapy, HBNDSs can passively target tumor tissues via the EPR effect, thus enhancing the efficacy of antitumor drugs. However, it remains to be validated whether similar nanoparticle enrichment mechanisms exist in other biomedical disease models. Secondly, it has been shown that the in vivo transport and distribution of HSA is regulated by ABPs. It is important to determine whether drug-loaded HSA nanoparticles are still recognized by cells and internalized through this pathway. Moreover, the in vivo transport mechanism of drug-loaded HSA nanoparticles also needs to be investigated. Further research on the structural changes, the distribution and transport of HSA nanoparticles after binding with drugs will play a key role in accelerating the development of HSA formulations. HSA also has its own defects as a drug carrier, such as the limited source and expensive cost. Recombinant HSA is a recently developed genetically engineered protein expressed by yeast cells and is promising as a potential candidate. The clinical challenge for HSA nanoformulations in future biomedical applications may be to look for raw materials with stable properties and highly reproducible preparation methods. Currently, research on HSA formulation-based approaches is ongoing. We expect that in the near future, more new HSA-based therapeutic and diagnostic products will be approved for clinical use, benefiting patients.
Author Contributions
Conceptualization, B.C.; software, D.Z. and Y.P.; validation, C.L.; writing—original draft preparation, D.Z.; writing—review and editing, C.L.; image plotting, D.Z. and Y.P.; supervision, C.L. and B.C.; funding acquisition, C.L. and B.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research & Development Program of China (2019YFE0113600), and the Scientific Research Funds of Huaqiao University (23BS108, 23BS113).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the Program for Innovative Research Team in Science and Technology in Fujian Province University.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ABPs | Albumin binding proteins. |
| AD | Alzheimer’s disease. |
| Ag2S | Silver sulfide. |
| AML | Acute myeloid leukemia. |
| AT1 | Angiotensin II type 1. |
| AzMMMan | Azidomethyl-methylmaleic anhydride. |
| Aβ | Amyloid-beta. |
| Bcl-2 | B-cell lymphoma-2. |
| CAT | Catalase. |
| Ce6 | Chlorine e6. |
| CIA | Collagen-induced arthritis. |
| CDs | Carbon dots. |
| CDT | Chemodynamic therapy. |
| CESAR | Copper peroxide-based tumor pH-responsive autocatalytic nanoreactor. |
| CU | Curcumin. |
| CuS | Copper sulfide. |
| Cy5.5 | Cyanine 5.5. |
| cys34 | Cysteine 34. |
| DBCO | Dibenzocyclooctyne. |
| DBCO-Ce6 | DBCO coupled Ce6. |
| DC | Dibenzocyclooctyne/chlorin. |
| DOX | Doxorubicin. |
| DSP | Dexamethasone sodium phosphate. |
| EPR | Enhanced permeability and retention. |
| EZH2 | Enhancer of Zeste Homolog 2. |
| FcRn | Fc receptor. |
| FDA | Food and Drug Administration. |
| G-CSF | Granulocyte colony-stimulating factor. |
| Gd2O3 | Gadolinium oxide. |
| GOD | Glucose oxidase. |
| gp60 | Glycoprotein 60 kDa. |
| GSH | Glutathione. |
| HSA | Human serum albumin. |
| HBNDSs | HSA-based nanodrug delivery systems. |
| HC | HSA conjugated with Ce6. |
| HO | HSA conjugated with oxaliplatin. |
| HCHOA | A nanoplatform by crosslinking azobenzene group between HC and HO. |
| HPTX | HSA conjugated with PTX-SS-C10-COOH. |
| HSA-BFP | A multifunctional nanoparticle based on basified HSA (HSA-B) by incorporating an Aβ fluorescent probe (F) and a cell-penetrating peptide (Penetratin, Pen). |
| HSAP | HSA-Pt (IV). |
| IL-10 | Interleukin-10. |
| INH | Isoniazid. |
| IR780 | IR780 iodide. |
| LDL-C | Low-density lipoprotein cholesterol. |
| MBS-A | Metal binding sites-A. |
| MBS-B | Metal binding sites-B. |
| MCP-1 | Monocyte chemotactic protein-1. |
| MI | Maleimide. |
| MMP | Matrix metalloproteinase. |
| MnO2 | Manganese dioxide. |
| MRN | Milrinone. |
| MTX | Methotrexate. |
| NCs | Nanocarriers. |
| NIR | Near-infrared. |
| NP(s) | Nanoparticle(s). |
| NTS | N-terminal binding site. |
| PA | Palmitic acid. |
| PCSK9 | Proprotein convertase subtilisin/kexin type 9. |
| PD | Prednisolone. |
| PD-L1 | Programmed cell death-ligand 1. |
| pDNA | Plasmid DNA. |
| PDT | Photodynamic therapy. |
| PDX | Patient-derived xenograft. |
| PEI | Polyethylenimine. |
| PEP | Albumin-binding peptide. |
| PFN | Photothermal Fenton nanocatalyst. |
| pHis | Polyhistidine. |
| PIC | Piceatannol. |
| PTT | Photothermal therapy. |
| Pt (IV)SA | Succinic acid-derivatized cisplatin prodrug. |
| PTX | Paclitaxel. |
| pVax | Peptide vaccine. |
| RA | Rheumatoid arthritis. |
| RB | Rose bengal. |
| RANTES | Regulated on activation, normal T cell expressed and secreted. |
| RGD | Arg-Gly-Asp. |
| RIF | Rifampicin. |
| ROS | Reactive oxygen species. |
| SCI | Spinal cord injury. |
| siRNA | Small interfering RNA. |
| SOD | Superoxide dismutase. |
| SPARC | Secreted protein acidic and rich in cysteine. |
| SPDP | N-succinimidyl-3-(2-pyridyldithio) propionate. |
| TAT | Trans-activator of transcription. |
| TME | Tumor microenvironment. |
| TMP | Tetramethylpyrazine. |
| 5-FU | 5-Fluorouracil. |
References
- Wang, Y.; Iqbal, H.; Ur-Rehman, U.; Zhai, L.; Yuan, Z.; Razzaq, A.; Lv, M.; Wei, H.; Ning, X.; Xin, J.; et al. Albumin-based nanodevices for breast cancer diagnosis and therapy. J. Drug Delivery Sci. Technol. 2023, 79, 104072. [Google Scholar] [CrossRef]
- Li, C.; Wang, X.; Song, H.; Deng, S.; Li, W.; Li, J.; Sun, J. Current multifunctional albumin-based nanoplatforms for cancer multi-mode therapy. Asian J. Pharm. Sci. 2020, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.; Zhu, H.; Wang, Z.; Hao, S.; Wang, B. Preparation of Drug-Loaded Albumin Nanoparticles and Its Application in Cancer Therapy. J. Nanomater. 2022, 2022, 3052175. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, L.; Ge, G.; Hu, K. Emerging Epigenetic-Based Nanotechnology for Cancer Therapy: Modulating the Tumor Microenvironment. Adv. Sci. 2023, 10, e2206169. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboezem, E.N.; Duvall, C.L. Harnessing albumin as a carrier for cancer therapies. Adv. Drug Delivery Rev. 2018, 130, 73–89. [Google Scholar] [CrossRef]
- Tao, H.Y.; Wang, R.Q.; Sheng, W.J.; Zhen, Y.S. The development of human serum albumin-based drugs and relevant fusion proteins for cancer therapy. Int. J. Biol. Macromol. 2021, 187, 24–34. [Google Scholar] [CrossRef]
- Hornok, V. Serum Albumin Nanoparticles: Problems and Prospects. Polymers 2021, 13, 3759. [Google Scholar] [CrossRef]
- Iqbal, H.; Yang, T.; Li, T.; Zhang, M.; Ke, H.; Ding, D.; Deng, Y.; Chen, H. Serum protein-based nanoparticles for cancer diagnosis and treatment. J. Control. Release 2021, 329, 997–1022. [Google Scholar] [CrossRef]
- Han, Y.; Pang, X.; Pi, G. Biomimetic and Bioinspired Intervention Strategies for the Treatment of Rheumatoid Arthritis. Adv. Funct. Mater. 2021, 31, 2104640. [Google Scholar] [CrossRef]
- Shen, X.; Liu, X.; Li, T.; Chen, Y.; Chen, Y.; Wang, P.; Zheng, L.; Yang, H.; Wu, C.; Deng, S.; et al. Recent Advancements in Serum Albumin-Based Nanovehicles Toward Potential Cancer Diagnosis and Therapy. Front. Chem. 2021, 9, 746646. [Google Scholar] [CrossRef]
- Bhushan, B.; Khanadeev, V.; Khlebtsov, B.; Khlebtsov, N.; Gopinath, P. Impact of albumin based approaches in nanomedicine: Imaging, targeting and drug delivery. Adv. Colloid Interface Sci. 2017, 246, 13–39. [Google Scholar] [CrossRef] [PubMed]
- Steinhardt, J.; Krijn, J.; Leidy, J.G. Differences between bovine and human serum albumins: Binding isotherms, optical rotatory dispersion, viscosity, hydrogen ion titration, and fluorescence effects. Biochemistry 1971, 10, 4005–4015. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, B.; Kratz, F. Impact of albumin on drug delivery-New applications on the horizon. J. Control. Release 2012, 157, 4–28. [Google Scholar] [CrossRef] [PubMed]
- Elzoghby, A.O.; Samy, W.M.; Elgindy, N.A. Albumin-based nanoparticles as potential controlled release drug delivery systems. J. Control. Release 2012, 157, 168–182. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; He, X.M.; Munson, S.H.; Twigg, P.D.; Gernert, K.M.; Broom, M.B.; Miller, T.Y. Three-dimensional structure of human serum albumin. Science 1989, 244, 1195–1198. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, X. Simple bioconjugate chemistry serves great clinical advances: Albumin as a versatile platform for diagnosis and precision therapy. Chem. Soc. Rev. 2016, 45, 1432–1456. [Google Scholar] [CrossRef]
- Kunde, S.S.; Wairkar, S. Targeted delivery of albumin nanoparticles for breast cancer: A review. Colloids Surf. B 2022, 213, 112422. [Google Scholar] [CrossRef]
- Kudarha, R.R.; Sawant, K.K. Albumin based versatile multifunctional nanocarriers for cancer therapy: Fabrication, surface modification, multimodal therapeutics and imaging approaches. Mater. Sci. Eng. C 2017, 81, 607–626. [Google Scholar] [CrossRef]
- Kianfar, E. Protein nanoparticles in drug delivery: Animal protein, plant proteins and protein cages, albumin nanoparticles. J. Nanobiotechnol. 2021, 19, 159. [Google Scholar] [CrossRef]
- Rudnik-Jansen, I.; Howard, K.A. FcRn expression in cancer: Mechanistic basis and therapeutic opportunities. J. Control. Release 2021, 337, 248–257. [Google Scholar] [CrossRef]
- Zhang, X.; Feng, L.; Dong, Z.; Xin, X.; Yang, Z.; Deng, D.; Wagner, E.; Liu, Z.; Liu, X. Protein-drug conjugate programmed by pH-reversible linker for tumor hypoxia relief and enhanced cancer combination therapy. Int. J. Pharm. 2020, 582, 119321. [Google Scholar] [CrossRef]
- Lu, Y.; Gong, Y.; Zhu, X.; Dong, X.; Zhu, D.; Ma, G. Design of Light-Activated Nanoplatform through Boosting “Eat Me” Signals for Improved CD47-Blocking Immunotherapy. Adv. Healthc. Mater. 2022, 11, e2102712. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Phua, S.Z.F.; Lim, W.Q.; Zhang, R.; Feng, L.; Liu, G.; Wu, H.; Bindra, A.K.; Jana, D.; Liu, Z.; et al. A hypoxia-responsive albumin-based nanosystem for deep tumor penetration and excellent therapeutic efficacy. Adv. Mater. 2019, 31, 1901513. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Wang, S.; Yang, H.; Luo, T.; Zhao, F.; Han, J.; Zhang, J. An albumin-based nanosystem for cocktail therapy of breast cancer amplifies the therapeutic efficacy of combination chemotherapy with photodynamic therapy. J. Mater. Sci. 2023, 58, 8952–8968. [Google Scholar] [CrossRef]
- Chen, H.; Huang, S.; Wang, H.; Chen, X.; Zhang, H.; Xu, Y.; Fan, W.; Pan, Y.; Wen, Q.; Lin, Z.; et al. Preparation and characterization of paclitaxel palmitate albumin nanoparticles with high loading efficacy: An in vitro and in vivo anti-tumor study in mouse models. Drug Deliv. 2021, 28, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Li, H.; Zhong, Z.; Zhou, M.; Lin, Y.; Tang, C.; Li, C. Co-Delivery of Prednisolone and Curcumin in Human Serum Albumin Nanoparticles for Effective Treatment of Rheumatoid Arthritis. Int. J. Nanomed. 2019, 14, 9113–9125. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mu, W.; Wei, D.; Zhang, Y.; Duan, Y.; Gao, J.X.; Gong, X.Q.; Wang, H.J.; Wu, X.L.; Tao, H.; et al. A Novel Targeted and High-Efficiency Nanosystem for Combinational Therapy for Alzheimer’s Disease. Adv. Sci. 2020, 7, 1902906. [Google Scholar] [CrossRef]
- Shojania, H.R.; Momeni-Moghaddam, M.; Hossini, S.E.; Armin, M.; Bidi, J.O. MicroRNA 155 downregulation by vitamin C-Loaded human serum albumin nanoparticles during cutaneous wound healing in mice. Int. J. Low. Extrem. Wounds 2019, 18, 143–152. [Google Scholar] [CrossRef]
- Zhang, D.G.; Chen, B.Q.; Pan, Y.J.; Liu, H.; Shi, Y.H.; Chen, L.F.; Kankala, R.K.; Wang, S.B.; Chen, A.Z. Albumin-based smart nanoplatform for ultrasound-mediated enhanced chemo-sonodynamic combination therapy. Mater. Des. 2023, 227, 111794. [Google Scholar] [CrossRef]
- Galiyeva, A.; Daribay, A.; Zhumagaliyeva, T.; Zhaparova, L.; Sadyrbekov, D.; Tazhbayev, Y. Human serum albumin nanoparticles: Synthesis, optimization and immobilization with antituberculosis drugs. Polymers 2023, 15, 2774. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Xing, H.; Liu, C.; Li, X. Redox-responsive paclitaxel-pentadecanoic acid conjugate encapsulated human serum albumin nanoparticles for cancer therapy. Int. J. Pharm. 2023, 635, 122761. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhang, L.; Cai, Y.; Yang, Y.; Qu, L.; Shen, Y.; Jin, J.; Zhou, J.; Chen, J. Bioengineered Human Serum Albumin Fusion Protein as Target/Enzyme/pH Three-Stage Propulsive Drug Vehicle for Tumor Therapy. ACS Nano 2020, 14, 17405–17418. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhang, L.; Zhang, Y.; Yue, C.W.; Lin, J.; Wang, H.; Fang, Z.J.; Wu, J. Smart albumin-loaded Rose Bengal and doxorubicin nanoparticles for breast cancer therapy. J. Microencapsul. 2019, 36, 728–737. [Google Scholar] [CrossRef]
- Xiong, F.; Ling, X.; Chen, X.; Chen, J.; Tan, J.; Cao, W.; Ge, L.; Ma, M.; Wu, J. Pursuing specific chemotherapy of orthotopic breast cancer with lung metastasis from docking nanoparticles driven by bioinspired exosomes. Nano Lett. 2019, 19, 3256–3266. [Google Scholar] [CrossRef]
- Liu, F.; Mu, J.; Xing, B. Recent Advances on the Development of Pharmacotherapeutic Agents on the Basis of Human Serum Albumin. Curr. Pharm. Des. 2015, 21, 1866–1888. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, S.; Lee, S. Albumin nanoscience: Homing nanotechnology enabling targeted drug delivery and therapy. Arch. Pharm. Res. 2020, 43, 118–133. [Google Scholar] [CrossRef]
- Bruelisauer, L.; Valentino, G.; Morinaga, S.; Cam, K.; Bukrinski, J.T.; Gauthier, M.A.; Leroux, J.-C. Bio-reduction of redox-sensitive albumin conjugates in FcRn-expressing cells. Angew. Chem. Int. Ed. 2014, 53, 8392–8396. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Wang, X.; Wang, C.; Feng, L.; Li, Y.; Liu, Z. Drug-induced self-assembly of modified albumins as nano-theranostics for tumor-targeted combination therapy. ACS Nano 2015, 9, 5223–5233. [Google Scholar] [CrossRef]
- Kuan, S.L.; Stoeckle, B.; Reichenwallner, J.; Ng, D.Y.W.; Wu, Y.; Doroshenko, M.; Koynov, K.; Hinderberger, D.; Muellen, K.; Weil, T. Dendronized albumin core-shell transporters with high drug loading capacity. Biomacromolecules 2013, 14, 367–376. [Google Scholar] [CrossRef]
- de Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Fu, L.; Li, C.; Lin, J.; Huang, P. Conquering the hypoxia limitation for photodynamic therapy. Adv. Mater. 2021, 33, 2103978. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Ni, D.; Cheng, W.; Ji, C.; Wang, Y.; Muellen, K.; Su, Z.; Liu, Y.; Chen, C.; Yin, M. Enzyme-triggered disassembly of perylene monoimide-based nanoclusters for activatable and deep photodynamic therapy. Angew. Chem. Int. Ed. 2020, 59, 14014–14018. [Google Scholar] [CrossRef] [PubMed]
- Piao, W.; Tsuda, S.; Tanaka, Y.; Maeda, S.; Liu, F.; Takahashi, S.; Kushida, Y.; Komatsu, T.; Ueno, T.; Terai, T.; et al. Development of azo-based fluorescent probes to detect different levels of hypoxia. Angew. Chem. Int. Ed. 2013, 52, 13028–13032. [Google Scholar] [CrossRef]
- Yuan, P.; Zhang, H.; Qian, L.; Mao, X.; Du, S.; Yu, C.; Peng, B.; Yao, S.Q. Intracellular delivery of functional native antibodies under hypoxic conditions by using a biodegradable silica nanoquencher. Angew. Chem. Int. Ed. 2017, 56, 12481–12485. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Bakshi, H.A.; Hakkim, F.L.; Haggag, Y.A.; Al-Batanyeh, K.M.; Al Zoubi, M.S.; Al-Trad, B.; Nasef, M.M.; Satija, S.; Mehta, M.; et al. Albumin Nano-Encapsulation of Piceatannol Enhances Its Anticancer Potential in Colon Cancer Via Downregulation of Nuclear p65 and HIF-1 alpha. Cancers 2020, 12, 113. [Google Scholar] [CrossRef]
- Li, R.; Ng, T.S.C.; Wang, S.J.; Prytyskach, M.; Rodell, C.B.; Mikula, H.; Kohler, R.H.; Garlin, M.A.; Lauffenburger, D.A.; Parangi, S.; et al. Therapeutically reprogrammed nutrient signalling enhances nanoparticulate albumin bound drug uptake and efficacy in KRAS-mutant cancer. Nat. Nanotechnol. 2021, 16, 830–839. [Google Scholar] [CrossRef]
- Kratz, F. Albumin as a drug carrier: Design of prodrugs, drug conjugates and nanoparticles. J. Control. Release 2008, 132, 171–183. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, H.; Li, Y. Stimuli-responsive nanomedicines for overcoming cancer multidrug resistance. Theranostics 2018, 8, 1059–1074. [Google Scholar] [CrossRef]
- Um, W.; Park, J.; Youn, A.; Cho, H.; Lim, S.; Lee, J.W.; Yoon, H.Y.; Lim, D.-K.; Park, J.H.; Kim, K. A comparative study on albumin-binding molecules for targeted tumor delivery through covalent and noncovalent approach. Bioconjug. Chem. 2019, 30, 3107–3118. [Google Scholar] [CrossRef]
- Bal, W.; Sokolowska, M.; Kurowska, E.; Faller, P. Binding of transition metal ions to albumin: Sites, affinities and rates. Biochim. Biophys. Acta Gen. Subj. 2013, 1830, 5444–5455. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Y.; Chen, S.; Wang, R.; Chen, Q.; Li, J.; Luo, Y.; Wang, X.; Chen, H. Photothermal Fenton Nanocatalysts for Synergetic Cancer Therapy in the Second Near-Infrared Window. ACS Appl. Mater. Interfaces 2020, 12, 30145–30154. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; He, T.; Gong, S.; Shen, M.; Ma, S.; Huang, X.; Li, L.; Wang, L.; Wu, Q.; Gong, C. A tumor pH-responsive autocatalytic nanoreactor as a H2O2 and O2 self-supplying depot for enhanced ROS-based chemo/photodynamic therapy. Acta Biomater. 2022, 154, 510–522. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, S.; Puli, L.; Patil, C.R. Role of reactive oxygen species in the progression of Alzheimer’s disease. Drug Discov. Today 2021, 26, 794–803. [Google Scholar] [CrossRef]
- Lipiec, E.; Perez-Guaita, D.; Kaderli, J.; Wood, B.R.; Zenobi, R. Direct Nanospectroscopic Verification of the Amyloid Aggregation Pathway. Angew. Chem. Int. Ed. 2018, 57, 8519–8524. [Google Scholar] [CrossRef] [PubMed]
- Blaszczyk, J.W. Pathogenesis of Dementia. Int. J. Mol. Sci. 2023, 24, 543. [Google Scholar] [CrossRef]
- Wang, W.; Lin, X.; Dong, X.; Sun, Y. A multi-target theranostic nano-composite against Alzheimer’s disease fabricated by conjugating carbon dots and triple-functionalized human serum albumin. Acta Biomater. 2022, 148, 298–309. [Google Scholar] [CrossRef]
- Dimitrov, D.S. Therapeutic proteins. In Therapeutic Proteins; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 899, pp. 1–26. [Google Scholar] [CrossRef]
- Wang, Z.; Mei, J.; Ni, D.; Li, S.; Ye, J.; Li, S.; Wang, Y.; Liu, Y. A nanoplatform self-assembled by coordination delivers siRNA for lung cancer therapy. Rare Metals 2023, 42, 1483–1493. [Google Scholar] [CrossRef]
- Zheng, X.; Yu, X.; Wang, C.; Liu, Y.; Jia, M.; Lei, F.; Tian, J.; Li, C. Targeted co-delivery biomimetic nanoparticles reverse macrophage polarization for enhanced rheumatoid arthritis therapy. Drug Delivery 2022, 29, 1025–1037. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, A.C.; Mohanbhai, S.J.; Sardoiwala, M.N.; Sood, A.; Karmakar, S.; Choudhury, S.R. Epigenetic Regulation of Bmi1 by Ubiquitination and Proteasomal Degradation Inhibit Bcl-2 in Acute Myeloid Leukemia. ACS Appl. Mater. Interfaces 2020, 12, 25633–25644. [Google Scholar] [CrossRef]
- Kaundal, B.; Kushwaha, A.C.; Srivastava, A.K.; Karmakar, S.; Choudhury, S.R. A non-viral nano-delivery system targeting epigenetic methyltransferase EZH2 for precise acute myeloid leukemia therapy. J. Mater. Chem. B 2020, 8, 8658–8670. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Chen, Z.; Sun, M.; Li, B.; Pan, F.; Ma, A.; Liao, J.; Yin, T.; Tang, X.; Huang, G.; et al. IL-12 nanochaperone-engineered CAR T cell for robust tumor-immunotherapy. Biomaterials 2022, 281, 121341. [Google Scholar] [CrossRef] [PubMed]
- Pham, L.M.; Poudel, K.; Ou, W.; Phung, C.D.; Nguyen, H.T.; Nguyen, B.L.; Karmacharya, P.; Pandit, M.; Chang, J.-H.; Jeong, J.-H.; et al. Combination chemotherapeutic and immune-therapeutic anticancer approach via anti-PD-L1 antibody conjugated albumin nanoparticles. Int. J. Pharm. 2021, 605, 120816. [Google Scholar] [CrossRef]
- Ji, H.; Wu, G.; Li, Y.; Wang, K.; Xue, X.; You, S.; Wu, S.; Ren, T.; He, B.; Shi, X. Self-albumin camouflage of carrier protein prevents nontarget antibody production for enhanced LDL-C immunotherapy. Adv. Healthc. Mater. 2020, 9, 1901203. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.J.; Ko, E.J.; Park, Y.-Y.; Park, S.S.; Ju, E.J.; Park, J.; Shin, S.H.; Suh, Y.-A.; Hong, S.-M.; Park, I.J.; et al. A novel nanoparticle-based theranostic agent targeting LRP-1 enhances the efficacy of neoadjuvant radiotherapy in colorectal cancer. Biomaterials 2020, 255, 120151. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Cen, L.; Liu, Q.; Chu, Y.; Feng, X.; Ke, Y.; Zhang, Z.; Dai, H.; Huang, S.; Liu, B.; et al. A dual-adjuvant neoantigen nanovaccine loaded with imiquimod and magnesium enhances anti-tumor immune responses of melanoma. Biomater. Sci. 2022, 10, 6740–6748. [Google Scholar] [CrossRef]
- Lomis, N.; Westfall, S.; Shum-Tim, D.; Prakash, S. Synthesis and characterization of peptide conjugated human serum albumin nanoparticles for targeted cardiac uptake and drug delivery. PLoS ONE 2021, 16, e0254305. [Google Scholar] [CrossRef]
- Li, J.; Wei, J.; Wan, Y.; Du, X.; Bai, X.; Li, C.; Lin, Y.; Liu, Z.; Zhou, M.; Zhong, Z. TAT-modified tetramethylpyrazine-loaded nanoparticles for targeted treatment of spinal cord injury. J. Control. Release 2021, 335, 103–116. [Google Scholar] [CrossRef]
- Chen, J.; Liang, C.; Song, X.; Yi, X.; Yang, K.; Feng, L.; Liu, Z. Hybrid protein nano-reactors enable simultaneous increments of tumor oxygenation and iodine-131 delivery for enhanced radionuclide therapy. Small 2019, 15, 1903628. [Google Scholar] [CrossRef]
- Zhong, J.; Zhang, Q.; Zhang, Z.; Shi, K.; Sun, Y.; Liu, T.; Lin, J.; Yang, K. Albumin mediated reactive oxygen species scavenging and targeted delivery of methotrexate for rheumatoid arthritis therapy. Nano Res. 2022, 15, 153–161. [Google Scholar] [CrossRef]
- Yang, G.; Wang, D.; Phua, S.Z.F.; Bindra, A.K.; Qian, C.; Zhang, R.; Cheng, L.; Liu, G.; Wu, H.; Liu, Z.; et al. Albumin-based therapeutics capable of glutathione consumption and hydrogen peroxide generation for synergetic chemodynamic and chemotherapy of cancer. ACS Nano 2022, 16, 2319–2329. [Google Scholar] [CrossRef] [PubMed]
- Her, Z.; Yong, K.S.M.; Paramasivam, K.; Tan, W.W.S.; Chan, X.Y.; Tan, S.Y.; Liu, M.; Fan, Y.; Linn, Y.C.; Hui, K.M.; et al. An improved pre-clinical patient-derived liquid xenograft mouse model for acute myeloid leukemia. J. Hematol. Oncol. 2017, 10, 162. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Ying, W.; Gray, F.; Yao, Y.; Simes, M.L.; Zhao, Q.; Miao, H.; Cho, H.J.; Gonzalez-Alonso, P.; Winkler, A.; et al. Small-molecule inhibitors targeting Polycomb repressive complex 1 RING domain. Nat. Chem. Biol. 2021, 17, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.L.L.; Pratt, A.G.G.; Dodds, R.; Sayer, A.A.A.; Isaacs, J.D.D. Rheumatoid sarcopenia: Loss of skeletal muscle strength and mass in rheumatoid arthritis. Nat. Rev. Rheumatol. 2023, 19, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.S.; Kim, G.C.; Lee, H.M.; Kim, B.; Kim, H.R.; Chung, S.W.; Chang, H.W.; Ko, Y.G.; Lee, Y.S.; Kim, S.W.; et al. Albumin metabolism targeted peptide-drug conjugate strategy for targeting pan-KRAS mutant cancer. J. Control. Release 2022, 344, 26–38. [Google Scholar] [CrossRef]
- Liu, L.; Hu, F.; Wang, H.; Wu, X.; Eltahan, A.S.; Stanford, S.; Bottini, N.; Xiao, H.; Bottini, M.; Guo, W.; et al. Secreted protein acidic and rich in cysteine mediated biomimetic delivery of methotrexate by albumin-based nanomedicines for rheumatoid arthritis therapy. ACS Nano 2019, 13, 5036–5048. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).