Effect of Nitrogen Arc Discharge Plasma Treatment on Physicochemical Properties and Biocompatibility of PLA-Based Scaffolds
Abstract
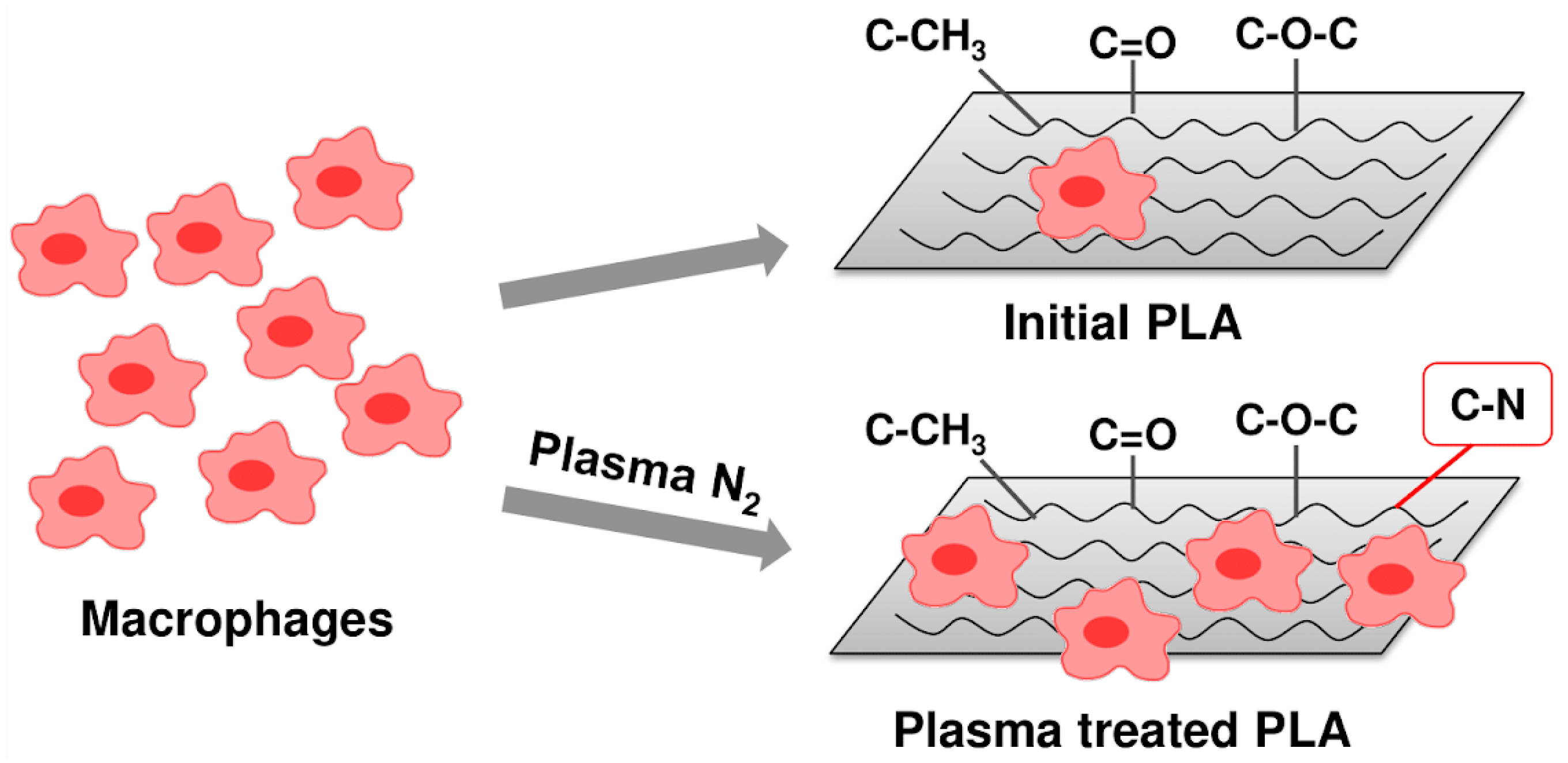
:1. Introduction
2. Materials and Methods
2.1. Obtainment of PLA-Based Scaffolds
2.2. Plasma Treatment of PLA-Based Scaffolds
2.3. Investigation Techniques
2.3.1. Chemical Composition
2.3.2. Wettability
2.3.3. Surface Morphology
2.3.4. Monocyte Isolation and Culture
2.3.5. Alamar Blue Assay
2.3.6. Statistics
3. Results
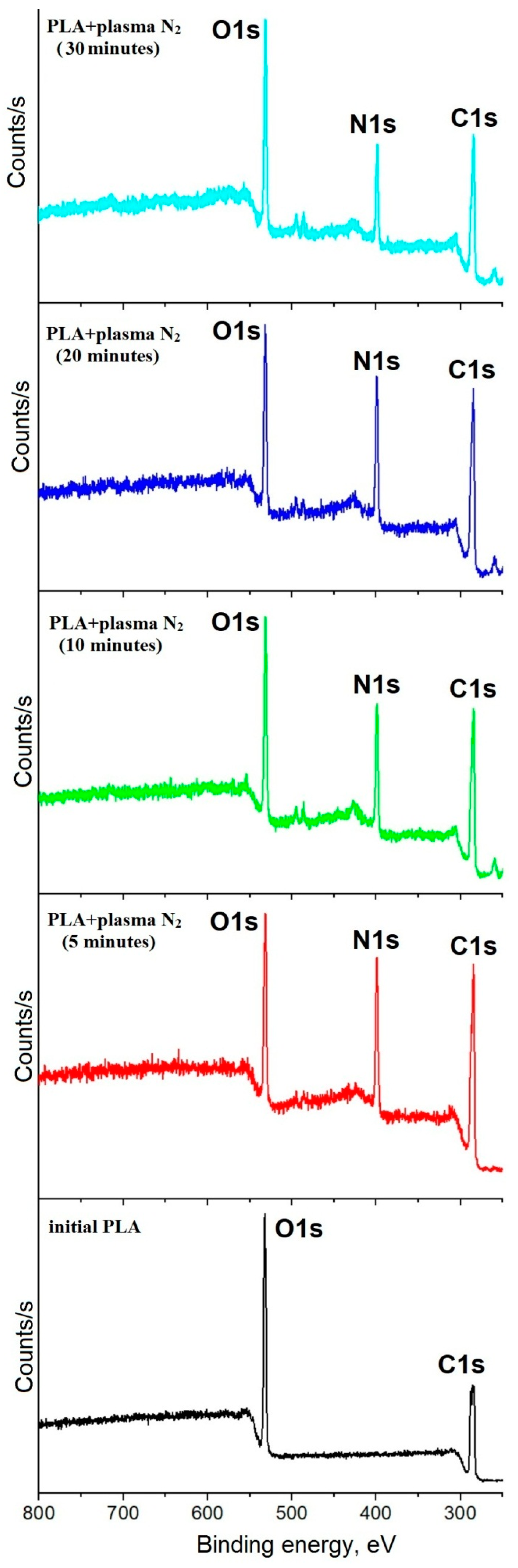
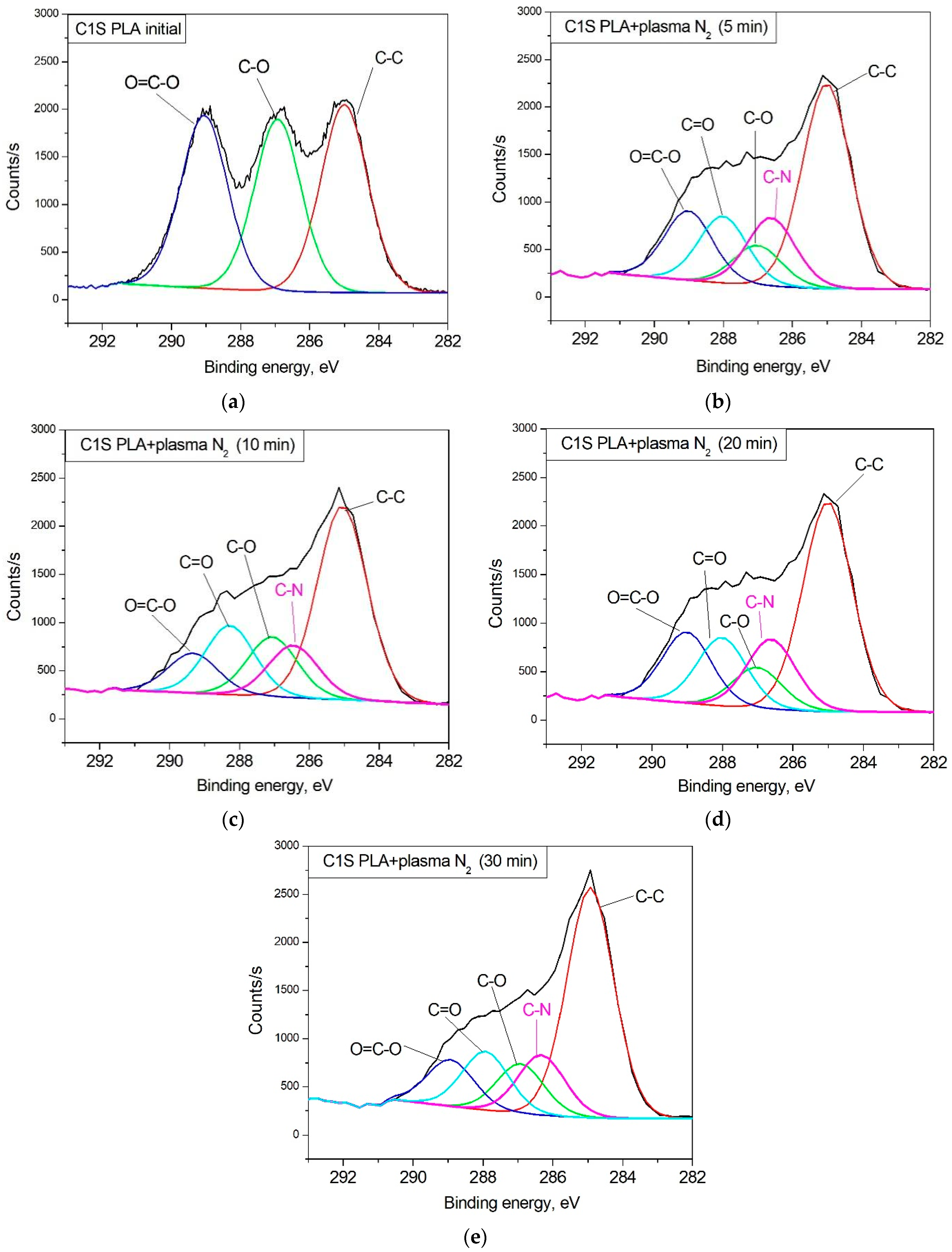
3.1. Surface Chemical Composition
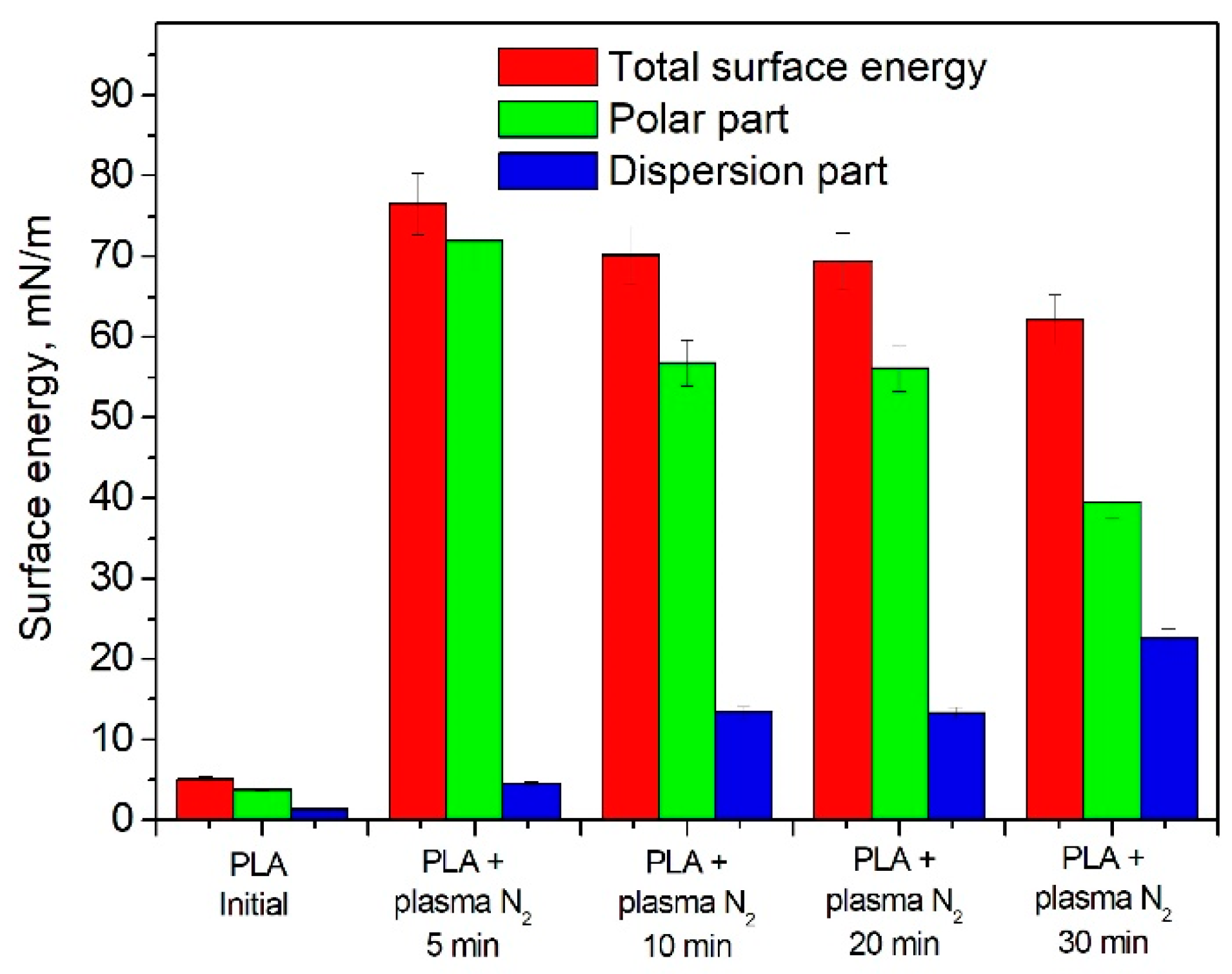
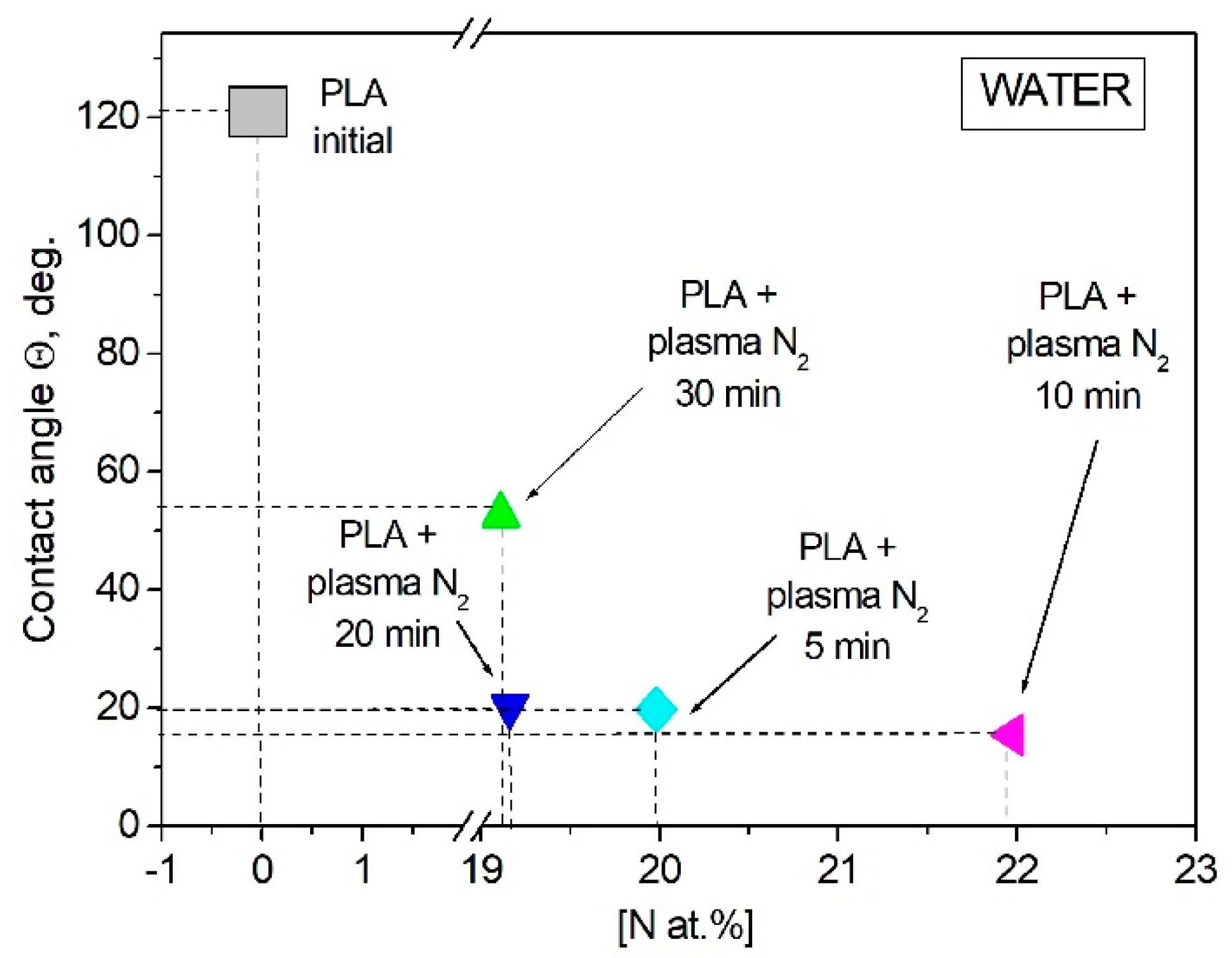
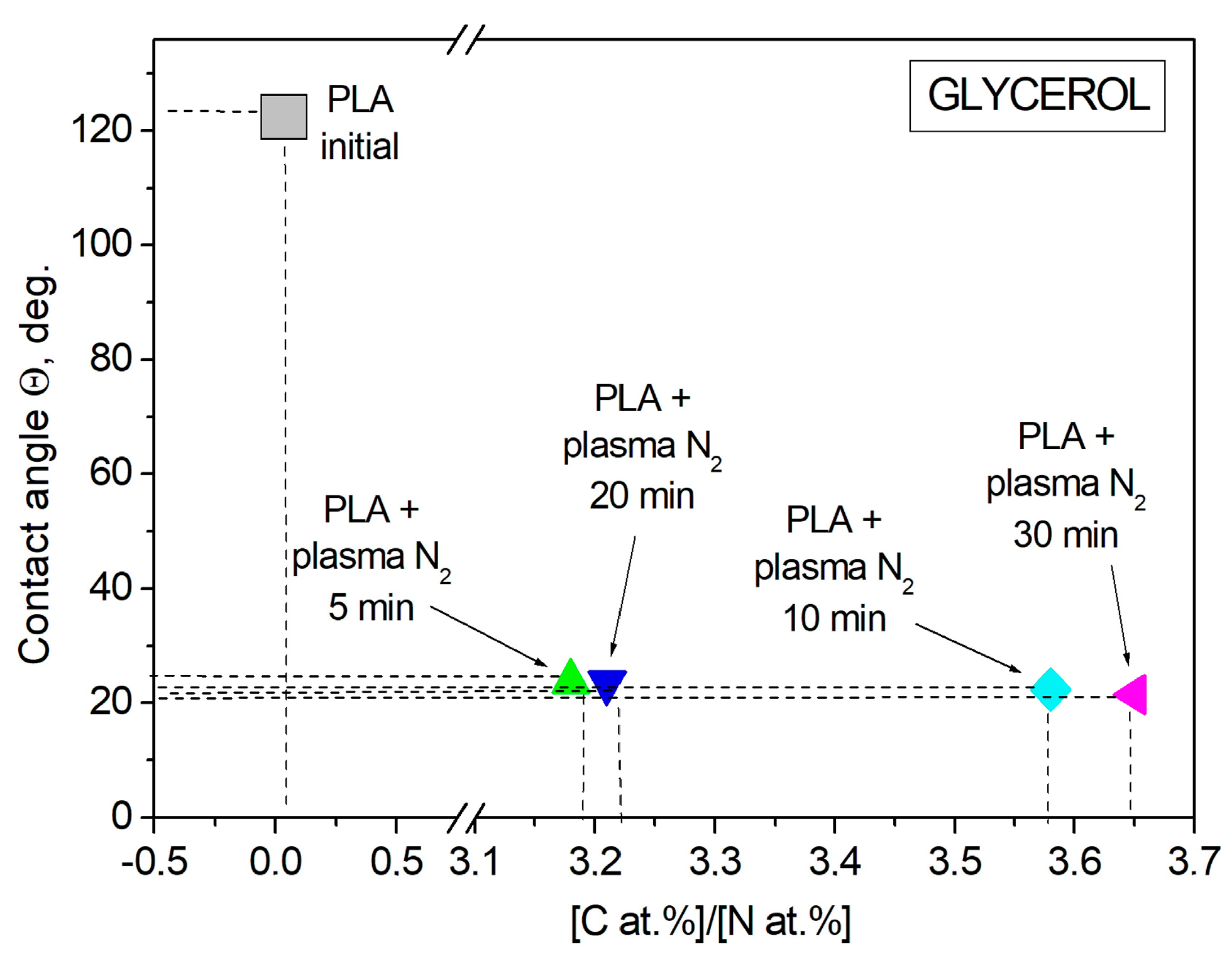
3.2. Wettability of PLA Scaffolds
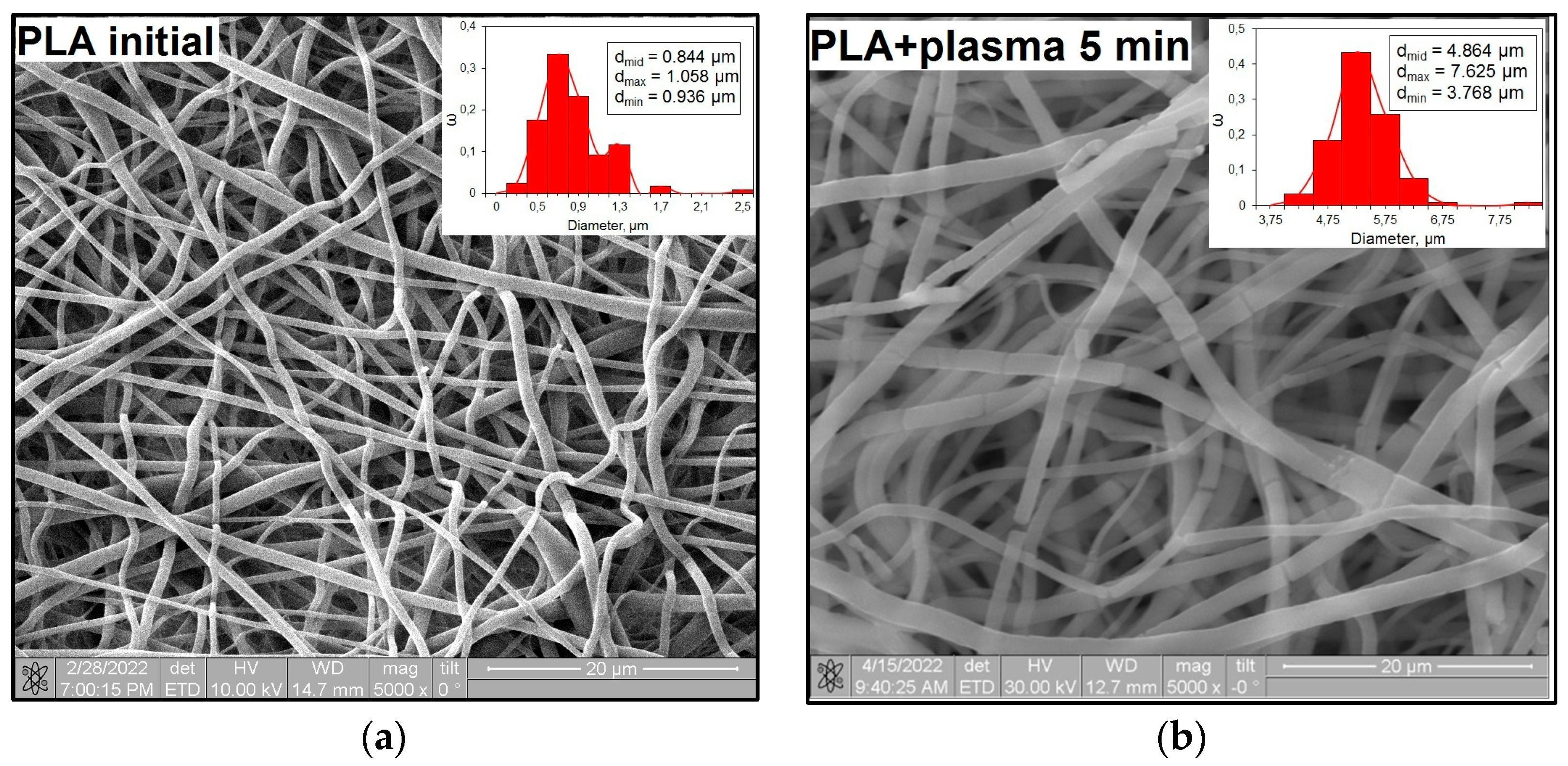
3.3. Microstructures of PLA Scaffolds
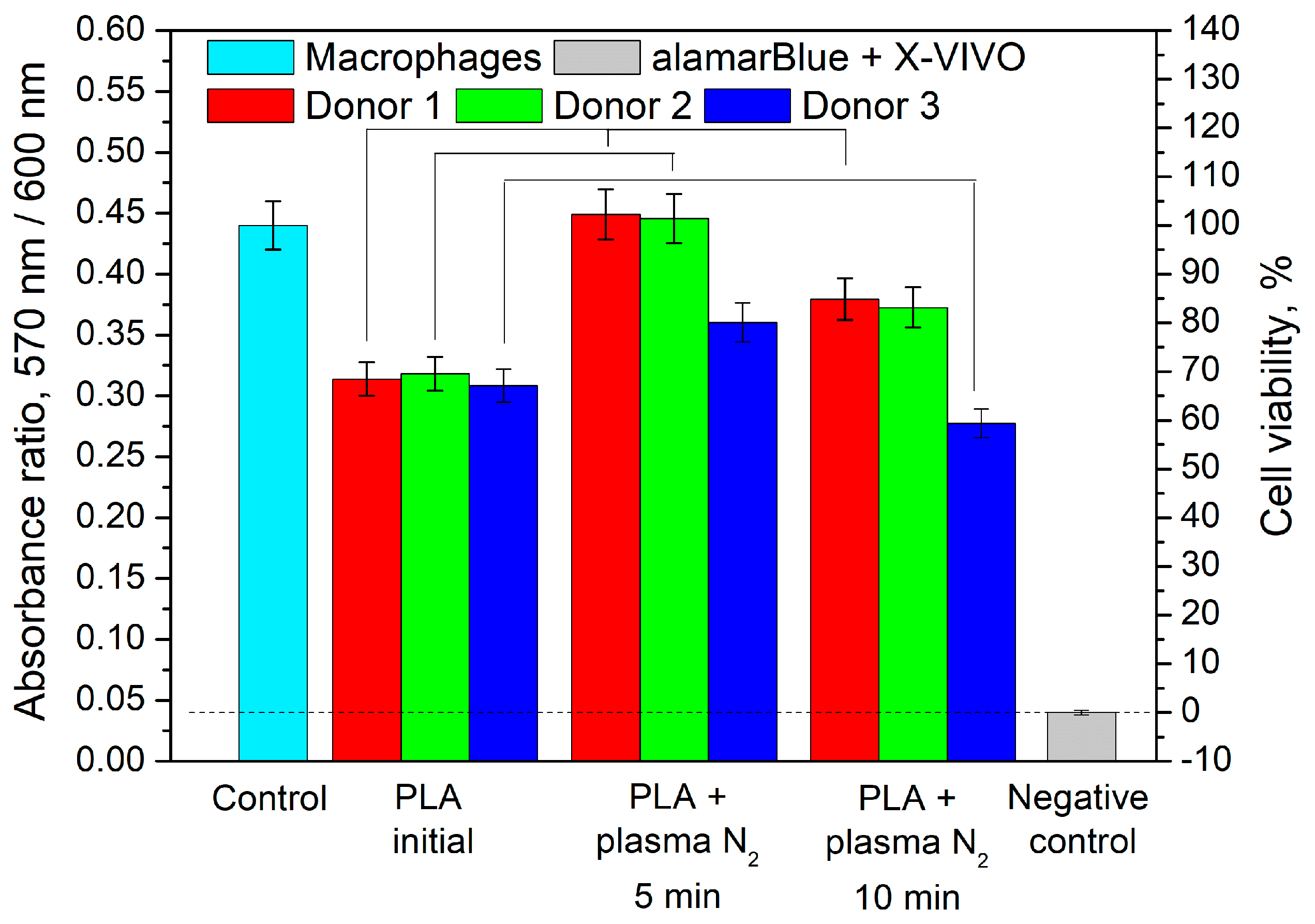
3.4. Effect of PLA Scaffolds on Macrophage Viability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mathias, E.; Murthy, M.S. Pediatric Thermal Burns and Treatment: A Review of Progress and Future Prospects. Medicines 2017, 4, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings—A review. BioMedicine 2015, 5, 24–28. [Google Scholar] [CrossRef]
- Cartmell, J.S.; Dunn, M.G. Effect of Chemical Treatment on Tendon Cellularity and Mechanical Properties. Biomed. Mater. Res. 2000, 49, 134–140. [Google Scholar] [CrossRef]
- Carletti, E.; Molta, A.; Migliaresi, C. Scaffolds for Tissue Engineering and 3D Cell Culture. Methods Mol. Biol. 2001, 75, 17–39. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C. Poly(lactic acid) Foaming. Prog. Colloid Polym. Sci. 2014, 30, 1721–1741. [Google Scholar] [CrossRef]
- Leonard, D.J.; Pick, L.T.; Farrar, D.F. The modification of PLA and PLGA using electron-beam radiation. J. Biomed. Mater. Res. 2009, 89A, 567–574. [Google Scholar] [CrossRef]
- Glowacki, J.; Mizuno, S. Collagen scaffolds for tissue engineering. Biopolymers 2008, 89, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, M.; Boroujeni, S.M.; Omidvarkordshouli, N.; Soleimani, M. Advances in Skin Regeneration: Application of Electrospun Scaffolds. Adv. Healthc. Mater. 2015, 4, 1114–1133. [Google Scholar] [CrossRef]
- Maleki, H.; Azimi, B.; Ismaeilimoghadam, S.; Danti, S. Poly(lactic acid)-Based Electrospun Fibrous Structures for Biomedical Applications. Appl. Sci. 2022, 12, 3192. [Google Scholar] [CrossRef]
- Michael, F.M.; Khalid, M.; Walvekar, R.; Siddiqui, H.; Balaji, A.B. Surface modification techniques of biodegradable and biocompatible polymers. In Biodegradable and Biocompatible Polymer Composites; Woodhead Publishing Series in Composites Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 34–54. [Google Scholar] [CrossRef]
- Techaikool, P.; Daranarong, D.; Kongsuk, J.; Boonyawan, D.; Haron, N.; Harley, W.S.; Thomson, K.A.; Foster, L.J.R.; Punyodom, W. Effects of plasma treatment on biocompatibility of poly[(L-lactide)- co-(ε-caprolactone)] and poly[(L-lactide)-co-glycolide] electrospun nanofibrous membranes. Soc. Chem. Ind. 2017, 66, 1640–1650. [Google Scholar] [CrossRef]
- Izdebska-Podsiadły, J.; Dorsam, E. Effects of argon low temperature plasma on PLA film surface and aging behaviors. Vacuum 2017, 145, 278–284. [Google Scholar] [CrossRef]
- Song, A.Y.; Oh, Y.A.; Roh, S.H.; Kim, J.H.; Min, S.C. Cold oxygen plasma treatments for the improvement of the physicochemical and biodegradable properties of polylactic acid films for food packaging. Inst. Food Technol. 2015, 81, 86–96. [Google Scholar] [CrossRef]
- Darvish, F.; Sarkari, N.M.; Khani, M.; Eslami, E.; Shokri, B.; Mohseni, M.; Ebrahimi, M.; Alizadeh, M.; Dee, C.F. Direct plasma treatment approach based on non-thermal gliding arc for surface modification of biaxially-oriented polypropylene with post-exposure hydrophilicity improvement and minus aging effects. Appl. Surf. Sci. 2020, 509, 144815. [Google Scholar] [CrossRef]
- Durán, I.R.; Vanslambrouck, S.; Chevallier, P.; Hoesli, C.A.; Laroche, G. Atmospheric pressure cold plasma versus wet-chemical surface treatments for carboxyl functionalization of polylactic acid: A first step toward covalent immobilization of bioactive molecules. Colloids Surf. B Biointerfaces 2020, 189, 110847. [Google Scholar] [CrossRef] [PubMed]
- Sauerbier, P.; Köhler, R.; Renner, G.; Militz, H. Surface Activation of Polylactic Acid-Based Wood-Plastic Composite by Atmospheric Pressure Plasma Treatment. Materials 2020, 13, 4673. [Google Scholar] [CrossRef]
- Mohsenimehr, S.; Khani, M.R.; Fani, N.; Eslaminejad, M.R.B.; Shokri, B.; Ghassami, A. Surface modification of PLA scaffold using radio frequency (RF) nitrogen plasma in tissue engineering application. Surf. Topogr. Metrol. Prop. 2020, 8, 015012. [Google Scholar] [CrossRef]
- Djordjevic, I.; Britcher, L.G.; Kumar, S. Morphological and surface compositional changes in poly (lactide-co-glycolide) tissue engineering scaffolds upon radio frequency glow discharge plasma treatment. Appl. Surf. Sci. 2008, 254, 1929–1935. [Google Scholar] [CrossRef]
- Camara-Torres, M.; Sinha, R.; Scopece, P.; Neubert, T.; Lachmann, K.; Patelli, A.; Mota, C.; Moroni, L. Tuning cell behavior on 3D scaffolds fabricated by atmospheric plasma-assisted additive manufacturing. ACS Appl. Mater. Interfaces 2021, 13, 3631–3644. [Google Scholar] [CrossRef]
- Laput, O.A.; Vasenina, I.V.; Shapovalova, Y.G.; Ochered’ko, A.N.; Chernyavskii, A.V.; Kudryashov, S.V.; Kurzina, I.A. Low-Temperature Barrier Discharge Plasma Modification of Scaffolds Based on Polylactic Acid. ACS Appl. Mater. Interfaces 2022, 14, 41742–41750. [Google Scholar] [CrossRef]
- Botvin, V.V.; Shapovalova, E.G.; Zenkova, E.V.; Pozdnyakov, M.A. Synthesis of Glycolic and Lactic Acid Oligomers. In Proceedings of the X International Conference of Students and Young Scientists “Prospects of Fundamental Sciences Development”, Tomsk, Russia, 23–26 April 2013; pp. 266–268. [Google Scholar]
- Devyatkov, V.N.; Ivanov, Y.F.; Krysina, O.V.; Koval, N.N.; Petrikova, E.A.; Shugurov, V.V. Equipment and Processes of Vacuum Electron-Ion Plasma Surface Engineering. Vacuum 2017, 143, 464–472. [Google Scholar] [CrossRef]
- Bico, J.; Thiele, U.; Quere, D. Wetting of Textured Surfaces. Colloids Surf. A 2002, 206, 41–46. [Google Scholar] [CrossRef]
- Gratchev, A.; Kzhyshkowska, J.; Köthe, K.; Muller-Molinet, I.; Kannookadan, S.; Utikal, J.; Goerdt, S. Mφ1 and Mφ2 Can be Re-polarized by Th2 or Th1 Cytokines Respectively, and Respond to Exogenous Danger Signals. Immunobiology 2006, 211, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Beamson, G.; Briggs, D. High Resolution Monochromated X-ray Photoelectron Spectroscopy of Organic Polymers: A Comparison between Solid State Data for Organic Polymers and Gas Phase Data for Small Molecules. Mol. Phys. 1992, 76, 919–936. [Google Scholar] [CrossRef]
- Laput, O.A.; Vasenina, I.V.; Botvin, V.V.; Kurzina, I.A. Surface Modification of Polylactic Acid by Ion, Electron Beams and Low-Temperature Plasma: A Review. J. Mater. Sci. 2022, 57, 2335–2361. [Google Scholar] [CrossRef]
- Yang, Y.-W.; Wu, J.-Y.; Liu, C.-T.; Liao, G.-C.; Huang, H.-Y.; Hsu, R.-Q.; Chiang, M.-H.; Wu, J.-S. Fast Incorporation of Primary Amine Group into Polylactide Surface for Improving C2C12 Cell Proliferation Using Nitrogen-based Atmospheric-pressure Plasma Jets. J. Biomed. Mater. Res. Part A 2014, 102, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Gao, J.; Gao, Q.; Chen, Y. Surface Modification of Biodegradable Poly(D,L-lactic acid) by Nitrogen and Nitrogen/Hydrogen Plasma for Improving Surface Hydrophilicity. Plasma Sci. Technol. 2011, 13, 230–234. [Google Scholar] [CrossRef]
- Seibold, A.; Nardin, M.; Schultz, J.; Walliser, A.; Oppliger, M. Effect of Dynamic Contact Angle on Capillary Rise Phenomena. Colloids Surf. A 2000, 161, 81–87. [Google Scholar] [CrossRef]





| Forming voltage | 20 kV |
| Solution feed rate | 3 mL/h |
| Solution volume | 10 mL |
| Scaffold width | 50 mm |
| Collector rotation | 50 rpm |
| Distance between the needle and collector | 130 mm |
| Needle diameter | 1.3 mm (18 G) |
| Frequency/needle cleaning interval | 10/0 min |
| Plasma-forming gas | N2 | |||
| Pressure, Pa | 0.3 | |||
| Treatment duration, min | 5 | 10 | 20 | 30 |
| Discharge current, A | 5 | 5 | 5 | 5 |
| Temperature in the chamber, °C | 35 | 46 | 59 | 80 |
| Sample | Ratio of Atomic Concentrations | Atomic Content of N, at.% | |
|---|---|---|---|
| [C, at.%]/[O, at.%] | [C, at.%]/[N, at.%] | ||
| PLA initial | 2.00 ± 0.2 | - | - |
| PLA + N2 plasma 5 min | 2.24 ± 0.2 | 3.21 ± 0.3 | 21.97 ± 0.2 |
| PLA + N2 plasma 10 min | 2.27 ± 0.1 | 3.58 ± 0.2 | 25.15 ± 0.3 |
| PLA + N2 plasma 20 min | 2.36 ± 0.5 | 3.18 ± 0.4 | 19.16 ± 0.3 |
| PLA + N2 plasma 30 min | 2.37 ± 0.4 | 3.65 ± 0.1 | 19.11 ± 0.2 |
 | Content of Bonds in C1s Spectrum, at.% | |||||
|---|---|---|---|---|---|---|
| CH3-CH- (1) | -O-CH- (2) | -O-C=O (3) | -C=O | -C-N | ||
| Sample | 285.00 | 286.98 | 289.06 | 288.00 | 286.40 | |
| Binding Energy, eV | ||||||
| PLA initial | 35.3 ± 0.4 | 32.3 ± 0.2 | 32.4 ± 0.4 | |||
| PLA + N2 plasma 5 min | 45.6 ± 0.3 | 8.9 ± 0.2 | 15.5 ± 0.2 | 14.8 ± 0.2 | 15.2 ± 0.7 | |
| PLA + N2 plasma 10 min | 51.6 ± 0.2 | 11.2 ± 0.2 | 10.5 ± 0.2 | 13.2 ± 0.4 | 13.5 ± 0.3 | |
| PLA + N2 plasma 20 min | 47.8 ± 0.1 | 11.9 ± 0.2 | 14.1 ± 0.2 | 13.5 ± 0.5 | 12.7 ± 0.4 | |
| PLA + N2 plasma 30 min | 46.7 ± 0.1 | 14.4 ± 0.4 | 9.6 ± 0.3 | 16.7 ± 0.3 | 12.6 ± 0.2 | |
| Sample | Surface Roughness Ra, μm | 3D Images of PLA Surface |
|---|---|---|
| PLA initial | 15 ± 3 |  |
| PLA + N2 plasma 5 min. | 129 ± 23 |  |
| PLA + N2 plasma 10 min. | 152 ± 27 |  |
| PLA + N2 plasma 20 min. | 211 ± 37 |  |
| PLA + N2 plasma 30 min. | 248 ± 41 |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laput, O.A.; Vasenina, I.V.; Korzhova, A.G.; Bryuzgina, A.A.; Khomutova, U.V.; Tuyakova, S.G.; Akhmadeev, Y.H.; Shugurov, V.V.; Bolbasov, E.N.; Tverdokhlebov, S.I.; et al. Effect of Nitrogen Arc Discharge Plasma Treatment on Physicochemical Properties and Biocompatibility of PLA-Based Scaffolds. Polymers 2023, 15, 3381. https://doi.org/10.3390/polym15163381
Laput OA, Vasenina IV, Korzhova AG, Bryuzgina AA, Khomutova UV, Tuyakova SG, Akhmadeev YH, Shugurov VV, Bolbasov EN, Tverdokhlebov SI, et al. Effect of Nitrogen Arc Discharge Plasma Treatment on Physicochemical Properties and Biocompatibility of PLA-Based Scaffolds. Polymers. 2023; 15(16):3381. https://doi.org/10.3390/polym15163381
Chicago/Turabian StyleLaput, Olesya A., Irina V. Vasenina, Alena G. Korzhova, Anastasia A. Bryuzgina, Ulyana V. Khomutova, Sitora G. Tuyakova, Yuriy H. Akhmadeev, Vladimir V. Shugurov, Evgeny N. Bolbasov, Sergei I. Tverdokhlebov, and et al. 2023. "Effect of Nitrogen Arc Discharge Plasma Treatment on Physicochemical Properties and Biocompatibility of PLA-Based Scaffolds" Polymers 15, no. 16: 3381. https://doi.org/10.3390/polym15163381
APA StyleLaput, O. A., Vasenina, I. V., Korzhova, A. G., Bryuzgina, A. A., Khomutova, U. V., Tuyakova, S. G., Akhmadeev, Y. H., Shugurov, V. V., Bolbasov, E. N., Tverdokhlebov, S. I., Chernyavskii, A. V., & Kurzina, I. A. (2023). Effect of Nitrogen Arc Discharge Plasma Treatment on Physicochemical Properties and Biocompatibility of PLA-Based Scaffolds. Polymers, 15(16), 3381. https://doi.org/10.3390/polym15163381










