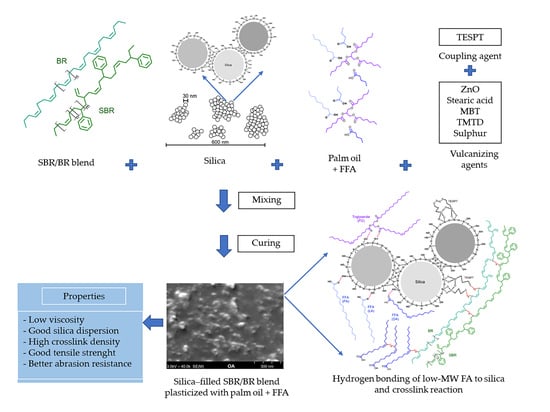
Property Improvements of Silica-Filled Styrene Butadiene Rubber/Butadiene Rubber Blend Incorporated with Fatty-Acid-Containing Palm Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Compound Preparation
2.3. Morphology, Viscosity, and Dynamic Mechanical Properties
2.4. Cure Characteristics and Sample Preparation
2.5. Mechanical Property Measurement
- A
- = abrasion loss (mm3),
- ∆mt
- = mass loss of the test specimen (mg),
- dt
- = density of the test specimen (mg/mm3),
- S0
- = normal abrasiveness (200 mg),
- S
- = abrasiveness of the abrasive paper (mg).
3. Results and Discussion
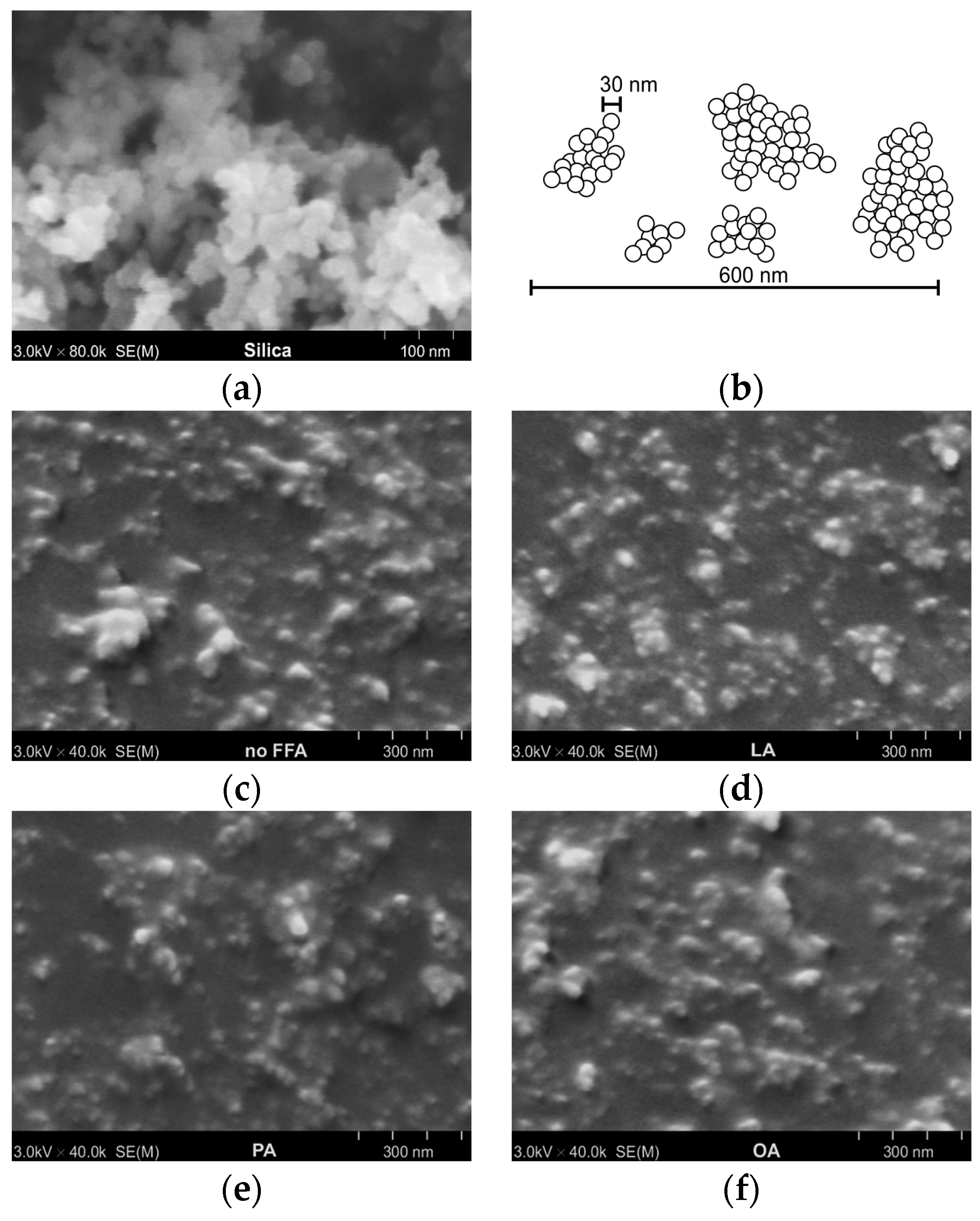
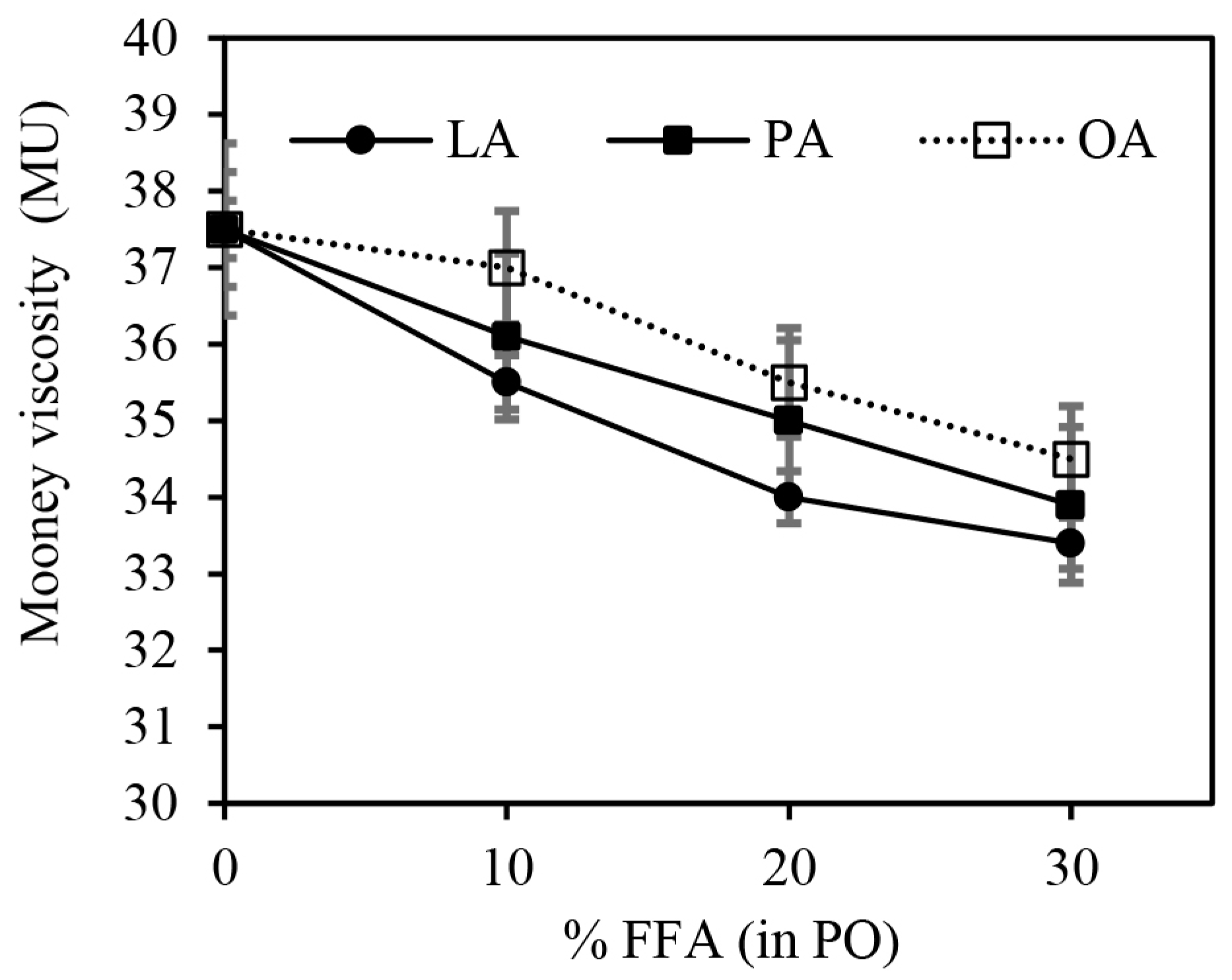
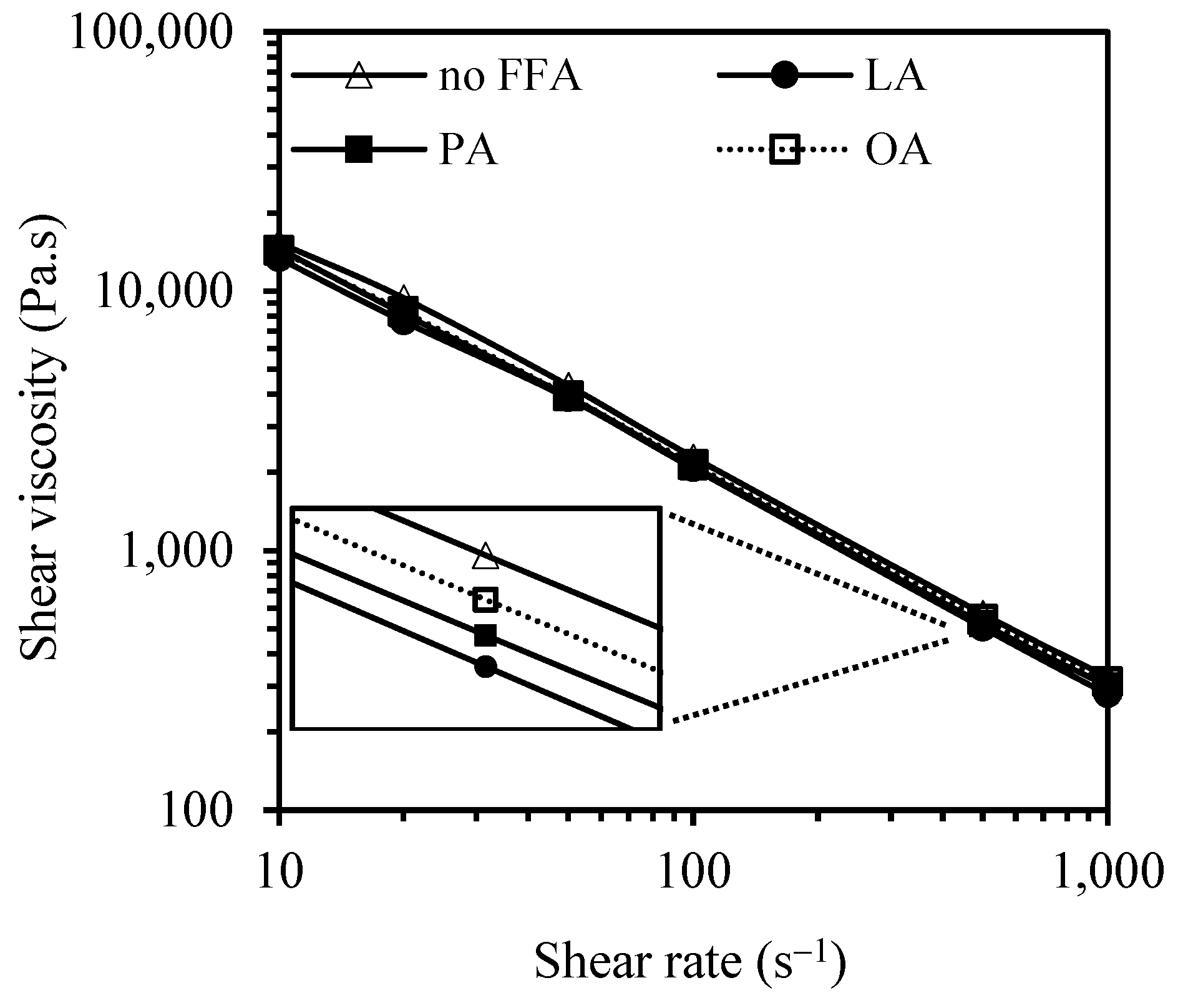
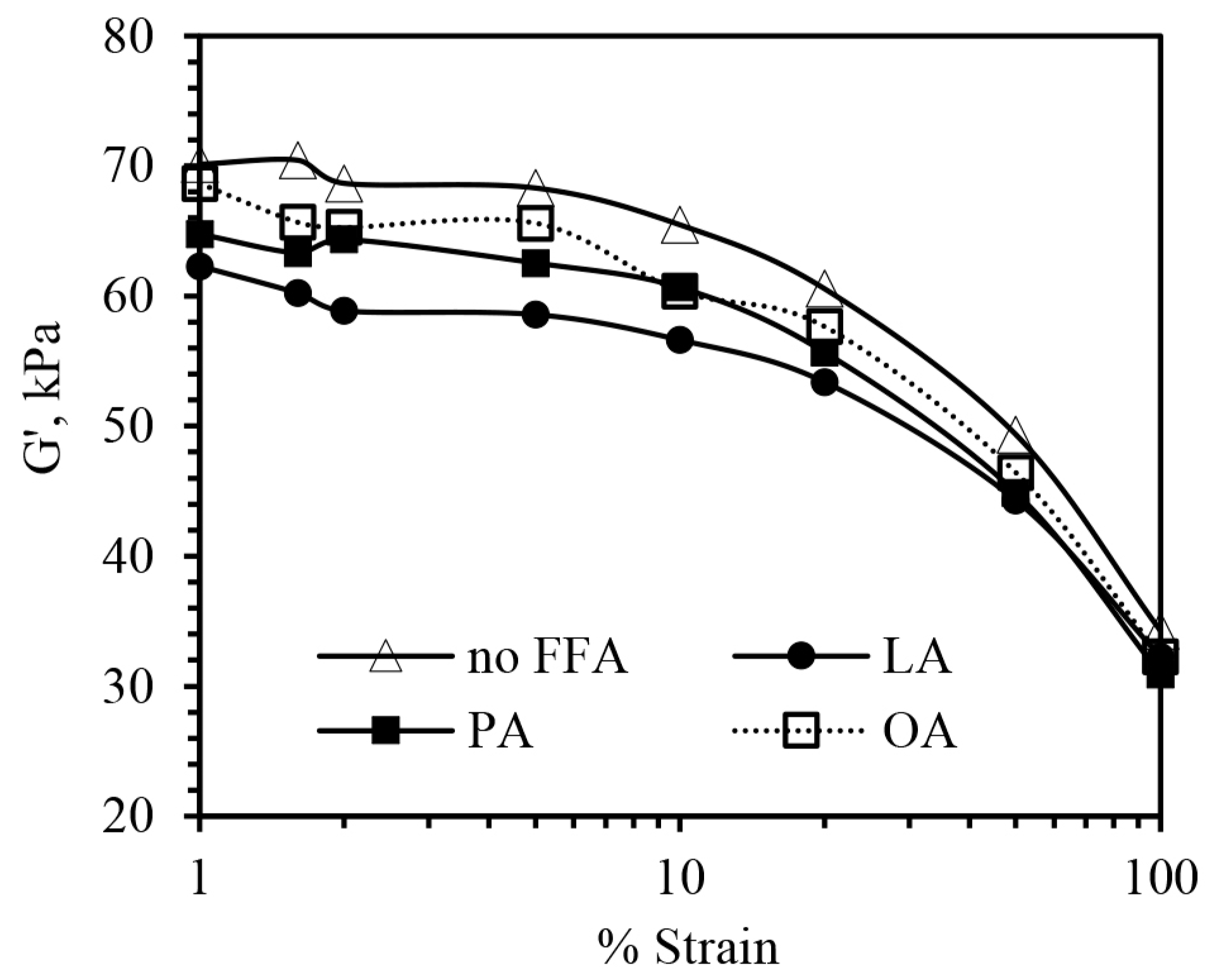
3.1. Effect of FFAs on Morphology, Viscosity, and Dynamic Mechanical Properties
3.2. Effect of FFAs on Cure Characteristics
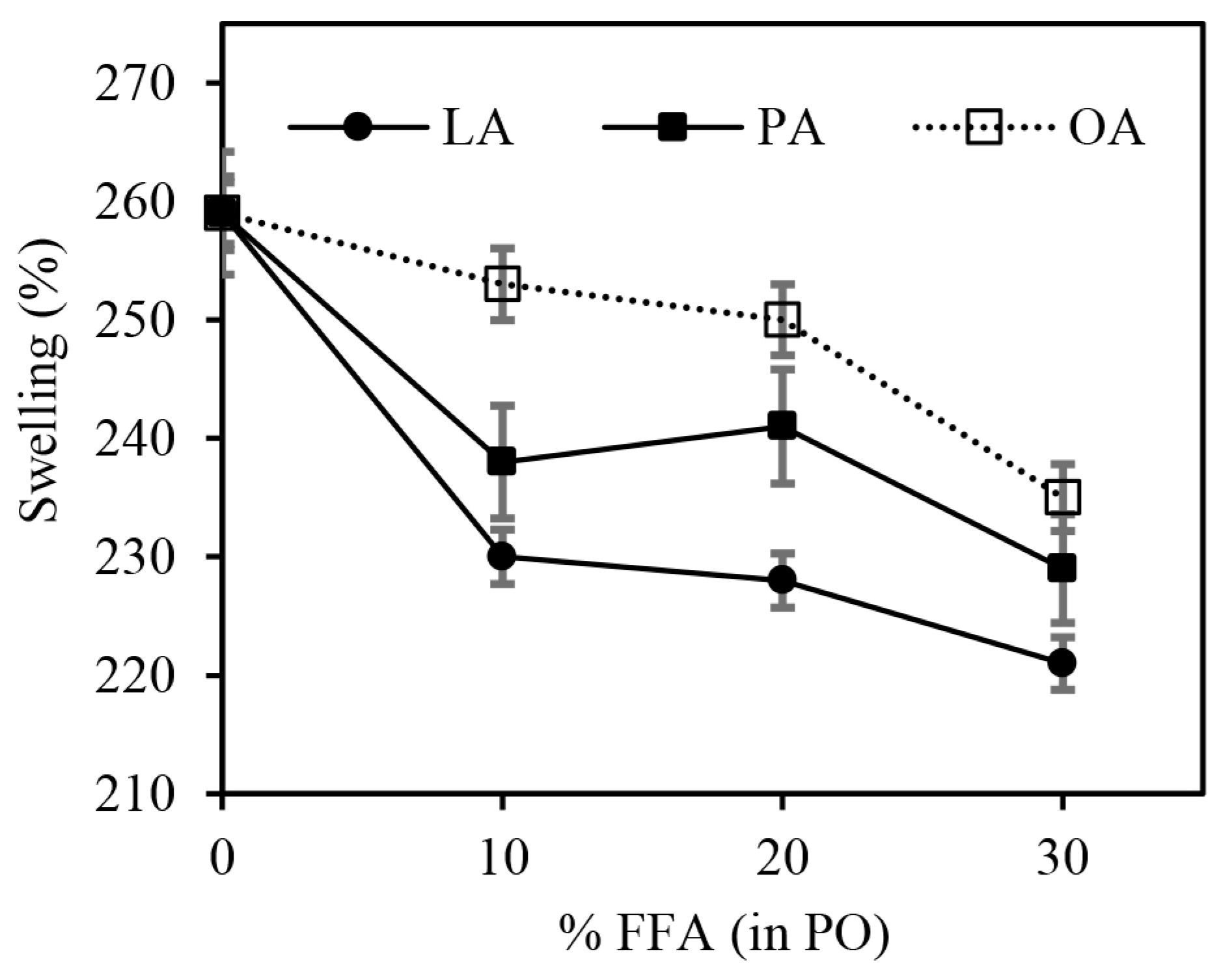
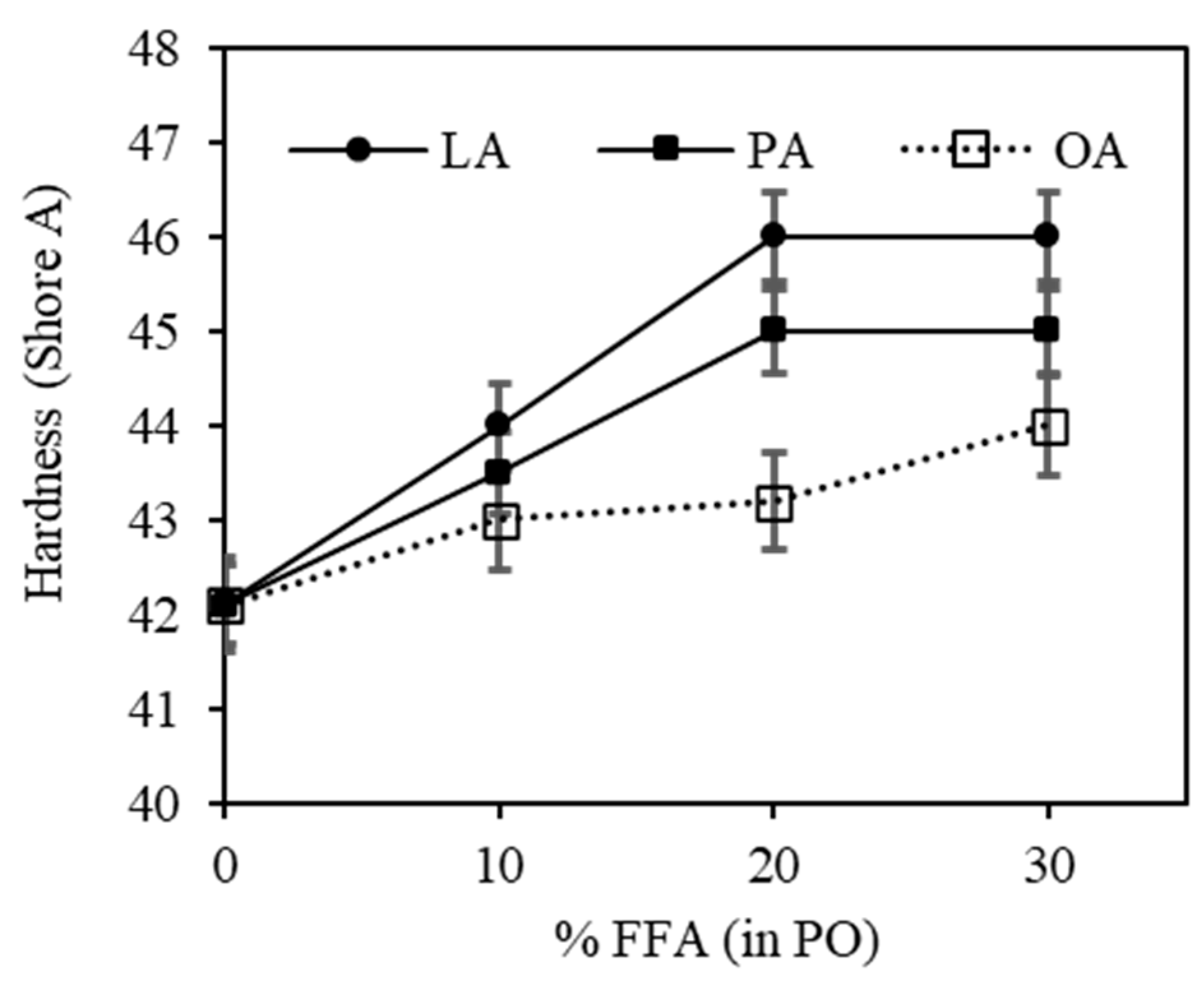
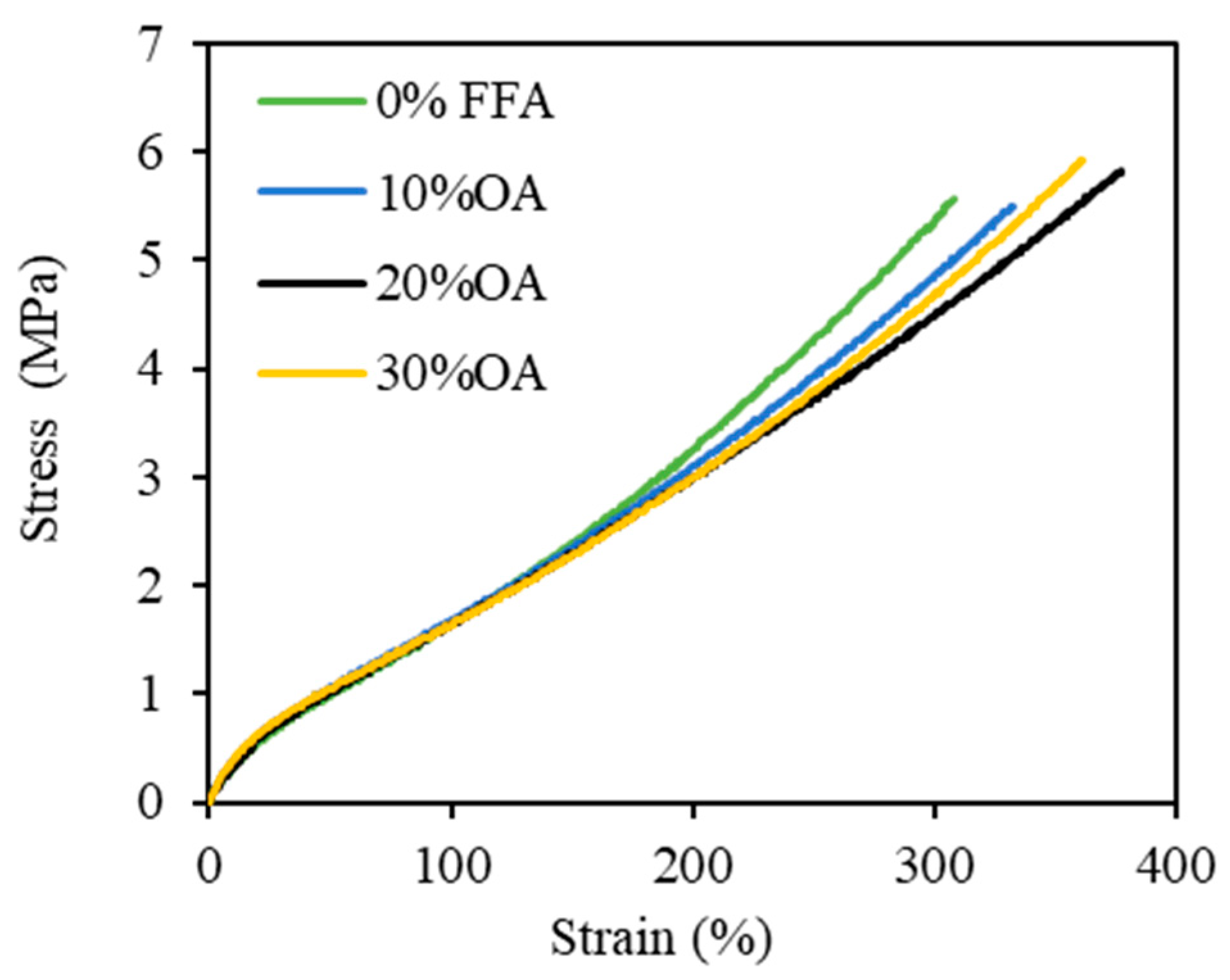
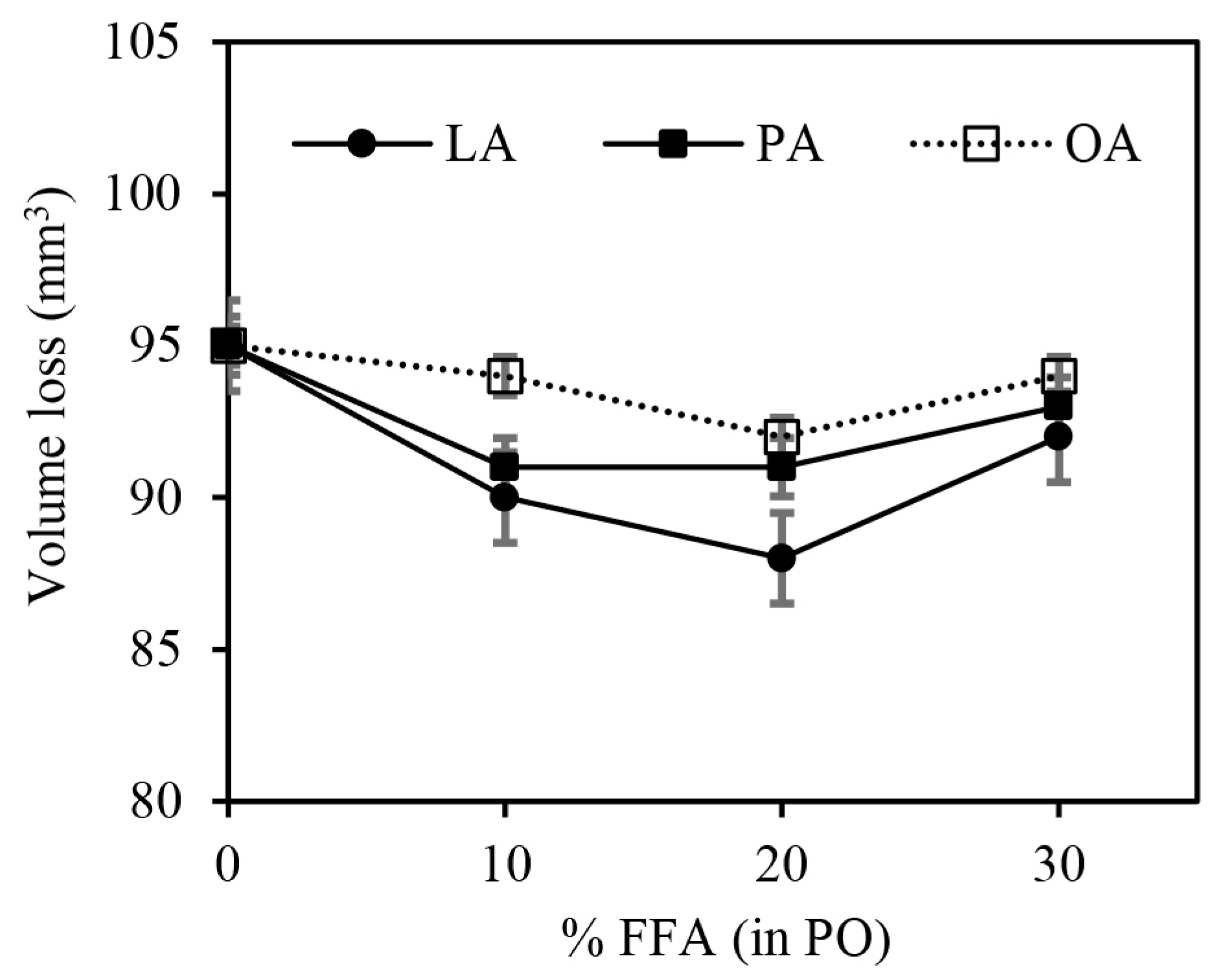
3.3. Effect of FFAs on Physical Properties of the Vulcanizates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, S.H. Influence of Eco-Friendly Processing Aids on Silica-Based Rubber Composites. Appl. Sci. 2020, 10, 7244. [Google Scholar] [CrossRef]
- Hofmann, W. Rubber Technology Handbook; Hanser Publishers: New York, NY, USA, 1989. [Google Scholar]
- Boonrasri, S.; Sae-Oui, P.; Reungsang, A.; Rachtanapun, P. New Vegetable Oils with Different Fatty Acids on Natural Rubber Composite Properties. Polymers 2021, 13, 1108. [Google Scholar] [PubMed]
- Mohamed, N.R.; Othman, N.; Shuib, R.K. Synergistic Effect of Sunflower Oil and Soybean Oil as Alternative Processing Oil in The Development of Greener Tyre Tread Compound. J. Rubber Res. 2022, 25, 239–249. [Google Scholar] [CrossRef]
- Hayichelaeh, C.; Boonkerd, K.; Nun-Anan, P.; Purbaya, M. Elucidation of the accelerated sulfur vulcanization of bio oil-extended natural rubber compounds. Polym. Adv. Technol. 2022, 33, 303–313. [Google Scholar] [CrossRef]
- Li, J.; Isayev, A.I. Recent Development in Application of Bio-Based Oils in Elastomers. Rubber Chem. Technol. 2018, 91, 719–728. [Google Scholar] [CrossRef]
- Sovtić, N.; Predrag, K.S.; Bera, O.J.; Pavličević, J.M.; Govedarica, O.M.; Jovičić, M.C.; Govedarica, D.D. A review of environmentally friendly rubber production using different vegetable oils. Polym. Eng. Sci. 2020, 60, 1097–1117. [Google Scholar] [CrossRef]
- Hayichelaeh, C.; Boonkerd, K. Enhancement of the properties of carbon-black-filled natural rubber compounds containing soybean oil cured with peroxide through the addition of coagents. Ind. Crops Prod. 2022, 187, 115306. [Google Scholar] [CrossRef]
- Roy, K.; Poompiew, N.; Pongwisuthiruchte, A.; Potiyaraj, P. Application of Different Vegetable Oils as Processing Aids in Industrial Rubber Composites: A Sustainable Approach. ACS Omega 2021, 6, 31384–31389. [Google Scholar] [CrossRef]
- Zhang, C.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Jayewardhana, W.G.D.; Perera, G.M.; Edirisinghe, D.G.; Karunanayake, L. Study on natural oils as alternative processing aids and activators in carbon black filled natural rubber. J. Natl. Sci. Found. Sri Lanka 2009, 37, 187–193. [Google Scholar] [CrossRef]
- Hayichelaeh, C.; Boonkerd, K. Utilization of palm oil as an alternative processing oil in carbon black-filled natural rubber compounds. Ind. Crops Prod. 2023, 194, 116270. [Google Scholar] [CrossRef]
- Shahbandeh, M. Vegetable Oils: Production Worldwide 2012/13–2022/23, by Type. Available online: https://www.statista.com/statistics/263933/production-of-vegetable-oils-worldwide-since-2000/ (accessed on 30 April 2023).
- Wang, Z.; Peng, Y.; Zhang, L.; Zhao, Y.; Vyzhimov, R.; Tan, T.; Fong, H. Investigation of Palm Oil as Green Plasticizer on the Processing and Mechanical Properties of Ethylene Propylene Diene Monomer Rubber. Ind. Eng. Chem. Res. 2016, 55, 2784–2789. [Google Scholar] [CrossRef]
- Siwarote, B.; Sae-Oui, P.; Wirasate, S.; Suchiva, K. Effects of Bio-based Oils on Processing Properties and Cure Characteristics of Silica-filled Natural Rubber Compounds. J. Rubber Res. 2017, 20, 1–19. [Google Scholar] [CrossRef]
- Abbas, K.; Ong, S.K. Investigation of Crude Palm Oil as an Alternative Processing Oils in Natural Rubber: Effect of the Unsaturated Fatty Acid. IOP Conf. Ser. Mater. Sci. Eng. 2019, 548, 012009. [Google Scholar] [CrossRef]
- Berchmans, H.J.; Hirata, S. Biodiesel production from crude Jatropha curcas L. seed oil with a high content of free fatty acids. Bioresour. Technol. 2008, 99, 1716–1721. [Google Scholar] [CrossRef]
- Nakpong, P.; Wootthikanokkhan, S. High free fatty acid coconut oil as a potential feedstock for biodiesel production in Thailand. Renew. Energy 2010, 35, 1682–1687. [Google Scholar] [CrossRef]
- Dayrit, F.M.; Buenafe, O.E.M.; Chainani, E.T.; de Vera, I.M.S. Analysis of Monoglycerides, Diglycerides, Sterols, and Free Fatty Acids in Coconut (Cocos nucifera L.) Oil by 31P NMR Spectroscopy. J. Agric. Food Chem. 2008, 56, 5765–5769. [Google Scholar] [CrossRef]
- Skiera, C.; Steliopoulos, P.; Kuballa, T.; Holzgrabe, U.; Diehl, B. Determination of free fatty acids in edible oils by 1H NMR spectroscopy. Lipid Technol. 2012, 24, 279–281. [Google Scholar] [CrossRef]
- Di Pietro, M.E.; Mannu, A.; Mele, A. NMR Determination of Free Fatty Acids in Vegetable Oils. Processes 2020, 8, 410. [Google Scholar]
- Che Man, Y.B.; Moh, M.H.; van de Voort, F.R. Determination of free fatty acids in crude palm oil and refined-bleached-deodorized palm olein using fourier transform infrared spectroscopy. J. Am. Oil Chem. Soc. 1999, 76, 485–490. [Google Scholar] [CrossRef]
- Hayichelaeh, C.; Reuvekamp, L.; Dierkes, W.; Blume, A.; Noordermeer, J.; Sahakaro, K. Silica-reinforced natural rubber tire tread compounds containing bio-based process oils. II: Influence of epoxide and amino functional groups. Rubber Chem. Technol. 2020, 93, 195–207. [Google Scholar]
- Mostoni, S.; Milana, P.; Di Credico, B.; D’Arienzo, M.; Scotti, R. Zinc-Based Curing Activators: New Trends for Reducing Zinc Content in Rubber Vulcanization Process. Catalysts 2019, 9, 664. [Google Scholar]
- Butuc, S.G.; van Leerdam, K.; Rossenaar, B.; Swart, J.; Geurts, F.; Bossinga-Geurts, B.; Verwer, P.; Talma, A.; Blume, A. Elucidation of the role of ZnO in sulfur cure in novel EPDM-CTS blends. Polym. Test. 2023, 117, 107843. [Google Scholar] [CrossRef]
- Junkong, P.; Morimoto, R.; Miyaji, K.; Tohsan, A.; Sakaki, Y.; Ikeda, Y. Effect of fatty acids on the accelerated sulfur vulcanization of rubber by active zinc/carboxylate complexes. RSC Adv. 2020, 10, 4772–4785. [Google Scholar] [CrossRef] [PubMed]
- Labatut, R.A.; Pronto, J.L. Chapter 4—Sustainable Waste-to-Energy Technologies: Anaerobic Digestion. In Sustainable Food Waste-to-Energy Systems; Trabold, T.A., Babbitt, C.W., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 47–67. [Google Scholar]
- Xiao, H.-M.; Rao, D.; Zhao, S.; Hussain, D.; Chen, J.-L.; Luo, D.; Wang, D.; Lv, X.; Wei, F.; Chen, H. Formation of medium- and long-chain fatty alcohols in long-term stored oil and biodiesels revealed by chemical isotope labeling-liquid chromatography-high resolution mass spectrometry. Ind. Crops Prod. 2023, 193, 116171. [Google Scholar] [CrossRef]
- ISO 289-1; Rubber, Unvulcanized—Determinations Using a Shearing-Disc Viscometer—Part 1: Determination of Mooney Viscosity. ISO: Geneva, Switzerland, 2015.
- ISO 6502-3; Rubber—Measurement of Vulcanization Characteristics Using Curemeters—Part 3: Rotorless Curemeter. ISO: Geneva, Switzerland, 2023.
- ISO 48-4; Rubber, Vulcanized or Thermoplastic—Determination of Hardness—Part 4: Indentation Hardness by Durometer Method (Shore Hardness). ISO: Geneva, Switzerland, 2018.
- ISO 37; Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. ISO: Geneva, Switzerland, 2017.
- ISO 4649:2017; Rubber, Vulcanized or Thermoplastic—Determination of Abrasion Resistance Using a Rotating Cylindrical Drum Device. ISO: Geneva, Switzerland, 2017.
- Yan, C.; Datta Sarma, A.; Moretto, E.; Thomann, J.-S.; Verge, P.; Schmidt, D.; Kayser, F.; Dieden, R. Semiquantitative Solid-State NMR Study of the Adsorption of Soybean Oils on Silica and Its Significance for Rubber Processing. Langmuir 2021, 37, 10298–10307. [Google Scholar] [CrossRef]
- Mensah, B.; Onwona-Agyeman, B.; Nyankson, E.; Bensah, D.Y. Effect of palm oil as plasticizer for compounding polar and non-polar rubber matrix reinforced carbon black composites. J. Polym. Res. 2023, 30, 67. [Google Scholar] [CrossRef]
- Siriwong, C.; Khansawai, P.; Boonchiangma, S.; Sirisinha, C.; Sae-Oui, P. The influence of modified soybean oil as processing aids in tire application. Polym. Bull. 2021, 78, 3589–3606. [Google Scholar] [CrossRef]
- Ismail, H. Effect of palm oil fatty acid additive (POFA) on curing characteristics and vulcanizate properties of silica filled natural rubber compounds. J. Elastomers Plast. 2000, 32, 33–45. [Google Scholar] [CrossRef]
- Xu, H.; Fan, T.; Ye, N.; Wu, W.; Huang, D.; Wang, D.; Wang, Z.; Zhang, L. Plasticization Effect of Bio-Based Plasticizers from Soybean Oil for Tire Tread Rubber. Polymers 2020, 12, 623. [Google Scholar] [CrossRef]
- Li, J.; Isayev, A.I.; Ren, X.; Soucek, M.D. Modified soybean oil-extended SBR compounds and vulcanizates filled with carbon black. Polymer 2015, 60, 144–156. [Google Scholar] [CrossRef]
- Hayichelaeh, C.; Nun-Anan, P.; Purbaya, M.; Boonkerd, K. Unfilled Natural Rubber Compounds Containing Bio-Oil Cured with Different Curing Systems: A Comparative Study. Polymers 2022, 14, 2479. [Google Scholar] [CrossRef] [PubMed]
- Nun-Anan, P.; Hayichelaeh, C.; Boonkerd, K. Effect of a Natural Processing Aid on the Properties of Acrylonitrile-Butadiene Rubber: Study on Soybean Oil Fatty Acid from Seed Crop. Polymers 2021, 13, 3459. [Google Scholar] [CrossRef] [PubMed]
- Nandanan, V.; Joseph, R.; George, K.E. Rubber seed oil: A multipurpose additive in NR and SBR compounds. J. Appl. Polym. Sci. 1999, 72, 487–492. [Google Scholar] [CrossRef]





| Ingredients | Content, phr a | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0% FFA in PO | %LA in PO | %PA in PO | %OA in PO | |||||||
| 10 | 20 | 30 | 10 | 20 | 30 | 10 | 20 | 30 | ||
| SBR 1502 | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 | 70.0 |
| BR 1220 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| ZnO | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Stearic acid | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Silica | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
| TESPT | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| MBT | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| TMTD | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Sulfur | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| PO | 20.0 | 18.0 | 16.0 | 14.0 | 18.0 | 16.0 | 14.0 | 18.0 | 16.0 | 14.0 |
| LA | 0.0 | 2.0 | 4.0 | 6.0 | - | - | - | - | - | - |
| PA | - | - | - | - | 2.0 | 4.0 | 6.0 | - | - | - |
| OA | - | - | - | - | - | - | - | 2.0 | 4.0 | 6.0 |
| No FFA | LA | PA | OA | |
|---|---|---|---|---|
| ΔG′ (kPa) | 34.41 | 26.60 | 30.43 | 30.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boonrasri, S.; Thipchai, P.; Sae-Oui, P.; Thanakkasaranee, S.; Jantanasakulwong, K.; Rachtanapun, P. Property Improvements of Silica-Filled Styrene Butadiene Rubber/Butadiene Rubber Blend Incorporated with Fatty-Acid-Containing Palm Oil. Polymers 2023, 15, 3429. https://doi.org/10.3390/polym15163429
Boonrasri S, Thipchai P, Sae-Oui P, Thanakkasaranee S, Jantanasakulwong K, Rachtanapun P. Property Improvements of Silica-Filled Styrene Butadiene Rubber/Butadiene Rubber Blend Incorporated with Fatty-Acid-Containing Palm Oil. Polymers. 2023; 15(16):3429. https://doi.org/10.3390/polym15163429
Chicago/Turabian StyleBoonrasri, Siwarote, Parichat Thipchai, Pongdhorn Sae-Oui, Sarinthip Thanakkasaranee, Kittisak Jantanasakulwong, and Pornchai Rachtanapun. 2023. "Property Improvements of Silica-Filled Styrene Butadiene Rubber/Butadiene Rubber Blend Incorporated with Fatty-Acid-Containing Palm Oil" Polymers 15, no. 16: 3429. https://doi.org/10.3390/polym15163429