A Lightweight and Low-Voltage-Operating Linear Actuator Based on the Electroactive Polymer Polypyrrole
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
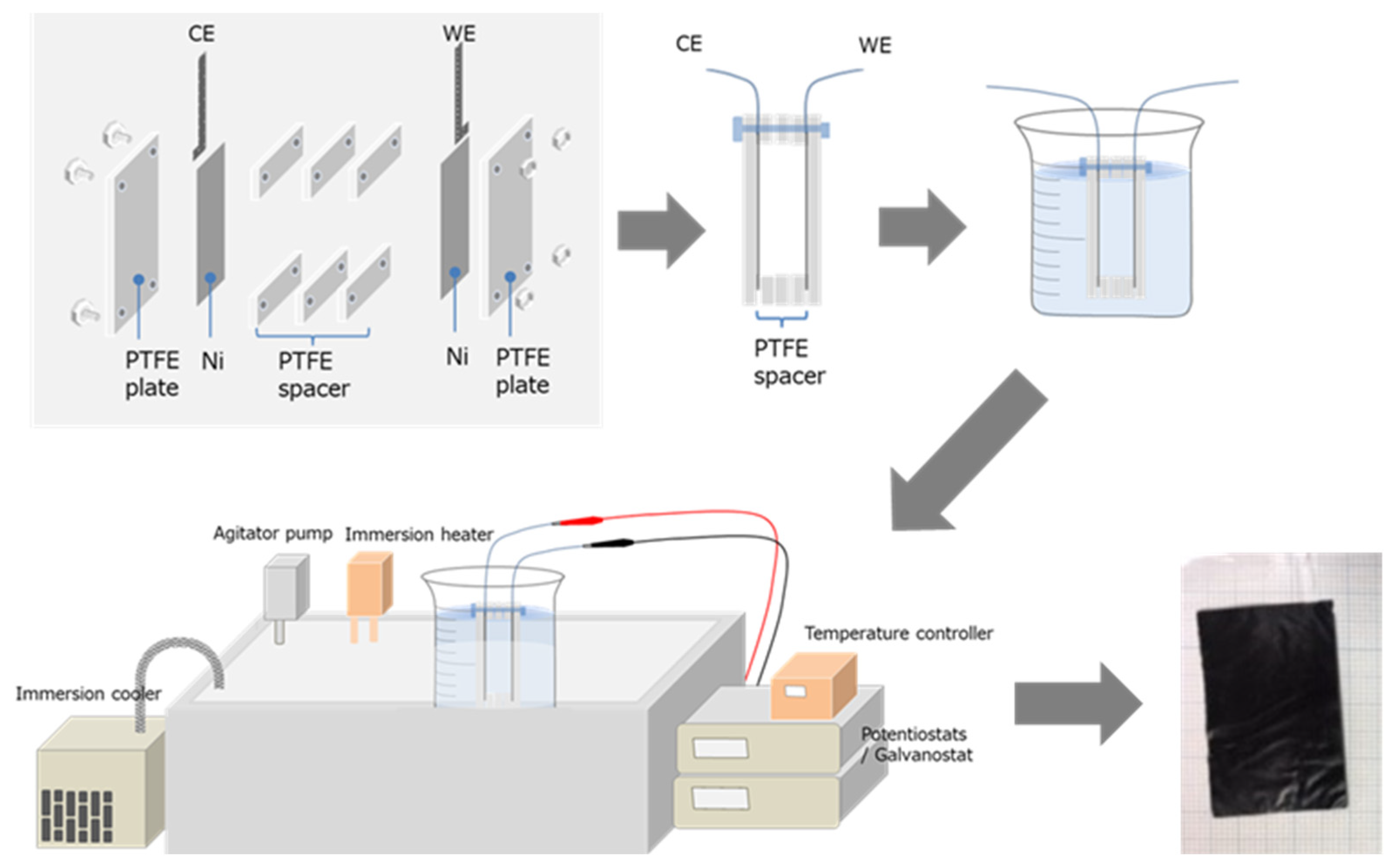
2.2. Preparation of PPy Films
2.3. Characterization of Actuator Films
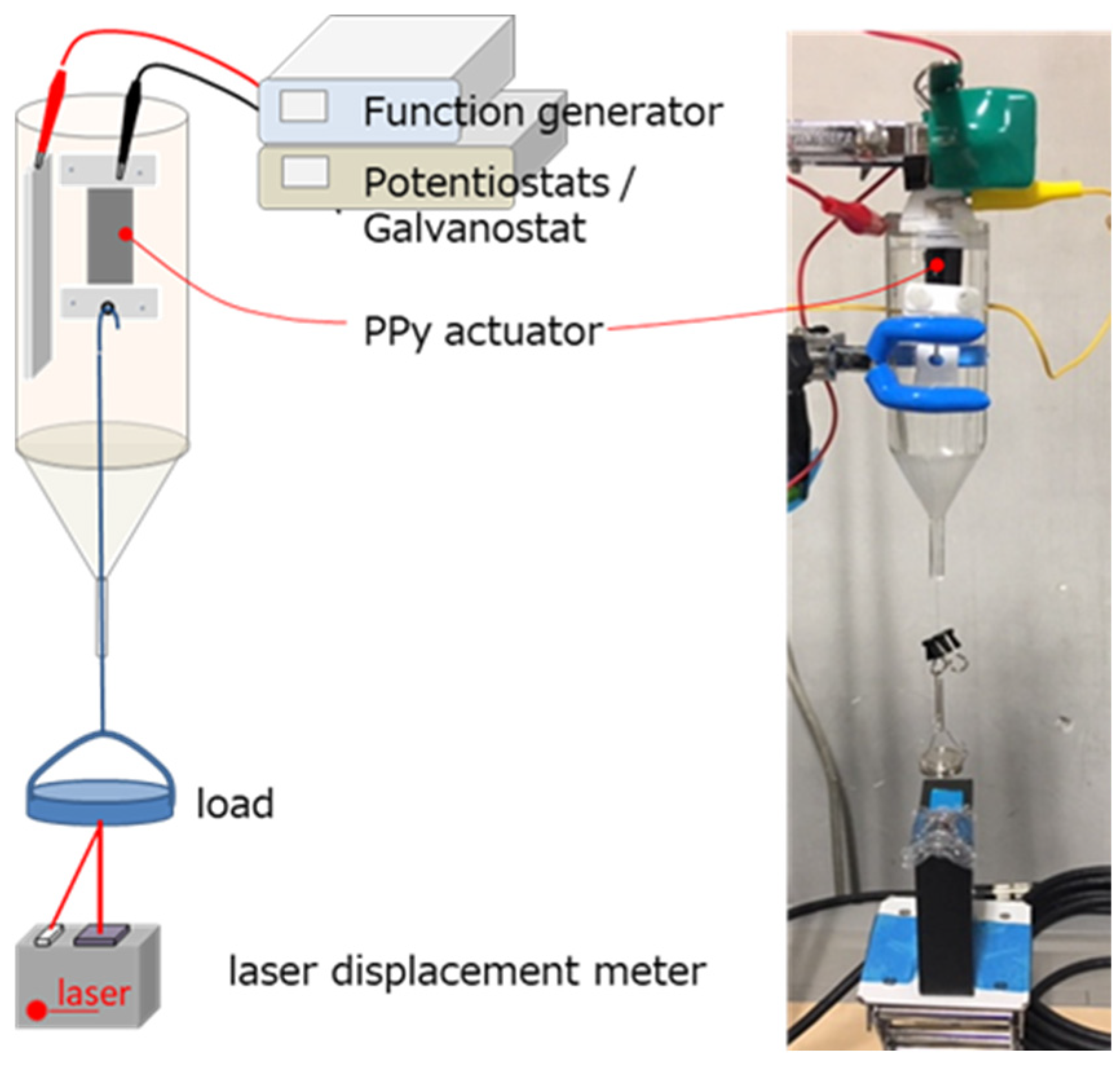
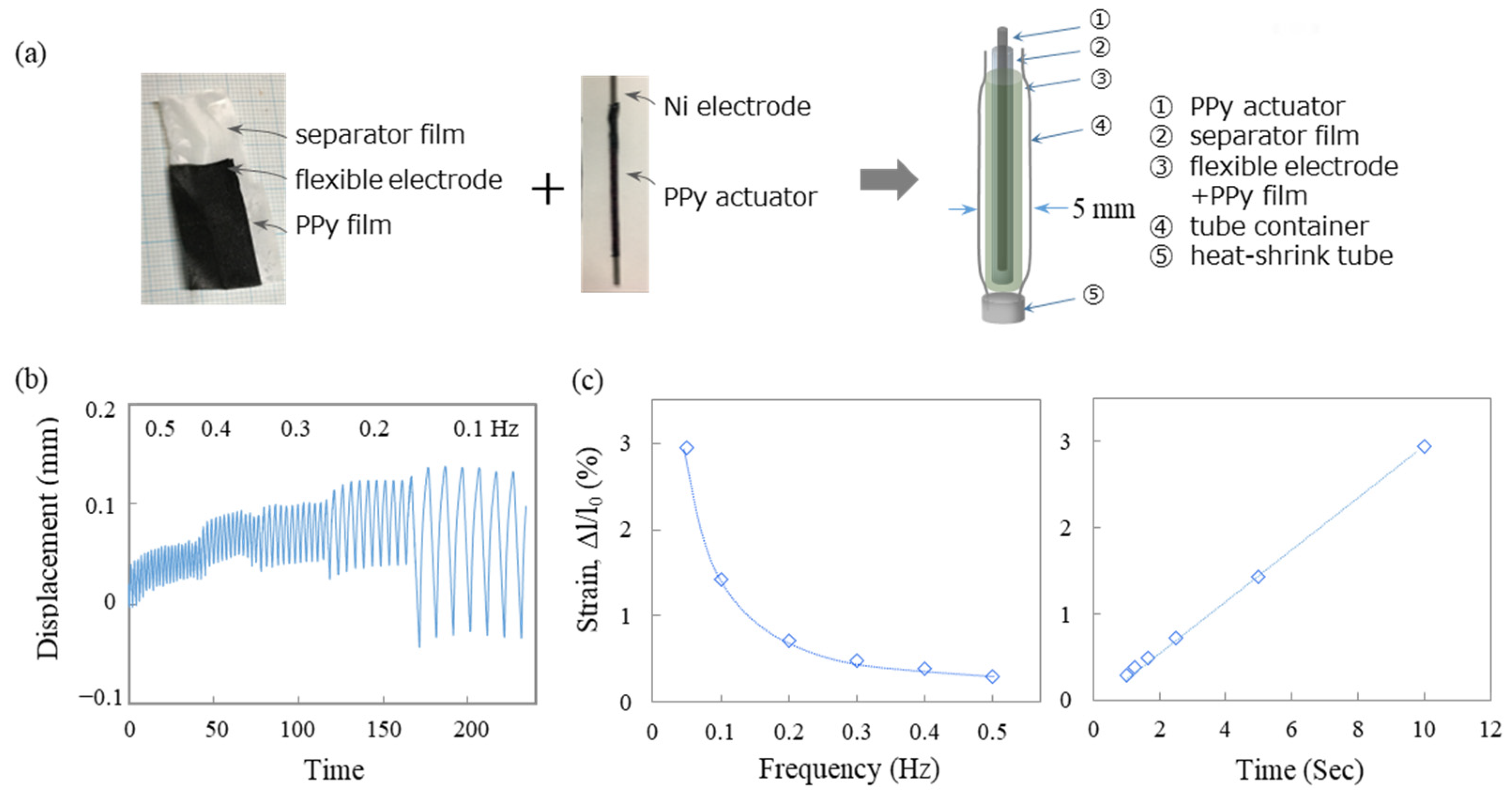
2.4. Preparation and Evaluation of Atmospheric-Operable Linear Actuators
3. Results
3.1. Effect of Polymerization Solution on Actuator Strain
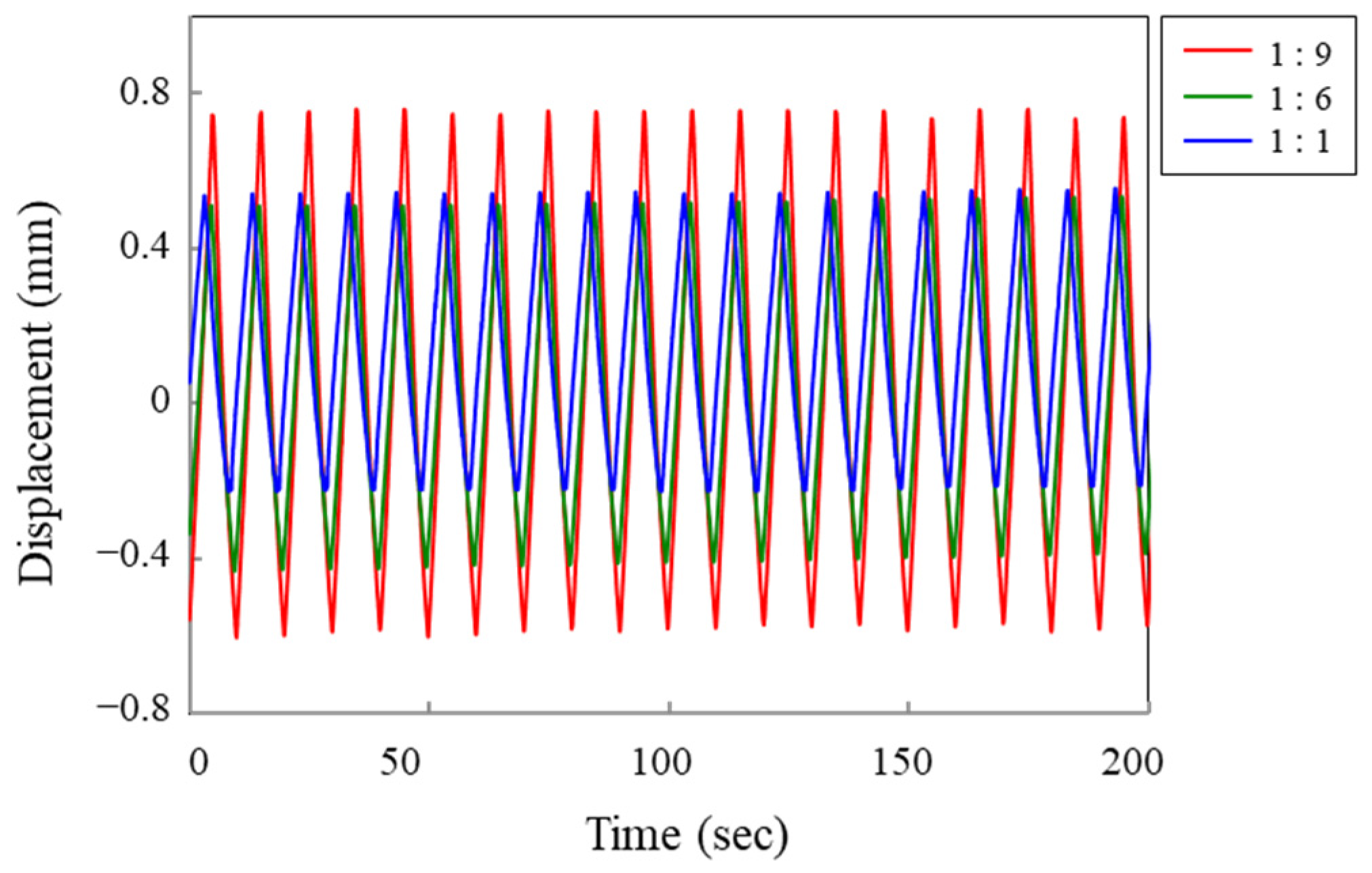
3.2. Effect of the Size of the Counter Electrode on Actuator Strain
3.3. Calculating the Generated Force of an Actuator
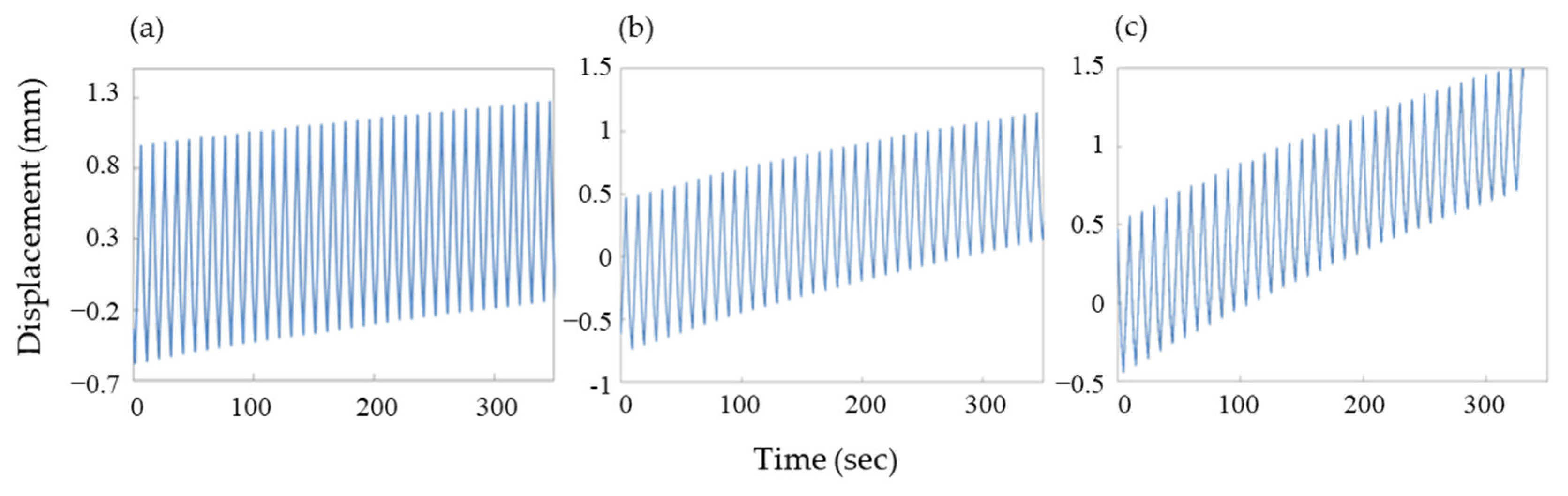
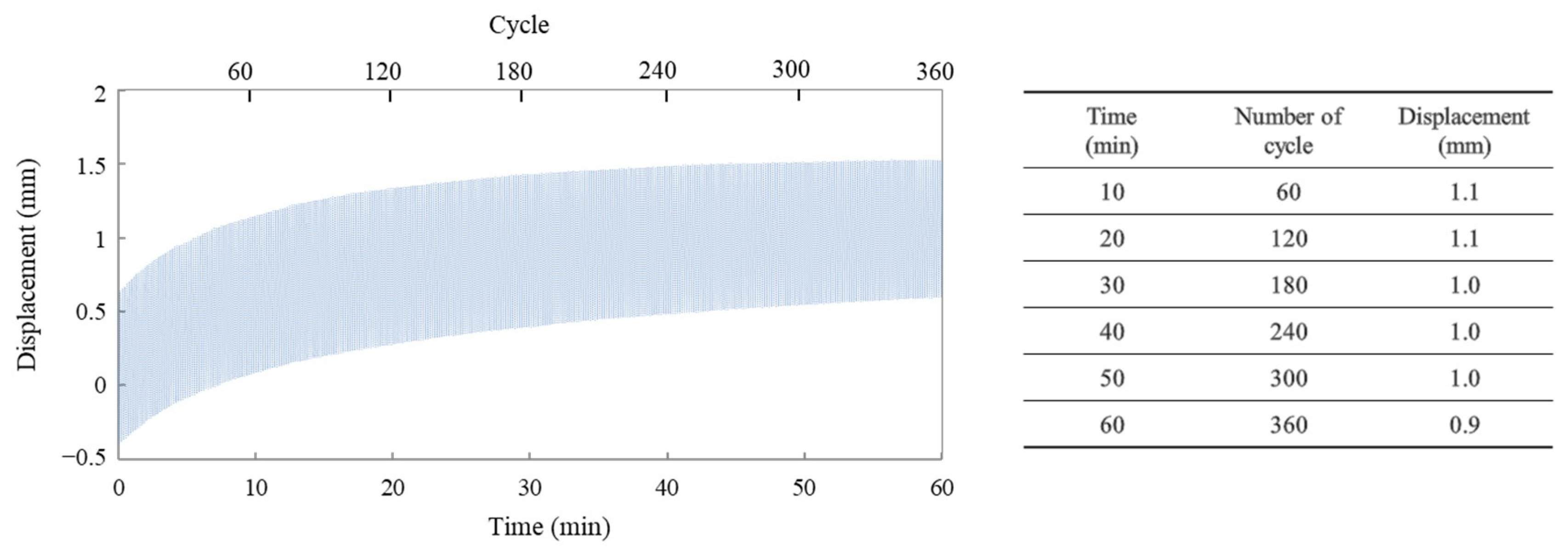
3.4. Cycle Characteristic Based on Expansion and Contraction of an Actuator
3.5. Fabrication of an Atmospheric-Operable Linear Actuator
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Eatock, D. Demographic Outlook for the European Union 2019; European Parliamentary Research Service: Brussels, Belgium; European Union: Maastricht, The Netherlands, 2019; ISBN 978-92-846-4922-8. [Google Scholar] [CrossRef]
- Suzman, R.; Beard, J. Global Health and Aging, World Health Organization; World Health: Geneva, Switzerland, 2011. Available online: https://www.nia.nih.gov/sites/default/files/2017-06/global_health_aging.pdf (accessed on 8 July 2023).
- Broadbent, E.; Stafford, R.; MacDonald, B. Investigating the Potential for Social Engagement with Robots among Older Adults in Assisted Living and Long-Term Care Facilities. Int. J. Soc. Robot. 2009, 1, 319–330. [Google Scholar] [CrossRef]
- Pasluosta, C.F.; Gassner, H.; Winkler, J.; Klucken, J.; Eskofier, B.M. Wearable acceleration sensors to assess Parkinsonian tremor and rigidity during free body movements. IEEE J. Biomed. Health Inform. 2015, 19, 1873–1881. [Google Scholar] [CrossRef]
- Wang, S.; Bolling, K.; Mao, W.; Reichstadt, J.; Jeste, D.; Kim, H.-C.; Nebeker, C. Technology-Mediated Activity, Leisure, and Social Participation in Older Adults with Depressive Symptoms: Secondary Analysis of a Randomized Controlled Trial. Healthcare 2019, 7, 60. [Google Scholar] [CrossRef]
- Kim, O.; Kim, H.; Choi, U.H.; Park, M.J. Reversible and Repeatable Deformation of Self-Assembled Hydrogel Shapes Induced by Uniaxial Stretch. Nat. Commun. 2016, 7, 13576. [Google Scholar] [CrossRef]
- Rasouli, H.; Naji, L.; Hosseini, M.G. Preparation of Novel Porphyrin-Silica Hybrid Materials via Sol-Gel Process for Dye Sensitized Solar Cells. N. J. Chem. 2018, 42, 12104–12118. [Google Scholar] [CrossRef]
- Hiraoka, M.; Nakamura, K.; Arase, H.; Asai, K.; Kaneko, Y.; John, S.W.; Tagashira, K.; Omote, A. Optical Properties of All-Inorganic CsPbX3 (X = Cl, Br, I) Perovskite Nanocrystals and Their Anion Exchange Reactions. Sci. Rep. 2016, 6, 36358. [Google Scholar] [CrossRef] [PubMed]
- Miriyev, A.; Stack, K.; Lipson, H. Soft Material for Soft Actuators. Nat. Commun. 2017, 8, 596. [Google Scholar] [CrossRef]
- Naka, Y.; Fuchiwaki, M.; Tanaka, K. A Dual-Stage, Two-Dimensional Cell Manipulation System Based on Electromagnetic Actuation. In Proceedings of the 2009 ICCAS-SICE, Fukuoka, Japan, 18–21 August 2009; pp. 4761–4766. [Google Scholar]
- Must, I.; Kaasik, F.; Põldsalu, I.; Mihkels, L.; Johanson, U.; Punning, A.; Aabloo, A. Nanocomposite Actuator Materials Based on Ionic Polymer-Metal Composites and Carbon Nanotubes. Adv. Eng. Mater. 2015, 17, 84–94. [Google Scholar] [CrossRef]
- Rus, D.; Tolley, M.T. Design, Fabrication and Control of Soft Robots. Nature 2015, 521, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Carpi, F.; De Rossi, D. Electroactive Polymer Actuators as Artificial Muscles: Are They Ready for Bioinspired Applications? IEEE Trans. Inf. Technol. Biomed. 2005, 9, 295–318. [Google Scholar] [CrossRef]
- Georgea, P.M.; Lyckman, A.W.; LaVan, D.A.; Hegde, A.; Leung, Y.; Avasare, R.; Testa, C.; Alexander, P.M.; Langer, R.; Sur, M. A quantum dot-labeled aptamer nanosensor for ATP detection. Biomaterials 2005, 26, 3511–3519. [Google Scholar]
- Hara, S.; Zama, T.; Sewa, S.; Takashima, W.; Kaneto, K. Chemical vapor deposition growth of polyaniline thin films and their application to gas sensors. Chem. Lett. 2003, 32, 576–577. [Google Scholar] [CrossRef]
- Dubal, D.P.; Lee, S.H.; Kim, J.G.; Kim, W.B.; Lokhande, C.D. Facile synthesis and electrochemical characterization of SnO2 nanoparticles thin film for supercapattery application. J. Mater. Chem. 2012, 22, 3044–3052. [Google Scholar] [CrossRef]
- Otero, T.F.; Tejada, R.; Elola, A.S. Formation and modification of polypyrrole films on platinum electrodes by cyclic voltammetry and anodic polarization. Polymer 1987, 28, 651–656. [Google Scholar] [CrossRef]
- Hara, S.; Zama, T.; Ametani, A.; Takashima, W.; Kaneto, K. Enhancement in electrochemical strain of a polypyrrole–metal composite film actuator. J. Mater. Chem. 2004, 14, 2724. [Google Scholar] [CrossRef]
- Kiefer, R.; Bowmaker, G.A.; Cooney, R.P.; Kilmartin, P.A.; Travas-Sejdic, J. Cation driven actuation for free standing PEDOT films prepared from propylene carbonate electrolytes containing TBACF3SO3. Electrochim. Acta 2008, 53, 2593–2599. [Google Scholar] [CrossRef]
- Liu, S.; Masurkar, N.; Varma, S.; Avrutsky, I.; Reddy Arava, L.M. Experimental Studies and Numerical Simulation of Polypyrrole Trilayer Actuators. ACS Omega 2019, 4, 6436–6442. [Google Scholar] [CrossRef]
- Noori, J.S.; Mortensen, J.; Geto, A. Recent Development on the Electrochemical Detection of Selected Pesticides: A Focused Review. Sensors 2020, 20, 2221. [Google Scholar] [CrossRef] [PubMed]
- Onoda, M.; Abe, Y.; Tada, K. New fabrication technique of conductive polymer/insulating polymer composite films and evaluation of biocompatibility in neuron cultures. Thin. Solid Film. 2009, 518, 743–749. [Google Scholar] [CrossRef]
- Hara, S.; Zama, T.; Tanaka, N.; Takashima, W.; Kaneto, K. Artificial Fibular Muscles with 20% Strain Based on Polypyrrole–Metal Coil Composites. Chem. Lett. 2005, 34, 784–785. [Google Scholar] [CrossRef]
- Yan, B.; Li, B.; Kunecke, F.; Gu, Z.; Guo, L. Biocompatible conjugated polymer nanoparticles for efficient photothermal tumor therapy. ACS Appl. Mater. Interfaces 2015, 7, 14563–14568. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bathaei, M.J.; Istif, E.; Beker, L. Development of a Novel Biosensor Based on PEDOT:PSS and Gold Nanoparticles for the Detection of Adenosine Triphosphate. Adv. Healthc. Mater. 2020, 9, 2000790. [Google Scholar] [CrossRef] [PubMed]
- Salmon, M.; Diaz, A.F.; Logan, A.J.; Krounbi, M.; Bargon, J. Chemical modification of conducting polypyrrole films. Mol. Cryst. Liq. Cryst. 1982, 83, 265–276. [Google Scholar] [CrossRef]
- Gupta, S. Template-free synthesis of conducting-polymer polypyrrole micro/nanostructures using electrochemistry. Appl. Phys. Lett. 2006, 88, 063108. [Google Scholar] [CrossRef]
- Brie, M.; Turcu, R.; Neamtu, C.; Pruneanu, S. The effect of initial conductivity and doping anions on gas sensitivity of conducting polypyrrole films to NH3. Sens. Actuators B 1996, 37, 119–122. [Google Scholar] [CrossRef]
- Maddison, D.S.; Jenden, C.M. Dopant exchange in conducting polypyrrole films. Polym. Int. 1992, 27, 231–235. [Google Scholar] [CrossRef]
- Arribas, C.; Rueda, D. Preparation of conductive polypyrrole-polystyrene sulfonate by chemical polymerization. Synth. Met. 1996, 79, 23–26. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, Y.; Ahn, K.J.; Huh, J.; Shim, H.W.; Sampath, G.; Im, W.B.; Huh, Y.I.; Yoon, H. Role of co-vapors in vapor deposition polymerization. Sci. Rep. 2015, 5, 8420. [Google Scholar] [CrossRef]
- Zondakaa, Z.; Keskülaa, A.; Tamma, T.; Kieferb, R. ZnO Nanoparticle Based Electrode for Mechanical Strain Sensing. Sens. Actuators B Chem. 2017, 247, 742–748. [Google Scholar]
- Zhang, X.; Zhang, J.; Song, W.; Liu, Z. Surfactant-assisted synthesis of single-crystal CdS nanowires and nanobelts. J. Phys. Chem. B 2006, 110, 1158–1165. [Google Scholar] [CrossRef]
- Ouyang, J.; Li, Y. Fabrication of Conducting Polymer Ultrathin Films and Their Use in Electroluminescent Devices. Polymer 1997, 38, 1971–1976. [Google Scholar] [CrossRef]
- Kiefer, R.; Chu, S.Y.; Kilmartin, P.A.; Bowmaker, G.A.; Cooney, R.P.; Travas-Sejdic, J. Electrochemical Properties of Poly (3,4-ethylenedioxythiophene) Films in Aqueous Sodium Perchlorate. Electrochim. Acta 2007, 52, 2386–2391. [Google Scholar] [CrossRef]
- Khadk, R.; Aydemir, N.; Kesküla, A.; Tamm, T.; T-Sejdic, J.; Kiefer, R. Synthesis and Characterization of Poly(3,4-ethylenedioxythiophene)-Based Hybrid Materials for Chemical Sensing Applications. Synth. Met. 2017, 232, 1–7. [Google Scholar]
- Lizarraga, L.; María Andrade, E.; Victor Molina, F. Swelling and volume changes of polyaniline upon redox switching. J. Electroanal. Chem. 2004, 561, 127–135. [Google Scholar] [CrossRef]
- Otero, T.F.; Angulo, E.; Rodríguez, J.; Santamaría, C. Electrochemomechanical properties from a bilayer: Polypyrrole/non-conducting and flexible material-artificial muscle. J. Electroanal. Chem. 1992, 341, 369–375. [Google Scholar] [CrossRef]
- Bay, L.; Jacobsen, T.; Skaarup, S.; West, K. Mechanism of actuation in conducting polymers: Osmotic expansion. J. Phys. Chem. B 2001, 105, 8492–8497. [Google Scholar] [CrossRef]
- Kilmartin, P.A.; Travas-Sejdic, J. Nanostructural aspects of conducting-polymer actuators. In Nanostructured Conductive Polymers, 1st ed.; Eftekhari, A., Ed.; John Wiley & Sons: New York, NY, USA, 2010; pp. 599–630. [Google Scholar]
- Wataru, T.; Shyam, S.P.; Masaki, F.; Keiichi, K. Cyclic step-voltammetric analysis of cation-driven and anion-driven actuation in polypyrrole films. Jpn. J. Appl. Phys. 2002, 41, 7532. [Google Scholar]
- Maw, S.; Smela, E.; Yoshida, K.; Stein, R.B. Effects of monomer and electrolyte concentrations on actuation of PPy(DBS) bilayers. Synth. Met. 2005, 155, 18–26. [Google Scholar] [CrossRef]
- Ding, J.; Liu, L.; Spinks, G.M.; Zhou, D.; Wallace, G.G.; Gillespie, J. Synthesis and Characterization of Polypyrrole/Single-Walled Carbon Nanotube Composite Material. Synth. Met. 2003, 138, 391–398. [Google Scholar] [CrossRef]
- Xi, B.; Truong, V.-T.; Mottaghitalab, V.; Whitten, P.G.; Spinks, G.M.; Wallace, G.G. Smart hydrogel-based biosensors: A review. Smart Mater. Struct. 2007, 16, 1549–1554. [Google Scholar] [CrossRef][Green Version]
- Hara, S.; Zama, T.; Takashima, W.; Kaneto, K. Synthesis and Characteristics of Novel Water-Soluble Polythiophene Derivatives. Synth. Met. 2004, 146, 47–55. [Google Scholar] [CrossRef]
- Plesse, C.; Vidal, F.; Teyssié, D.; Chevrot, C. Conducting polymer artificial muscle fibres: Toward an open air linear actuation. Chem. Commun. 2010, 46, 2910–2912. [Google Scholar] [CrossRef] [PubMed]
- Fannir, A.; Temmer, R.; Nguyen, G.T.M.; Cadiergues, L.; Laurent, E.; Madden, J.D.W.; Vidal, F.; Plesse, C. Linear Artificial Muscle Based on Ionic Electroactive Polymer: A Rational Design for Open-Air and Vacuum Actuation. Adv. Mater. Technol. 2019, 4, 1800519. [Google Scholar] [CrossRef]
- Naficy, S.; Stoboi, N.; Whitten, P.G.; Spinks, G.M.; Wallace, G.G. Evaluation of encapsulating coatings on the performance of polypyrrole actuators. Smart Mater. Struct. 2013, 22, 075005. [Google Scholar] [CrossRef]


| Electrolyte | Solvent | (Abbreviation) |
|---|---|---|
| TBABF4 | PE + EA | I-1 |
| PE + DEP | I-2 | |
| TEATFSI | PE + EA | II-1 |
| PE + DEP | II-2 | |
| TBABF4 + TEATFSI | PE + EA | III-1 |
| PE + DEP | III-2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Yoshida, Y. A Lightweight and Low-Voltage-Operating Linear Actuator Based on the Electroactive Polymer Polypyrrole. Polymers 2023, 15, 3455. https://doi.org/10.3390/polym15163455
Kim Y, Yoshida Y. A Lightweight and Low-Voltage-Operating Linear Actuator Based on the Electroactive Polymer Polypyrrole. Polymers. 2023; 15(16):3455. https://doi.org/10.3390/polym15163455
Chicago/Turabian StyleKim, Yeji, and Yasukazu Yoshida. 2023. "A Lightweight and Low-Voltage-Operating Linear Actuator Based on the Electroactive Polymer Polypyrrole" Polymers 15, no. 16: 3455. https://doi.org/10.3390/polym15163455
APA StyleKim, Y., & Yoshida, Y. (2023). A Lightweight and Low-Voltage-Operating Linear Actuator Based on the Electroactive Polymer Polypyrrole. Polymers, 15(16), 3455. https://doi.org/10.3390/polym15163455





