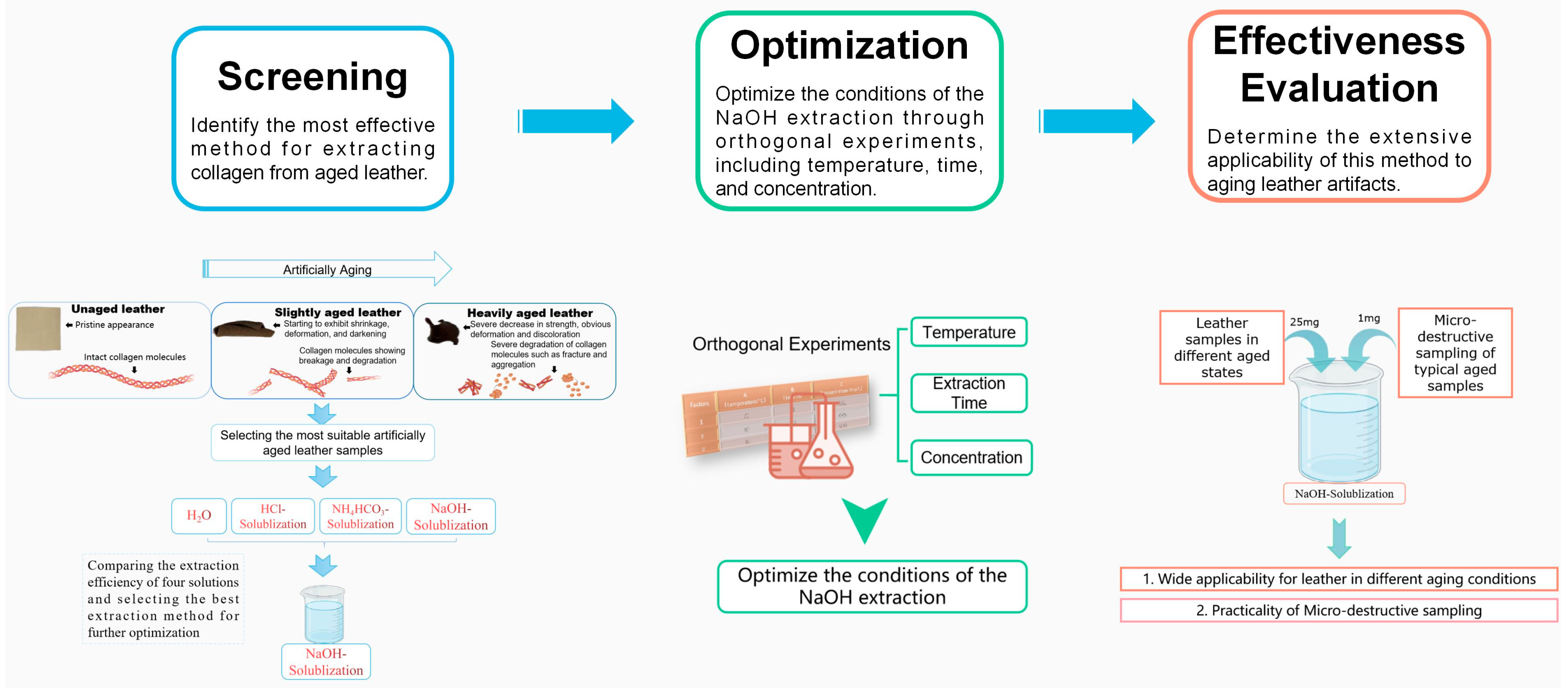
The Efficient Extraction Method of Collagen from Deteriorated Leather Artifacts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
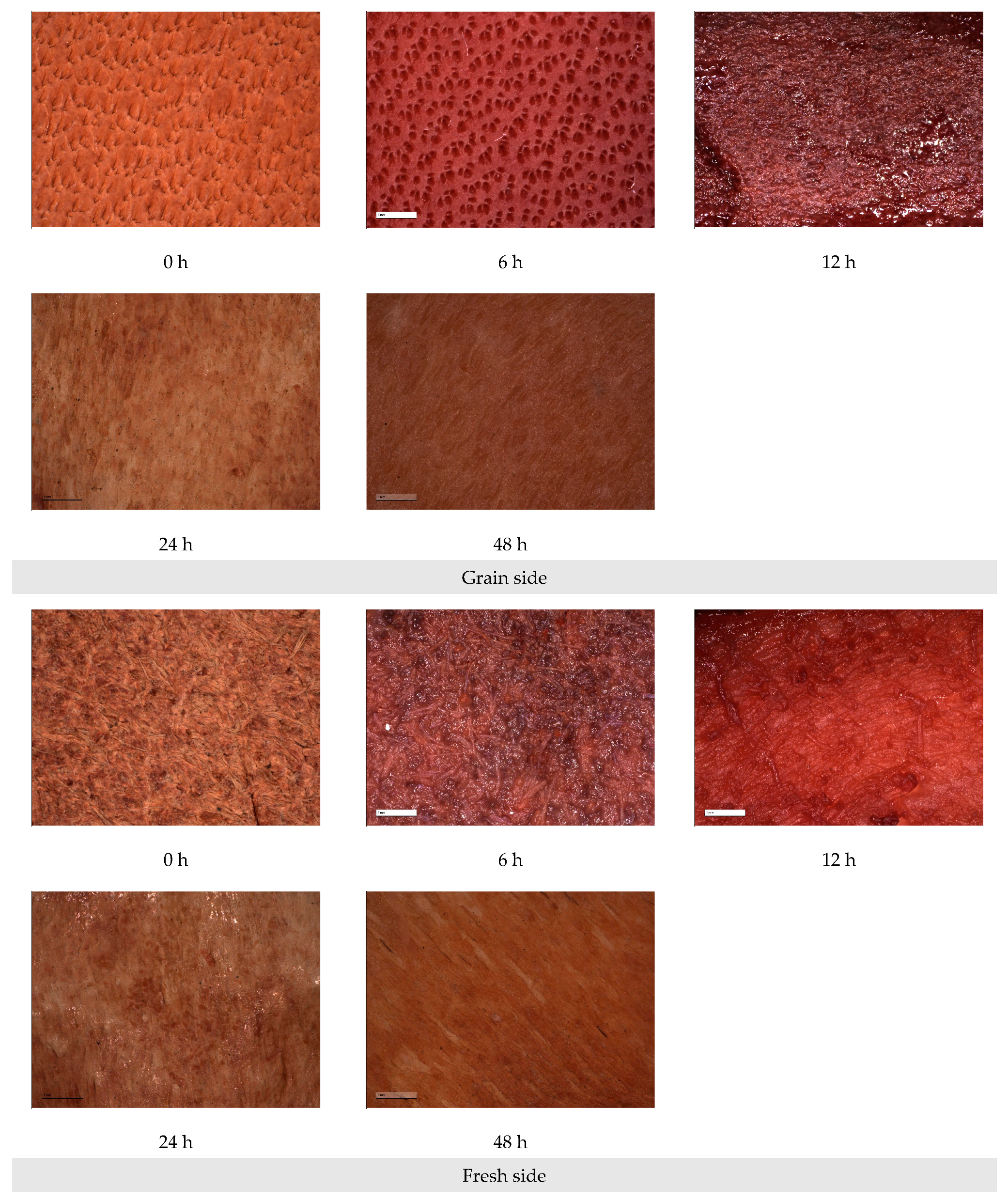
2.2.1. Preparation of Aged Leather Samples
2.2.2. Extraction of Collagen from Aged Leather Samples
2.2.3. Optimization of Experimental Conditions for the Extraction of Collagen from Aged Leather Samples by NaOH-Solublization (NS)
Making Bradford Standard Protein Concentration Curve
Orthogonal Experiment
2.2.4. Effectiveness Evaluation of the Modified Collagen Extraction Method
Collagen Extraction of Leather Samples with Different Aging Degrees
Collagen Extraction with Micro-Destructive Sampling
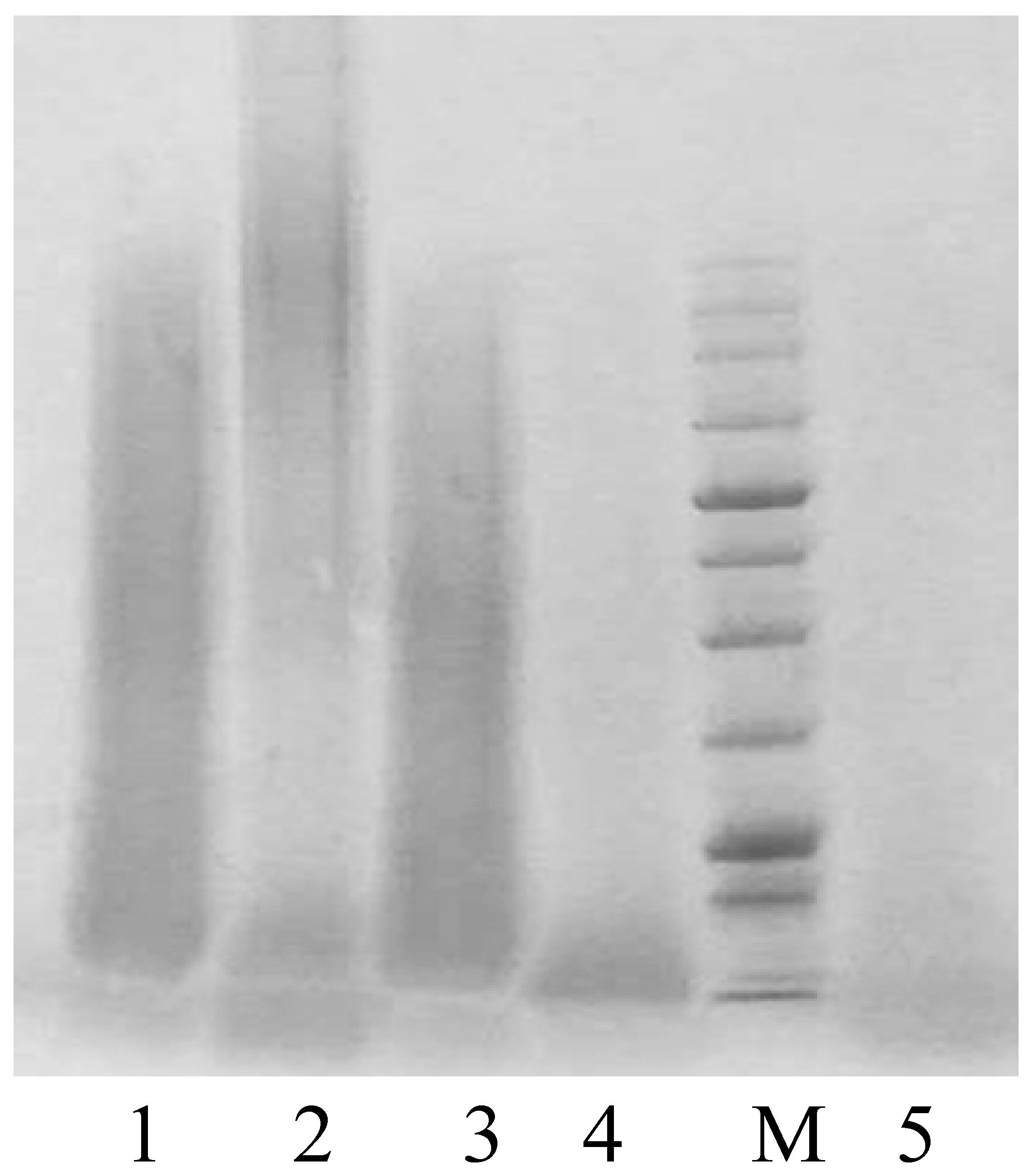
SDS-PAGE
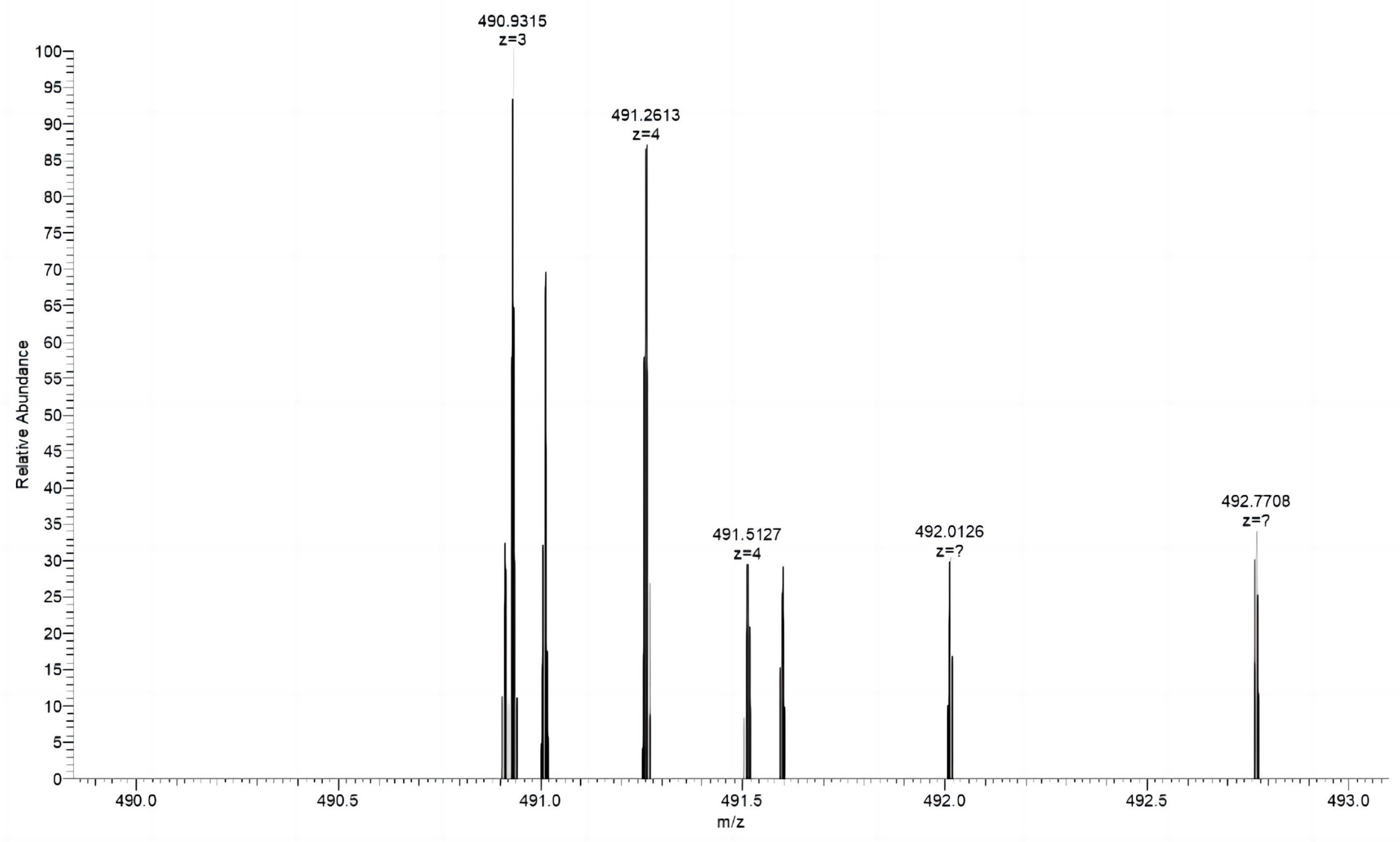
Biological Mass Spectrometry Analysis
3. Results and Discussion
3.1. Preparation of Artificial Aging Samples
3.2. Comparison of Collagen Extraction Methods from the Aging Leather Samples
3.3. Optimization of Experimental Parameters for NaOH Extraction Method
3.4. Evaluation of the Effectiveness of NaOH Solubilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Heth, C.L. The Skin They Were In: Leather and Tanning in Antiquity. In Chemical Technology in Antiquity; American Chemical Society: Washington, DC, USA, 2015; pp. 181–196. [Google Scholar] [CrossRef]
- Zhang, M.; Hu, Y.; Liu, J.; Pei, Y.; Tang, K.; Lei, Y. Biodeterioration of collagen-based cultural relics: A review. Fungal Biol. Rev. 2022, 39, 46–59. [Google Scholar] [CrossRef]
- Hendy, J. Ancient protein analysis in archaeology. Sci. Adv. 2021, 7, eabb9314. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, C.A. Clark Alexander. In Animal Hide Processing: Impact on Collagen Structure-ProQuest; Cardiff University: Cardiff, Wales, 2007. [Google Scholar]
- Kennedy, C.J.; Wess, T.J. The Structure of Collagen within Parchment–A Review. De Gruyter 2003, 24, 61–80. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Zhou, S.; Yao, J. Research on leather artifacts from Inner Mongolia: Classification, composition and analysis of the properties related to the technology of manufacture. Sci. Conserv. Archaeol. 2012, 24, 33–44. [Google Scholar] [CrossRef]
- Plavan, V.; Miu, L.; Gavrilyuk, N. Evaluation of the Amino Acid Composition, Structure and Properties of Archaeological Leather. Procedia Chem. 2013, 8, 279–283. [Google Scholar] [CrossRef]
- Kirby, D.P.; Buckley, M.; Promise, E.; Trauger, S.A.; Holdcraft, T.R. Identification of collagen-based materials in cultural heritage. Analyst R. Soc. Chem. 2013, 138, 4849–4858. [Google Scholar] [CrossRef]
- Warinner, C.; Richter, K.K.; Collins, M.J. Paleoproteomics. Chem. Rev. 2022, 122, 13401–133446. [Google Scholar] [CrossRef]
- Ebsen, J.A.; Haase, K.; Larsen, R.; Sommer, D.V.P.; Brandt, L.Ø. Identifying archaeological leather–discussing the potential of grain pattern analysis and zooarchaeology by mass spectrometry (ZooMS) through a case study involving medieval shoe parts from Denmark. J. Cult. Herit. 2019, 39, 21–31. [Google Scholar] [CrossRef]
- Chen, L.; Bu, Q.; Qiang, T.; Zhu, J. Research advance of historical leather and its restoration materials. China Leather 2020, 49, 14–19. [Google Scholar] [CrossRef]
- Gong, D.; Yang, Z.; Huang, W. Identification of waterlogged leather unearthed from a Warring States Period tomb in Yiyuan, Shandong Province. Sci. Conserv. Archaeol. 2015, 27, 59–64. [Google Scholar] [CrossRef]
- Vest, M.; Jacobsen, J.; Larsen, R. Accelerated ageing: Effect of heat and relative humidity. In Improved Damage Assess Parchment IDAP; Larsen, R., Ed.; European Commission, Directorate General Research: Brussels, Belgium, 2007; pp. 67–68. [Google Scholar]
- Axelsson, K.M. Oxidative and Hydrolytic Degradation of Collagen in Parchment: A Deeper Insight to the Degradation Mechanisms at Microscopic and Molecular Levels; Det Kongelige Danske Kunstakademis Skoler for Arkitektur, Design og Konservering. Kunstakademiets Konservatorskole: København, Denmark, 2014. [Google Scholar]
- Smith, G.J. New trends in photobiology (invited review) photodegradation of keratin and other structural proteins. J. Photochem. Photobiol. B 1995, 27, 187–198. [Google Scholar] [CrossRef]
- Plavan, V.; Miu, L.; Gordienko, I.; Ibragimova, A.; Gavrilyuk, N. Determination of the Amino acid Composition, Structure and Properties of the Archaeological Leather Before and After Restoration. Rev. Chim. 2013, 64, 603–605. [Google Scholar]
- Larsen, R.; Sommer, D.V.P.; Vest, M.; Jensen, A.L. Amino acid analysis of new and historical parchments. In Microanal Parchment; Larsen, R., Ed.; Archetype Publications: London, UK, 2002; pp. 93–99. [Google Scholar]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Larsen, R.; Poulsen, D.V.; Minddal, K.; Dahlstrøm, N.; Fazlic, N. Molecular damage of parchment studied by amino acid analysis. In Improv Damage Assess Parchment IDAP; Larsen, R., Ed.; European Commission, Directorate General Research: Brussels, Belgium, 2007; pp. 111–114. [Google Scholar]
- Harris, S.; Piquette, K.E. Reflectance Transformation Imaging (RTI) for visualising leather grain surface morphology as an aid to species identification: A pilot study. Archaeol. Leather Group Newslett. 2015, 42, 13–18. [Google Scholar]
- Zhang, Y.; Chen, Z.; Liu, X.; Shi, J.; Chen, H.; Gong, Y. SEM, FTIR and DSC Investigation of Collagen Hydrolysate Treated Degraded Leather. J. Cult. Herit. 2021, 48, 205–210. [Google Scholar] [CrossRef]
- Yang, Z.; Chen, Z.; Wang, C.; Tian, Y.; Gong, D. Studies of structure changes of archeological leather by FTIR spectroscopy. J. Soc. Leather Technol. Chem. 2018, 102, 262–267. [Google Scholar]
- Vyskočilová, G.; Kopecká, R.; Pavliňák, D.; Laichmanová, M.; Sedláček, I.; Orlita, A.; Příhoda, J.; Miu, L. The influence of soil environment on the degradation of archaeological leather. Archaeometry 2022, 64, 483–499. [Google Scholar] [CrossRef]
- Kumazawa, Y.; Taga, Y.; Iwai, K.; Koyama, Y.-I. A Rapid and Simple LC-MS Method Using Collagen Marker Peptides for Identification of the Animal Source of Leather. J. Agric. Food Chem. Am. Chem. Soc. 2016, 64, 6051–6057. [Google Scholar] [CrossRef]
- Dyer, J.M.; Plowman, J.E.; Krsinic, G.L.; Deb-Choudhury, S.; Koehn, H.; Millington, K.R.; Clerens, S. Proteomic evaluation and location of UVB-induced photo-oxidation in wool. J. Photochem. Photobiol. B 2010, 98, 118–127. [Google Scholar] [CrossRef]
- Buckley, M. Species Identification of Bovine, Ovine and Porcine Type 1 Collagen; Comparing Peptide Mass Fingerprinting and LC-Based Proteomics Methods. Int. J. Mol. Sci. Multidiscip. Digit. Publ. Inst. 2016, 17, 445. [Google Scholar] [CrossRef]
- Noorzai, S.; Verbeek, C.J.R.; Lay, M.C.; Swan, J. Collagen Extraction from Various Waste Bovine Hide Sources. Waste Biomass Valoriz. 2020, 11, 5687–5698. [Google Scholar] [CrossRef]
- Meyer, M.; Mühlbach, R.; Harzer, D. Solubilisation of cattle hide collagen by thermo-mechanical treatment. Polym. Degrad. Stab. 2005, 87, 137–142. [Google Scholar] [CrossRef]
- Cucos, A.; Budrugeac, P. The impact of natural ageing on the hydrothermal stability of new and artificially aged parchment and leather samples. Thermochim. Acta 2018, 669, 40–44. [Google Scholar] [CrossRef]
- Vyskočilová, G.; Ebersbach, M.; Kopecká, R.; Prokeš, L.; Příhoda, J. Model study of the leather degradation by oxidation and hydrolysis. Herit. Sci. 2019, 7, 26. [Google Scholar] [CrossRef]
- Sebestyén, Z.; Czégény, Z.; Badea, E.; Carsote, C.; Şendrea, C.; Barta-Rajnai, E.; Bozi, J.; Miu, L.; Jakab, E. Thermal characterization of new, artificially aged and historical leather and parchment. J. Anal. Appl. Pyrolysis 2015, 115, 419–427. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Candiano, G.; Bruschi, M.; Musante, L.; Santucci, L.; Ghiggeri, G.M.; Carnemolla, B.; Orecchia, P.; Zardi, L.; Righetti, P.G. Blue silver: A very sensitive colloidal Coomassie G-250 staining for proteome analysis. Electrophoresis 2004, 25, 1327–1333. [Google Scholar] [CrossRef]
- Malea, E.; Vogiatzi, T.; Watkinson, D. Assessing the Physical Condition of Waterlogged Archaeological Leather. In Proceedings of the 11th ICOM-CC Group on Wet Organic Archaeological Materials Conference, Greenville, NC, USA, 24–28 May 2010. [Google Scholar]
- Elnaggar, A.; Leona, M.; Nevin, A.; Heywood, A. The Characterization of Vegetable Tannins and Colouring Agents in Ancient Egyptian Leather from the Collection of the Metropolitan Museum of Art. Archaeometry 2017, 59, 133–147. [Google Scholar] [CrossRef]
- Johnson, J.S.; Wills, B. Review of Leather Wet and Dry: Current Treatments in the Conservation of Waterlogged and Desiccated Archaeological Leather. J. Am. Inst. Conserv. 2002, 41, 189–191. [Google Scholar] [CrossRef]
- Rijkelijkhuizen, M. Leather gloves and mittens–examples recovered from the Netherlands. Archaeol. Leather. Group Newslett. 2013, 38, 5–9. [Google Scholar]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef] [PubMed]


| No. | Method | Temperature/°C | Time/h | Volume/mL |
|---|---|---|---|---|
| 1 | 0.1 mol/L NaOH | 80 | 12 | 10 |
| 2 | 0.01 mol/L HCl | 80 | 12 | 10 |
| 3 | 0.05 mol/L NH4HCO3 | 80 | 12 | 10 |
| 4 | Water | 100 | 12 | 10 |
| Factors. | A (Temperature/°C) | B (Time/h) | C (Concentration/mol/L) |
|---|---|---|---|
| 1 | 70 | 9 | 0.01 |
| 2 | 80 | 12 | 0.05 |
| 3 | 90 | 15 | 0.10 |
| No. | A (Temperature/°C) | B (Time/h) | C (Concentration/mol/L) | Protein Concentration μg/mL |
|---|---|---|---|---|
| 1 | 1 (70) | 1 (9) | 1 (0.01) | 560 |
| 2 | 1 (70) | 2 (12) | 2 (0.05) | 2678 |
| 3 | 1 (70) | 3 (15) | 3 (0.10) | 1590 |
| 4 | 2 (80) | 1 (9) | 2 (0.05) | 2329 |
| 5 | 2 (80) | 2 (12) | 3 (0.10) | 1145 |
| 6 | 2 (80) | 3 (15) | 1 (0.01) | 2022 |
| 7 | 3 (90) | 1 (9) | 3 (0.10) | 1398 |
| 8 | 3 (90) | 2 (12) | 1 (0.01) | 1794 |
| 9 | 3 (90) | 3 (15) | 2 (0.05) | 1917 |
| K1 | 1609 | 1429 | 1459 | |
| K2 | 1832 | 1872 | 2308 | |
| K3 | 1703 | 1843 | 1378 | |
| Variance | 223 | 443 | 930 |
| No. | Aging Time (h) | Weight (mg) | Extract Concentration (μg/mL) | Protein Extraction Rate * (%) |
|---|---|---|---|---|
| 1 | 6 | 25 | 1942 | 3.88 |
| 2 | 12 | 25 | 1489 | 2.98 |
| 3 | 24 | 25 | 2693 | 5.39 |
| 4 | 48 | 10 | 647 | 3.24 |
| 5 | 24 | 1 | 102 | 5.10 |
| Organism | Protein IDs | Protein | Total Peptides |
|---|---|---|---|
| Bos taurus | sp|P02453.3|CO1A1_BOVIN; NP_001029211.1; DAA18573.1; AAI05185.1; XP_024835395.1 | Collagen alpha-1(I) chain | 30 |
| NP_001068594.1 | collagen alpha-2(VI) chain precursor | 7 | |
| sp|P02465.2|CO1A2_BOVIN | Collagen alpha-2(I) chain | 17 | |
| sp|P21793.2|PGS2_BOVIN | Decorin | 10 | |
| XP_002697097.2 | collagen alpha-1(VII) chain | 3 | |
| NP_001106695.1 | collagen alpha-1(II) chain isoform 2 precursor | 4 | |
| XP_024846034.1 | collagen alpha-3(VI) chain isoform X5 | 25 | |
| NP_001137337.1 | collagen alpha-1(VI) chain precursor | 9 | |
| prf||0910139A; AAA30436.2 | collagen alpha1(I)CN8 | 6 | |
| XP_005202110.1 | collagen alpha-2(VI) chain isoform X1 | 10 | |
| XP_024835542.1 | collagen alpha-2(V) chain | 3 |
| Sequences | |
|---|---|
| Specific peptides shared by cattle and buffalo skin collagen | IGQPGAVGPAGIR |
| SGDRGETGPAGPAGPIGPVGAR | |
| DGRIGQPGAVGPAGIR | |
| Peptide sequences unique to cattle skin collagen | VIDELDVKPEGTR |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Zhang, M. The Efficient Extraction Method of Collagen from Deteriorated Leather Artifacts. Polymers 2023, 15, 3459. https://doi.org/10.3390/polym15163459
Li L, Zhang M. The Efficient Extraction Method of Collagen from Deteriorated Leather Artifacts. Polymers. 2023; 15(16):3459. https://doi.org/10.3390/polym15163459
Chicago/Turabian StyleLi, Li, and Meng Zhang. 2023. "The Efficient Extraction Method of Collagen from Deteriorated Leather Artifacts" Polymers 15, no. 16: 3459. https://doi.org/10.3390/polym15163459
APA StyleLi, L., & Zhang, M. (2023). The Efficient Extraction Method of Collagen from Deteriorated Leather Artifacts. Polymers, 15(16), 3459. https://doi.org/10.3390/polym15163459






