Influence of Pyrolytic Carbon Black Derived from Waste Tires at Varied Temperatures within an Industrial Continuous Rotating Moving Bed System
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Device
2.3. CBp Production
2.4. Production of CBp/NR Composites
2.5. Characterization
3. Results and Discussion
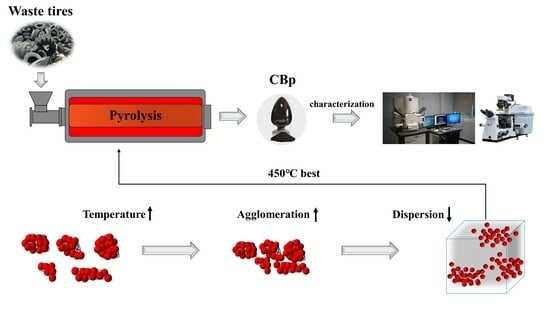
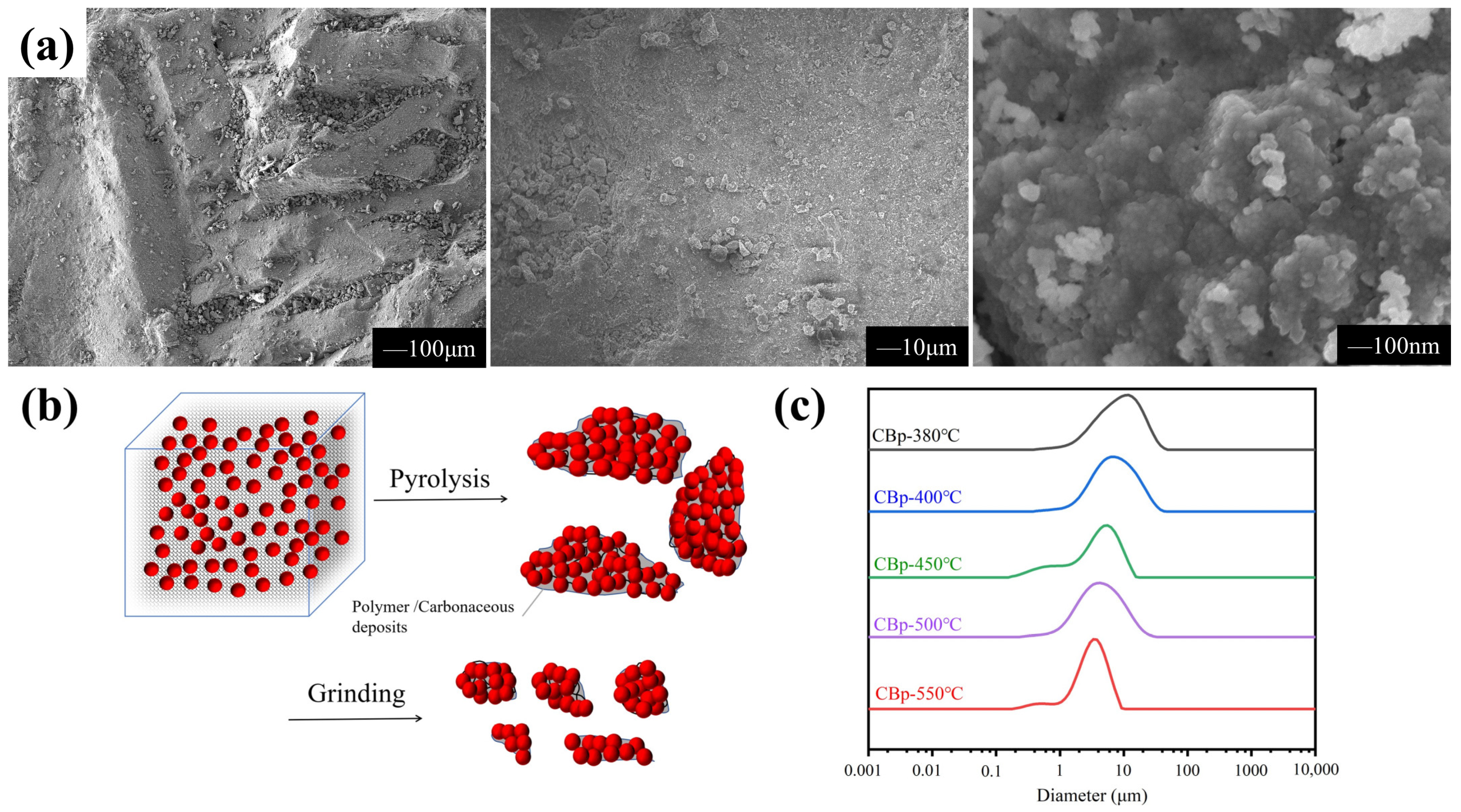
3.1. Pyrolysis Process and Formation of CBp
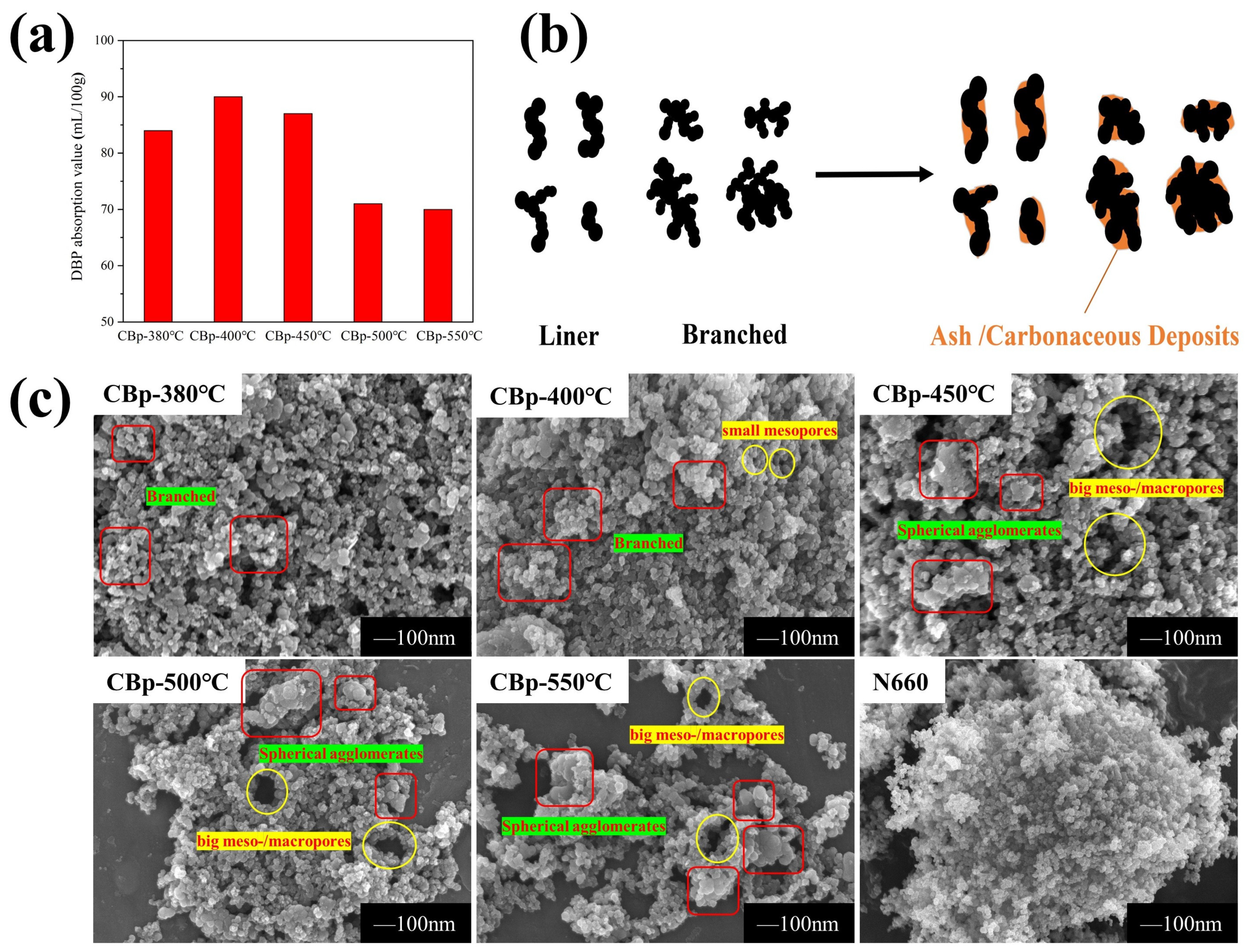
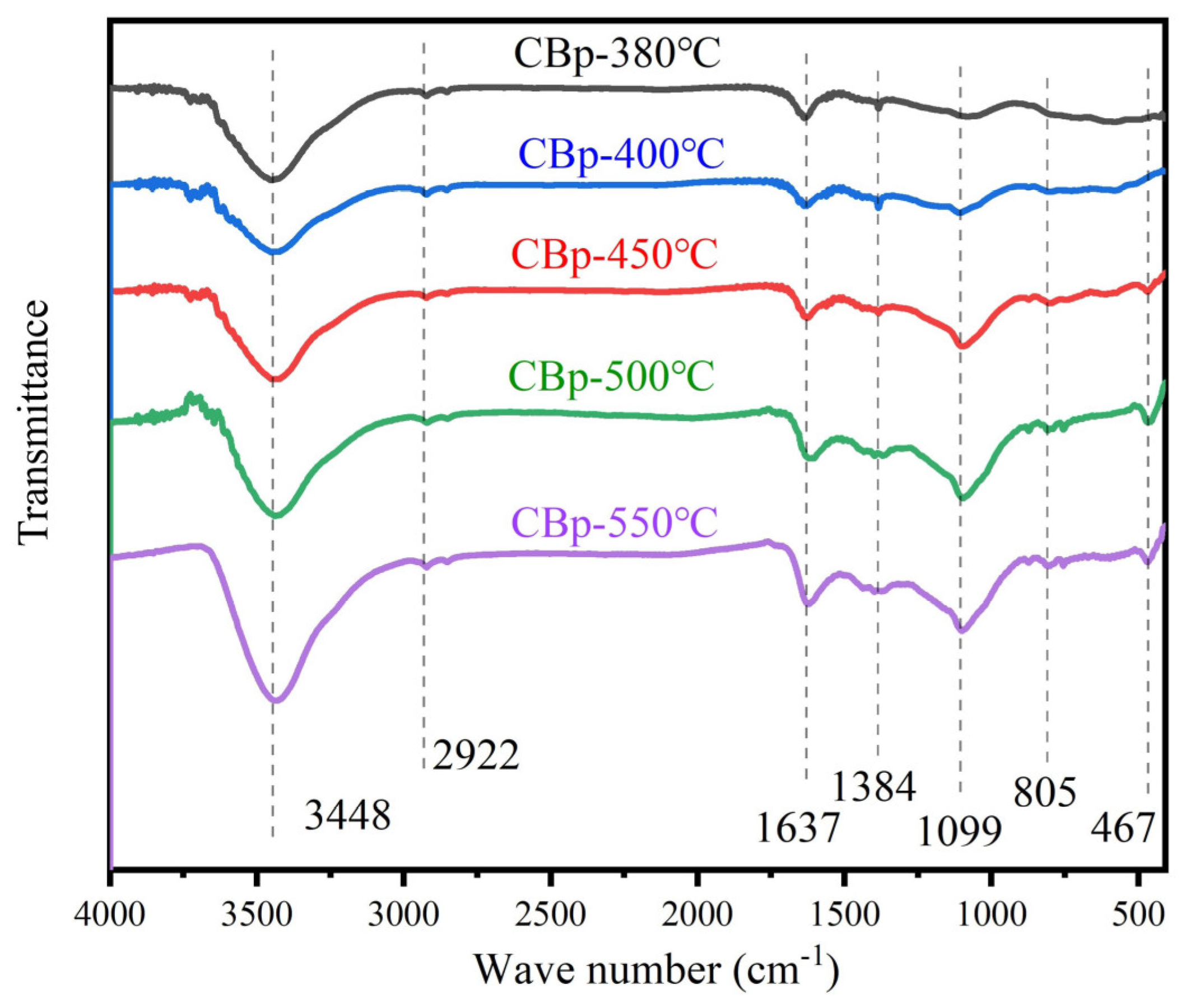
3.2. Surface Physical/Chemical Characteristics of CBps with Different Temperatures
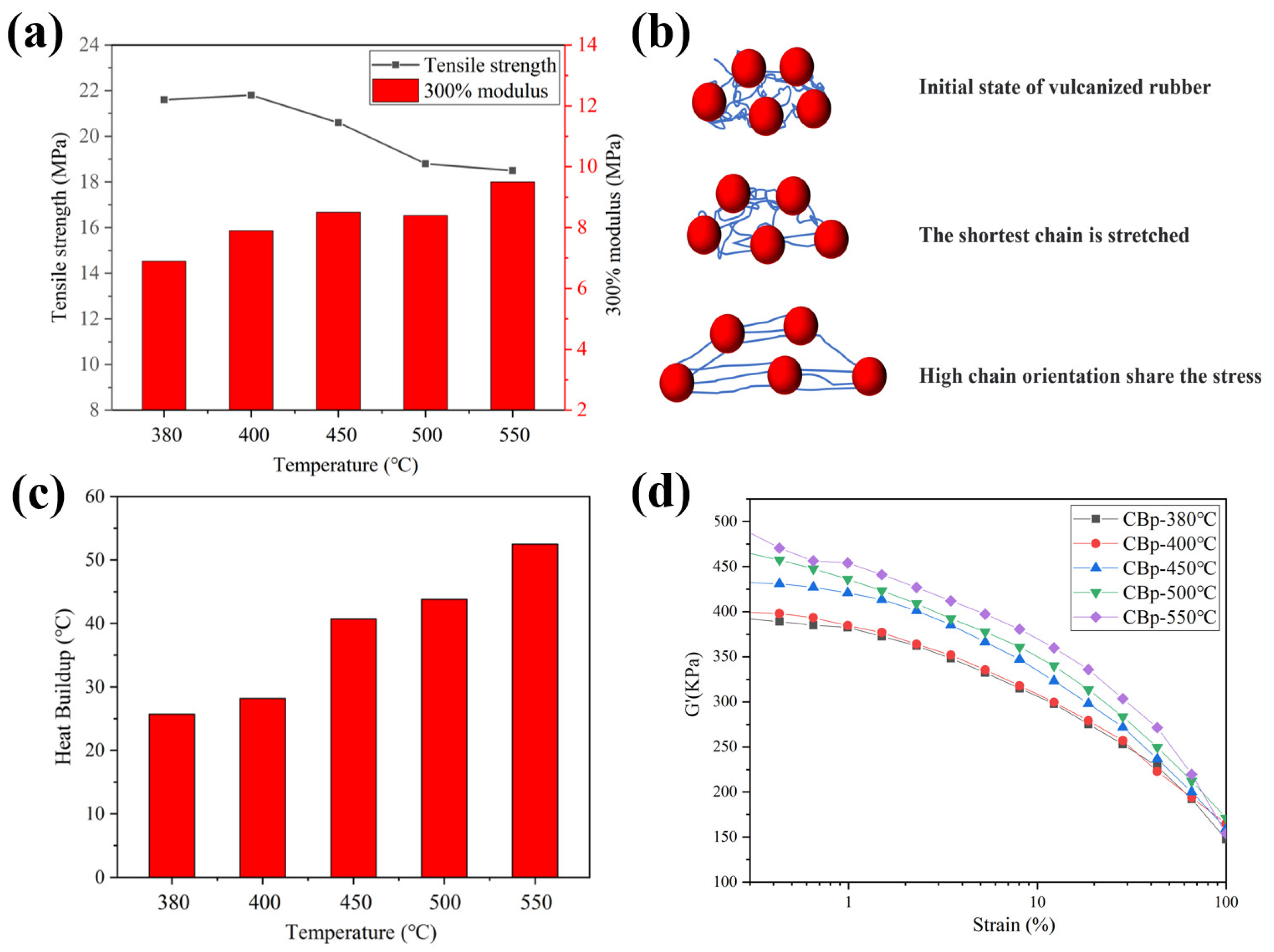
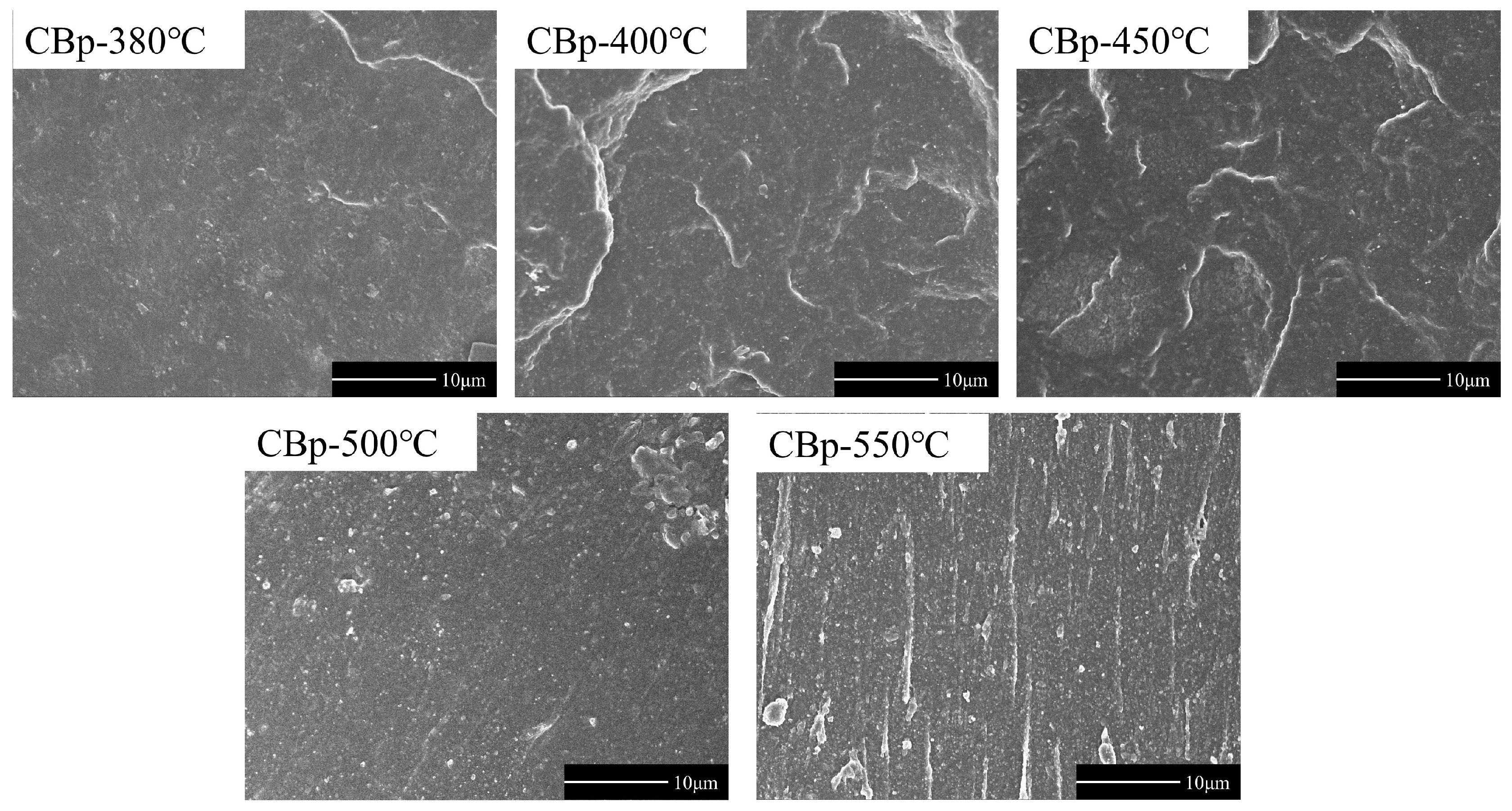
3.3. Performance of NR Filled by CBps at Different Pyrolysis Temperatures
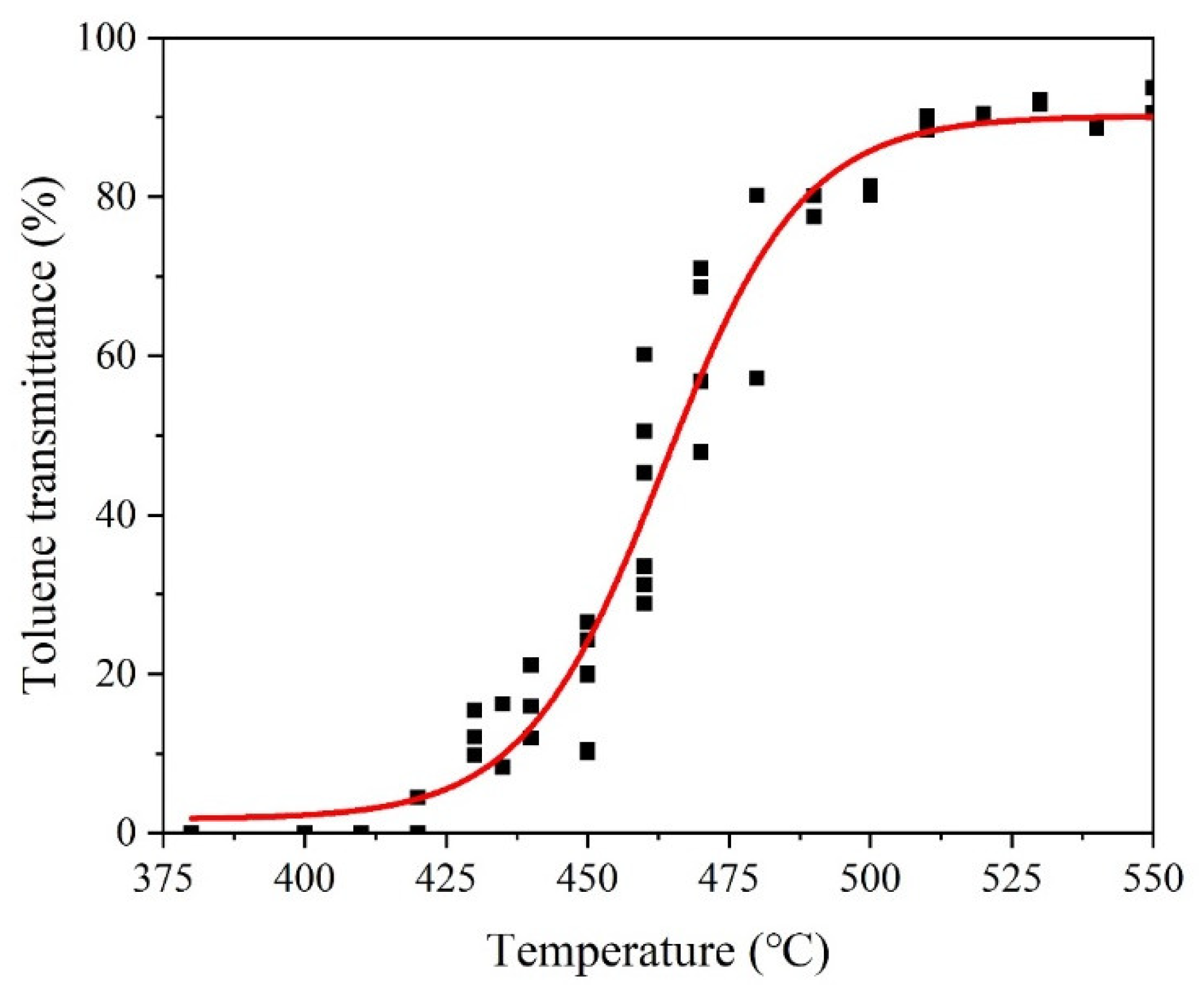
3.4. Granulation Characteristics of CBp
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Thomas, B.S.; Gupta, R.C. A comprehensive review on the applications of waste tire rubber in cement concrete. Renew. Sustain. Energy Rev. 2016, 54, 1323–1333. [Google Scholar] [CrossRef]
- Zabaniotou, A.; Antoniou, N.; Bruton, G. Analysis of good practices, barriers and drivers for ELTs pyrolysis industrial application. Waste Manag. 2014, 34, 2335–2346. [Google Scholar] [CrossRef] [PubMed]
- Idoia, H.; Miriam, A.; Martin, O.; Javier, B.; Jose, A.; Pedro, C. Opportunities and barriers for producing high quality fuels from the pyrolysis of scrap tires. Renew. Sustain. Energy Rev. 2016, 56, 745–759. [Google Scholar]
- Machin, E.B.; Pedroso, D.T.; de Carvalho, J.A. Energetic valorization of waste tires. Renew. Sustain. Energy Rev. 2017, 68, 306–315. [Google Scholar] [CrossRef]
- Oboirien, B.O.; North, B.C. A review of waste tyre gasification. J. Environ. Chem. Eng. 2017, 5, 5169–5178. [Google Scholar] [CrossRef]
- Costa, S.M.; Fowler, D.; Carreira, G.A.; Portugal, I.; Silva, C.M. Production and Upgrading of Recovered Carbon Black from the Pyrolysis of End-of-Life Tires. Materials 2022, 15, 2030. [Google Scholar] [CrossRef] [PubMed]
- Teng, H.; Lin, Y.C.; Hsu, L.Y. Production of activated carbons from pyrolysis of waste tires impregnated with potassium hydroxide. J. Air Waste Manag. Assoc. 2000, 50, 1940–1946. [Google Scholar] [CrossRef]
- Martínez, J.D.; Puy, N.; Murillo, R.; García, T.; Navarro, M.V.; Mastral, A.M. Waste tyre pyrolysis—A review. Renew. Sustain. Energy Rev. 2013, 23, 179–213. [Google Scholar] [CrossRef]
- Yaqoob, H.; Teoh, Y.H.; Sher, F.; Jamil, M.A.; Murtaza, D.; Al Qubeissi, M.; Ul Hassan, M.; Mujtaba, M. Current status and potential of tire pyrolysis oil production as an alternative fuel in developing countries. Sustainability 2021, 13, 3214. [Google Scholar] [CrossRef]
- Ren, Q.; Zhang, J.; Hu, S.; Ma, S.; Huang, R.; Su, S.; Wang, Y.; Jiang, L.; Xu, J.; Xiang, J. Novel photothermal pyrolysis on waste tire to generate high-yield limonene. Fuel 2022, 329, 125482. [Google Scholar] [CrossRef]
- Antoniou, N.; Zabaniotou, A. Features of an efficient and environmentally attractive used tyres pyrolysis with energy and material recovery. Renew. Sustain. Energy Rev. 2013, 20, 539–558. [Google Scholar] [CrossRef]
- Williams, P.T. Pyrolysis of waste tyres: A review. Waste Manag. 2013, 33, 1714–1728. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.T.; Besler, S.; Taylor, D.T. The pyrolysis of scrap automotive tyres: The influence of temperature and heating rate on product composition. Fuel 1990, 69, 1474–1482. [Google Scholar] [CrossRef]
- Cunliffe, A.M.; Williams, P.T. Composition of oils derived from the batch pyrolysis of tyres. J. Anal. Appl. Pyrolysis 1998, 44, 131–152. [Google Scholar] [CrossRef]
- Zabaniotou, A.A.; Stavropoulos, G. Pyrolysis of used automobile tires and residual char utilization. J. Anal. Appl. Pyrolysis 2003, 70, 711–722. [Google Scholar] [CrossRef]
- Roy, C.; Chaala, A.; Darmstadt, H. The vacuum pyrolysis of used tires: End-uses for oil and carbon black products. J. Anal. Appl. Pyrolysis 1999, 51, 201–221. [Google Scholar] [CrossRef]
- López, G.; Olazar, M.; Aguado, R.; Bilbao, J. Continuous pyrolysis of waste tyres in a conical spouted bed reactor. Fuel 2010, 89, 1946–1952. [Google Scholar] [CrossRef]
- Butler, E.; Devlin, G.; Meier, D.; McDonnell, K. A review of recent laboratory research and commercial developments in fast pyrolysis and upgrading. Renew. Sustain. Energy Rev. 2011, 15, 4171–4186. [Google Scholar] [CrossRef]
- Mkhize, N.; Danon, B.; Alvarez, J.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M.; van der Gryp, P.; Görgens, J.F. Influence of reactor and condensation system design on tyre pyrolysis products yields. J. Anal. Appl. Pyrolysis 2019, 143, 104683. [Google Scholar] [CrossRef]
- Ayanoğlu, A.; Yumrutaş, R. Rotary kiln and batch pyrolysis of waste tire to produce gasoline and diesel like fuels. Energy Convers. Manag. 2016, 111, 261–270. [Google Scholar] [CrossRef]
- Donatelli, A.; Garzone, P.; Iovane, P. Discharging granular material from a rotary kiln in a slumping regime: Theoretical and experimental studies. Particuology 2015, 23, 56–61. [Google Scholar] [CrossRef]
- Li, S.Q.; Yao, Q.; Chi, Y.; Yan, J.H.; Cen, K.F. Pilot-scale pyrolysis of scrap tires in a continuous rotary kiln reactor. Ind. Eng. Chem. Res. 2004, 43, 5133–5145. [Google Scholar] [CrossRef]
- Lewandowski, W.M.; Januszewicz, K.; Kosakowski, W. Efficiency and proportions of waste tyre pyrolysis products depending on the reactor type—A review. J. Anal. Appl. Pyrolysis 2019, 140, 25–53. [Google Scholar] [CrossRef]
- Zhang, Y.T.; Ji, G.Z.; Chen, C.S.; Wang, Y.X.; Wang, W.J.; Li, A.M. Liquid oils produced from pyrolysis of plastic wastes with heat carrier in rotary kiln. Fuel Process. Technol. 2020, 206, 106455. [Google Scholar] [CrossRef]
- Xu, J.Q.; Yu, J.X.; He, W.Z.; Huang, J.W.; Xu, J.S.; Li, G.M. Recovery of carbon black from waste tire in continuous commercial rotary kiln pyrolysis reactor. Sci. Total Environ. 2021, 772, 145507. [Google Scholar] [CrossRef]
- Yazdani, E.; Hashemabadi, S.H.; Taghizadeh, A. Study of waste tire pyrolysis in a rotary kiln reactor in a wide range of pyrolysis temperature. Waste Manag. 2019, 85, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, S.; Sathiskumar, C.; Moorthy, R.S. Effect of process parameters on tire pyrolysis: A review. J. Sci. Ind. Res. 2012, 71, 309–315. [Google Scholar]
- Fan, Y.; Fowler, G.; Zhao, M. The past, present and future of carbon black as a rubber reinforcing filler–A review. J. Clean. Prod. 2020, 247, 119115. [Google Scholar] [CrossRef]
- Zhong, R.P.; Xu, J.J.; Hui, D.; Bhosale, S.S.; Hong, R. Morphology of carbon-black aggregates: Fractal versus euclidean geometry. Waste Manag. Res. 2020, 38, 35–43. [Google Scholar] [CrossRef]
- Zhang, X.H.; Wang, T.J.; Ma, L.L.; Chang, J. Vacuum pyrolysis of waste tires with basic additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef]
- Berrueco, C.; Esperanza, E.; Mastral, F.J.; Ceamanos, J.; García-Bacaicoa, P. Pyrolysis of waste tyres in an atmospheric static-bed batch reactor: Analysis of the gases obtained. J. Anal. Appl. Pyrolysis 2005, 74, 245–253. [Google Scholar] [CrossRef]
- Helleur, R.; Popovic, N.; Ikura, M.; Stanciulescu, M.; Liu, D. Characterization and potential applications of pyrolytic char from ablative pyrolysis of used tires. J. Anal. Appl. Pyrolysis 2001, 58, 813–824. [Google Scholar] [CrossRef]
- Herd, C.R.; Mcdonald, G.C.; Hess, W.M. Morphology of carbon-black aggregates: Fractal versus euclidean geometry. Rubber Chem. Technol. 1992, 65, 107–129. [Google Scholar] [CrossRef]
- Gao, N.B.; Wang, F.C.; Quan, C.; Santamaria, L.; Lopez, G.; Williams, P.T. Tire pyrolysis char: Processes, properties, upgrading and applications. Prog. Energy Combust. Sci. 2022, 93, 101022. [Google Scholar] [CrossRef]
- Fernández, A.M.; Barriocanal, C.; Alvarez, R. Pyrolysis of a waste from the grinding of scrap tyres. J. Hazard. Mater. 2012, 203, 236–243. [Google Scholar] [CrossRef]
- Mui, E.L.K.; Cheung, W.H.; McKay, G. Tyre char preparation from waste tyre rubber for dye removal from effluents. J. Hazard. Mater. 2010, 175, 151–158. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Cao, Q.; Jin, L.; Wang, F. Upgrading pyrolytic residue from waste tires to commercial carbon black. Waste Manag. Res. 2018, 36, 436–444. [Google Scholar] [CrossRef]
- Xie, L.J.; Sun, G.H.; Su, F.Y.; Guo, X.Q.; Kong, Q.Q.; Li, X.M.; Huang, X.H.; Wan, L.; Li, K.X.; Lv, C.X. Hierarchical porous carbon microtubes derived from willow catkins for supercapacitor applications. J. Mater. Chem. A 2016, 4, 1637–1646. [Google Scholar] [CrossRef]
- Hossain, M.K.; Strezov, V.; Chan, K.Y.; Ziolkowski, A.; Nelson, P.F. Influence of pyrolysis temperature on production and nutrient properties of wastewater sludge biochar. J. Environ. Manag. 2011, 92, 223–228. [Google Scholar] [CrossRef]
- Kumar, R.; Sharma, A. Morphologically tailored activated carbon derived from waste tires as high-performance anode for Li-ion battery. J. Appl. Electrochem. 2018, 48, 1–13. [Google Scholar]
- Zerda, T.; Xu, W.; Yang, H.; Gerspacher, M. The effects of heating and cooling rates on the structure of carbon black particles. Rubber Chem. Technol. 1998, 71, 26–37. [Google Scholar] [CrossRef]
- Wang, M.J. Effect of Polymer-Filler and Filler-Filler Interactions on Dynamic Properties of Filled Vulcanizates. Rubber Chem. Technol. 1998, 71, 520–589. [Google Scholar] [CrossRef]
- Danmaliki, G.; Saleh, T. Influence of conversion parameters of waste tires to activated carbon on adsorption of dibenzothiophene from model fuels. J. Clean. Prod. 2016, 117, 50–55. [Google Scholar] [CrossRef]
- Rofiqul, I.M.; Haniu, H.; Rafiqul, A.B.M. Limonene-rich liquids from pyrolysis of heavy automotive tire wastes. J. Environ. Eng. 2007, 2, 681–695. [Google Scholar] [CrossRef][Green Version]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]




| Furnace Temperature (°C) | 450~500 | 500~550 | 550~650 | 650~750 | 750~850 |
| Pyrolysis Temperature (°C) | 380 | 400 | 450 | 500 | 550 |
| Material | Weight Ratio (phr) | Weight (g) |
|---|---|---|
| Natural rubber (NR) | 100 | 200 |
| Stearic acid (Sa) | 3 | 6 |
| Zinc oxide (ZnO) | 5 | 10 |
| Accelerator M | 0.6 | 1.2 |
| Sulphur (S) | 2.5 | 5 |
| Pyrolytic carbon black (CBp) | 50 | 100 |
| Carbon Black | DBP Absorption/(mL/100 g) |
|---|---|
| N234 | 124 |
| N330 | 100 |
| N326 | 72 |
| N375 | 114 |
| N660 | 91 |
| Sample | D-Bands | G-Bands | ID/IG | ||
|---|---|---|---|---|---|
| Position | FWHM | Position | FWHM | ||
| CBp-380 °C | 1382.5 | 381.2 | 1591.9 | 88.6 | 1.005 |
| CBp-450 °C | 1367.4 | 247.5 | 1585.1 | 112.6 | 0.851 |
| CBp-550 °C | 1393.7 | 168.4 | 1588.1 | 78.5 | 0.474 |
| Functional Group | Peaks/cm−1 | Vibration |
|---|---|---|
| -OH | 3448 | Stretching vibration [43] |
| -C-H (-CH2, -CH3) | 2885 2992 | Stretching vibration [43] |
| -C=C- (Aromatic) | 1637 | Stretching vibration [44] |
| Si-O-Si | 467 805 1099 | bending vibration [45] stretching vibration [45] stretching vibration [45] |
| Temperature | |||||
|---|---|---|---|---|---|
| Sample | CBp-380 °C | CBp-400 °C | CBp-450 °C | CBp-500 °C | CBp-550 °C |
| ts1 (min) | 1.12 | 1.31 | 1.45 | 1.55 | 1.51 |
| tc90 (min) | 13.45 | 13.61 | 16.37 | 16.99 | 16.93 |
| MH (dN·m) | 13.13 | 13.73 | 13.81 | 14.19 | 14.22 |
| ML (dN·m) | 1.98 | 1.47 | 1.72 | 2.20 | 2.12 |
| ∆M (dN·m) | 11.15 | 12.26 | 12.09 | 11.99 | 12.10 |
| Characteristics | Value | |||||
|---|---|---|---|---|---|---|
| Toluene transmittance (%) | 7.3 | 15.2 | 22.6 | 44.8 | 69.8 | 93.7 |
| Gr (%) | / | 17.8 | 67.2 | 69.5 | 69.1 | 75.8 |
| ω (%) | 100 | 78.4 | 3.22 | 4.73 | 5.61 | 3.40 |
| Particle crushing strength (cN) | / | 32 | 34 | 35.7 | 46.0 | 57.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, H.; Hou, Z.; Shan, L.; Cai, X.; Xin, Z. Influence of Pyrolytic Carbon Black Derived from Waste Tires at Varied Temperatures within an Industrial Continuous Rotating Moving Bed System. Polymers 2023, 15, 3460. https://doi.org/10.3390/polym15163460
Fang H, Hou Z, Shan L, Cai X, Xin Z. Influence of Pyrolytic Carbon Black Derived from Waste Tires at Varied Temperatures within an Industrial Continuous Rotating Moving Bed System. Polymers. 2023; 15(16):3460. https://doi.org/10.3390/polym15163460
Chicago/Turabian StyleFang, Haibin, Zhanfeng Hou, Lingdi Shan, Xiaohui Cai, and Zhenxiang Xin. 2023. "Influence of Pyrolytic Carbon Black Derived from Waste Tires at Varied Temperatures within an Industrial Continuous Rotating Moving Bed System" Polymers 15, no. 16: 3460. https://doi.org/10.3390/polym15163460
APA StyleFang, H., Hou, Z., Shan, L., Cai, X., & Xin, Z. (2023). Influence of Pyrolytic Carbon Black Derived from Waste Tires at Varied Temperatures within an Industrial Continuous Rotating Moving Bed System. Polymers, 15(16), 3460. https://doi.org/10.3390/polym15163460