Potential Application of Yeast Cell Wall Biopolymers as Probiotic Encapsulants
Abstract
:1. Introduction
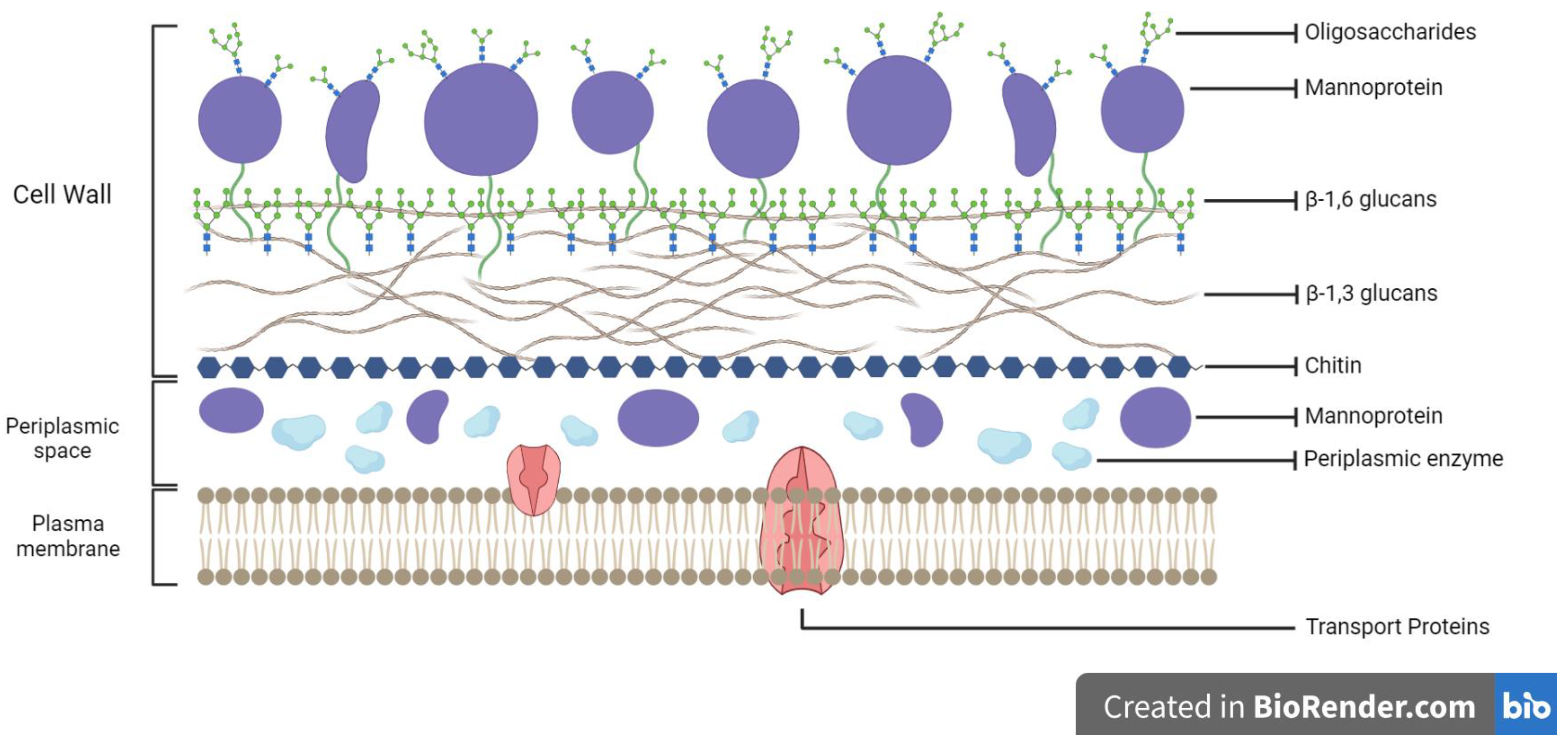
2. Yeast Cell Walls: Structures and Compositions
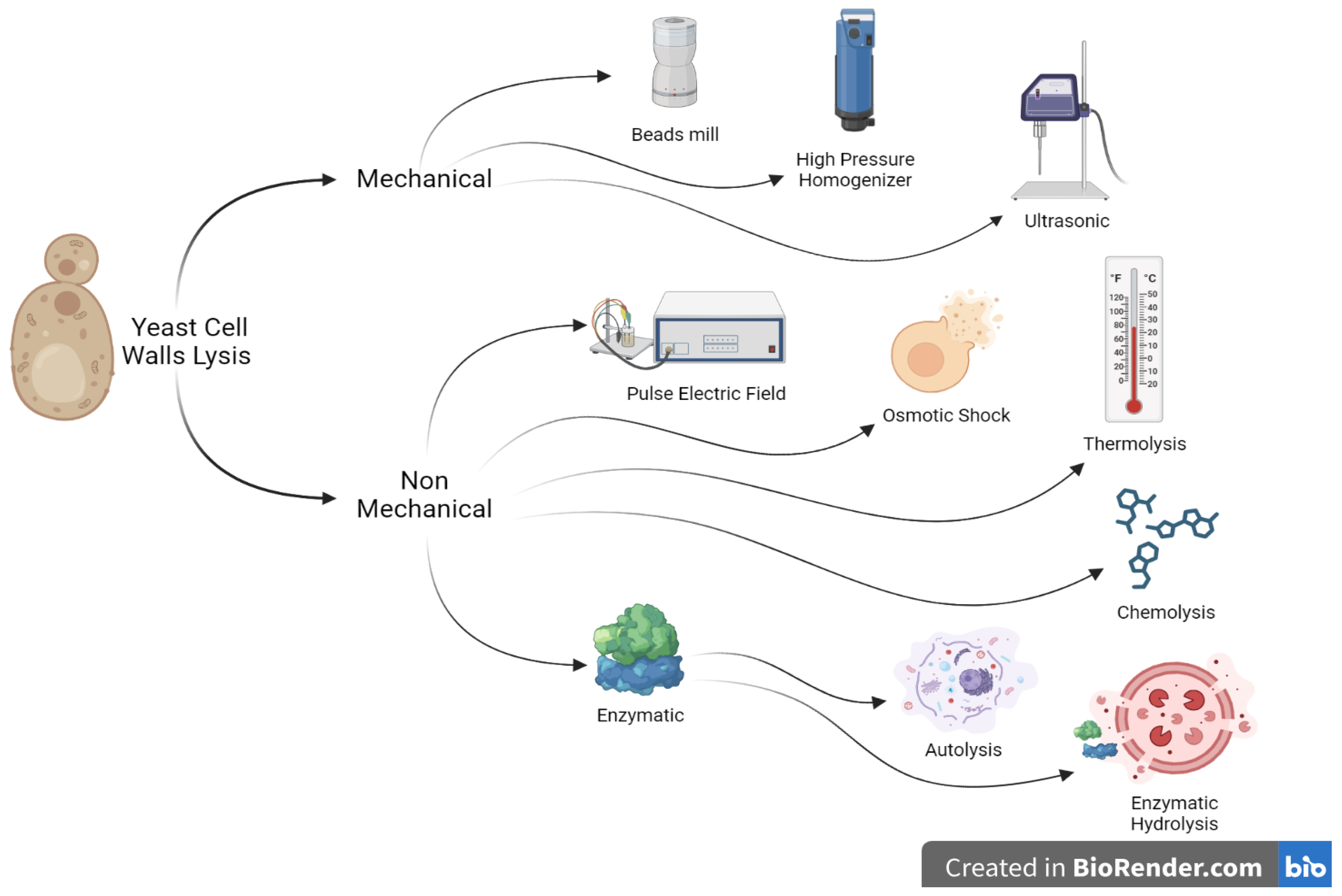
3. Lysis and Extraction of Yeast Cell Walls
3.1. β-Glucan Extraction
3.2. Mannoprotein Extraction
3.3. Chitin Extraction
4. Separation and Purification of Yeast Cell Walls
5. Compatibility of Encapsulant Biopolymers from Yeast Cell Walls
| Characteristics | Description | References |
|---|---|---|
| Psychochemical properties | ||
| Mechanical strength |
| [72] |
| [73] | |
| Thermal stability |
| [6] |
| [74] | |
| Cryoprotectant |
| [14] |
| Protection |
| [75,76] |
| Toxicology | ||
| Non-toxic |
| [2] |
| Generally recognized as safe |
| [6,11] |
| Functional properties | ||
| Anti-pathogenic |
| [77] |
| Adhesion |
| [26] |
| [79] | |
| Functionality |
| [78] |
5.1. β-Glucan as a Probiotic Encapsulant
5.2. Mannoprotein as a Probiotic Encapsulant
5.3. Chitin as a Probiotic Encapsulant
6. Role of Yeast Cell Wall Biopolymers as Encapsulants in Protecting Probiotics
| Probiotics | Formula | Results | References |
|---|---|---|---|
| L. acidophilus and B. bifidum | Probiotic bacteria were encapsulated with calcium alginate using the emulsion method; then, the microbeads were covered by the S. cerevisiae cell wall and then re-encapsulated with the final layer of calcium alginate. | S. cerevisiae cell wall compounds provide a protective barrier for delivering viable bacterial cells to the colon. They improve acid tolerance for L. acidophilus but not for B. bifidum, making their protective ability dependent on microbe type. | [10] |
| L. acidophilus LA-05, L. plantarum 49, and L. plantarum 201 | β-glucan from yeasts mixed with cell suspensions were kept for 1 h at room temperature. The suspensions were divided into 1 mL aliquots, transferred aseptically into 5 mL containers, and frozen at 20 °C for 24 h. The samples were freeze-dried in a benchtop lyophiliser for 40 h at 55 2 °C and 1 mm/h. After freeze-drying, the containers were sealed and refrigerated for 120 days at 0.5 °C. | β-glucan is a potential cryoprotectant for probiotic lactobacilli, providing similar protection to fructooligosaccharides after freeze-drying, storage, and exposure to simulated gastrointestinal conditions. It offers potential applications as a functional food ingredient and can be obtained from by-products of the beer industry, which reduces environmental impacts. | [14] |
| L. acidophilus | L. acidophilus were dissolved in saline and mixed with yeast cell walls, agitated on an orbital shaker, and optimized for encapsulation. Filtration was improved with vacuum-filtered glass funnels and filter holders; then, the filtrate was centrifuged at 5000 rpm for 15 min. | The viability of the encapsulated cells was 19.048 ± 2.701%, while the majority of free cells could not survive 150 min of treatment with SGJ at pH 2. Encapsulated L. acidophilus were enhanced, with greater survival at 56.338 5.094%. | [113] |
| S. boulardii | Briefly, 1 g of chitosan in 100 mL of distilled water is acidified with 0.4 mL glacial acetic acid to 3.6. Chitosan solution was autoclaved (121 °C for 15 min) before use. A magnetic bar swirled alginate particles in chitosan solution for 30 min. Probiotic cells were suspended, filtered, and rinsed with distilled water. | Low-cost external ionic gelation and drying at 40 °C maintain S. boulardii survival, with chitosan coating providing increased resistance to yeasts and protection against simulated gastric and intestinal fluids. | [101] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V. Technology and potential applications of probiotic encapsulation in fermented milk products. J. Food Sci. Technol. 2015, 52, 4679–4696. [Google Scholar] [CrossRef] [PubMed]
- Huq, T.; Khan, A.; Khan, R.A.; Riedl, B.; Lacroix, M. Encapsulation of Probiotic Bacteria in Biopolymeric System. Crit. Rev. Food Sci. Nutr. 2013, 53, 909–916. [Google Scholar] [CrossRef]
- Shori, A.B. Microencapsulation Improved Probiotics Survival During Gastric Transit. HAYATI J. Biosci. 2017, 24, 1–5. [Google Scholar] [CrossRef]
- Chavarri, M.; Maranon, I.; Carmen, M. Encapsulation Technology to Protect Probiotic Bacteria. In Probiotics; Rigobelo, E., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0776-7. [Google Scholar]
- Pech-Canul, A.D.L.C.; Ortega, D.; García-Triana, A.; González-Silva, N.; Solis-Oviedo, R.L. A Brief Review of Edible Coating Materials for the Microencapsulation of Probiotics. Coatings 2020, 10, 197. [Google Scholar] [CrossRef]
- Paramera, E.I.; Karathanos, V.T.; Konteles, S.J. Yeast Cells and Yeast-Based Materials for Microencapsulation. In Microencapsulation in the Food Industry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 267–281. ISBN 978-0-12-404568-2. [Google Scholar]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Shi, G.; Rao, L.; Xie, Q.; Li, J.; Li, B.; Xiong, X. Characterization of yeast cells as a microencapsulation wall material by Fourier-transform infrared spectroscopy. Vib. Spectrosc. 2010, 53, 289–295. [Google Scholar] [CrossRef]
- Pham-hoang, B.N.; Voilley, A.; Waché, Y. Molecule structural factors influencing the loading of flavoring compounds in a natural-preformed capsule: Yeast cells. Colloids Surf. B Biointerfaces 2016, 148, 220–228. [Google Scholar] [CrossRef]
- Mokhtari, S.; Jafari, S.M.; Khomeiri, M.; Maghsoudlou, Y.; Ghorbani, M. The cell wall compound of Saccharomyces cerevisiae as a novel wall material for encapsulation of probiotics. Food Res. Int. 2017, 96, 19–26. [Google Scholar] [CrossRef]
- Mokhtari, S.; Khomeiri, M.; Jafari, S.M.; Maghsoudlou, Y.; Ghorbani, M. Descriptive analysis of bacterial profile, physicochemical and sensory characteristics of grape juice containing Saccharomyces cerevisiae cell wall-coated probiotic microcapsules during storage. Int. J. Food Sci. Technol. 2017, 52, 1042–1048. [Google Scholar] [CrossRef]
- Nakhaee Moghadam, M.; Khameneh, B.; Fazly Bazzaz, B.S. Saccharomyces cervisiae as an Efficient Carrier for Delivery of Bioactives: A Review. Food Biophys. 2019, 14, 346–353. [Google Scholar] [CrossRef]
- Paramera, E.I.; Konteles, S.J.; Karathanos, V.T. Stability and release properties of curcumin encapsulated in Saccharomyces cerevisiae, β-cyclodextrin and modified starch. Food Chem. 2011, 125, 913–922. [Google Scholar] [CrossRef]
- Da Silva Guedes, J.; Pimentel, T.C.; Diniz-Silva, H.T.; Tayse Da Cruz Almeida, E.; Tavares, J.F.; Leite De Souza, E.; Garcia, E.F.; Magnani, M. Protective effects of β-glucan extracted from spent brewer yeast during freeze-drying, storage and exposure to simulated gastrointestinal conditions of probiotic lactobacilli. LWT 2019, 116, 108496. [Google Scholar] [CrossRef]
- Vélez-Erazo, E.M.; Saturno, R.P.; Marson, G.V.; Hubinger, M.D. Spent brewer’s yeast proteins and cell debris as innovative emulsifiers and carrier materials for edible oil microencapsulation. Food Res. Int. 2021, 140, 109853. [Google Scholar] [CrossRef]
- Sultana, A.; Tanaka, Y.; Fushimi, Y.; Yoshii, H. Stability and release behavior of encapsulated flavor from spray-dried Saccharomyces cerevisiae and maltodextrin powder. Food Res. Int. 2018, 106, 809–816. [Google Scholar] [CrossRef]
- Ganan, M.; Carrascosa, A.V.; De Pascual-Teresa, S.; Martinez-Rodriguez, A.J. Effect of Mannoproteins on the Growth, Gastrointestinal Viability, and Adherence to Caco-2 Cells of Lactic Acid Bacteria. J. Food Sci. 2012, 77, M176–M180. [Google Scholar] [CrossRef] [PubMed]
- Aravamudhan, A.; Ramos, D.M.; Nada, A.A.; Kumbar, S.G. Natural Polymers. In Natural and Synthetic Biomedical Polymers; Elsevier: San Diego, CA, USA, 2014; pp. 67–89. ISBN 978-0-12-396983-5. [Google Scholar]
- Călinoiu, L.-F.; Ştefănescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan Coating Applications in Probiotic Microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef]
- Lipke, P.N.; Ovalle, R. Cell Wall Architecture in Yeast: New Structure and New Challenges. J. Bacteriol. 1998, 180, 3735–3740. [Google Scholar] [CrossRef]
- McLellan, W.L.; McDaniel, L.E.; Lampen, J.O. Purification of Phosphomannanase and Its Action on the Yeast Cell Wall. J. Bacteriol. 1970, 102, 261–270. [Google Scholar] [CrossRef]
- Cabib, E.; Roh, D.-H.; Schmidt, M.; Crotti, L.B.; Varma, A. The Yeast Cell Wall and Septum as Paradigms of Cell Growth and Morphogenesis. J. Biol. Chem. 2001, 276, 19679–19682. [Google Scholar] [CrossRef]
- Liu, H.-Z.; Liu, L.; Hui, H.; Wang, Q. Structural Characterization and Antineoplastic Activity of Saccharomyces cerevisiae Mannoprotein. Int. J. Food Prop. 2015, 18, 359–371. [Google Scholar] [CrossRef]
- Kollár, R.; Petráková, E.; Ashwell, G.; Robbins, P.W.; Cabib, E. Architecture of the Yeast Cell Wall. J. Biol. Chem. 1995, 270, 1170–1178. [Google Scholar] [CrossRef]
- Zlotnik, H.; Fernandez, M.P.; Bowers, B.; Cabib, E. Saccharomyces cerevisiae mannoproteins form an external cell wall layer that determines wall porosity. J. Bacteriol. 1984, 159, 1018–1026. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.D.; Chaffin, W.L. Oral Colonization By Candida Albicans. Crit. Rev. Oral Biol. Med. 1999, 10, 359–383. [Google Scholar] [CrossRef]
- Bowman, S.M.; Free, S.J. The structure and synthesis of the fungal cell wall. Bioessays 2006, 28, 799–808. [Google Scholar] [CrossRef]
- Ciamponi, F.; Duckham, C.; Tirelli, N. Yeast cells as microcapsules. Analytical tools and process variables in the encapsulation of hydrophobes in S. cerevisiae. Appl. Microbiol. Biotechnol. 2012, 95, 1445–1456. [Google Scholar] [CrossRef]
- De Nobel, J.G.; Dijkers, C.; Hooijberg, E.; Klis, F.M. Increased Cell Wall Porosity in Saccharomyces cerevisiae after Treatment with Dithiothreitol or EDTA. Microbiology 1989, 135, 2077–2084. [Google Scholar] [CrossRef]
- Gow, N.A.R.; Latge, J.-P.; Munro, C.A. The Fungal Cell Wall: Structure, Biosynthesis, and Function. In The Fungal Kingdom; Heitman, J., Howlett, B.J., Crous, P.W., Stukenbrock, E.H., James, T.Y., Gow, N.A.R., Eds.; ASM Press: Washington, DC, USA, 2017; pp. 267–292. ISBN 978-1-68367-082-7. [Google Scholar]
- De Groot, P.W.J.; Ruiz, C.; Vázquez De Aldana, C.R.; Duenas, E.; Cid, V.J.; Del Rey, F.; Rodríquez-Peña, J.M.; Pérez, P.; Andel, A.; Caubín, J.; et al. A Genomic Approach for the Identification and Classification of Genes Involved in Cell Wall Formation and Its Regulation in Saccharomyces cerevisiae. Comp. Funct. Genom. 2001, 2, 124–142. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Fleet, G.H.; Rogers, P.L. Composition of the cell walls of several yeast species. Appl. Microbiol. Biotechnol. 1998, 50, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Javmen, A.; Grigiškis, S.; Gliebutė, R. β-glucan extraction from Saccharomyces cerevisiae yeast using Actinomyces rutgersensis 88 yeast lyzing enzymatic complex. Biologija 2012, 58, 51–59. [Google Scholar] [CrossRef]
- Shokri, H.; Asadi, F.; Khosravi, A.R. Isolation of β -glucan from the cell wall of Saccharomyces cerevisiae. Nat. Prod. Res. 2008, 22, 414–421. [Google Scholar] [CrossRef]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast cell disruption strategies for recovery of intracellular bio-active compounds—A review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Posch, A. Proteomic Profiling: Methods and Protocols; Methods in Molecular Biology; Posch, A., Ed.; Springer: New York, NY, USA, 2015; Volume 1295, ISBN 978-1-4939-2549-0. [Google Scholar]
- Kleinig, A.R.; Middelberg, A.P.J. On the mechanism of microbial cell disruption in high-pressure homogenisation. Chem. Eng. Sci. 1998, 53, 891–898. [Google Scholar] [CrossRef]
- Rincz, L.A. Application of the ultrasound hyperthermia model for a multi-layered tissue system. J. Phys. Conf. Ser. 2004, 1, 224–229. [Google Scholar] [CrossRef]
- Saulis, G. Electroporation of Cell Membranes: The Fundamental Effects of Pulsed Electric Fields in Food Processing. Food Eng. Rev. 2010, 2, 52–73. [Google Scholar] [CrossRef]
- Espinasse, V.; Perrier-Cornet, J.-M.; Marecat, A.; Gervais, P. High gas pressure effects on yeast. Biotechnol. Bioeng. 2008, 101, 729–738. [Google Scholar] [CrossRef]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): A comparative study. World J. Microbiol. Biotechnol. 2020, 36, 68. [Google Scholar] [CrossRef]
- Wang, J.; Li, M.; Zheng, F.; Niu, C.; Liu, C.; Li, Q.; Sun, J. Cell wall polysaccharides: Before and after autolysis of brewer’s yeast. World J. Microbiol. Biotechnol. 2018, 34, 137. [Google Scholar] [CrossRef]
- Loaces, I.; Bottini, G.; Moyna, G.; Fabiano, E.; Martínez, A.; Noya, F. EndoG: A novel multifunctional halotolerant glucanase and xylanase isolated from cow rumen. J. Mol. Catal. B: Enzym. 2016, 126, 1–9. [Google Scholar] [CrossRef]
- Xu, W.; Wang, J.; Li, Q. Microarray studies on lager brewer’s yeasts reveal cell status in the process of autolysis. FEMS Yeast Res. 2014, 14, 714–728. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Rodríguez, A.J.; Polo, M.C.; Carrascosa, A.V. Structural and ultrastructural changes in yeast cells during autolysis in a model wine system and in sparkling wines. Int. J. Food Microbiol. 2001, 71, 45–51. [Google Scholar] [CrossRef]
- Teimouri, I.; Naderi-Ahranjani, R.; Babaei, K.; Tadayyon, S.; Taheri, E.; Diddar, N.; Vaghari, H.; Jafarizadeh-Malmiri, H. Yeast Extracts: Production, Properties and Application. In Proceedings of the 6th National Congress on Strategic Research in Chemical and Chemical Engineering with Emphasis on Iranian Native Technologies, Tehran, Iran, 20 January 2020; Indian Institute of Technology, Iran: Tehran, Iran, 2020. [Google Scholar]
- Huang, G.L. Extraction of Two Active Polysaccharides from the Yeast Cell Wall. Zeitschrift für Naturforschung C 2008, 63, 919–921. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S.; Kawarska, A.; Stasiak-Różańska, L.; Gientka, I.; Majewska, E. Evaluation of the Efficiency of Different Disruption Methods on Yeast Cell Wall Preparation for β-Glucan Isolation. Molecules 2014, 19, 20941–20961. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Cui, S.; Liu, H. A new isolation method of β-d-glucans from spent yeast Saccharomyces cerevisiae. Food Hydrocoll. 2008, 22, 239–247. [Google Scholar] [CrossRef]
- Chang, H.H.; Hoon, C.; Yun, C.W.; Paik, H.D.; Kim, S.W.; Kang, C.W.; Hwang, H.J.; Chang, H.I. Preparation and Analysis of Yeast Cell Wall Mannoproteins, Immune Enhancing Materials, from Cell Wall Mutant Saccharomyces cerevisiae. J. Microbiol. Biotechnol. 2006, 16, 247–255. [Google Scholar]
- Hajhosseini, A.; Sharifan, A.; Eftekhari, Z.; Alavi, A.; Doroud, D. Optimal Extraction and Deproteinization Method for Mannoprotein Purification from Kluyveromyces marxianus. Iran. Biomed. J. 2023, 27, 6. [Google Scholar]
- Silva Araújo, V.B.D.; Melo, A.N.F.D.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; Souza, E.L.D.; Magnani, M. Followed extraction of β-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- Ferreira, C.; Silva, S.; van Voorst, F.; Aguiar, C.; Kielland-Brandt, M.C.; Brandt, A.; Lucas, C. Absence of Gup1p in Saccharomyces cerevisiae results in defective cell wall composition, assembly, stability and morphology. FEMS Yeast Res. 2006, 6, 1027–1038. [Google Scholar] [CrossRef] [PubMed]
- Utama, G.L.; Dio, C.; Sulistiyo, J.; Yee Chye, F.; Lembong, E.; Cahyana, Y.; Kumar Verma, D.; Thakur, M.; Patel, A.R.; Singh, S. Evaluating comparative β-glucan production aptitude of Saccharomyces cerevisiae, Aspergillus oryzae, Xanthomonas campestris, and Bacillus natto. Saudi J. Biol. Sci. 2021, 28, 6765–6773. [Google Scholar] [CrossRef]
- Varelas, V.; Tataridis, P.; Liouni, M.; Nerantzis, E.T. Valorization of Winery Spent Yeast Waste Biomass as a New Source for the Production of β-Glucan. Waste Biomass Valor. 2016, 7, 807–817. [Google Scholar] [CrossRef]
- Varelas, V.; Tataridis, P.; Liouni, M.; Nerantzis, E.T. Application of different methods for the extraction of yeast β-glucan. E-J. Sci. Technol. 2016, 11, 75–89. [Google Scholar]
- Larson, J.W. Handbook of Brewing, 3rd ed.; Stewart, G.G., Russell, I., Anstruther, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; ISBN 978-1-351-22833-6. [Google Scholar]
- Liu, Y.; Huang, G. The derivatization and antioxidant activities of yeast mannan. Int. J. Biol. Macromol. 2018, 107, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Karboune, S. A comparative study for the isolation and characterization of mannoproteins from Saccharomyces cerevisiae yeast cell wall. Int. J. Biol. Macromol. 2018, 119, 654–661. [Google Scholar] [CrossRef]
- Orlean, P. Architecture and Biosynthesis of the Saccharomyces cerevisiae Cell Wall. Genetics 2012, 192, 775–818. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Karboune, S.; Sedman, J.; Ismail, A. Characterization of the structural properties of mannoproteins isolated from selected yeast-based products upon the enzymatic treatment. LWT 2020, 131, 109596. [Google Scholar] [CrossRef]
- Squeglia, F.; Berisio, R.; Shibuya, N.; Kaku, H. Defense Against Pathogens: Structural Insights into the Mechanism of Chitin Induced Activation of Innate Immunity. CMC 2017, 24, 3980–3986. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, L.; Soetemans, L.; D’Hondt, E.; Elst, K. Sources of Chitin and Chitosan and their Isolation. In Chitin and Chitosan; Broek, L.A.M., Boeriu, C.G., Eds.; Wiley: West Sussex, UK, 2019; pp. 1–34. ISBN 978-1-119-45043-6. [Google Scholar]
- Sun, C.; Fu, D.; Jin, L.; Chen, M.; Zheng, X.; Yu, T. Chitin isolated from yeast cell wall induces the resistance of tomato fruit to Botrytis cinerea. Carbohydr. Polym. 2018, 199, 341–352. [Google Scholar] [CrossRef]
- Lee, J.-N.; Lee, D.-Y.; Ji, I.-H.; Kim, G.-E.; Kim, H.N.; Sohn, J.; Kim, S.; Kim, C.-W. Purification of Soluble β-Glucan with Immune-enhancing Activity from the Cell Wall of Yeast. Biosci. Biotechnol. Biochem. 2001, 65, 837–841. [Google Scholar] [CrossRef]
- Farahnejad, Z.; Rasaee, M.J.; Yadegari, H.; Moghadam, M.F. Purification And Characterization Of Cell Wall Mannoproteins Of Candidaalbicans Using Intact Cell Method. Med. J. Islam. Repub. Iran 2004, 18, 167–172. [Google Scholar]
- Aguilar-Uscanga, B.; Francois, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- Li, J. Mannoproteins and Beta Glucan from Saccharomyces Cerevisiae Yeast Based Products: Isolation and Characterization of Their Properties. Ph.D. Thesis, McGill University, Montréal, QC, Canada, 2018. [Google Scholar]
- Li, J.; Karboune, S. Characterization of the composition and the techno-functional properties of mannoproteins from Saccharomyces cerevisiae yeast cell walls. Food Chem. 2019, 297, 124867. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Li, X.-Y.; Liu, B.-J.; Meng, X.-H. Microencapsulation of Lactobacillus bulgaricus and survival assays under simulated gastrointestinal conditions. J. Funct. Foods 2017, 29, 248–255. [Google Scholar] [CrossRef]
- Jannah, S.R.; Rahayu, E.S.; Yanti, R.; Suroto, D.A.; Wikandari, R. Study of Viability, Storage Stability, and Shelf Life of Probiotic Instant Coffee Lactiplantibacillus plantarum Subsp. plantarum Dad-13 in Vacuum and Nonvacuum Packaging at Different Storage Temperatures. Int. J. Food Sci. 2022, 2022, 1663772. [Google Scholar] [CrossRef]
- Gani, A.; Shah, A.; Ahmad, M.; Ashwar, B.A.; Masoodi, F.A. β-d-glucan as an enteric delivery vehicle for probiotics. Int. J. Biol. Macromol. 2018, 106, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.S.; Cha, D.S.; Park, H.J. Survival of Freeze-Dried Lactobacillus bulgaricus KFRI 673 in Chitosan-Coated Calcium Alginate Microparticles. J. Agric. Food Chem. 2004, 52, 7300–7305. [Google Scholar] [CrossRef]
- Shah, A.; Gani, A.; Ahmad, M.; Ashwar, B.A.; Masoodi, F.A. β-Glucan as an encapsulating agent: Effect on probiotic survival in simulated gastrointestinal tract. Int. J. Biol. Macromol. 2016, 82, 217–222. [Google Scholar] [CrossRef]
- Magnani, M.; Calliari, C.M.; De Macedo, F.C.; Mori, M.P.; De Syllos Cólus, I.M.; Castro-Gomez, R.J.H. Optimized methodology for extraction of (1 → 3)(1 → 6)-β-d-glucan from Saccharomyces cerevisiae and in vitro evaluation of the cytotoxicity and genotoxicity of the corresponding carboxymethyl derivative. Carbohydr. Polym. 2009, 78, 658–665. [Google Scholar] [CrossRef]
- Romano, N.; Schebor, C.; Mobili, P.; Gómez-Zavaglia, A. Role of mono- and oligosaccharides from FOS as stabilizing agents during freeze-drying and storage of Lactobacillus delbrueckii subsp. bulgaricus. Food Res. Int. 2016, 90, 251–258. [Google Scholar] [CrossRef]
- Santin, E.; Maiorka, A.; Macari, M.; Grecco, M.; Sanchez, J.C.; Okada, T.M.; Myasaka, A.M. Performance and Intestinal Mucosa Development of Broiler Chickens Fed Diets Containing Saccharomyces cerevisiae Cell Wall. J. Appl. Poult. Res. 2001, 10, 236–244. [Google Scholar] [CrossRef]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial applications of crustacean by-products (chitin, chitosan, and chitooligosaccharides): A review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Ganan, M.; Carrascosa, A.V.; de Pascual-Teresa, S.; Martinez-Rodriguez, A.J. Inhibition by yeast-derived mannoproteins of adherence to and invasion of Caco-2 cells by Campylobacter jejuni. J. Food Prot. 2009, 72, 55–59. [Google Scholar] [CrossRef]
- Rodrigues, F.J.; Cedran, M.F.; Bicas, J.L.; Sato, H.H. Encapsulated probiotic cells: Relevant techniques, natural sources as encapsulating materials and food applications—A narrative review. Food Res. Int. 2020, 137, 109682. [Google Scholar] [CrossRef] [PubMed]
- Zuidam, N.J.; Shimoni, E. Overview of Microencapsulates for Use in Food Products or Processes and Methods to Make Them. In Encapsulation Technologies for Active Food Ingredients and Food Processing; Zuidam, N.J., Nedovic, V., Eds.; Springer: New York, NY, USA, 2010; pp. 3–29. ISBN 978-1-4419-1008-0. [Google Scholar]
- Dadkhodazade, E.; Mohammadi, A.; Shojaee-Aliabadi, S.; Mortazavian, A.M.; Mirmoghtadaie, L.; Hosseini, S.M. Yeast Cell Microcapsules as a Novel Carrier for Cholecalciferol Encapsulation: Development, Characterization and Release Properties. Food Biophys. 2018, 13, 404–411. [Google Scholar] [CrossRef]
- Wu, J.; Guan, Y.; Zhong, Q. Yeast mannoproteins improve thermal stability of anthocyanins at pH 7.0. Food Chem. 2015, 172, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Numata, M.; Shinkai, S. ‘Supramolecular wrapping chemistry’ by helix-forming polysaccharides: A powerful strategy for generating diverse polymeric nano-architectures. Chem. Commun. 2011, 47, 1961. [Google Scholar] [CrossRef]
- Utama, G.L.; Kurniawan, M.O.; Cahyana, Y.; Balia, R.L. Chapter 18—β-Glucan production through bioconversion of sugarcane bagasse by Saccharomyces cerevisiae and Aspergillus niger. In Innovations in Fermentation and Phytopharmaceutical Technologies; Thatoi, H., Mohapatra, S., Das, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 397–416. ISBN 978-0-12-821877-8. [Google Scholar]
- Gani, A.; Masoodi, F.A.; Shah, U.; Asima, S. Food Hydrocolloids as Encapsulating Agents in Delivery Systems; CRC Press: Boca Raton, FL, USA, 2019; ISBN 978-0-429-89416-9. [Google Scholar]
- Veverka, M.; Dubaj, T.; Gallovič, J.; Jorík, V.; Veverková, E.; Mičušík, M.; Šimon, P. Beta-glucan complexes with selected nutraceuticals: Synthesis, characterization, and stability. J. Funct. Foods 2014, 8, 309–318. [Google Scholar] [CrossRef]
- Normand, V.; Dardelle, G.; Bouquerand, P.-E.; Nicolas, L.; Johnston, D.J. Flavor Encapsulation in Yeasts: Limonene Used as a Model System for Characterization of the Release Mechanism. J. Agric. Food Chem. 2005, 53, 7532–7543. [Google Scholar] [CrossRef]
- Stack, H.M.; Kearney, N.; Stanton, C.; Fitzgerald, G.F.; Ross, R.P. Association of Beta-Glucan Endogenous Production with Increased Stress Tolerance of Intestinal Lactobacilli. Appl. Environ. Microbiol. 2010, 76, 500–507. [Google Scholar] [CrossRef]
- Lee, I.-C.; Caggianiello, G.; Van Swam, I.I.; Taverne, N.; Meijerink, M.; Bron, P.A.; Spano, G.; Kleerebezem, M. Strain-Specific Features of Extracellular Polysaccharides and Their Impact on Lactobacillus plantarum-Host Interactions. Appl. Environ. Microbiol. 2016, 82, 3959–3970. [Google Scholar] [CrossRef] [PubMed]
- Chaffin, W.L.; López-Ribot, J.L.; Casanova, M.; Gozalbo, D.; Martínez, J.P. Cell Wall and Secreted Proteins of Candida albicans: Identification, Function, and Expression. Microbiol. Mol. Biol. Rev. 1998, 62, 130–180. [Google Scholar] [CrossRef]
- Ibe, C.; Munro, C.A. Fungal Cell Wall Proteins and Signaling Pathways Form a Cytoprotective Network to Combat Stresses. J. Fungi 2021, 7, 739. [Google Scholar] [CrossRef]
- Sun, J.; Le, G.-W.; Shi, Y.-H.; Su, G.-W. Factors involved in binding of Lactobacillus plantarum Lp6 to rat small intestinal mucus. Lett. Appl. Microbiol. 2007, 44, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhou, T.-T.; Le, G.-W.; Shi, Y.-H. Association of Lactobacillus acidophilus with mice Peyer’s patches. Nutrition 2010, 26, 1008–1013. [Google Scholar] [CrossRef]
- Parhi, R. Drug delivery applications of chitin and chitosan: A review. Environ. Chem. Lett. 2020, 18, 577–594. [Google Scholar] [CrossRef]
- Muhamad, I.A.; Selvakumaran, S.; Lazim, N.A.M. Designing Polymeric Nanoparticles for Targeted Drug Delivery System. In Nanomedicine; One Central Press (OCP): Manchester, UK, 2014; pp. 287–313. [Google Scholar]
- Estevinho, B.N.; Rocha, F.; Santos, L.; Alves, A. Microencapsulation with chitosan by spray drying for industry applications—A review. Trends Food Sci. Technol. 2013, 31, 138–155. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Groboillot, A.F.; Champagne, C.P.; Darling, G.D.; Poncelet, D.; Neufeld, R.J. Membrane formation by interfacial cross-linking of chitosan for microencapsulation of Lactococcus lactis. Biotechnol. Bioeng. 1993, 42, 1157–1163. [Google Scholar] [CrossRef]
- Ansari, F.; Pourjafar, H.; Jodat, V.; Sahebi, J.; Ataei, A. Effect of Eudragit S100 nanoparticles and alginate chitosan encapsulation on the viability of Lactobacillus acidophilus and Lactobacillus rhamnosus. AMB Expr. 2017, 7, 144. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.A.S.; Machado, M.T.C. Coated alginate–chitosan particles to improve the stability of probiotic yeast. Int. J. Food Sci. Technol. 2021, 56, 2122–2131. [Google Scholar] [CrossRef]
- Suvarna, S.; Dsouza, J.; Ragavan, M.L.; Das, N. Potential probiotic characterization and effect of encapsulation of probiotic yeast strains on survival in simulated gastrointestinal tract condition. Food Sci. Biotechnol. 2018, 27, 745–753. [Google Scholar] [CrossRef]
- Khosravi Zanjani, M.A.; Ghiassi Tarzi, B.; Sharifan, A.; Mohammadi, N. Microencapsulation of Probiotics by Calcium Alginate-gelatinized Starch with Chitosan Coating and Evaluation of Survival in Simulated Human Gastro-intestinal Condition. Iran. J. Pharm. Res. 2014, 13, 843–852. [Google Scholar]
- Krasaekoopt, W.; Bhandari, B.; Deeth, H. The influence of coating materials on some properties of alginate beads and survivability of microencapsulated probiotic bacteria. Int. Dairy J. 2004, 14, 737–743. [Google Scholar] [CrossRef]
- Bâati, L.; Fabre-Gea, C.; Auriol, D.; Blanc, P.J. Study of the cryotolerance of Lactobacillus acidophilus: Effect of culture and freezing conditions on the viability and cellular protein levels. Int. J. Food Microbiol. 2000, 59, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D. Chapter 3—The Interaction Between Insoluble and Soluble Fiber. In Dietary Fiber for the Prevention of Cardiovascular Disease; Samaan, R.A., Ed.; Academic Press: London, UK, 2017; pp. 35–59. ISBN 978-0-12-805130-6. [Google Scholar]
- Chávarri, M.; Marañón, I.; Ares, R.; Ibáñez, F.C.; Marzo, F.; Villarán, M. del C. Microencapsulation of a probiotic and prebiotic in alginate-chitosan capsules improves survival in simulated gastro-intestinal conditions. Int. J. Food Microbiol. 2010, 142, 185–189. [Google Scholar] [CrossRef] [PubMed]
- Rajam, R.; Kumar, S.B.; Prabhasankar, P.; Anandharamakrishnan, C. Microencapsulation of Lactobacillus plantarum MTCC 5422 in fructooligosaccharide and whey protein wall systems and its impact on noodle quality. J. Food Sci. Technol. 2015, 52, 4029–4041. [Google Scholar] [CrossRef] [PubMed]
- Ayama, H.; Sumpavapol, P.; Chanthachum, S. Effect of encapsulation of selected probiotic cell on survival in simulated gastrointestinal tract condition. Songklanakarin J. Sci. Technol. 2014, 36, 291–299. [Google Scholar]
- Cheow, W.S.; Kiew, T.Y.; Hadinoto, K. Controlled release of Lactobacillus rhamnosus biofilm probiotics from alginate-locust bean gum microcapsules. Carbohydr. Polym. 2014, 103, 587–595. [Google Scholar] [CrossRef]
- Wu, J.; Zhao, L.; Li, J.; Jin, S.; Wu, S. Aggregation and gelation of oat β -glucan in aqueous solution probed by NMR relaxometry. Carbohydr. Polym. 2017, 163, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Gbassi, G.K.; Vandamme, T. Probiotic Encapsulation Technology: From Microencapsulation to Release into the Gut. Pharmaceutics 2012, 4, 149–163. [Google Scholar] [CrossRef]
- Han, L.N.; Van, L.H.H.; Duc, T.V.; Dao, D.T.A. Encapsulation of Lactobacillus acidophilus in yeast cell walls (Saccharomyces cerevisiae) for improving survival in gastrointestinal conditions. Vietnam. J. Sci. Technol. 2016, 54, 533–544. [Google Scholar] [CrossRef]
- Hernandez-Hernandez, O.; Muthaiyan, A.; Moreno, F.J.; Montilla, A.; Sanz, M.L.; Ricke, S.C. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol. 2012, 30, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.B.; Vaidyanathan, M.; Radhakrishnan, K.; Raichur, A.M. Enhanced viability of probiotic Saccharomyces boulardii encapsulated by layer-by-layer approach in pH responsive chitosan–dextran sulfate polyelectrolytes. J. Food Eng. 2014, 136, 1–8. [Google Scholar] [CrossRef]
- Ramos, P.E.; Cerqueira, M.A.; Teixeira, J.A.; Vicente, A.A. Physiological protection of probiotic microcapsules by coatings. Crit. Rev. Food Sci. Nutr. 2018, 58, 1864–1877. [Google Scholar] [CrossRef]
- De Prisco, A.; Maresca, D.; Ongeng, D.; Mauriello, G. Microencapsulation by vibrating technology of the probiotic strain Lactobacillus reuteri DSM 17938 to enhance its survival in foods and in gastrointestinal environment. LWT—Food Sci. Technol. 2015, 61, 452–462. [Google Scholar] [CrossRef]
- Kanmani, P.; Satish Kumar, R.; Yuvaraj, N.; Paari, K.A.; Pattukumar, V.; Arul, V. Effect of cryopreservation and microencapsulation of lactic acid bacterium Enterococcus faecium MC13 for long-term storage. Biochem. Eng. J. 2011, 58–59, 140–147. [Google Scholar] [CrossRef]
- Li, X.Y.; Chen, X.G.; Sun, Z.W.; Park, H.J.; Cha, D.-S. Preparation of alginate/chitosan/carboxymethyl chitosan complex microcapsules and application in Lactobacillus casei ATCC 393. Carbohydr. Polym. 2011, 83, 1479–1485. [Google Scholar] [CrossRef]
| Resources | Extraction Processes | Results | References |
|---|---|---|---|
| Yeast cell walls S.cereviseae | The yeast cell walls were extracted with NaOH, dissolved in distilled water, and collected via centrifugation. The insoluble material was recovered, washed three times, and extracted twice with phosphoric acid. The insoluble residue, representing cell wall β-d-glucan, was separated, resuspended in distilled water, and decanted with water until pH 7. | (1→3)-β-d-glucan with yield 13.5% | [47] |
| S. cerevisiae | Yeast cell exposure to hot water (autoclaving), thermally induced autolysis, homogenization in a bead mill, sonication and their combinations. | 13–14% of β(1,3)/(1,6)-glucans | [48] |
| Spent brewer’s yeast (S. cerevisiae) slurry, a brewery by-product with 18% solids | Preliminary purification, induced autolysis, hot water treatment, homogenization, organic solvent treatment, protease treatment, and spray drying. | β-d-glucan, with 93% purity and 11.2% yield | [49] |
| S. cerevisiae K48L3, K48L4, YPH499 | Mannoproteins were isolated and extracted using SDS and laminarinase. The late logarithmic phase cells were harvested, washed twice, and digested with 1200 units of glucanase for 3 h. The extract was centrifuged, and glucanase-extracted mannoproteins were purified using ion exchange and affinity chromatographies. | 725–2255 µg mannoprotein/100 mg dry weight of yeasts | [50] |
| K. marxianus | The yeast cell precipitate was re-suspended in a buffer solution, washed with acetic acid, and precipitated. The supernatant was incubated overnight and centrifuged. Hexadecyltrimethylammonium bromide was used for selective precipitation and purification of the mannoprotein. The precipitate was then dialyzed against deionized water for 48 h. | 8.42 ± 0.06% crude mannoprotein | [51] |
| S. uvarum | The insoluble material from autolyzed brewer’s yeasts slurry was diluted, heated, and washed three times with distilled water. Sonication, lipid extraction, and proteolysis were performed, and the insoluble residue was washed five times. Mannoprotein was precipitated, washed, dialyzed, and lyophilized. | 4.16% yield of mannoprotein | [52] |
| S. cerevisiae W301-1A | Cell walls were lyophilized and subjected to alkaline and acidic extractions. After centrifugation, the extracts were collected and used for subsequent steps. The samples were then resuspended in HCl, neutralized with NaOH, dialysed, and lyophilized. | 2.4% of chitin | [53] |
| Strain | Methods | Yield | Other Results | References |
|---|---|---|---|---|
| S. cereviseae | DEAE chromatography | 10.36% β-glucan | Protein (0.004%) Carbohydrate (0.090%) Glucose (0.022%) Mannose (0.069%) | [34] |
| S. cereviseae | DEAE chromatography | 13.00% β-glucan | Protein (0.3%) Glucan/Mannan ratio (30/70) | [68] |
| S. cereviseae | Concanavalin-A chromatography | 0.32% β-glucan | Glucose (0.014%) Mannose (0.000%) | [34] |
| S. cereviseae | Concanavalin-A chromatography | 4.00% β-glucan | Glucan/Mannan ratio (100/0) | [68] |
| Baker’s yeast S. cereviseae | SDS extraction followed by Concanavalin-A chromatography | 0.98% Mannoprotein | Mannan/Protein ratio (31/100) | [59] |
| C.albicans | Mercaptoethanol and sodium dodecyl sulfate followed by Concanavalin-A chromatography | 1.5mg/13g Mannoprotein | 30–55 kDa | [69] |
| Saccharomyces cerevisiae | Zymolase followed by Concanavalin-A chromatography and dialysis | 127.4 ± 3.2 μg/mg β-glucan 6.2 ± 0.55% Chitin | Mannan (93.3 ± 3.2 μg/mg) | [67] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utama, G.L.; Oktaviani, L.; Balia, R.L.; Rialita, T. Potential Application of Yeast Cell Wall Biopolymers as Probiotic Encapsulants. Polymers 2023, 15, 3481. https://doi.org/10.3390/polym15163481
Utama GL, Oktaviani L, Balia RL, Rialita T. Potential Application of Yeast Cell Wall Biopolymers as Probiotic Encapsulants. Polymers. 2023; 15(16):3481. https://doi.org/10.3390/polym15163481
Chicago/Turabian StyleUtama, Gemilang Lara, Lidya Oktaviani, Roostita Lobo Balia, and Tita Rialita. 2023. "Potential Application of Yeast Cell Wall Biopolymers as Probiotic Encapsulants" Polymers 15, no. 16: 3481. https://doi.org/10.3390/polym15163481
APA StyleUtama, G. L., Oktaviani, L., Balia, R. L., & Rialita, T. (2023). Potential Application of Yeast Cell Wall Biopolymers as Probiotic Encapsulants. Polymers, 15(16), 3481. https://doi.org/10.3390/polym15163481







