Self-Assembled Chitosan/Dialdehyde Carboxymethyl Cellulose Hydrogels: Preparation and Application in the Removal of Complex Fungicide Formulations from Aqueous Media
Abstract
:1. Introduction
- (i)
- Macroporosity: The adsorbents need to have a porous structure with interconnected channels or void spaces that allow water to pass through while retaining contaminants, thus ensuring a high flow rate and preventing clogging.
- (ii)
- Mechanical stability: Adsorbents should be able to withstand compression forces without losing their filtration efficiency. This feature is important for maintenance, cleaning, and reusability purposes.
- (iii)
- Reusability: To minimize waste and cost, it is desirable for PEC adsorbents to be reusable through multiple sorption/desorption cycles without significant loss in their performance. This implies that the adsorbents should maintain their adsorption capacity and structural integrity over time.
2. Materials and Methods
2.1. Materials
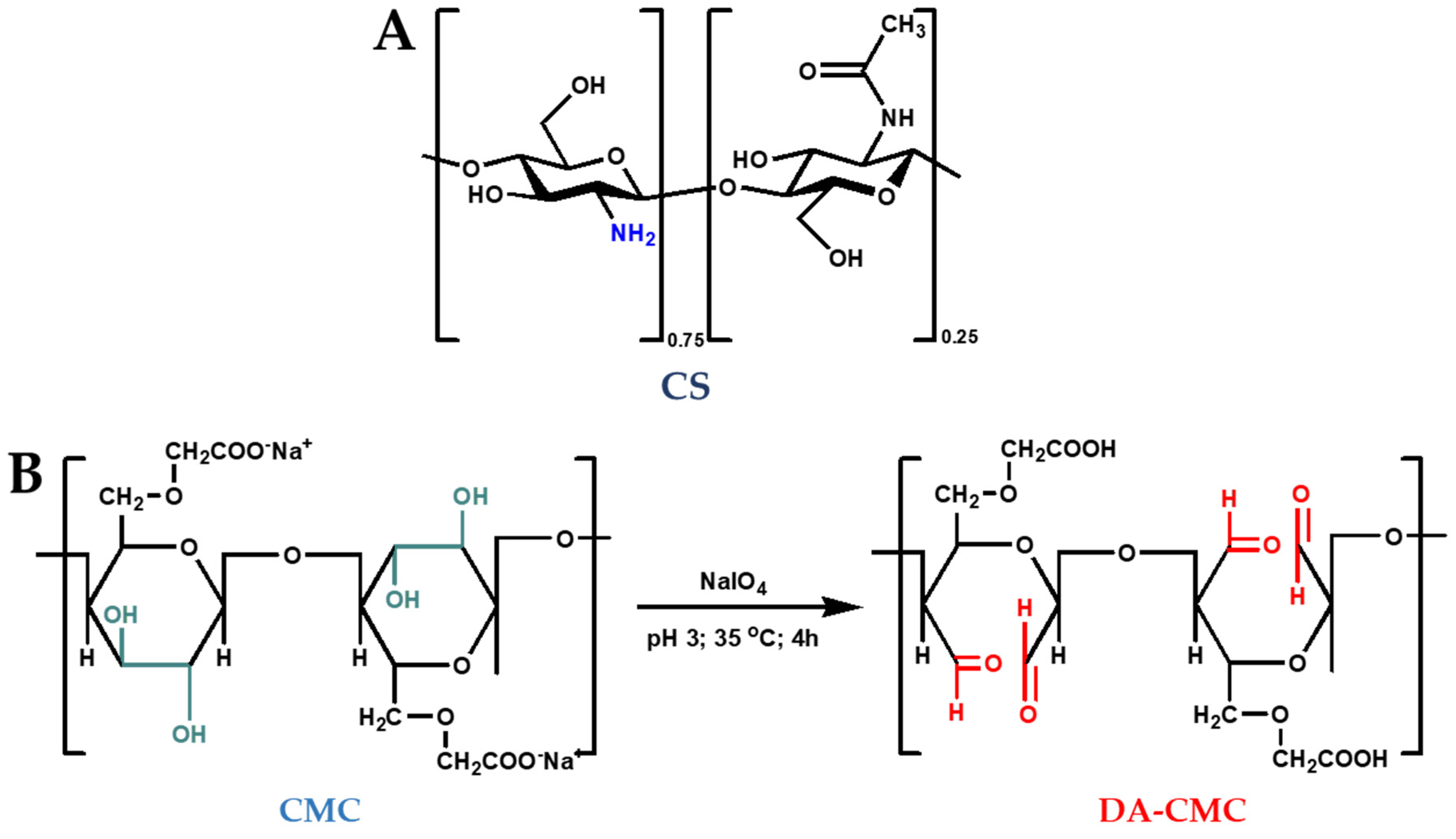
2.2. Oxidation of CMC
2.3. Hydrogels Preparation
2.4. Characterization Methods
2.4.1. Aldehyde Content
2.4.2. Carboxyl Groups Content
2.4.3. Structural Characterization
2.4.4. Gel Fraction Yield
2.4.5. Morphology of Sponges
2.4.6. Swelling Properties
2.4.7. Mechanical Tests
2.4.8. Erosion Study
2.5. Sorption of Fungicide Formulations
3. Results and Discussion
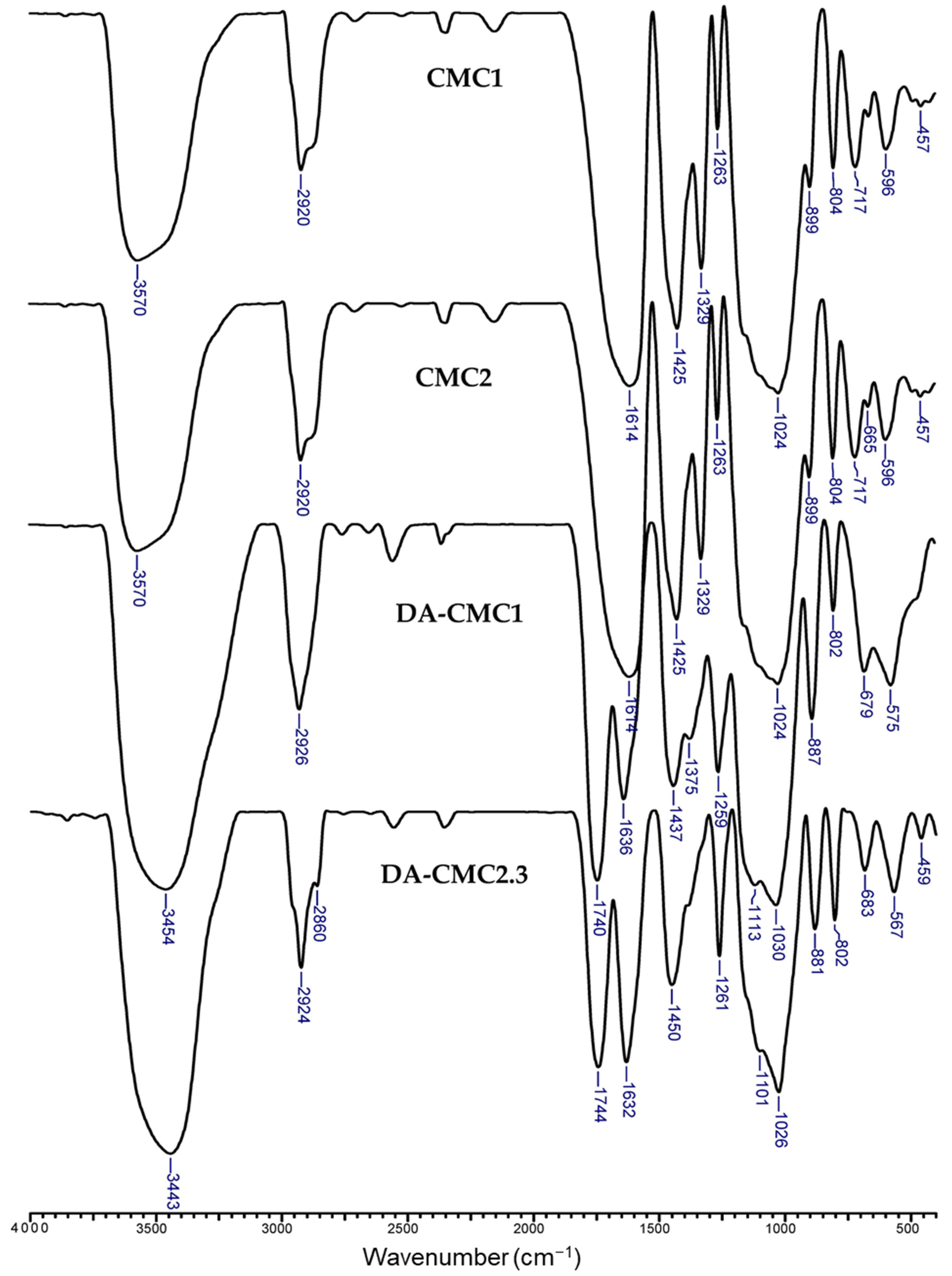
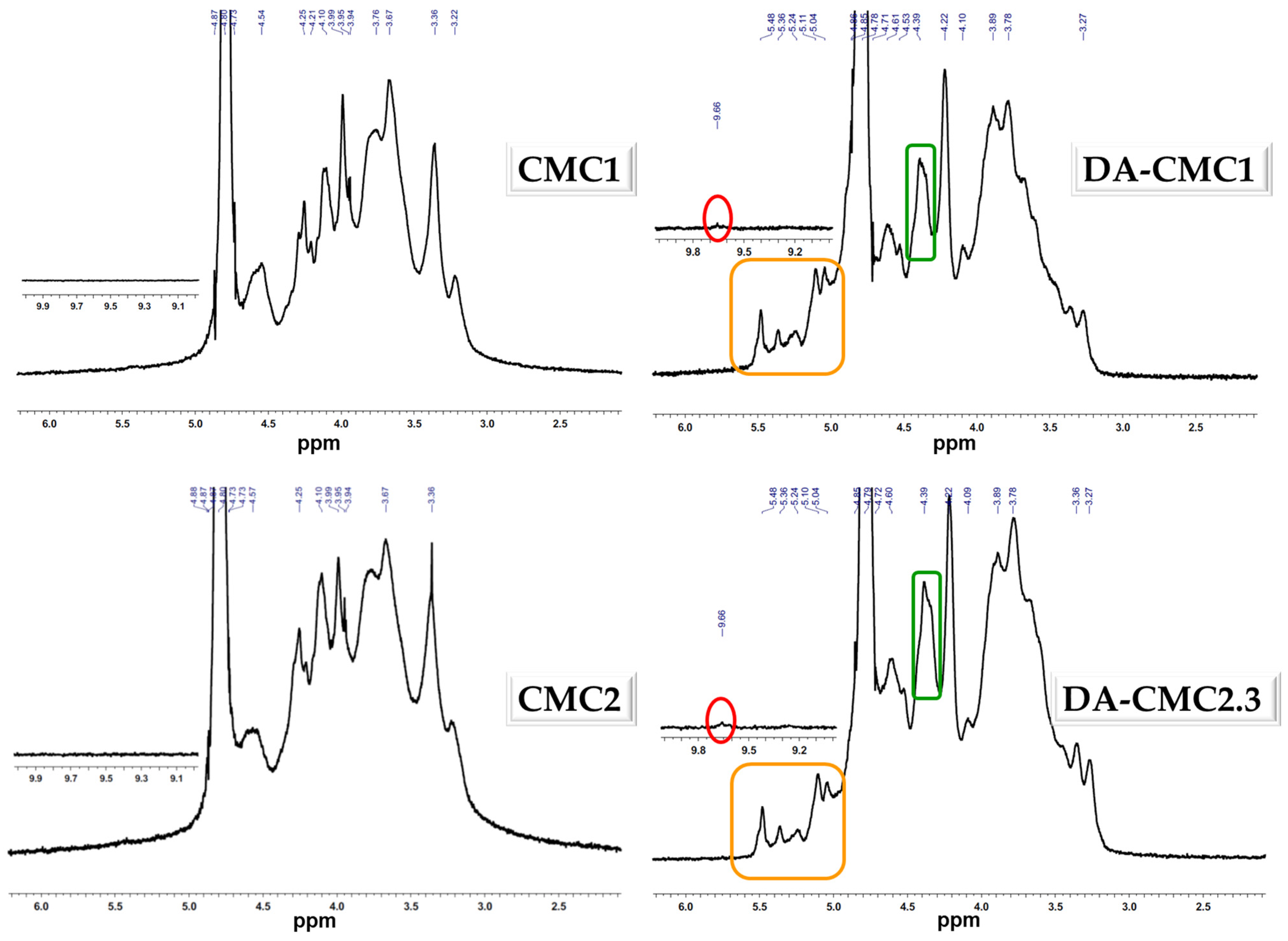
3.1. Oxidation of CMC
- (i)
- (ii)
- The disappearance of the bands at 1329 cm−1, which was associated with the protonation of carboxylate groups (-COO− → -COOH) [62];
- (iii)
- Blue-shift of the band at 3570 cm−1 to 3441–3456 cm−1, which could be correlated with the decrease in hydroxyl group content following the oxidation reaction [57].
- (iv)
- Red-shift of the band at 1614 cm−1 to 1636 cm−1; 1637 cm−1; and 1632 cm−1, for DA-CMC1, DA-CMC2.2 and DA-CMC2.3, respectively, that are assigned to conjugated carboxylate groups [57].
- (v)
- Red-shift of the bands at 1425 cm−1 to 1437 cm−1; 1431 cm−1; and 1450 cm−1, in the spectra of DA-CMC1, DA-CMC2.2 and DA-CMC2.3, respectively, that were also attributed to structural changes in carboxylate C=O groups.
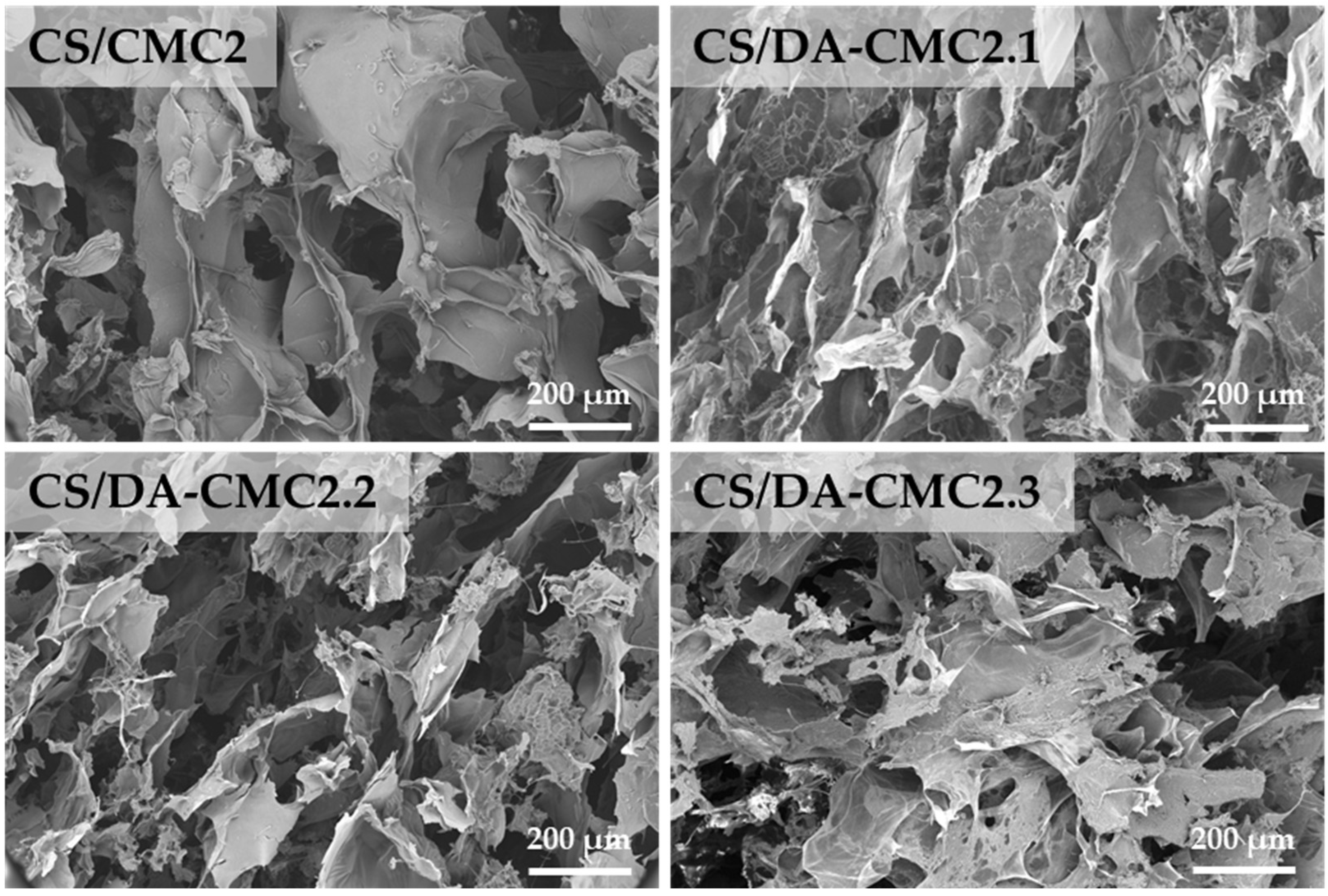
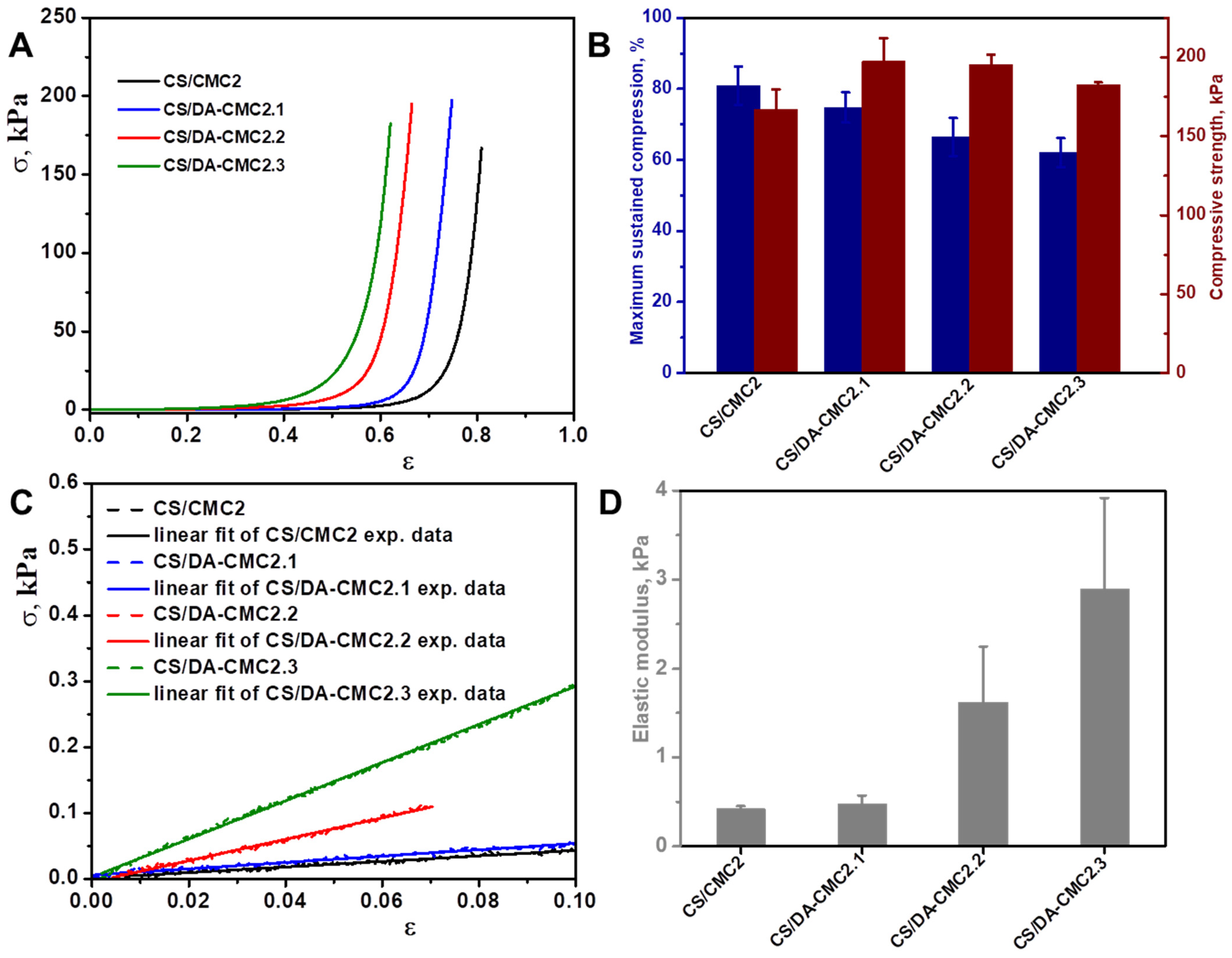
3.2. Preparation and Characterization of Hydrogels
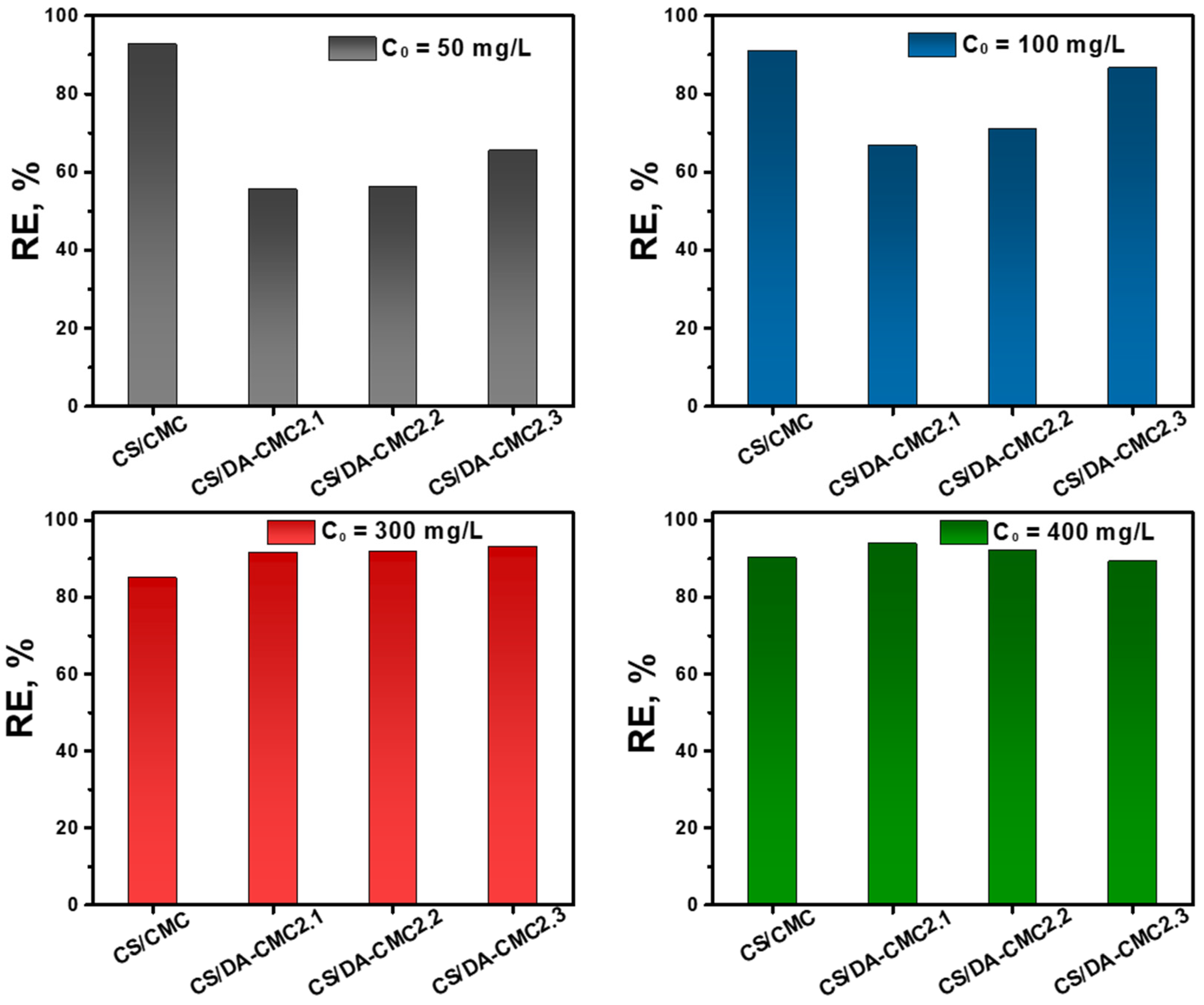
3.3. Sorption of Fungicide Formulations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maksymiv, I. Pesticides: Benefits and hazards. J. Vasyl Stefanyk Precarpathian Natl. Univ. 2015, 2, 70–76. [Google Scholar] [CrossRef]
- Sharma, A.; Kumar, V.; Shahzad, B.; Tanveer, M.; Sidhu, G.P.S.; Handa, N.; Kohli, S.K.; Yadav, P.; Bali, A.S.; Parihar, R.D.; et al. Worldwide pesticide usage and its impacts on ecosystem. SN Appl. Sci. 2019, 1, 1446. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar]
- Merel, S.; Benzing, S.; Gleiser, C.; Di Napoli-Davis, G.; Zwiener, C. Occurrence and overlooked sources of the biocide carbendazim in wastewater and surface water. Environ. Pollut. 2018, 239, 512–521. [Google Scholar] [CrossRef]
- Rizzi, V.; Gubitosa, J.; Fini, P.; Romita, R.; Agostiano, A.; Nuzzo, S.; Cosma, P. Commercial bentonite clay as low-cost and recyclable “natural” adsorbent for the Carbendazim removal/recover from water: Overview on the adsorption process and preliminary photodegradation considerations. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125060. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Ollier, R.P.; Pereira, A.E.S.; Fraceto, L.F.; Alvarez, V.A. Pesticide removal from industrial effluents using biopolymeric materials. In Biopolymer Membranes and Films; De Moraes, M.A., da Silva, C.F., Vieira, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 359–382. [Google Scholar]
- Baigorria, E.; Fraceto, L.F. Novel nanostructured materials based on polymer/organic-clay composite networks for the removal of carbendazim from waters. J. Clean. Prod. 2022, 331, 129867. [Google Scholar] [CrossRef]
- Ozcan, A.; Hamid, F.; Ozcan, A.A. Synthesizing of a nanocomposite based on the formation of silver nanoparticles on fumed silica to develop an electrochemical sensor for carbendazim detection. Talanta 2021, 222, 121591. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo-Ruiz, J.L.; Romero-Gonzalez, R.; Vidal, J.L.M.; Frenich, A.G. Monitoring of polar pesticides and contaminants in edible oils and nuts by liquid chromatography-tandem mass spectrometry. Food Chem. 2021, 343, 128495. [Google Scholar] [CrossRef] [PubMed]
- Golge, O.; Cinpolat, S.; Kabak, B. Quantification of pesticide residues in gherkins by liquid and gas chromatography coupled to tandem mass spectrometry. J. Food Compos. Anal. 2021, 96, 103755. [Google Scholar] [CrossRef]
- Marican, A.; Durán-Lara, E.F. A review on pesticide removal through different processes. Environ. Sci. Pollut. Res. 2018, 25, 2051–2064. [Google Scholar] [CrossRef]
- Ghimici, L.; Ghiorghiță, C.A.; Năfureanu, M.M. Abatement of some commercial fungicide content from model dispersions by a new thiourea-graft-polyethyleneimine derivative. Environ. Sci. Pollut. Res. 2023, 30, 67539–67551. [Google Scholar] [CrossRef]
- Vela, N.; Fenoll, J.; Garrido, I.; Pérez-Lucas, G.; Flores, P.; Hellín, P.; Navarro, S. Reclamation of agro-wastewater polluted with pesticide residues using sunlight activated persulfate for agricultural reuse. Sci. Total Environ. 2019, 660, 923–930. [Google Scholar] [CrossRef]
- Skaggs, C.S.; Alluhayb, A.H.; Logue, B.A. Comparison of the extraction efficiency of ice concentration linked with extractive stirrer, stir bar sorptive extraction, and solid-phase micro-extraction for pesticides from drinking water. J. Chromatogr. A 2020, 1622, 461102. [Google Scholar] [CrossRef]
- Orazbayeva, D.; Koziel, J.A.; Trujillo-Rodriguez, M.J.; Anderson, J.L.; Kenessov, B. Polymeric ionic liquid sorbent coatings in headspace solid-phase micro-extraction: A green sample preparation technique for the determination of pesticides in soil. Microchem. J. 2020, 157, 104996. [Google Scholar] [CrossRef]
- Al-Saad, K.; Issa, A.A.; Idoudi, S.; Shomar, B.; Al-Ghouti, M.A.; Al-Hashimi, N.; El-Azazy, M. Smart synthesis of trimethyl ethoxysilane (TMS) functionalized core-shell magnetic nanosorbents Fe3O4@SiO2: Process optimization and application for extraction of pesticides. Molecules 2020, 25, 4827. [Google Scholar] [CrossRef] [PubMed]
- Hassanzadeh-Afruzi, F.; Maleki, A.; Zare, E.N. Efficient remediation of chlorpyrifos pesticide from contaminated water by superparamagnetic adsorbent based on Arabic gum-grafted-polyamidoxime. Int. J. Biol. Macrom. 2022, 203, 445–456. [Google Scholar] [CrossRef]
- Musarurwa, H.; Tavengwa, N.T. Sustainable extraction of pesticides in food and environmental samples using emerging green adsorbents. Sustain. Chem. Pharm. 2021, 24, 100545. [Google Scholar] [CrossRef]
- Azelee, N.I.W.; Manas, N.H.A.; Hanapi, S.Z.; Sarip, S.H.M.; Malek, R.A.; El-Enshasy, H.A.; Dailin, D.J.; Ngah, M.F. Removal of pesticides from water and wastewater by agricultural biomass-based adsorbents. In Pesticides Remediation Technologies from Water and Wastewater, 2nd ed.; Dehghani, M.H., Karri, R.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 431–436. [Google Scholar]
- Singh, S.; Kumar, V.; Datta, S.; Dhanjal, D.S.; Sharma, K.; Samuel, J.; Singh, J. Current advancement and future prospect of biosorbents for bioremediation. Sci. Total Environ. 2020, 709, 135895. [Google Scholar] [CrossRef]
- Aziz, K.; El Achaby, M.; Mamouni, R.; Saffaj, N.; Aziz, F. A novel hydrogel beads based copper-doped Cerastoderma edule shells@Alginate biocomposite for highly fungicide sorption from aqueous medium. Chemosphere 2023, 311, 136932. [Google Scholar] [CrossRef]
- Wypij, M.; Trzcińska-Wencel, J.; Golińska, P.; Avila-Quezada, G.D.; Ingle, A.P.; Rai, M. The strategic applications of natural polymer nanocomposites in food packaging and agriculture: Chances, challenges, and consumers’ perception. Front. Chem. 2023, 10, 1106230. [Google Scholar] [CrossRef]
- Ali, N.; Funmilayo, O.R.; Khan, A.; Ali, F.; Bilal, M.; Yang, Y.; Akhter, M.S.; Zhou, C.; Wenjie, Y.; Iqbal, H.M.N. Nanoarchitectonics: Porous hydrogel as bio-sorbent for effective remediation of hazardous contaminants. J. Inorg. Organom. Polym. Mater. 2022, 32, 3301–3320. [Google Scholar] [CrossRef]
- Zagni, C.; Dattilo, S.; Mecca, T.; Gugliuzzo, C.; Scamporrino, A.A.; Privitera, V.; Puglisi, R.; Carroccio, S.C. Single and dual polymeric sponges for emerging pollutants removal. Eur. Polym. J. 2022, 179, 111556. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; El-Naggar, M.E.; Radwan, E.K.; Mohamed, I.M.; Azaam, M.; Kenawy, E.-R. High-performance mixed-matrix membranes enabled by organically/inorganic modified montmorillonite for the treatment of hazardous textile wastewater. Chem. Eng. J. 2021, 405, 126964. [Google Scholar] [CrossRef]
- Carmalin, S.A.; Lima, E.C. Removal of emerging contaminants from the environment by adsorption. Ecotoxicol. Environ. Saf. 2018, 150, 1–17. [Google Scholar]
- Tanaka, F.C.; Junior, C.R.F.; Fernandes, R.S.; de Moura, M.R.; Aouada, F.A. Correlating pH and swelling degree parameters to understand the sorption and desorption process of diquat herbicide from nanocomposites based on polysaccharide and clinoptilolite. J. Polym. Environ. 2021, 29, 3389–3400. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Lima-Tenório, M.K.; Tenório-Neto, E.T.; Guilherme, M.R.; Muniz, E.C.; Rubira, A.F. Chitosan-based hydrogels: From preparation to biomedical applications. Carbohydr. Polym. 2018, 196, 233–245. [Google Scholar] [CrossRef]
- Takeshita, S.; Zhao, S.; Malfait, W.J.; Koebel, M.M. Chemistry of chitosan aerogels: Three-dimensional pore control for tailored applications. Angew. Chem. Int. Ed. 2021, 60, 9828–9851. [Google Scholar] [CrossRef]
- Ghimici, L.; Brunchi, C.-E.; Diaconu, A. Removal of some commercial pesticides containing a-Cypermethrin, Deltamethrin and Mancozeb as active ingredients by chitosan solution. Cellulose 2016, 23, 3837–3846. [Google Scholar] [CrossRef]
- Ghimici, L.; Dinu, I.A. Removal of some commercial pesticides from aqueous dispersions using as flocculant a thymine-containing chitosan derivative. Sep. Purif. Technol. 2019, 209, 698–706. [Google Scholar] [CrossRef]
- Kanmani, P.; Kamaraj, J.; Sureshbabu, P.; Karthikeyan, S. Environmental applications of chitosan and cellulosic biopolymers: A comprehensive outlook. Bioresour. Technol. 2017, 242, 295–303. [Google Scholar] [CrossRef]
- Silvestro, I.; Fernández-García, M.; Ciarlantini, C.; Francolini, I.; Girelli, A.; Piozzi, A. molecularly imprinted polymers based on chitosan for 2,4-dichlorophenoxyacetic acid removal. Int. J. Mol. Sci. 2022, 23, 13192. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Dinu, M.V. Advances in porous chitosan-based composite hydrogels: Synthesis and applications. React. Funct. Polym. 2020, 146, 104372. [Google Scholar] [CrossRef]
- Humelnicu, D.; Lazar, M.M.; Ignat, M.; Dinu, I.A.; Dragan, E.S.; Dinu, M.V. Removal of heavy metal ions from multi-component aqueous solutions by eco-friendly and low-cost composite sorbents with anisotropic pores. J. Hazard. Mater. 2020, 381, 120980. [Google Scholar] [CrossRef]
- Dinu, I.A.; Ghimici, L.; Raschip, I.E. Macroporous 3D chitosan cryogels for Fastac 10EC pesticide adsorption and antibacterial applications. Polymers 2022, 14, 3145. [Google Scholar] [CrossRef] [PubMed]
- Dragan, E.S.; Humelnicu, D.; Dinu, M.V. Designing smart triple-network cationic cryogels with outstanding efficiency and selectivity for deep cleaning of phosphate. Chem. Eng. J. 2021, 426, 131411. [Google Scholar] [CrossRef]
- Sharma, G.; Thakur, B.; Kumar, A.; Sharma, S.; Naushad, M.; Stadler, F.J. Atrazine removal using chitin-cl-poly(acrylamide-co-itaconic acid) nanohydrogel: Isotherms and pH responsive nature. Carbohydr. Polym. 2020, 241, 116258. [Google Scholar] [CrossRef]
- Valdés, C.; Valdés, O.; Bustos, D.; Abril, D.; Cabrera-Barjas, G.; Pereira, A.; Villaseñor, J.; Polo-Cuadrado, E.; Carreño, G.; Durán-Lara, E.F.; et al. Use of poly(vinyl alcohol)-malic acid (CLHPMA) hydrogels and chitosan coated calcium alginate (CCCA) microparticles as potential sorbent phases for the extraction and quantitative determination of pesticides from aqueous solutions. Polymers 2021, 13, 3993. [Google Scholar] [CrossRef]
- Carneiro, R.T.A.; Taketa, T.B.; Neto, R.J.G.; Oliveira, J.L.; Campos, E.V.; de Moraes, M.A.; da Silva, C.M.; Beppu, M.M.; Fraceto, L.F. Removal of glyphosate herbicide from water using biopolymer membranes. J. Environ. Manag. 2015, 151, 353–360. [Google Scholar] [CrossRef]
- Philipp, B.; Dautzenberg, H.; Linov, K.J.; Koetz, J.; Dawydoff, W. Polyelectrolyte complexes—Recent developments and open problems. Prog. Polym. Sci. 1989, 14, 91–172. [Google Scholar] [CrossRef]
- Dragan, S.; Cristea, M.; Luca, C.; Simionescu, B.C. Polyelectrolyte complexes. I. Synthesis and characterization of some insoluble polyanion-polycation complexes. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 3485–3494. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Construction of self-assembled polyelectrolyte complex hydrogel based on oppositely charged polysaccharides for sustained delivery of green tea polyphenols. Food Chem. 2020, 306, 125632. [Google Scholar] [CrossRef]
- Hu, X.; Wang, Y.; Zhang, L.; Xu, M. Formation of self-assembled polyelectrolyte complex hydrogel derived from salecan and chitosan for sustained release of Vitamin C. Carbohydr. Polym. 2020, 234, 115920. [Google Scholar] [CrossRef]
- Stiglic, A.D.; Kargl, R.; Beaumont, M.; Strauss, C.; Makuc, D.; Egger, D.; Plavec, J.; Rojas, O.J.; Kleinschek, K.S.; Mohan, T. Influence of charge and heat on the mechanical properties of scaffolds from ionic complexation of chitosan and carboxymethyl cellulose. ACS Biomater. Sci. Eng. 2021, 7, 3618–3632. [Google Scholar] [CrossRef] [PubMed]
- Ghiorghita, C.A.; Humelnicu, D.; Dinu, M.V.; Ignat, M.; Bonardd, S.; Díaz, D.D.; Dragan, E.S. Polyelectrolyte complex composite cryogels with self-antibacterial properties and wide window for simultaneous removal of multiple contaminants. Chem. Eng. J. 2023, 459, 141562. [Google Scholar] [CrossRef]
- Dragan, E.S.; Ghiorghita, C.A.; Dinu, M.V.; Duceac, I.A.; Coseri, S. Fabrication of self-antibacterial chitosan/oxidized starch polyelectrolyte complex sponges for controlled delivery of curcumin. Food Hydrocoll. 2023, 135, 108147. [Google Scholar] [CrossRef]
- Dragan, E.S.; Dinu, M.V.; Ghiorghita, C.A. Chitosan-based polyelectrolyte complex cryogels with elasticity, toughness and delivery of curcumin engineered by polyions pair and cryostructuration steps. Gels 2022, 8, 240. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, L.; Shao, S.; Chang, X.; Li, M. Periodate Oxidation of Carboxymethyl Cellulose Under Controlled Conditions. ChemistrySelect 2020, 5, 6765–6773. [Google Scholar] [CrossRef]
- Zheng, T.; Yu, X.; Pilla, S. Mechanical and Moisture Sensitivity of Fully Bio-Based Dialdehyde Carboxymethyl Cellulose Cross-Linked Soy Protein Isolate Films. Carbohydr. Polym. 2017, 157, 1333–1340. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Y.; Yao, Y.; Tong, Z.R.; Li, F.; Yang, Z.; Jin, S. Preparation of the polyelectrolyte complex hydrogel of biopolymers via a semi-dissolution acidification sol-gel transition method and its application in solid-state supercapacitors. J. Power Sources 2018, 378, 603–609. [Google Scholar] [CrossRef]
- Li, H.; Wu, B.; Mu, C.; Lin, W. Concomitant degradation in periodate oxidation of carboxymethyl cellulose. Carbohydr. Polym. 2011, 84, 881–886. [Google Scholar] [CrossRef]
- Sheikhi, A.; Yang, H.; Alam, M.N.; van de Ven, T.G.M. Highly stable, functional hairy nanoparticles and biopolymers from wood fibers: Towards sustainable nanotechnology. J. Vis. Exp. 2016, 113, e54133. [Google Scholar]
- Platon, I.-V.; Ghiorghita, C.-A.; Lazar, M.M.; Raschip, I.E.; Dinu, M.V. Chitosan sponges with instantaneous shape recovery and multistrain antibacterial activity for controlled release of plant-derived polyphenols. Int. J. Mol. Sci. 2023, 24, 4452. [Google Scholar] [CrossRef] [PubMed]
- Chartier, C.; Buwalda, S.; Van Den Berghe, H.; Nottelet, B.; Budtova, T. Tuning the properties of porous chitosan: Aerogels and cryogels. Int. J. Biol. Macromol. 2022, 202, 215–223. [Google Scholar] [CrossRef]
- Li, S.; Li, Y.; Fu, Z.; Lu, L.; Cheng, J.; Fei, Y. A ‘top modification’ strategy for enhancing the ability of a chitosan aerogel to efficiently capture heavy metal ions. J. Colloid Interface Sci. 2021, 594, 141–149. [Google Scholar] [CrossRef]
- Dellali, M.; Iurciuc (Tincu), C.E.; Savin, C.L.; Spahis, N.; Djennad, M.; Popa, M. Hydrogel films based on chitosan and oxidized carboxymethylcellulose optimized for the controlled release of curcumin with applications in treating dermatological conditions. Molecules 2021, 26, 2185. [Google Scholar] [CrossRef]
- Çelikçi, N.; Ziba, C.A.; Dolaz, M. Synthesis and characterization of carboxymethyl cellulose (cmc) from different waste sources containing cellulose and investigation of its use in the construction industry. Cellul. Chem. Technol. 2022, 56, 55–68. [Google Scholar] [CrossRef]
- Dou, Y.; Zhang, L.; Zhang, B.; He, M.; Shi, W.; Yang, S.; Cui, Y.; Yin, G. Preparation and characterization of edible dialdehyde carboxymethyl cellulose crosslinked feather keratin films for food packaging. Polymers 2020, 12, 158. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; He, H.; Cai, R.; Tao, G.; Yang, M.; Zuo, H.; Umar, A.; Wang, Y. Cross-linking of dialdehyde carboxymethyl cellulose with silk sericin to reinforce sericin film for potential biomedical application. Carbohydr. Polym. 2019, 212, 403–411. [Google Scholar] [CrossRef]
- Calvini, P.; Gorassini, A.; Luciano, G.; Franceschi, E. FTIR and WAXS analysis of periodate oxycellulose: Evidence for a cluster mechanism of oxidation. Vib. Spectrosc. 2006, 40, 177–183. [Google Scholar] [CrossRef]
- Capanema, N.S.V.; Mansur, A.A.P.; Carvalho, I.C.; Carvalho, S.M.; Mansur, H.S. Bioengineered water-responsive carboxymethyl cellulose/poly(vinyl alcohol) hydrogel hybrids for wound dressing and skin tissue engineering applications. Gels 2023, 9, 166. [Google Scholar] [CrossRef]
- Kono, H. 1H and 13C Chemical shift assignment of the monomers that comprise carboxymethyl cellulose. Carbohydr. Polym. 2013, 97, 384–390. [Google Scholar] [CrossRef]
- Hu, X.; Yan, L.; Wang, Y.; Xu, M. Self-Assembly of binary oppositely charged polysaccharides into polyelectrolyte complex hydrogel film for facile and efficient Pb2+ removal. Chem. Eng. J. 2020, 388, 124189. [Google Scholar] [CrossRef]
- Sharma, S.; Jain, P.; Tiwari, S. Dynamic imine bond based chitosan smart hydrogel with magnified mechanical strength for controlled drug delivery. Int. J. Biol. Macromol. 2020, 160, 489–495. [Google Scholar] [CrossRef]
- Dimzon, I.K.D.; Knepper, T.P. Degree of deacetylation of chitosan by infrared spectroscopy and partial least squares. Int. J. Biol. Macromol. 2015, 72, 939–945. [Google Scholar] [CrossRef]
- Lau, C.; Cooney, M.J. Conductive macroporous composite chitosan-carbon nanotube scaffolds. Langmuir 2008, 24, 7004–7010. [Google Scholar] [CrossRef]
- Groult, S.; Buwalda, S.; Budtova, T. Pectin hydrogels, aerogels, cryogels and xerogels: Influence of drying on structural and release properties. Eur. Polym. J. 2021, 149, 110386. [Google Scholar] [CrossRef]
- Ghiorghita, C.-A.; Dinu, M.V.; Lazar, M.M.; Dragan, E.S. Polysaccharide-based composite hydrogels as sustainable materials for removal of pollutants from wastewater. Molecules 2022, 27, 8574. [Google Scholar] [CrossRef]
- Seida, Y.; Tokuyama, H. Hydrogel adsorbents for the removal of hazardous pollutants—Requirements and available functions as adsorbent. Gels 2022, 8, 220. [Google Scholar] [CrossRef]
- Dragan, E.S.; Perju, M.M. Preparation and swelling behavior of chitosan/poly(N-2-aminoethyl acrylamide) composite hydrogels. Soft Mater. 2010, 8, 49–62. [Google Scholar] [CrossRef]
- Karoyo, A.H.; Wilson, L.D. A Review on the design and hydration properties of natural polymer-based hydrogels. Materials 2021, 14, 1095. [Google Scholar] [CrossRef]
- Foudazi, R.; Zowada, R.; Manas-Zloczower, I.; Feke, D.L. Porous hydrogels: Present challenges and future opportunities. Langmuir 2023, 39, 2092–2111. [Google Scholar] [CrossRef]
- Dinu, M.V.; Gradinaru, A.C.; Lazar, M.M.; Dinu, I.A.; Raschip, I.E.; Ciocarlan, N.; Aprotosoaie, A.C. Physically cross-linked chitosan/dextrin cryogels entrapping thymus vulgaris essential oil with enhanced mechanical, antioxidant and antifungal properties. Int. J. Biol. Macromol. 2021, 184, 898–908. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Anghel, N.; Dinu, M.V.; Spiridon, I. Dextran-chitosan composites: Antioxidant and anti-inflammatory properties. Polymers 2023, 15, 1980. [Google Scholar] [CrossRef]
- Felfel, R.M.; Gideon-Adeniyi, M.J.; Zakir Hossain, K.M.; Roberts, G.A.F.; Grant, D.M. Structural, mechanical and swelling characteristics of 3D scaffolds from chitosan-agarose blends. Carbohydr. Polym. 2019, 204, 59–67. [Google Scholar] [CrossRef]
- Goodwin, L.; Carra, I.; Campo, P.; Soares, A. Treatment options for reclaiming wastewater produced by the pesticide industry. Int. J. Water Wastewater Treat. 2017, 4, 1–15. [Google Scholar]
- Rana, A.; Mishra, Y.K.; Gupta, V.K.; Thakur, V.K. Sustainable materials in the removal of pesticides from contaminated water: Perspective on macro to nanoscale cellulose. Sci. Total. Environ. 2021, 797, 149129. [Google Scholar] [CrossRef]
- Persson, I. Hydrated metal ions in aqueous solution: How regular are their structures? Pure Appl. Chem. 2010, 82, 1901–1917. [Google Scholar] [CrossRef]
- Yanyang, L.; Yangly, K.; Shang, Q.; Yang, X.; Wang, D.; Liao, G. Fabrication of multifunctional biomass-based aerogel with 3D hierarchical porous structure from waste reed for the synergetic adsorption of dyes and heavy metal ions. Chem. Eng. J. 2023, 451, 138934. [Google Scholar]






| PEC | mCS, g | Cpolyanion, % | mpolyanion, g | Notes |
|---|---|---|---|---|
| CS/CMC1–0.5/1 | 0.05 | 1 | 0.1 | Partial decomplexation during washing |
| CS/CMC1–1/1 | 0.1 | 1 | 0.1 | Partial decomplexation during drying |
| CS/CMC1–1.5/1 | 0.15 | 1 | 0.1 | Partial decomplexation during washing |
| CS/DA-CMC1 | 0.1 | 1 | 0.1 | Does not form gel |
| CS/CMC2–0.5/1 | 0.1 | 2 | 0.2 | Gel stable during washing |
| CS/CMC2–1/1 | 0.4 | 2 | 0.4 | Gel stable during washing |
| CS/CMC2–1.5/1 | 0.3 | 2 | 0.2 | Gel stable during washing |
| CS/DA-CMC2.1–1.1 | 0.24 | 2 | 0.24 | Gel stable during washing; Brittle after drying |
| CS/DA-CMC2.2–1.1 | 0.24 | 2 | 0.24 | Gel stable during washing; Brittle after drying |
| CS/DA-CMC2.3–0.5/1 | 0.2 | 2 | 0.4 | Gel stable during washing; Brittle after drying |
| CS/DA-CMC2.3–1/1 | 0.4 | 2 | 0.4 | Gel stable during washing; Brittle after drying |
| CS/DA-CMC2.3–1.5/1 | 0.3 | 2 | 0.2 | Gel stable during washing; Brittle after drying |
| Sample | CMC, mmol | NaIO4, mmol | AC, % | CCOOH, mmol/g |
|---|---|---|---|---|
| CMC1 | - | - | - | 3.36 |
| DA-CMC1 | 22.90 | 22.90 | 68.9 | 3.78 |
| CMC2 | - | - | - | 3.72 |
| DA-CMC2.1 | 21.37 | 3.562 | 23.8 | 3.77 |
| DA-CMC2.2 | 21.37 | 8.905 | 40.7 | 3.84 |
| DA-CMC2.3 | 21.37 | 21.37 | 60.2 | 4.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghiorghita, C.-A.; Lazar, M.M.; Ghimici, L.; Dinu, M.V. Self-Assembled Chitosan/Dialdehyde Carboxymethyl Cellulose Hydrogels: Preparation and Application in the Removal of Complex Fungicide Formulations from Aqueous Media. Polymers 2023, 15, 3496. https://doi.org/10.3390/polym15173496
Ghiorghita C-A, Lazar MM, Ghimici L, Dinu MV. Self-Assembled Chitosan/Dialdehyde Carboxymethyl Cellulose Hydrogels: Preparation and Application in the Removal of Complex Fungicide Formulations from Aqueous Media. Polymers. 2023; 15(17):3496. https://doi.org/10.3390/polym15173496
Chicago/Turabian StyleGhiorghita, Claudiu-Augustin, Maria Marinela Lazar, Luminita Ghimici, and Maria Valentina Dinu. 2023. "Self-Assembled Chitosan/Dialdehyde Carboxymethyl Cellulose Hydrogels: Preparation and Application in the Removal of Complex Fungicide Formulations from Aqueous Media" Polymers 15, no. 17: 3496. https://doi.org/10.3390/polym15173496





