Strong Bioactive Glass-Based Hybrid Implants with Good Biomineralization Activity Used to Reduce Formation Duration and Improve Biomechanics of Bone Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
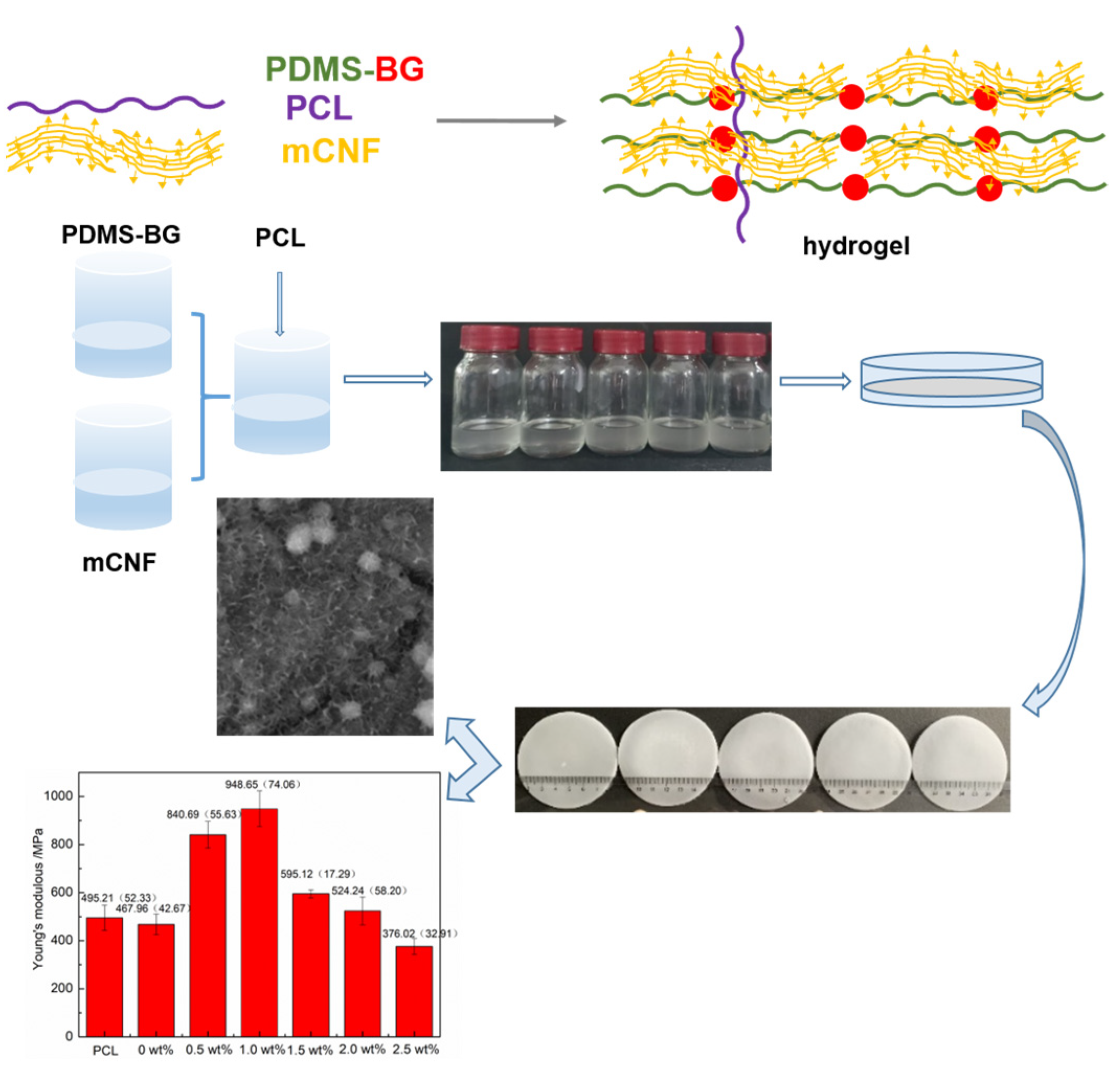
2.2. Synthesis of mCNF–BP Hybrids
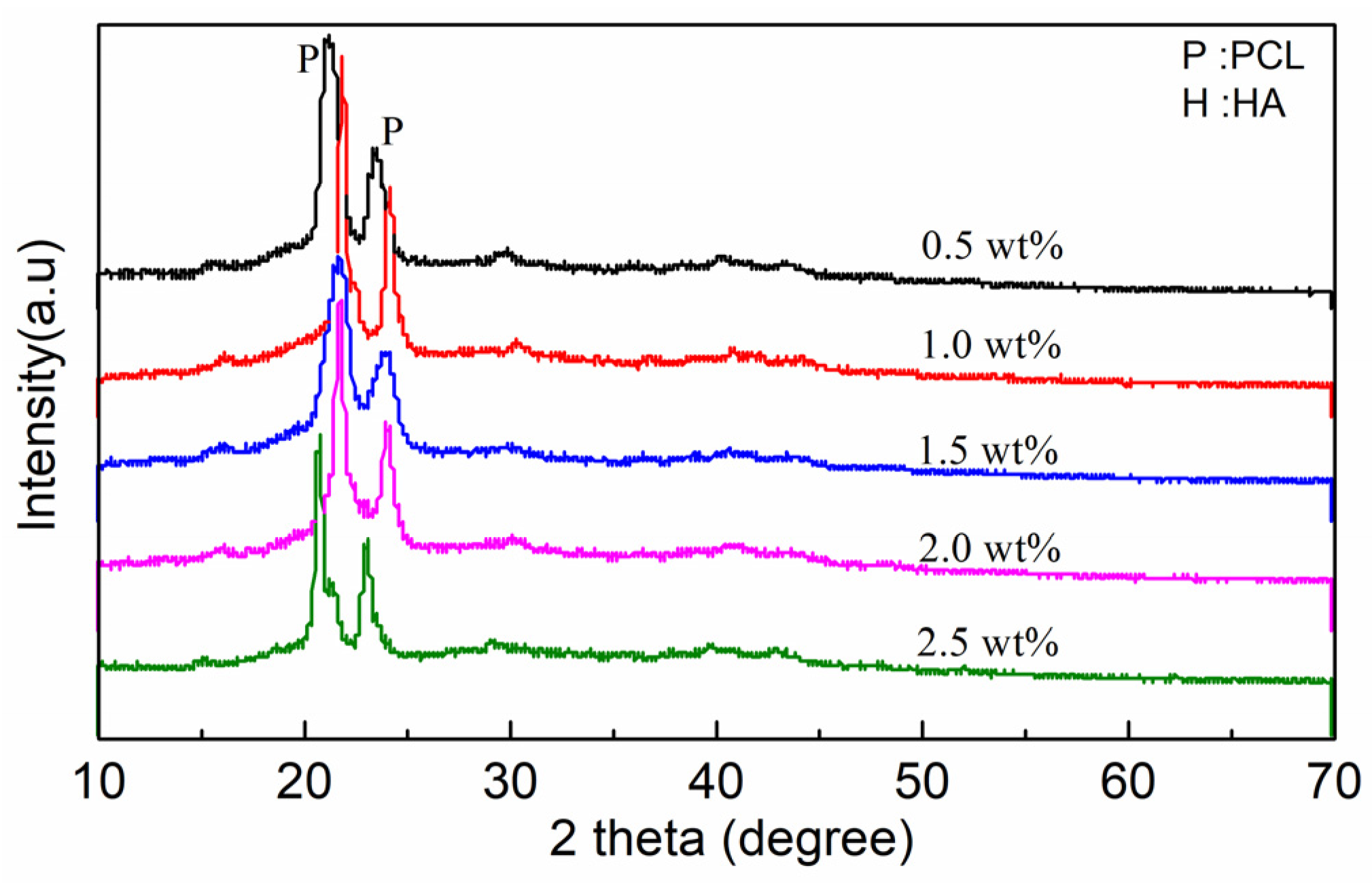
2.3. Characterization
2.4. Biomineralization Activity Test
2.5. Mechanical Property Test
2.6. Hydrophilicity Measurement
2.7. In Vitro Cytotoxicity Tests
2.8. Statistics
3. Results
3.1. Mechanical Performance Test
3.2. mCNF–BP Hydrophilicity Test
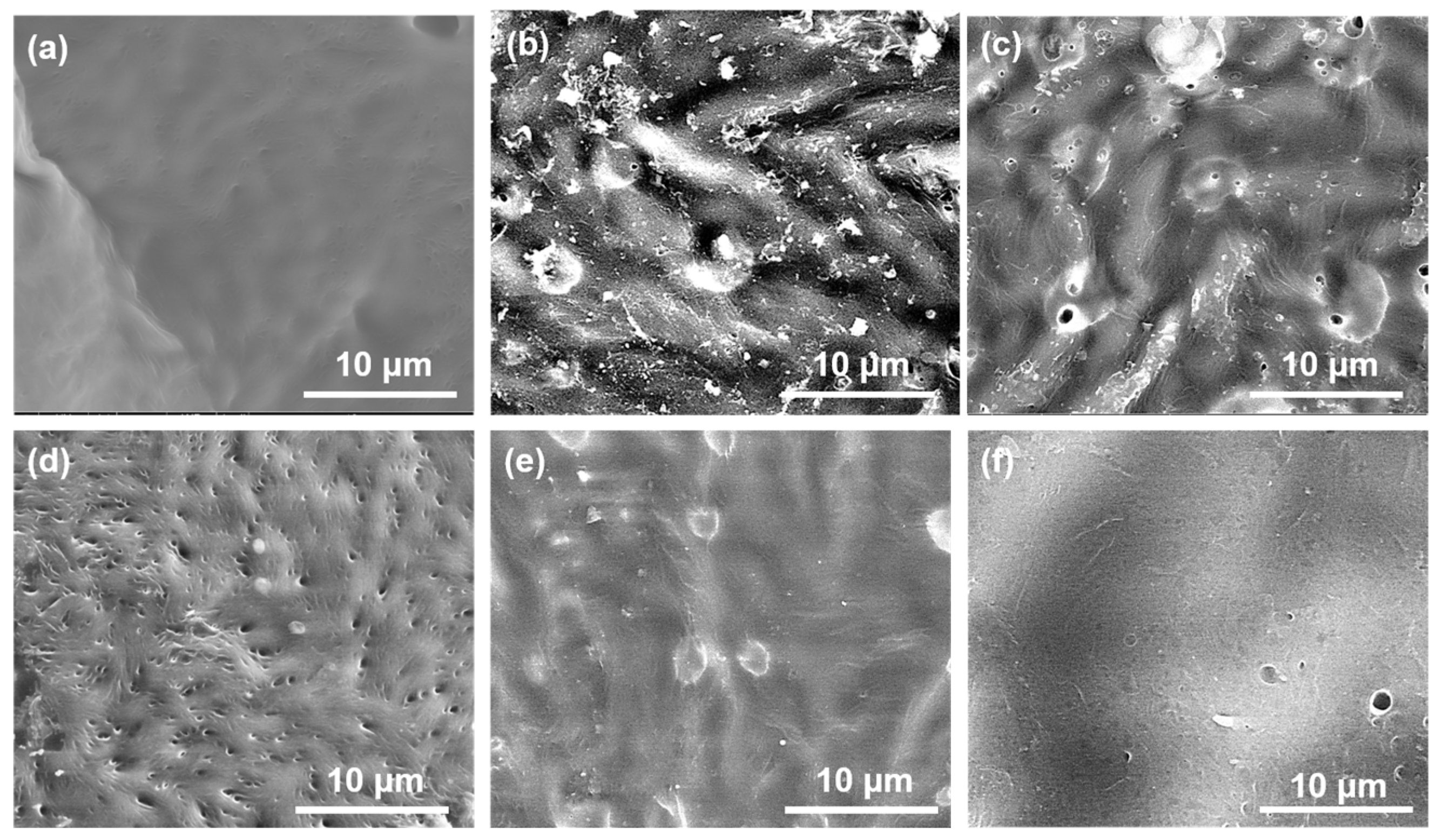
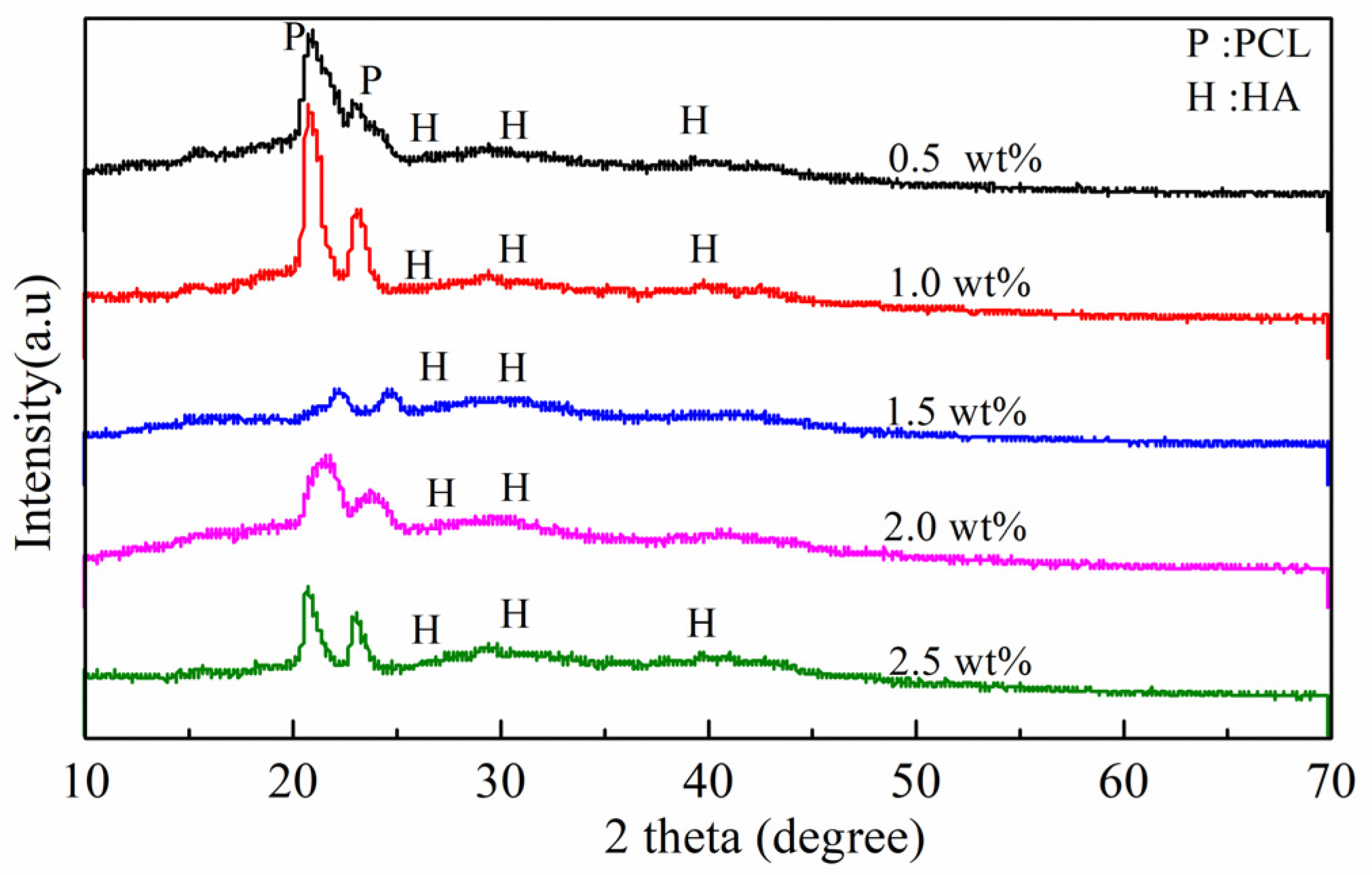
3.3. Biomineralization Activity Measurement
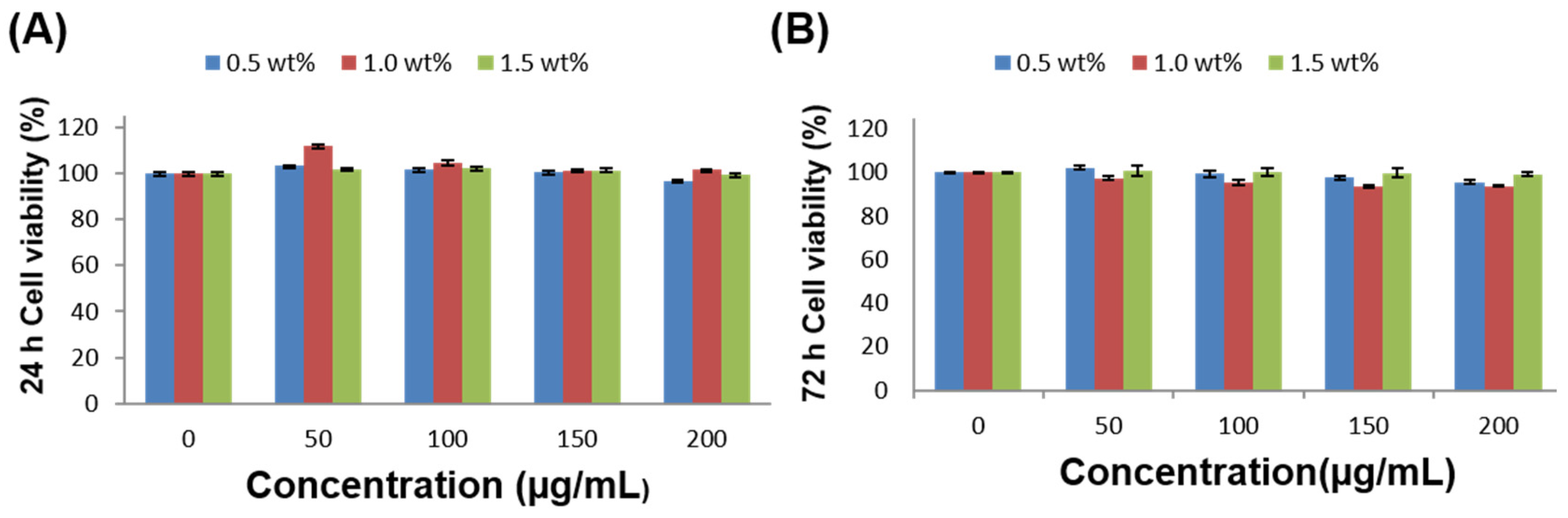
3.4. In Vitro Biocompatibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Amini, A.R.; Adams, D.J.; Laurencin, C.T.; Nukavarapu, S.P. Optimally Porous and Biomechani-cally Compatible Scaffolds for Large-Area Bone Regeneration Tissue. Eng. Part A 2012, 18, 1376–1388. [Google Scholar] [CrossRef]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Perić Kačarević, Ž.; Rider, P.; Alkildani, S.; Retnasingh, S.; Pejakić, M.; Schnettler, R.; Gosau, M.; Smeets, R.; Jung, O.; Barbeck, M. An introduction to bone tissue engineering. Int. J. Artif. Organs. 2019, 43, 69–86. [Google Scholar] [CrossRef]
- Singh, N.B.; Agarwal, S. Nanocomposites: An overview. Emerg. Mater. Res. 2016, 5, 5–43. [Google Scholar] [CrossRef]
- Chen, Q.; Kamitakahara, M.; Miyata, N.; Kokubo, T. Preparation of Bioactive PDMS-Modified CaO-SiO2-TiO2 Hybrids by the Sol-Gel Method. J. Sol.-Gel. Sci. Technol. 2000, 19, 101–105. [Google Scholar] [CrossRef]
- Sonatkar, J.; Kandasubramanian, B. Bioactive glass with biocompatible polymers for bone applications. Eur. Polym. J. 2021, 160, 110801. [Google Scholar] [CrossRef]
- Loty, C.; Sautier, J.M.; Boulekbache, H.; Kokubo, T.; Kim, H.M.; Forest, N. In vitro bone formation on a bone-like apatite layer prepared by a biomimetic process on a bioactive glass–ceramic. J. Biomed. Mater. Res. B Appl. Biomater. 2000, 49, 423. [Google Scholar] [CrossRef]
- Zhang, X.; Nguyen, H.; SonBinh, M.D.; Nguyen, T.; Espinosa, H.D. Nanoscale toughening of ultrathin graphene oxide-polymer composites: Mechanochemical insights into hydrogen-bonding/van der Waals interactions, polymer chain alignment, and steric parameters. Nanoscale 2019, 11, 12305–12316. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Que, W.; Xing, Y.; Lei, B. Content-dependent biomineralization activity and mechanical properties based on polydimethylsiloxane–bioactive glass- poly (caprolactone) hybrids monoliths for bone tissue regeneration. RSC Adv. 2015, 5, 61309–61317. [Google Scholar] [CrossRef]
- Chen, J.; Que, W.; Xing, Y.; Lei, B. Highly bioactive polysiloxane modified bioactive glass-poly (ethyleneglycol) hybrids monoliths with controlled surface structure for bone tissue regeneration. Appl. Surf. Sci. 2015, 332, 542–548. [Google Scholar] [CrossRef]
- Berglund, L.; Forsberg, F.; Jonoobi, M.; Oksman, K. Promoted hydrogel formation of lignin-containing arabinoxylan aerogel using cellulose nanofibers as a functional biomaterial. RSC. Adv. 2018, 8, 38219–38228. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, Y.; Etienne, M.; Sibottier, E.; Walcarius, A. Microscale Controlled Electrogeneration of Patterned Mesoporous Silica Thin Films. Chem. Mater. 2011, 23, 5313–5322. [Google Scholar] [CrossRef]
- Lowery, J.L.; Datta, N.; Rutledge, G.C. Effect of fiber diameter, pore size and seeding method on growth of human dermal fibroblasts in electrospun poly (ε-caprolactone) fibrous mats. Biomaterials 2010, 31, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhan, J.; Su, Y.; Wu, T.; Wu, C.; Ramakrishna, S.; Mo, X.; Al-Deyab, S.S.; El-Newehy, M. Effects of plasma treatment to nanofibers on initial cell adhesion and cell morphology. Colloids Surf. B 2014, 113, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Sagarika, V.K.; Sabu, C.; Kalarikkal, N.; Thomas, S. Cellulose nanocomposites: Fabrication and biomedical applications. J. Bioresour. Bioprod. 2020, 5, 223–237. [Google Scholar] [CrossRef]
- Shimizu, Y.; Sakakibara, K.; Akimoto, S.; Tsujii, Y. Effective reinforcement of Poly (methyl methacrylate) composites with a well-Defined Bacterial cellulose nanofiber network. ACS Sustain. Chem. Eng. 2019, 7, 13351–13358. [Google Scholar] [CrossRef]
- Wang, C.; Wang, L.; Zhang, Q.; Cheng, L.; Yue, H.; Xia, X.; Zhou, H. Preparation and characterization of apoacynum venetum cellulose nanofibers reinforced chitosan-based composite hydrogels. Colloids Surf. B 2021, 199, 111441. [Google Scholar] [CrossRef]
- De France, K.J.; Hoare, T.; Cranston, E.D. A Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, G.; Guan, G. Nanocellulose: Extraction and application. Carbon. Resour. Convers. 2018, 1, 32–43. [Google Scholar] [CrossRef]
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nanocomposites—A review. Biotechnol. Rep. 2019, 21, e00316. [Google Scholar] [CrossRef]
- Nascimento, D.M.; Nunes, Y.L.; Figueirêdo, M.C.B.; De Azeredo, H.M.C.; Aouada, F.A.; Feitosa, J.P.A.; Rosa, M.F.; Dufresne, A. Nanocellulose nanocomposite hydrogels: Technological and environmental issues. Green Chem. 2018, 20, 2428–2448. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Habibi, Y.; Adhikari, B. Surface modifications of nanocellulose: From synthesis to high-performance nanocomposites. Prog. Polym. Sci. 2021, 119, 101418. [Google Scholar] [CrossRef]
- Yang, Z.; Cai, J.; Zhou, C.; Zhou, D.; Chen, B.; Yang, H.; Cheng, R. Effects of the Content of Silane Coupling Agent KH-560 on the Properties of LLDPE /Magnesium Hydroxide Composites. J. Appl. Polym. Sci. 2010, 118, 2634–2641. [Google Scholar] [CrossRef]
- Pan, Y.; Wang, M.Z.; Xiao, H. Biocomposites containing cellulose fibers treated with nanosized elastomeric latex for enhancing impact strength. Compos. Sci. Technol. 2013, 77, 81–86. [Google Scholar] [CrossRef]
- Hossain, M.A.; Roy, C.K.; Sarkar, S.D.; Roy, H.; Howlader, A.H.; Firoz, S.H. Improvement of Strength of Poly(acrylic acid) Hydrogels by the Incorporation of Functionally Modified Nanocrystalline Cellulose. Mater. Adv. 2020, 1, 2107–2116. [Google Scholar] [CrossRef]
- Lei, B.; Guo, B.; Rambhia, K.J.; Ma, P.X. Hybrid polymer biomaterials for bone tissue regeneration. Front. Med. 2019, 13, 189–201. [Google Scholar] [CrossRef]
- Si, J.; Cui, Z.; Wang, Q.; Liu, Q.; Liu, C. Biomimetic composite scaffolds based on mineralization of hydroxyapatite on electrospun poly(rmvarepsilon-caprolactone)/nanocellulose fibers. Carbohyd. Polym. 2016, 143, 270–278. [Google Scholar] [CrossRef]
- Li, J.J.; Chen, Y.P.; Yin, Y.J.; Yao, F.L.; Yao, K.D. Modulation of nano-hydroxyapatite size via formation on chitosan-gelatin network film in situ. Biomaterials 2007, 28, 781. [Google Scholar] [CrossRef]
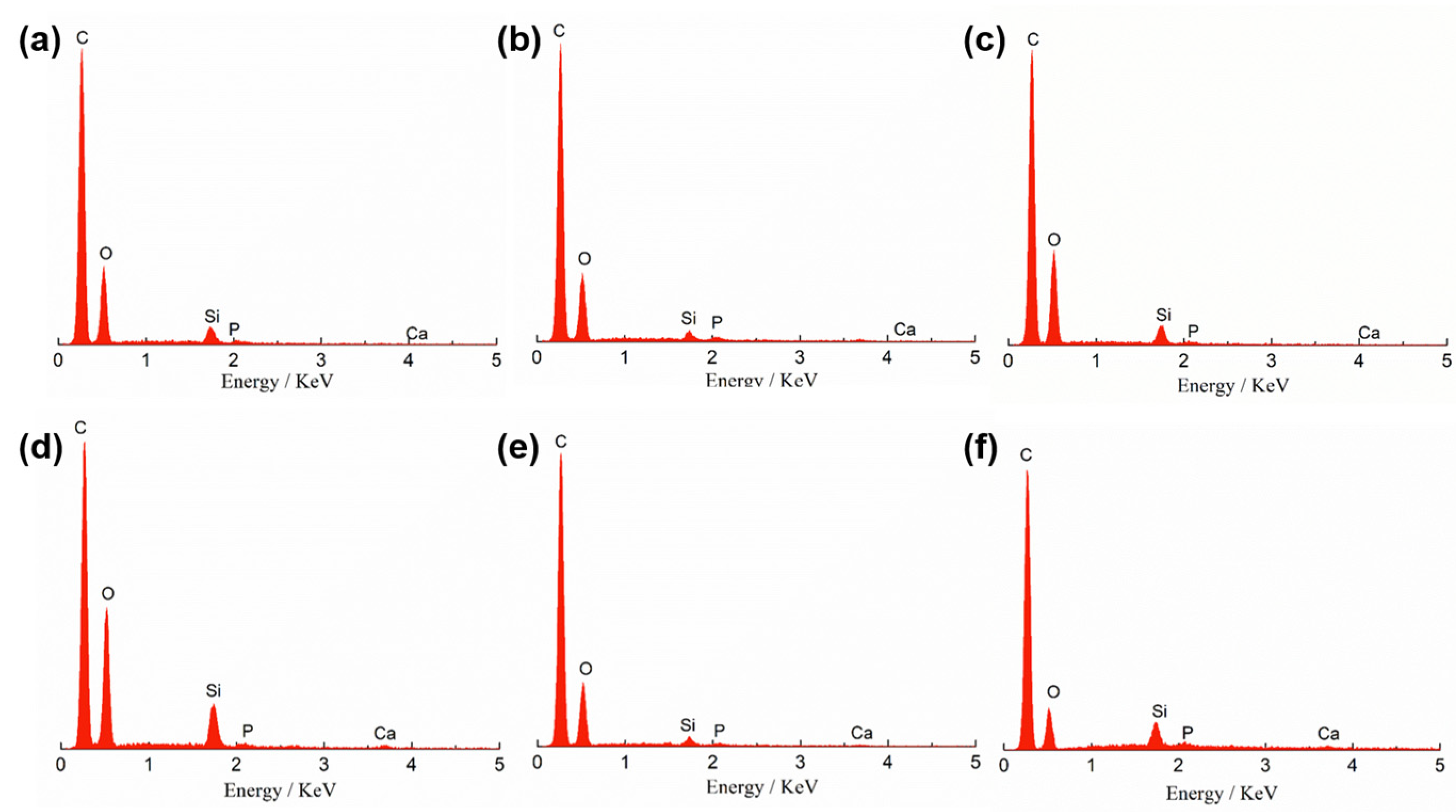



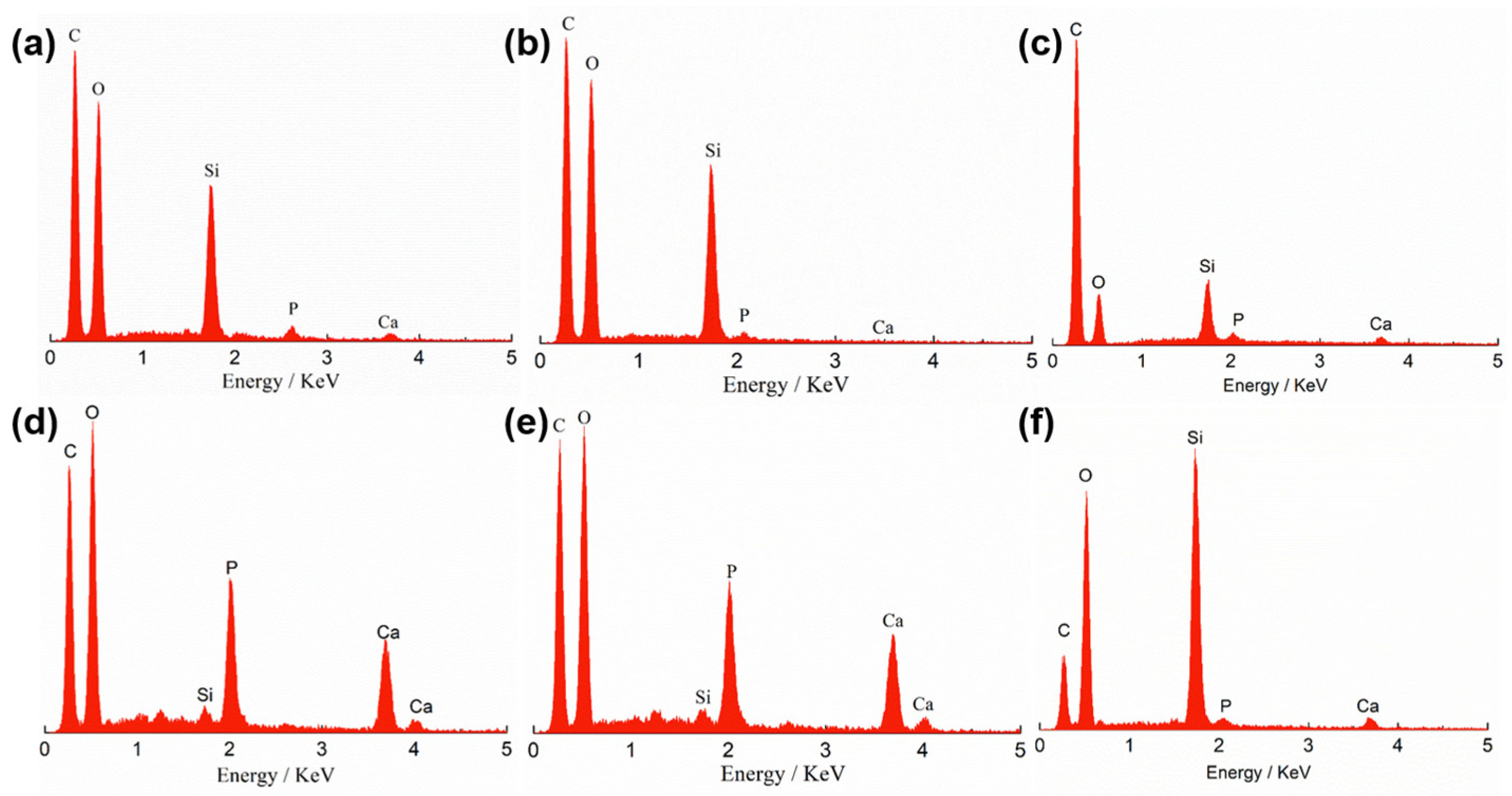
| Si | Ca | P | C | O | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mass (%) | Atom (%) | Mass (%) | Atom (%) | Mass (%) | Atom (%) | Mass (%) | Atom (%) | Mass (%) | Atom (%) | |
| 0.5 wt%-0d | 2.52 | 1.17 | 0.19 | 0.06 | 0.3 | 0.13 | 72 | 78.25 | 24.99 | 20.39 |
| 1.0 wt%-0d | 2.97 | 1.39 | 0.33 | 0.11 | 0.54 | 0.23 | 70.2 | 76.91 | 25.97 | 21.36 |
| 1.5 wt%-0d | 5.54 | 2.7 | 1.23 | 0.42 | 0.45 | 0.2 | 60.46 | 68.99 | 32.32 | 27.69 |
| 2.0 wt%-0d | 1.42 | 0.66 | 0.8 | 0.26 | 0.43 | 0.18 | 73.22 | 79.29 | 24.12 | 19.61 |
| 0.5 wt%-3d | 3.08 | 1.43 | 0.64 | 0.21 | 0.55 | 0.23 | 73.89 | 80.31 | 21.83 | 17.82 |
| 1.0 wt%-3d | 1.6 | 0.75 | 1.07 | 0.35 | 0.76 | 0.32 | 71.8 | 78.3 | 24.78 | 20.28 |
| 1.5 wt%-3d | 1.21 | 0.75 | 22.07 | 9.66 | 11.33 | 6.42 | 31.4 | 45.88 | 34 | 37.29 |
| 2.0 wt%-3d | 1.11 | 0.7 | 21.76 | 9.56 | 11.76 | 6.7 | 30.48 | 44.67 | 34.87 | 38.37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Xing, Y.; Bai, X.; Xue, M.; Shi, Q.; Li, B. Strong Bioactive Glass-Based Hybrid Implants with Good Biomineralization Activity Used to Reduce Formation Duration and Improve Biomechanics of Bone Regeneration. Polymers 2023, 15, 3497. https://doi.org/10.3390/polym15173497
Chen J, Xing Y, Bai X, Xue M, Shi Q, Li B. Strong Bioactive Glass-Based Hybrid Implants with Good Biomineralization Activity Used to Reduce Formation Duration and Improve Biomechanics of Bone Regeneration. Polymers. 2023; 15(17):3497. https://doi.org/10.3390/polym15173497
Chicago/Turabian StyleChen, Jing, Yonglei Xing, Xiaozhuan Bai, Min Xue, Qi Shi, and Beibei Li. 2023. "Strong Bioactive Glass-Based Hybrid Implants with Good Biomineralization Activity Used to Reduce Formation Duration and Improve Biomechanics of Bone Regeneration" Polymers 15, no. 17: 3497. https://doi.org/10.3390/polym15173497
APA StyleChen, J., Xing, Y., Bai, X., Xue, M., Shi, Q., & Li, B. (2023). Strong Bioactive Glass-Based Hybrid Implants with Good Biomineralization Activity Used to Reduce Formation Duration and Improve Biomechanics of Bone Regeneration. Polymers, 15(17), 3497. https://doi.org/10.3390/polym15173497







