Synthesis of Poly (2-Acrylamido-2-methylpropanesulfnoinc Salt) Modified Carbon Spheres
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
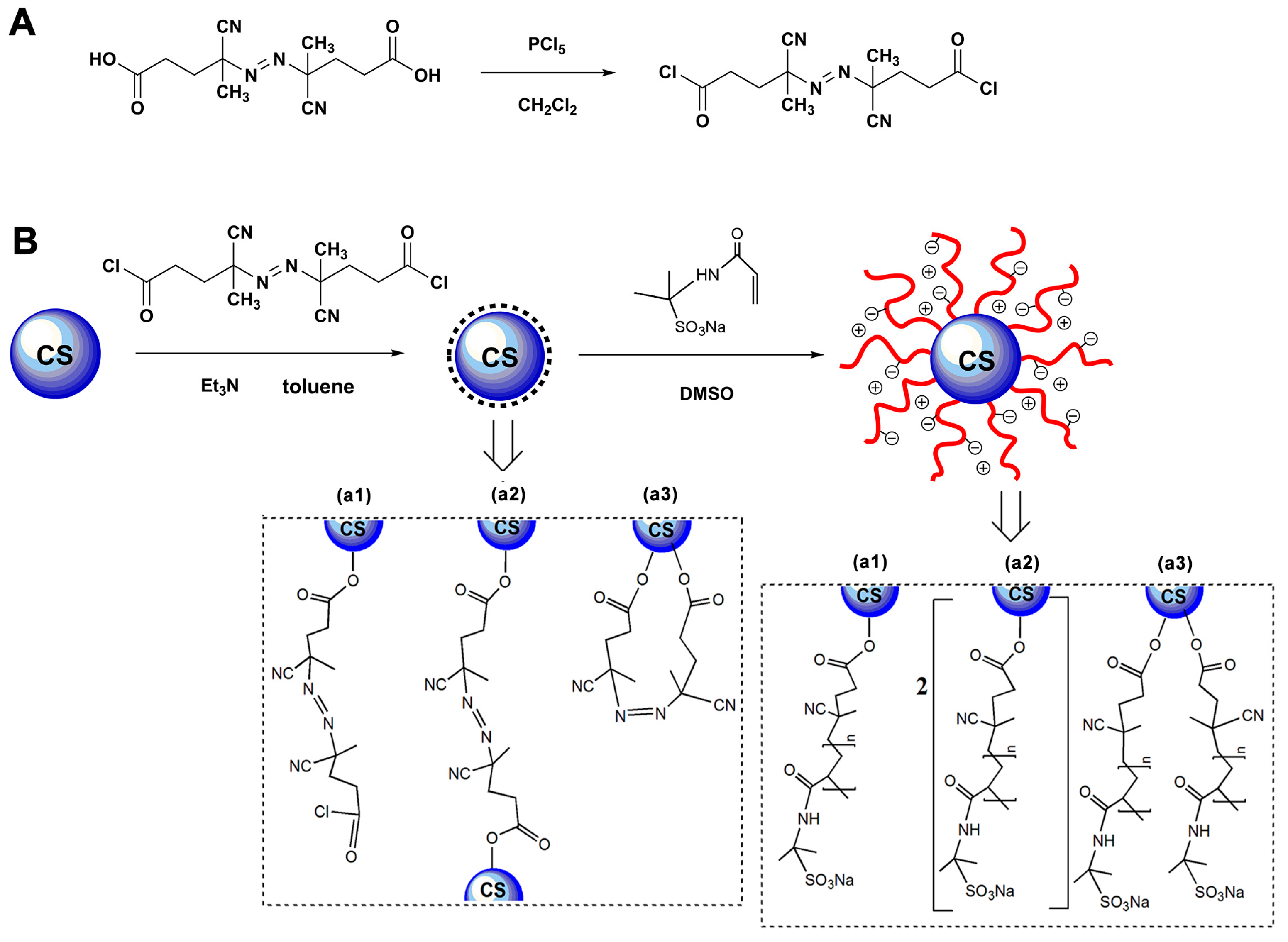
2.2. Preparation of Anionic Spherical Polyelectrolyte Brushes
2.3. Characterization
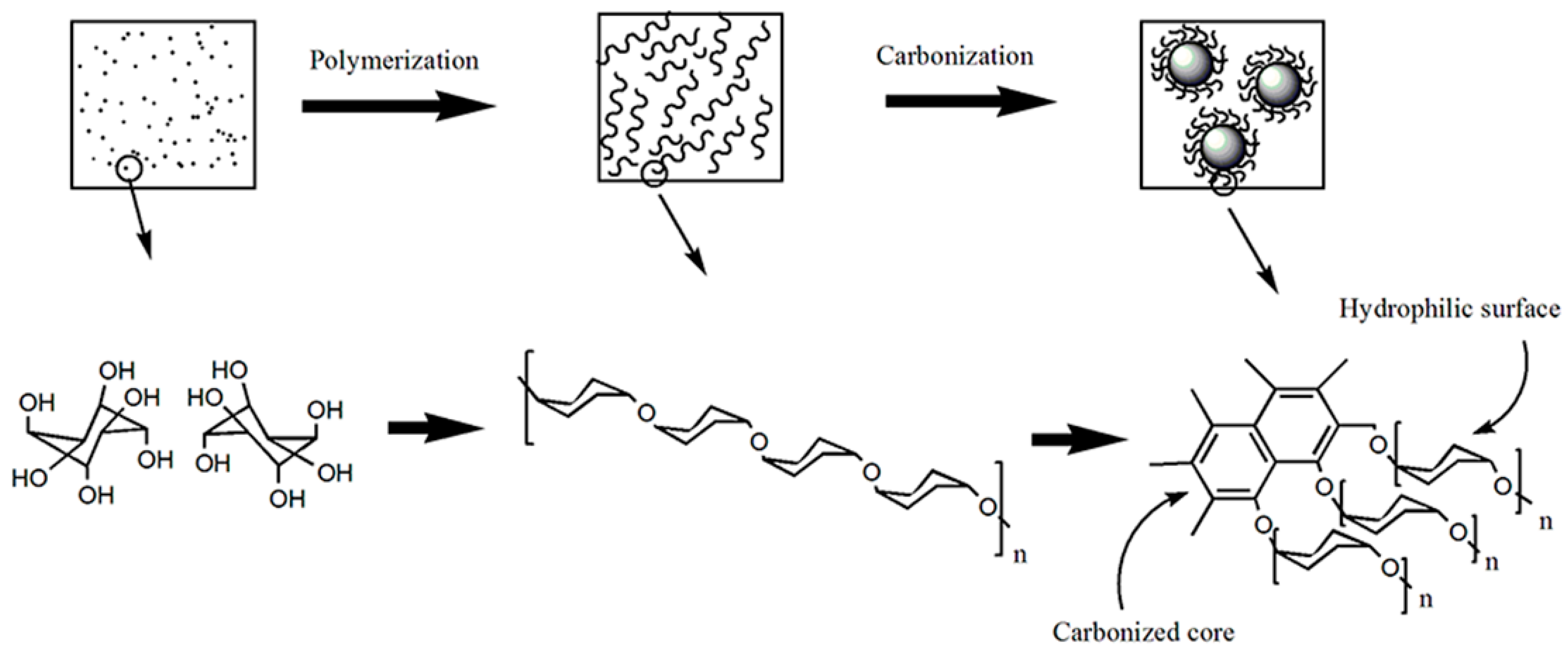
3. Results and Discussion
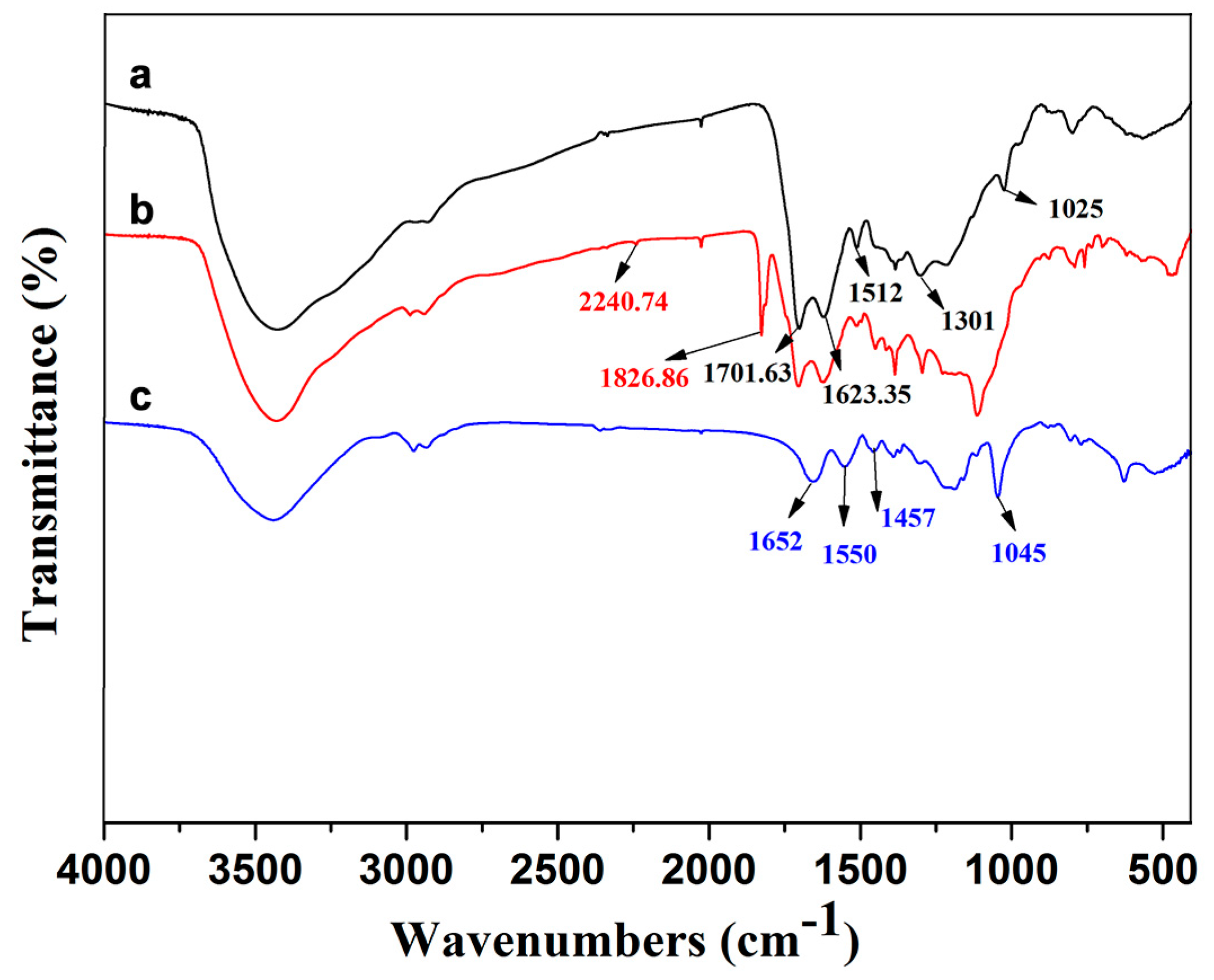
3.1. FTIR and XPS Analyses
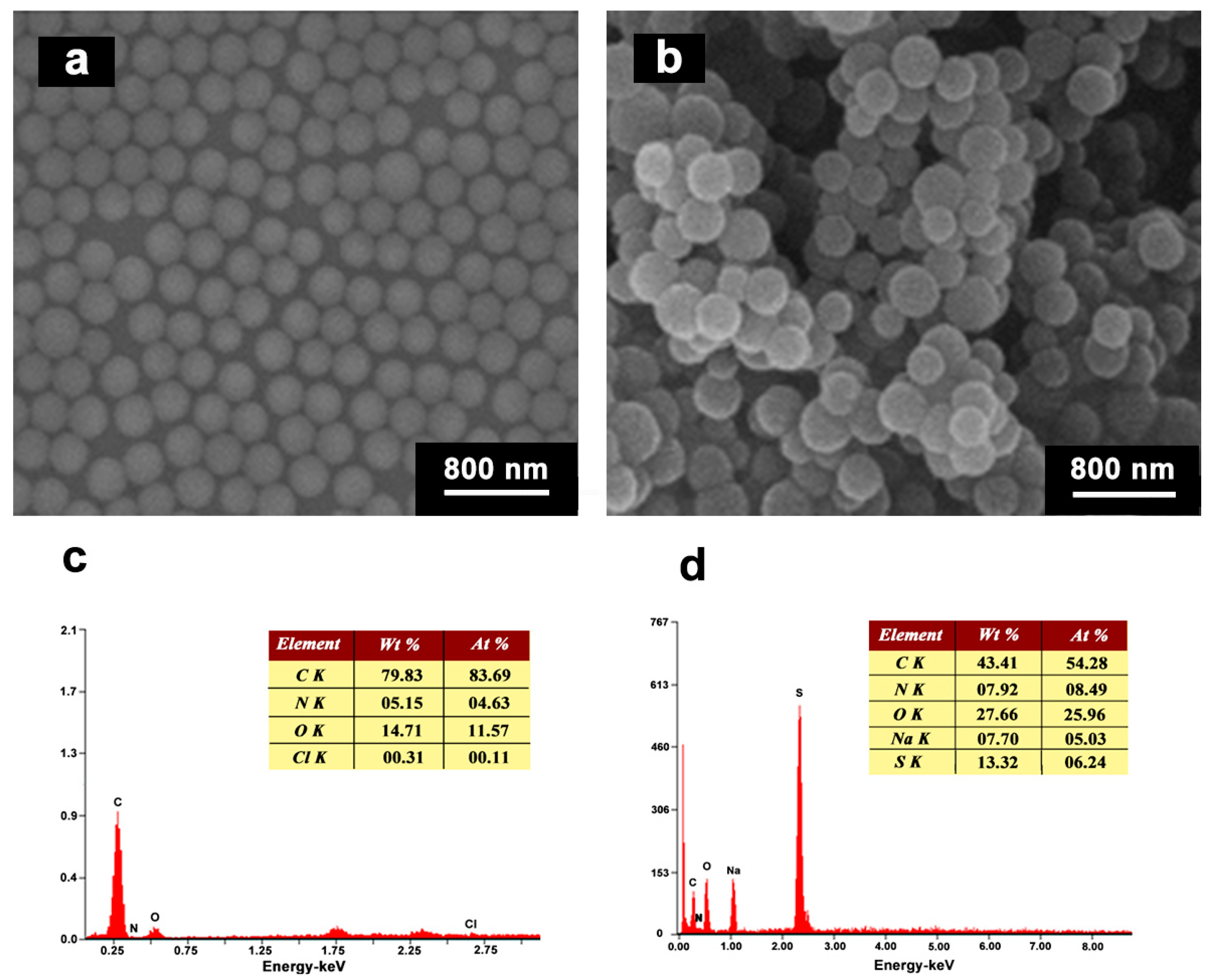
3.2. Morphologies and EDX Analyses
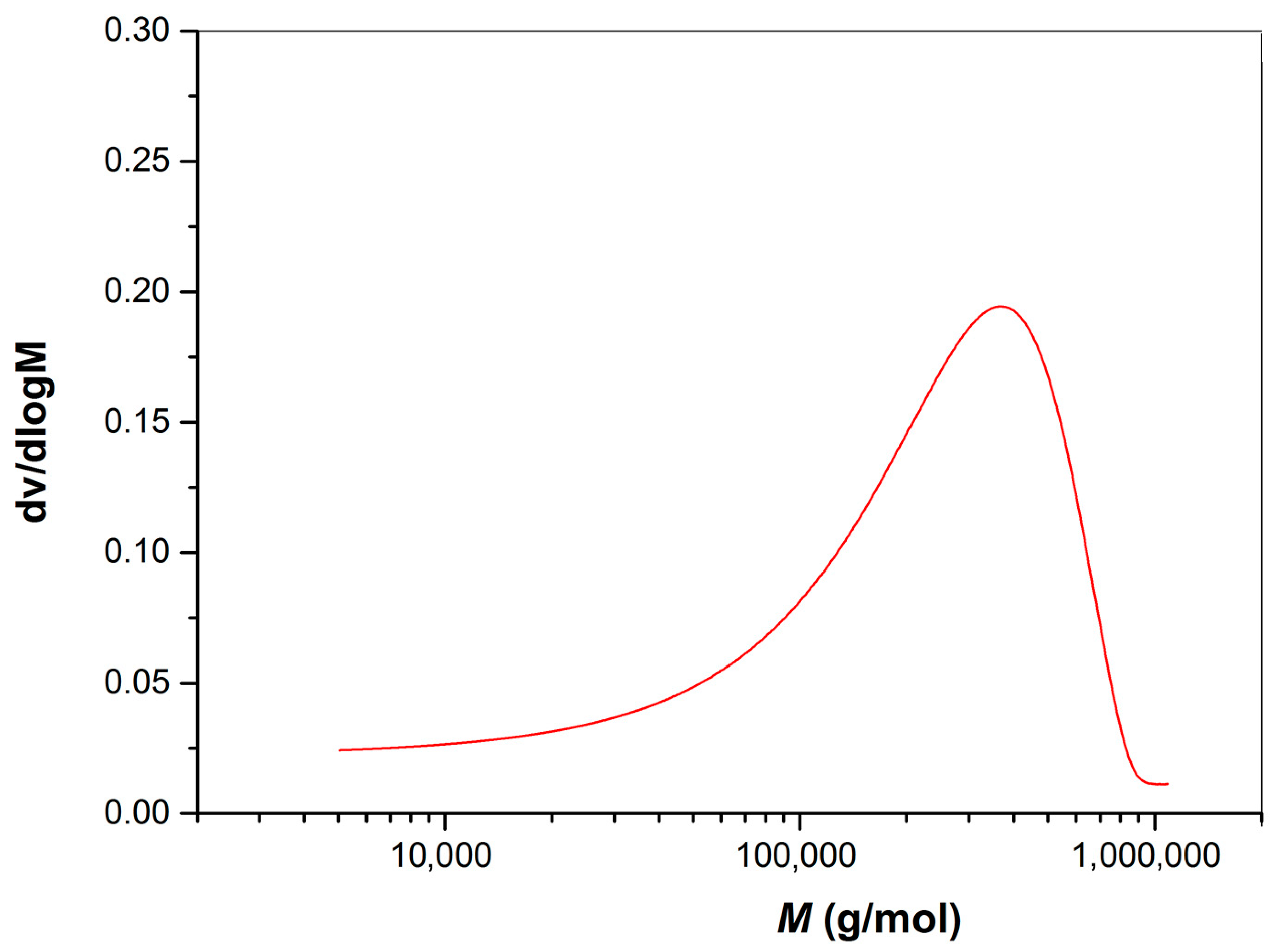
3.3. Molecular Weight
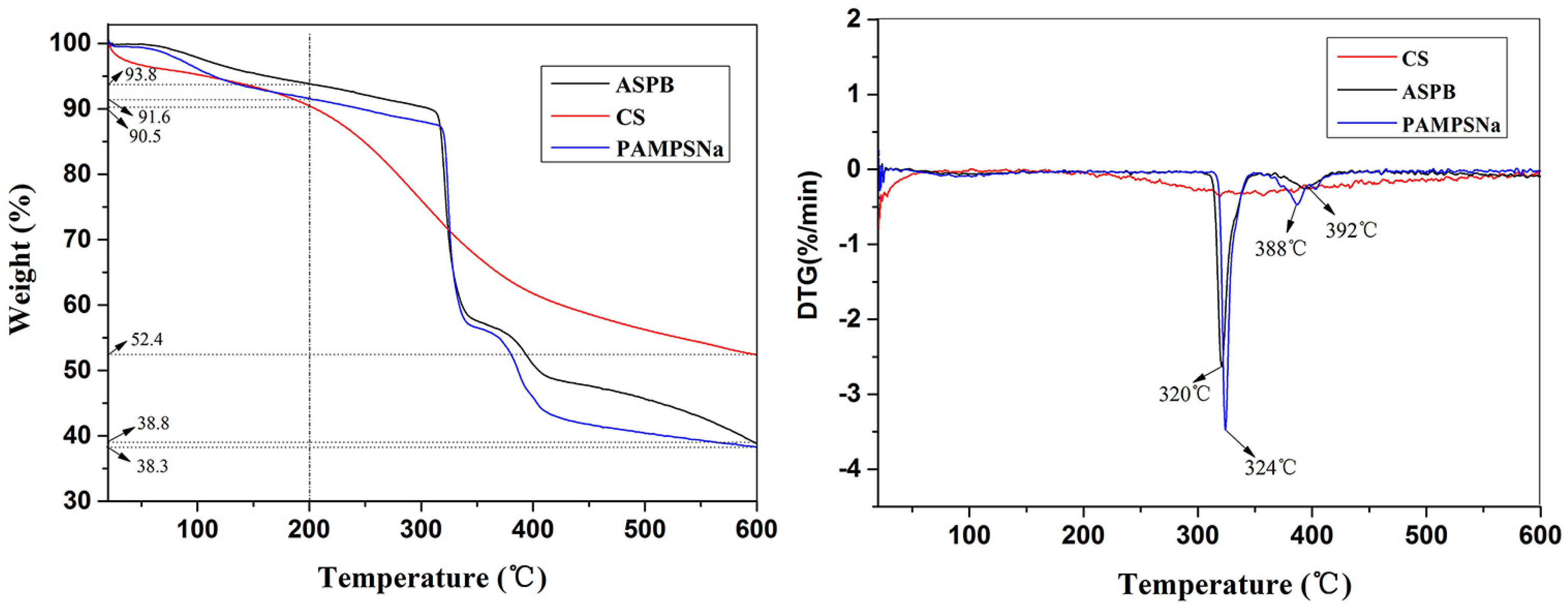
3.4. TGA Curves
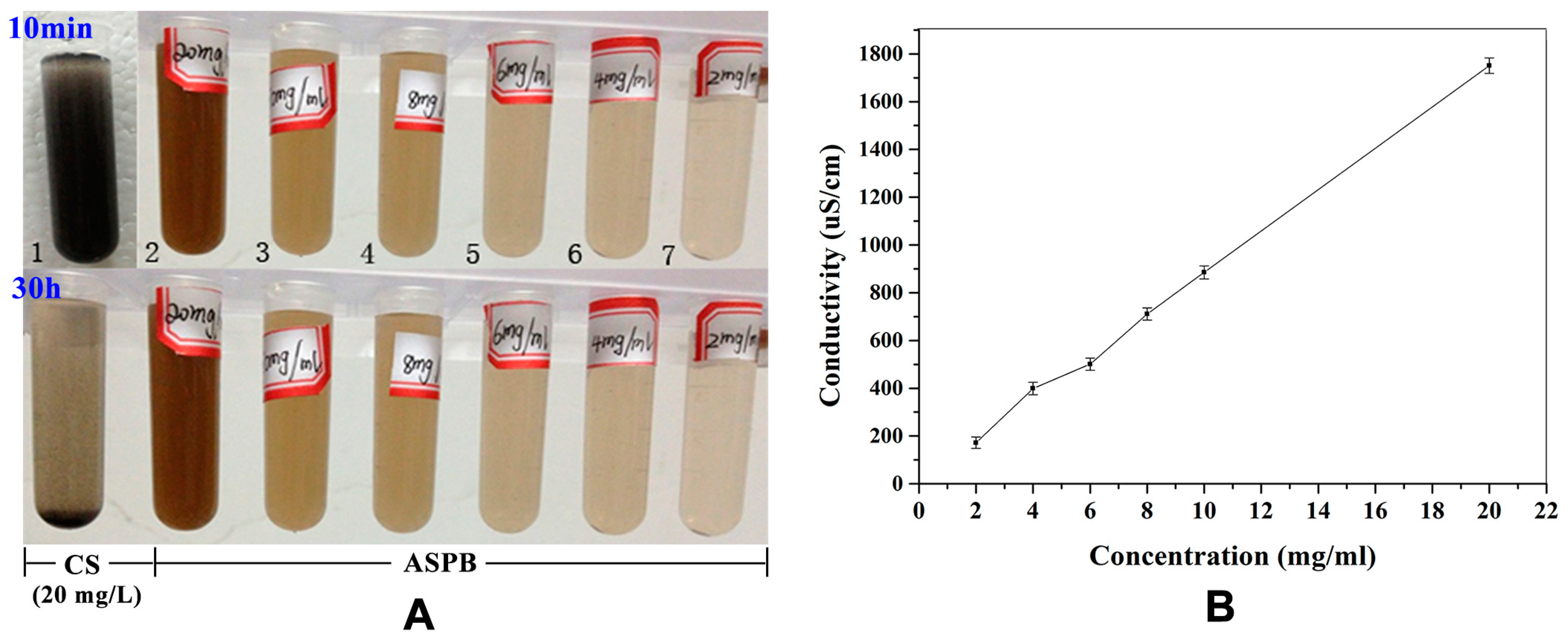
3.5. Solution Conductivity
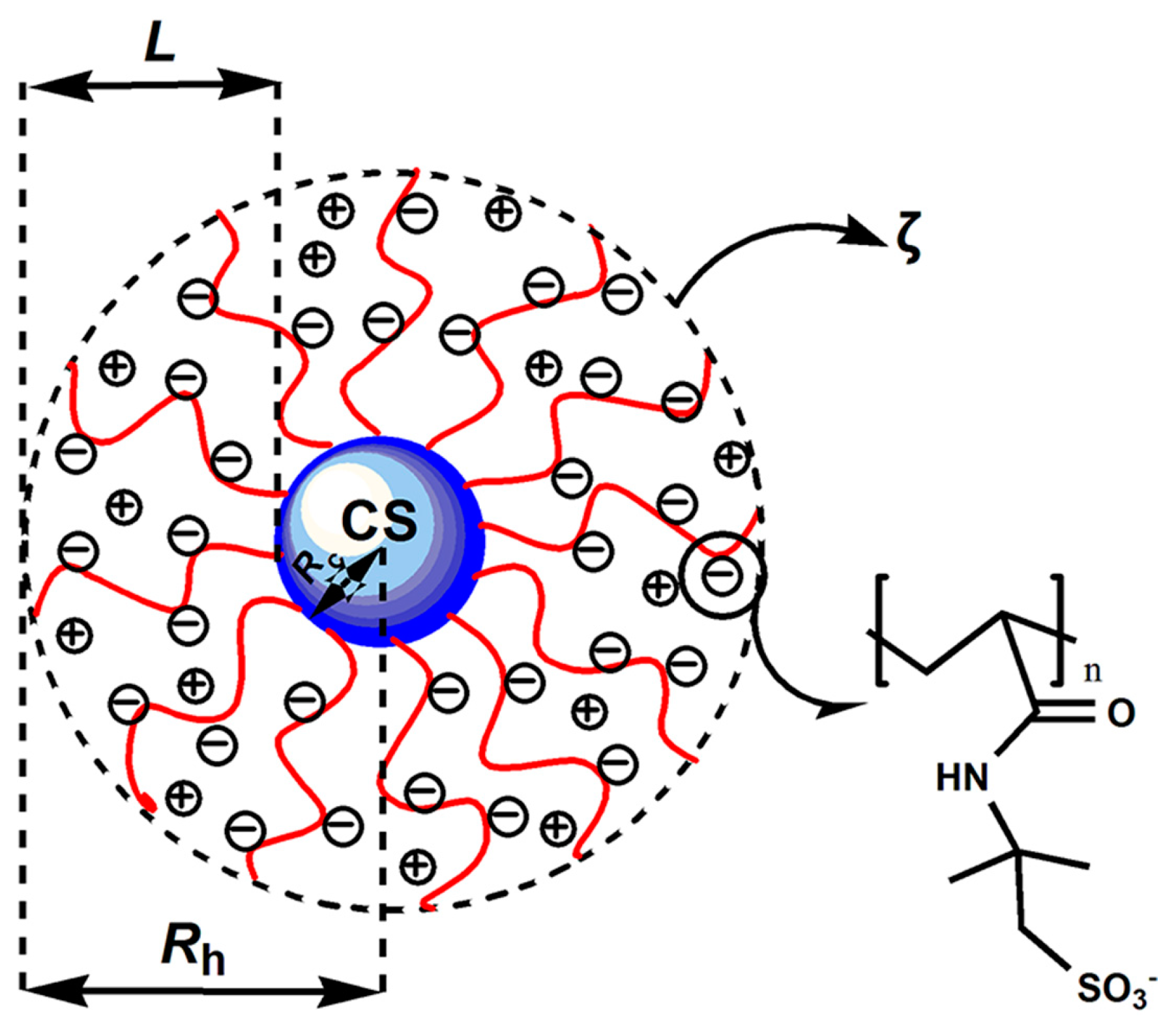
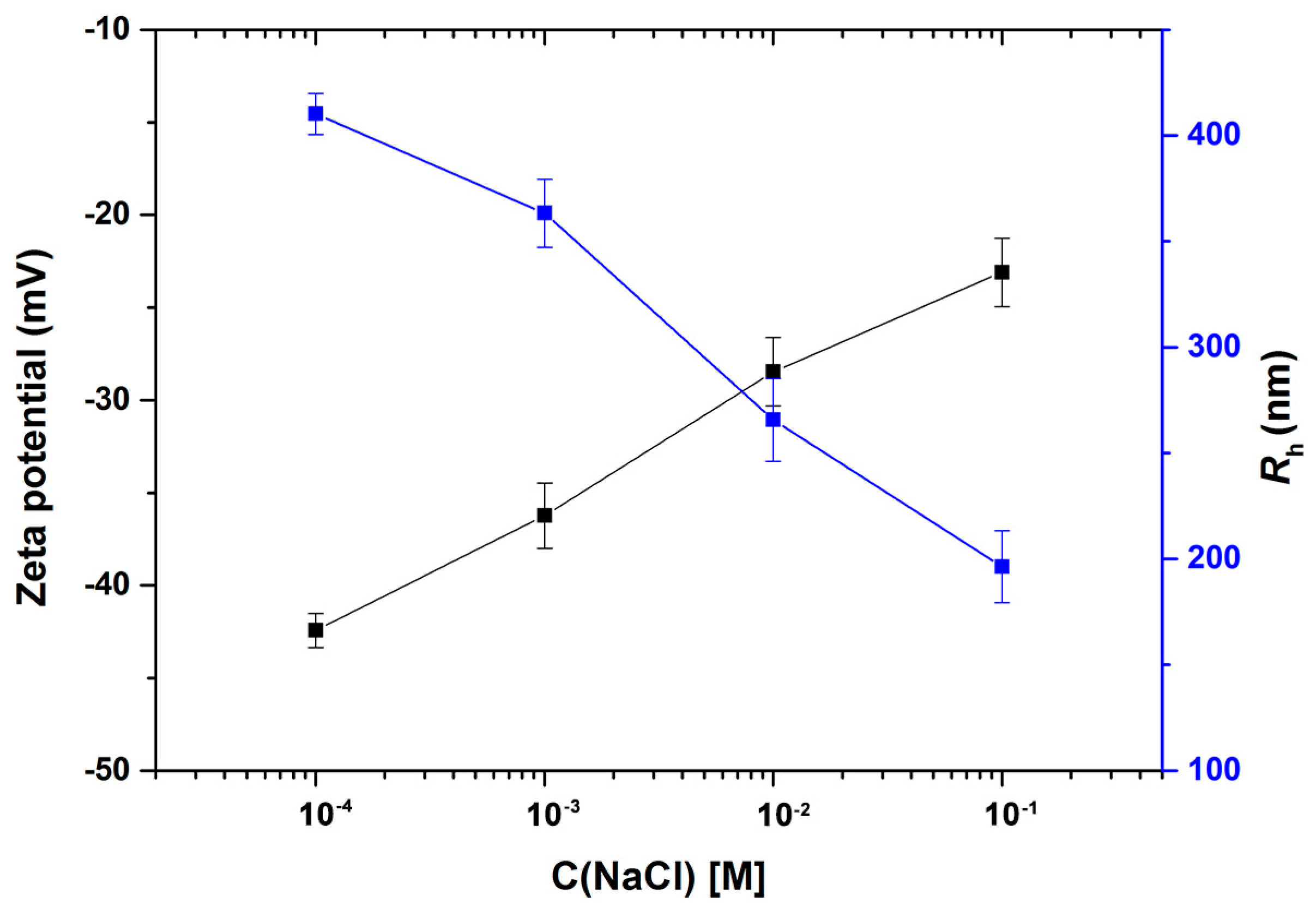
3.6. Zeta Potential and Rh
4. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ballauff, M. Spherical polyelectrolyte brushes. Prog. Polym. Sci. 2007, 32, 1135–1151. [Google Scholar] [CrossRef]
- Yang, Q.; Li, L.; Zhao, F.; Wang, Y.; Ye, Z.; Guo, X. Generation of MnO2 nanozyme in spherical polyelectrolyte brush for colorimetric detection of glutathione. Mater. Lett. 2019, 248, 89–104. [Google Scholar] [CrossRef]
- Lu, Y.; Ballauff, M. Spherical polyelectrolyte brushes as nanoreactors for the generation of metallic and oxidic nanoparticles: Synthesis and application in catalysis. Prog. Polym. Sci. 2016, 59, 86–90. [Google Scholar] [CrossRef]
- Hasani, S.; Mohamadnia, Z.; Kazem, F. Preparation of microbeads grafted with poly (2-(acryloyloxy)ethyl]trimethylammonium chloride) cationic polyelectrolyte as recyclable and effective adsorbents for organic dyes. React. Funct. Polym. 2021, 169, 105087. [Google Scholar] [CrossRef]
- Suchithra, P.S.; Vazhayal, L.; Mohamed, A.P.; Ananthakumar, S. Mesoporous organic-inorganic hybrid aerogels through ultrasonic assisted sol–gel intercalation of silica-PEG in bentonite for effective removal of dyes, volatile organic pollutants and petroleum products from aqueous solution. Chem. Eng. J. 2012, 200–202, 589–600. [Google Scholar] [CrossRef]
- Wang, W.H.; Li, L.; Henzler, K.; Lu, Y.; Wang, J.Y.; Han, H.; Tian, Y.; Wang, Y.; Zhou, Z.; Lotze, G.; et al. Protein Immobilization onto Cationic Spherical Polyelectrolyte Brushes Studied by Small Angle X-ray Scattering. Biomacromolecules 2017, 18, 1574–1581. [Google Scholar] [CrossRef]
- Han, L.; Yan, B.; Zhang, L.; Wu, M.; Wang, J.; Huang, J.; Deng, Y.; Zeng, H. Tuning protein adsorption on charged polyelectrolyte brushes via salinity adjustment. Colloid Surf. A 2018, 539, 37–45. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Huang, Y.; Fu, K.Q.; Yuan, S.J.; Huang, C.; Li, H.B. Preparation and performance of cationic flocculant for papermaking based on the graft polymerization of cationic chains from colloidal silica particles. Colloid Surf. A 2016, 491, 29–36. [Google Scholar] [CrossRef]
- Mei, Y.; Abetz, C.; Birkert, O.; Schȁdler, V.; Leyrer, R.J.; Ballauff, M. Interaction of Spherical Polyelectrolyte Brushes with Calcium Carbonate and Cellulose Fibers: Mechanistic Studies and Their Application in Papermaking. J. Appl. Polym. Sci. 2006, 102, 233–241. [Google Scholar] [CrossRef]
- Su, N. Polyaniline-Doped Spherical Polyelectrolyte Brush Nanocomposites with Enhanced Electrical Conductivity, Thermal Stability, and Solubility Property. Polymers 2015, 7, 1599–1616. [Google Scholar] [CrossRef]
- Su, N. Improving Electrical Conductivity, Thermal Stability, and Solubility of Polyaniline-Polypyrrole Nanocomposite by Doping with Anionic Spherical Polyelectrolyte Brushes. Nanoscale Res. Lett. 2015, 10, 301. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamaguchi, H.; Terayama, Y.; Wang, Z.; Ishihara, K.; Hino, M.; Takahara, A. Structure and surface properties of high-density polyelectrolyte brushes at the interface of aqueous solution. Macromol. Symp. 2009, 279, 79–87. [Google Scholar] [CrossRef]
- Xiang, L.; Qiu, M.; Shang, M.J.; Su, Y.H. Continuous synthesis of star polymers with RAFT polymerization in cascade microreactor systems. Polymer 2021, 222, 123669. [Google Scholar] [CrossRef]
- Riley, J.K.; Matyjaszewski, K.; Tilton, R.D. Friction and adhesion control between adsorbed layers of polyelectrolyte brush-grafted nanoparticles via pH-triggered bridging interactions. J. Colloid Interf. Sci. 2018, 526, 114–123. [Google Scholar] [CrossRef]
- Slowikowska, M.; Wojcik, A.J.; Wolski, K.; Hatalak, A.; Zapotoczny, S. Light-promoted synthesis of surface-grafted polymers bearing pyridine groups by metal-free ATRP in microliter volumes. Polymer 2021, 234, 124244. [Google Scholar] [CrossRef]
- Gao, T.; Nie, M.; Luo, J.; Huang, Z.; Sun, H.; Guo, P.; Xue, Z.; Liao, J.; Li, Q.; Teng, L. Nickel sulfides supported by carbon spheres as efficient catalysts for hydrogen evolution reaction. Electrochem. Commun. 2021, 129, 107076. [Google Scholar] [CrossRef]
- Norouzi, Z.; Mahmoudi Najafi, S.H.; Mozaffari, S.A. Silver-loaded carbon sphere-in-rod 3D nano-architectures as electrode material for supercapacitors. Diam. Relat. Mater. 2022, 121, 108734. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, X.; Kou, L.; Zhang, X.; Wang, R.; Xie, L.; Fan, C. Facile synthesis of nitrogen-rich porous carbon spheres assisted by NaNH2 as a bifunctional activator and nitrogen source for CO2 capture. J. Environ. Chem. Eng. 2021, 9, 6106605. [Google Scholar] [CrossRef]
- Chen, X.; Wu, Y.; Gu, W.; Zhou, M.; Tang, S.; Cao, J.; Zou, Z.; Ji, G. Research progress on nanostructure design and composition regulation of carbon spheres for the microwave absorption. Carbon 2022, 189, 617–633. [Google Scholar] [CrossRef]
- Afrifah, V.A.; Phiri, I.; Hamenu, L.; Madzvamuse, A.; Lee, K.S.; Ko, J.M. Electrochemical properties of poly (2-acrylamido-2-methylpropane sulfonic acid) polyelectrolyte containing zwitterionic silica sulfobetaine for supercapacitors. J. Power Sources 2020, 479, 228657. [Google Scholar] [CrossRef]
- Ashames, A.; Pervaiz, F.; Al-Tabakha, M.; Khalid, K.; Hassan, N.; Shoukat, H.; Buabeid, M.; Murtaza, G. Synthesis of cross-linked carboxymethyl cellulose and poly (2-acrylamido-2-methylpropane sulfonic acid) hydrogel for sustained drug release optimized by Box-Behnken Design. J. Saudi Chem. Soc. 2022, 26, 101541. [Google Scholar] [CrossRef]
- Mkhari, O.; Ntuli, T.D.; Coville, N.J.; Nxumalo, E.N.; Maubane-Nkadimeng, M.S. A comparison of fluorescent N-doped carbon dots supported on the surface of hollow and solid carbon spheres, and solid silica spheres. Diam. Relat. Mater. 2021, 118, 108500. [Google Scholar] [CrossRef]
- Su, N.; Li, H.B.; Hang, Y.; Zhang, X.Z. Synthesis of Salt Responsive Spherical Polymer Brushes. J. Nanomater. 2015, 2015, 956819. [Google Scholar] [CrossRef]
- Nikolaou, V.; Simula, A.; Droesbeke, M.; Risangud, N.; Anastasaki, A.; Kempe, K.; Wilson, P.; Haddleton, D.M. Polymerisation of 2-acrylamido-2-methylpropane sulfonic acid sodium salt (NaAMPS) and acryloyl phosphatidylcholine (APC) via aqueous Cu (0)-mediated radical polymerization. Polym. Chem. 2016, 7, 2452–2456. [Google Scholar] [CrossRef]
- Zhang, H.; Rȕhe, J. Swelling of poly (methacrylic acid) brushes: Influence of monovalent salts in the environment. Macromolecules 2005, 38, 4855–4860. [Google Scholar] [CrossRef]
- Asifjaved, H.M.; Ahmad, M.I.; Que, W.X.; Qureshi, A.A.; Sarfaraz, M.; Hussain, S.; Lqbal, M.Z.; Nisar, M.Z.; Shahid, M.; Algarni, T.S. Encapsulation of TiO2 nanotubes with Cs nanoparticles to enhance electron injection and thermal stability of perovskite solar cells. Surf. Interfaces 2021, 23, 101033. [Google Scholar]
- Hariharan, R.; Biver, C.; Russel, W.B. Ionic strength effects in polyelectrolyte brushes: The counterion correction. Macromolecules 1998, 31, 7514–7518. [Google Scholar] [CrossRef]
- Kao, J.Y.; Cheng, W.T. Study on Dispersion of TiO2 Nanopowder in Aqueous Solution via Near Supercritical Fluids. ACS Omega 2020, 5, 1832–1839. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, N. Synthesis of Poly (2-Acrylamido-2-methylpropanesulfnoinc Salt) Modified Carbon Spheres. Polymers 2023, 15, 3510. https://doi.org/10.3390/polym15173510
Su N. Synthesis of Poly (2-Acrylamido-2-methylpropanesulfnoinc Salt) Modified Carbon Spheres. Polymers. 2023; 15(17):3510. https://doi.org/10.3390/polym15173510
Chicago/Turabian StyleSu, Na. 2023. "Synthesis of Poly (2-Acrylamido-2-methylpropanesulfnoinc Salt) Modified Carbon Spheres" Polymers 15, no. 17: 3510. https://doi.org/10.3390/polym15173510



