Chitosan Resin-Modified Glass Ionomer Cement Containing Epidermal Growth Factor Promotes Pulp Cell Proliferation with a Minimum Effect on Fluoride and Aluminum Release
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
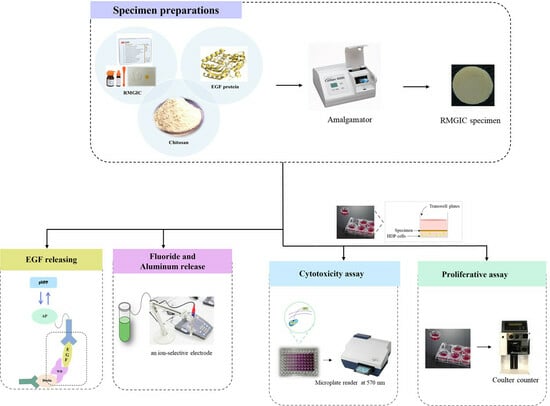
2.2. Specimen Preparation
2.3. Determination of EGF Releasing
2.4. Fluoride and Aluminum Release Measurement
2.4.1. Fluoride Analysis
2.4.2. Aluminum Analysis
2.5. Ethical Statement
2.6. Cell Culture
2.7. Cytotoxicity Assay
2.8. Proliferative Assay
2.9. Statistical Analysis
3. Results
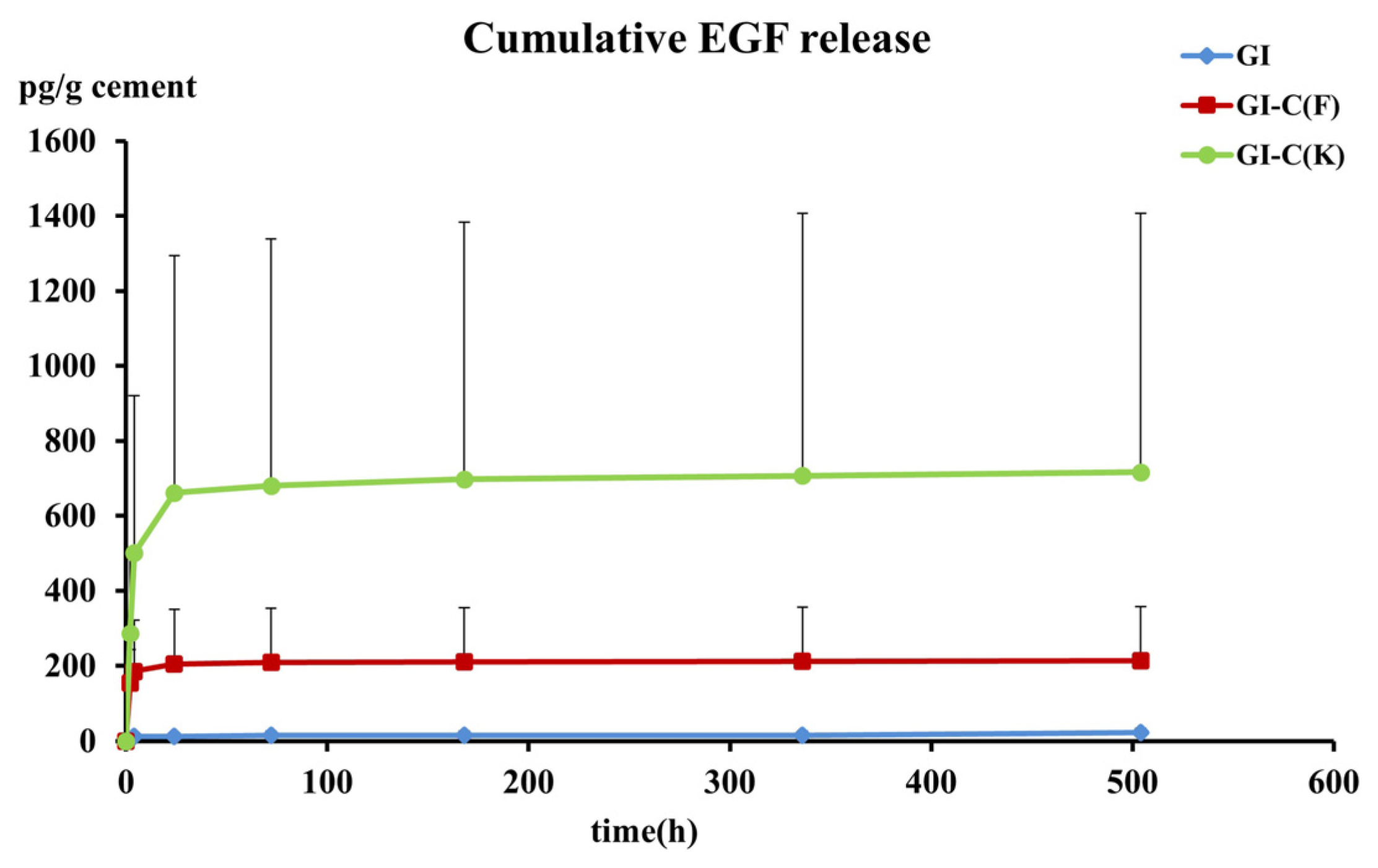
3.1. EGF Release
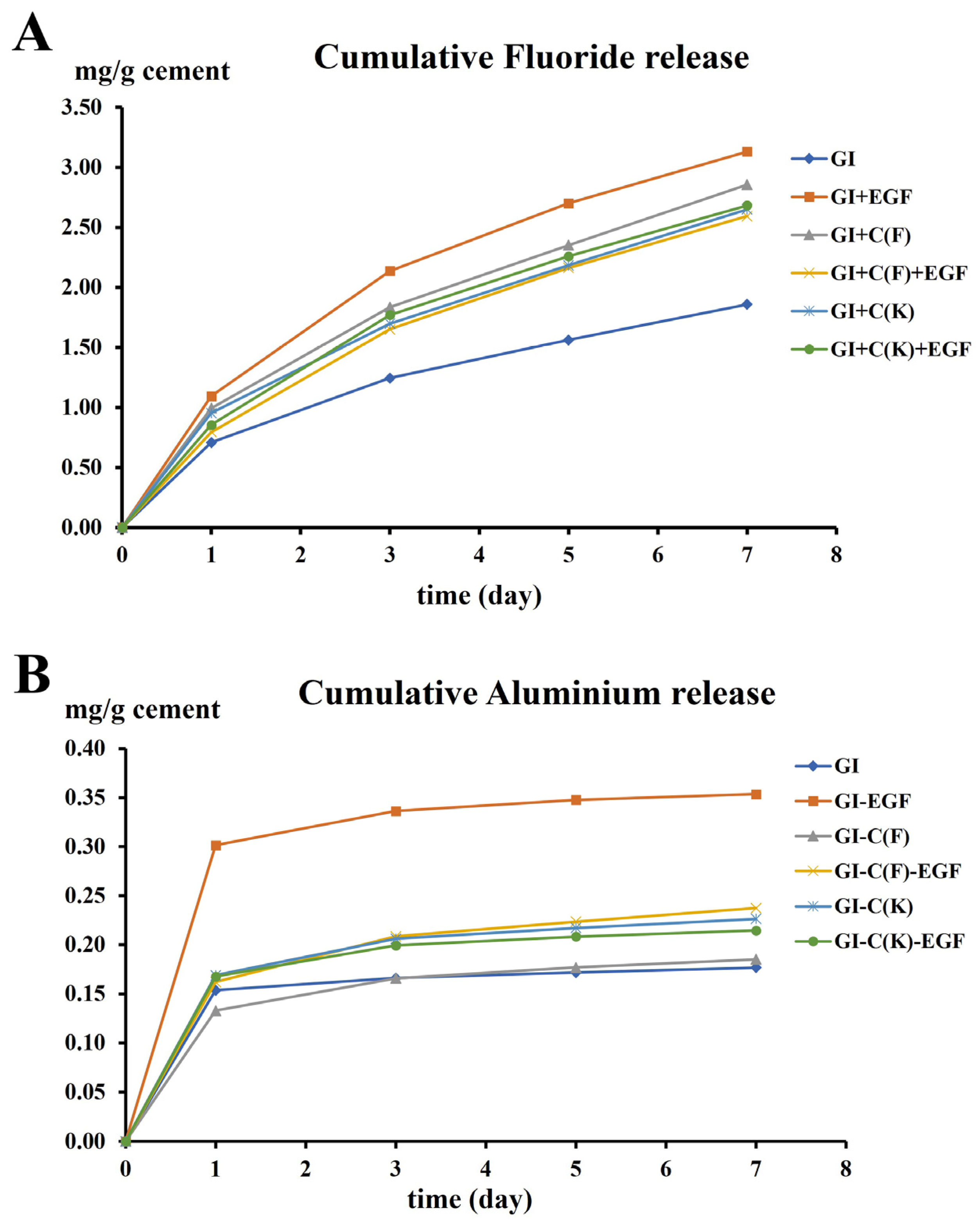
3.2. Fluoride and Aluminum Release
3.3. Cytotoxicity Assay
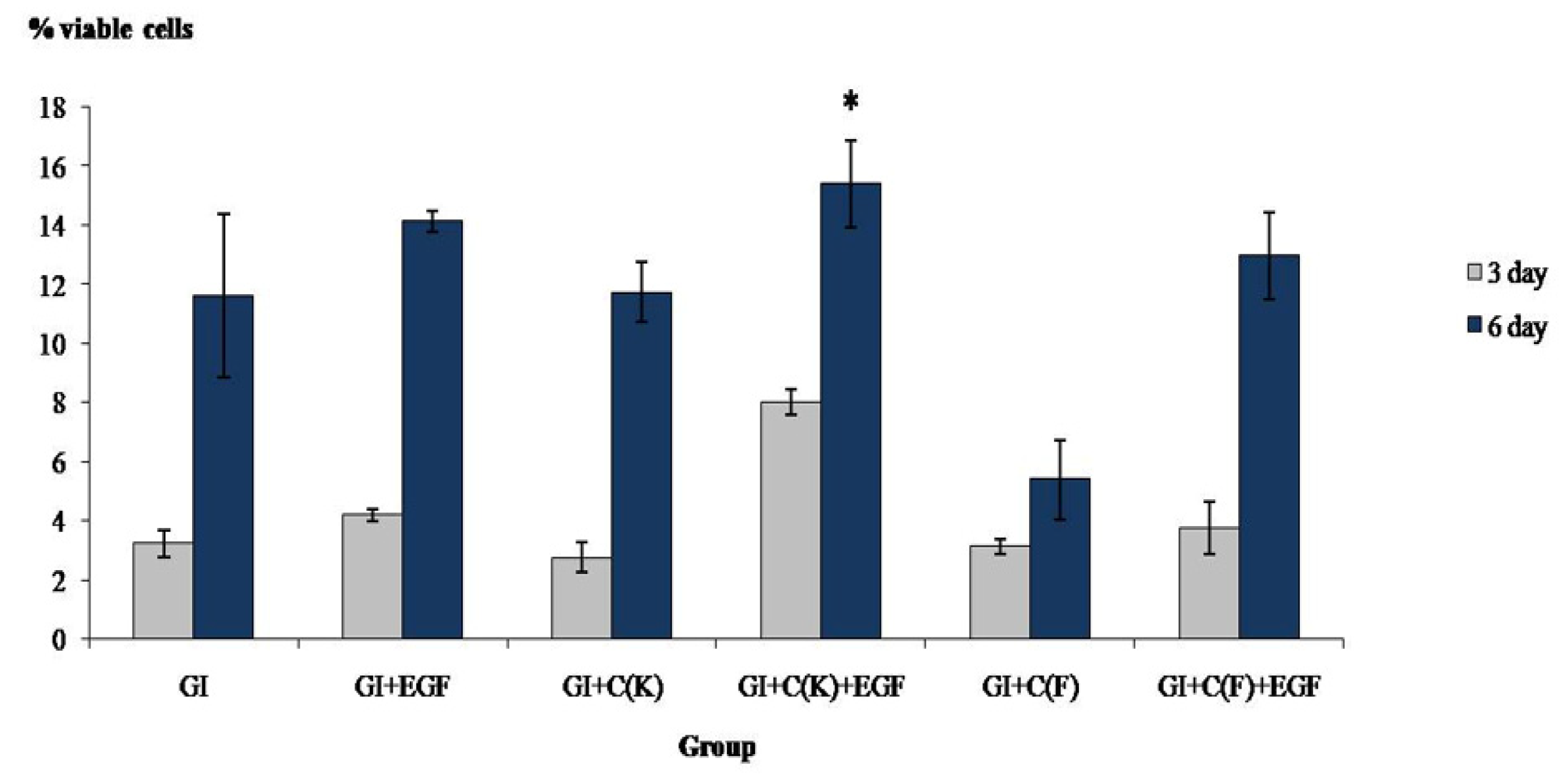
3.4. Proliferative Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, N.; Maher, N.; Amin, F.; Ghabbani, H.; Zafar, M.S.; Rodríguez-Lozano, F.J.; Oñate-Sánchez, R.E. Biomimetic Approaches in Clinical Endodontics. Biomimetics 2022, 7, 229. [Google Scholar] [CrossRef]
- Yu, S.; Zheng, Y.; Guo, Q.; Li, W.; Ye, L.; Gao, B. Mechanism of Pulp Regeneration Based on Concentrated Growth Factors Regulating Cell Differentiation. Bioengineering 2023, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Hafshejani, T.M.; Zamanian, A.; Venugopal, J.R.; Rezvani, Z.; Sefat, F.; Saeb, M.R.; Vahabi, H.; Zarrintaj, P.; Mozafari, M. Antibacterial glass-ionomer cement restorative materials: A critical review on the current status of extended release formulations. J. Control. Release 2017, 262, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Sobh, E.G.; Hamama, H.H.; Palamara, J.; Mahmoud, S.H.; Burrow, M.F. Effect of CPP-ACP modified-GIC on prevention of demineralization in comparison to other fluoride-containing restorative materials. Aust. Dent. J. 2022, 67, 220–229. [Google Scholar] [CrossRef]
- de Castilho, A.R.; Duque, C.; Negrini Tde, C.; Sacono, N.T.; de Paula, A.B.; de Souza Costa, C.A.; Spolidorio, D.M.; Puppin-Rontani, R.M. In vitro and in vivo investigation of the biological and mechanical behaviour of resin-modified glass-ionomer cement containing chlorhexidine. J. Dent. 2013, 41, 155–163. [Google Scholar] [CrossRef]
- Tomiyama, K.; Ishizawa, M.; Watanabe, K.; Kawata, A.; Hamada, N.; Mukai, Y. Antibacterial effects of surface pre-reacted glass-ionomer (S-PRG) filler eluate on polymicrobial biofilms. Am. J. Dent. 2023, 36, 91–94. [Google Scholar]
- Limapornvanich, A.; Jitpukdeebodintra, S.; Hengtrakool, C.; Kedjarune-Leggat, U. Bovine serum albumin release from novel chitosan-fluoro-aluminosilicate glass ionomer cement: Stability and cytotoxicity studies. J. Dent. 2009, 37, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Lu, N.; Pi, X.; Jin, Z.; Tian, R. Bovine Serum Albumin as a Potential Carrier for the Protection of Bioactive Quercetin and Inhibition of Cu(II) Toxicity. Chem. Res. Toxicol. 2022, 35, 529–537. [Google Scholar] [CrossRef]
- Hoang, H.T.; Jo, S.H.; Phan, Q.T.; Park, H.; Park, S.H.; Oh, C.W.; Lim, K.T. Dual pH-/thermo-responsive chitosan-based hydrogels prepared using “click” chemistry for colon-targeted drug delivery applications. Carbohydr. Polym. 2021, 260, 117812. [Google Scholar] [CrossRef]
- Budiarso, I.J.; Rini, N.D.W.; Tsalsabila, A.; Birowosuto, M.D.; Wibowo, A. Chitosan-Based Smart Biomaterials for Biomedical Applications: Progress and Perspectives. ACS Biomater. Sci. Eng. 2023, 9, 3084–3115. [Google Scholar] [CrossRef]
- Paker, E.S.; Senel, M. Polyelectrolyte Multilayers Composed of Polyethyleneimine-Grafted Chitosan and Polyacrylic Acid for Controlled-Drug-Delivery Applications. J. Funct. Biomater. 2022, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J.H. Chitosan based polyelectrolyte complexes as potential carrier materials in drug delivery systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.A.; Neo, J.; Esguerra, R.J.; Fawzy, A.S. Characterization of antibacterial and adhesion properties of chitosan-modified glass ionomer cement. J. Biomater. Appl. 2015, 30, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Debnath, A.; Kesavappa, S.B.; Singh, G.P.; Eshwar, S.; Jain, V.; Swamy, M.; Shetty, P. Comparative Evaluation of Antibacterial and Adhesive Properties of Chitosan Modified Glass Ionomer Cement and Conventional Glass Ionomer Cement: An in Vitro Study. J. Clin. Diagn. Res. 2017, 11, ZC75–ZC78. [Google Scholar] [CrossRef] [PubMed]
- Ranjani, M.S.; Kavitha, M.; Venkatesh, S. Comparative Evaluation of Osteogenic Potential of Conventional Glass-ionomer Cement with Chitosan-modified Glass-ionomer and Bioactive Glass-modified Glass-ionomer Cement An in vitro Study. Contemp. Clin. Dent. 2021, 12, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Elshenawy, E.A.; El-Ebiary, M.A.; Kenawy, E.R.; El-Olimy, G.A. Modification of glass-ionomer cement properties by quaternized chitosan-coated nanoparticles. Odontology 2023, 111, 328–341. [Google Scholar] [CrossRef]
- Wanachottrakul, N.; Chotigeat, W.; Kedjarune-Leggat, U. Effect of novel chitosan-fluoroaluminosilicate resin modified glass ionomer cement supplemented with translationally controlled tumor protein on pulp cells. J. Mater. Sci. Mater. Med. 2014, 25, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Hoshikawa, E.; Saito, T.; Suebsamarn, O.; Naito, E.; Suzuki, A.; Ishihara, S.; Haga, H.; Tomihara, K.; Izumi, K. The EGF/EGFR axis and its downstream signaling pathways regulate the motility and proliferation of cultured oral keratinocytes. FEBS Open Bio 2023, 13, 1469–1484. [Google Scholar] [CrossRef]
- Lott, K.; Collier, P.; Ringor, M.; Howard, K.M.; Kingsley, K. Administration of Epidermal Growth Factor (EGF) and Basic Fibroblast Growth Factor (bFGF) to Induce Neural Differentiation of Dental Pulp Stem Cells (DPSC) Isolates. Biomedicines 2023, 11, 255. [Google Scholar] [CrossRef]
- Tyler, J.E.; Poole, D.F. The rapid measurement of fluoride concentrations in stored human saliva by means of a differential electrode cell. Arch. Oral Biol. 1989, 34, 995–998. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Analysis; Pearson: Boston, MA, USA, 2007; pp. 98–99. [Google Scholar]
- Cao, J.; Wang, Y.; He, C.; Kang, Y.; Zhou, J. Ionically crosslinked chitosan/poly(acrylic acid) hydrogels with high strength, toughness and antifreezing capability. Carbohydr. Polym. 2020, 242, 116420. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, H.C.; Fonseca, J.L.; Pereira, M.R. Chitosan-poly(acrylic acid) polyelectrolyte complex membranes: Preparation, characterization and permeability studies. J. Biomater. Sci. Polym. Ed. 2008, 19, 143–160. [Google Scholar] [CrossRef]
- Murray, J.B.; Brown, L.; Langer, R.; Klagsburn, M. A micro sustained release system for epidermal growth factor. In Vitro 1983, 19, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Billington, R.W.; Pearson, G.J. A long term study of fluoride release from metal-containing conventional and resin-modified glass-ionomer cements. J. Oral Rehabil. 2001, 28, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Burgess, J.O. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials 2003, 24, 2451–2461. [Google Scholar] [CrossRef]
- Rolim, F.G.; de Araújo Lima, A.D.; Lima Campos, I.C.; de Sousa Ferreira, R.; da Cunha Oliveira-Júnior, C.; Gomes Prado, V.L.; Vale, G.C. Fluoride Release of Fresh and Aged Glass Ionomer Cements after Recharging with High-Fluoride Dentifrice. Int. J. Dent. 2019, 2019, 9785364. [Google Scholar] [CrossRef]
- Savarino, L.; Cervellati, M.; Stea, S.; Cavedagna, D.; Donati, M.E.; Pizzoferrato, A.; Visentin, M. In vitro investigation of aluminum and fluoride release from compomers, conventional and resin-modified glass-ionomer cements: A standardized approach. J. Biomater. Sci. Polym. Ed. 2000, 11, 289–300. [Google Scholar] [CrossRef]
- Renke, G.; Almeida, V.B.P.; Souza, E.A.; Lessa, S.; Teixeira, R.L.; Rocha, L.; Sousa, P.L.; Starling-Soares, B. Clinical Outcomes of the Deleterious Effects of Aluminum on Neuro-Cognition, Inflammation, and Health: A Review. Nutrients 2023, 15, 2221. [Google Scholar] [CrossRef]
- Hunt, C.D.; Meacham, S.L. Aluminum, boron, calcium, copper, iron, magnesium, manganese, molybdenum, phosphorus, potassium, sodium, and zinc: Concentrations in common western foods and estimated daily intakes by infants; toddlers; and male and female adolescents, adults, and seniors in the United States. J. Am. Diet. Assoc. 2001, 101, 1058–1060. [Google Scholar] [CrossRef]
- Malik, J.; Frankova, A.; Drabek, O.; Szakova, J.; Ash, C.; Kokoska, L. Aluminium and other elements in selected herbal tea plant species and their infusions. Food Chem. 2013, 139, 728–734. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Czarnecka, B. Review paper: Role of aluminum in glass-ionomer dental cements and its biological effects. J. Biomater. Appl. 2009, 24, 293–308. [Google Scholar] [CrossRef]
- Hayacibara, M.F.; Rosa, O.P.; Koo, H.; Torres, S.A.; Costa, B.; Cury, J.A. Effects of fluoride and aluminum from ionomeric materials on S. mutans biofilm. J. Dent. Res. 2003, 82, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Dhupar, J.K.; Jajoo, S.S.; Shah, P.; Chaudhary, S. Evaluation of Adhesive Bond Strength, and the Sustained Release of Fluoride by Chitosan-infused Resin-modified Glass Ionomer Cement: An in Vitro Study. Int. J. Clin. Pediatr. Dent. 2021, 14, 254–257. [Google Scholar] [CrossRef]
- Nishanthine, C.; Miglani, R.; Indira, R.; Poorni, S.; Srinivasan, M.R.; Robaian, A.; Albar, N.H.M.; Alhaidary, S.F.R.; Binalrimal, S.; Almalki, A.; et al. Evaluation of Fluoride Release in Chitosan-Modified Glass Ionomer Cements. Int. Dent. J. 2022, 72, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Lee, I.H.; Lee, E.; Kim, H.; Kim, Y.C.; Jon, S. Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan. J. Control. Release 2013, 170, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Affes, S.; Aranaz, I.; Acosta, N.; Heras, Á.; Nasri, M.; Maalej, H. Chitosan derivatives-based films as pH-sensitive drug delivery systems with enhanced antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2021, 182, 730–742. [Google Scholar] [CrossRef]

| Group of Specimens | Powder Compositions |
|---|---|
| GI | Fluoroaluminosilicate glass. |
| GI+EGF | Fluoroaluminosilicate glass with added EGF during mixing the cement. |
| GI+C(F) | Fluoroaluminosilicate glass, 15% chitosan Mw 545 kDa, and 5% BSA. |
| GI+C(F)+EGF | Fluoroaluminosilicate glass, 15% chitosan Mw 545 kDa, and 5% BSA with added EGF during cement mixing. |
| GI+C(K) | Fluoroaluminosilicate glass, 15% chitosan Mw 62 kDa, and 5% BSA. |
| GI+C(K)+EGF | Fluoroaluminosilicate glass, 15% chitosan Mw 62 kDa, and 5% BSA with added EGF during cement mixing. |
| Time (h) | Type of Material | ||
|---|---|---|---|
| GI+EGF a | GI-C(F)+EGF a,b | GI-C(K)+EGF b | |
| 2 | 3.362 | 131 | 253.31 |
| (0.00–6.38) | (50.06–277.62) | (31.44–529.67) | |
| 4 | 3.865 | 2.788 | 70.296 |
| (0.00–13.56) | (0.91–74.44) | (5.75–238.50) | |
| 24 | 0 | 0.773 | 1.71 |
| (0.00–0.01) | (0.16–2.42) | (0.27–23.82) | |
| 72 | 0 | 0.03 | 0 |
| (0.00–0.38) | (0.00–0.31) | (0.00–1.68) | |
| 168 | 0 | 0.004 | 0 |
| (0.00–0.00) | (0.00–0.06) | (0.00–0.61) | |
| 336 | 0 | 0.001 | 0.001 |
| (0.00–0.00) | (0–0.01)1 | (0.00–0.02) | |
| 504 | 0.008 | 0.01 | 0.001 |
| (0.00–0.22) | (0.00–0.05) | (0.00–0.33) | |
| Day1 | Day3 | Day5 | Day7 | |
|---|---|---|---|---|
| Rate of fluoride release (mg/g cement/day) | ||||
| GI a | 0.71 (0.08) | 0.25 (0.03) | 0.15 (0.00) | 0.14 (0.01) |
| GI+EGF d | 1.09 (0.08) | 0.53 (0.02) | 0.29 (0.01) | 0.22 (0.01) |
| GI+C(F) c | 0.99 (0.07) | 0.41 (0.02) | 0.25 (0.01) | 0.24 (0.01) |
| GI+C(F)+EGF b | 0.79 (0.03) | 0.42 (0.02) | 0.25 (0.01) | 0.21 (0.01) |
| GI+C(K) b,c | 0.95 (0.08) | 0.37 (0.02) | 0.24 (0.02) | 0.23 (0.01) |
| GI+C(K)+EGF b,c | 0.86 (0.11) | 0.46 (0.06) | 0.25 (0.01) | 0.21 (0.01) |
| Rate of aluminum release (mg/g cement/day) | ||||
| GI a | 0.15 (0.01) | 0.01 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
| GI+EGF b | 0.30 (0.01) | 0.02 (0.00) | 0.01 (0.00) | 0.00 (0.00) |
| GI+C(F) a | 0.16 (0.02) | 0.02 (0.00) | 0.01 (0.00) | 0.01 (0.00) |
| GI+C(F)+EGF a | 0.13 (0.01) | 0.02 (0.00) | 0.01 (0.00) | 0.00 (0.00) |
| GI+C(K) a | 0.17 (0.02) | 0.02 (0.00) | 0.01 (0.00) | 0.00 (0.00) |
| GI+C(K)+EGF a | 0.17 (0.04) | 0.02 (0.00) | 0.00 (0.00) | 0.00 (0.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hengtrakool, C.; Wanichpakorn, S.; Kedjarune-Leggat, U. Chitosan Resin-Modified Glass Ionomer Cement Containing Epidermal Growth Factor Promotes Pulp Cell Proliferation with a Minimum Effect on Fluoride and Aluminum Release. Polymers 2023, 15, 3511. https://doi.org/10.3390/polym15173511
Hengtrakool C, Wanichpakorn S, Kedjarune-Leggat U. Chitosan Resin-Modified Glass Ionomer Cement Containing Epidermal Growth Factor Promotes Pulp Cell Proliferation with a Minimum Effect on Fluoride and Aluminum Release. Polymers. 2023; 15(17):3511. https://doi.org/10.3390/polym15173511
Chicago/Turabian StyleHengtrakool, Chanothai, Supreya Wanichpakorn, and Ureporn Kedjarune-Leggat. 2023. "Chitosan Resin-Modified Glass Ionomer Cement Containing Epidermal Growth Factor Promotes Pulp Cell Proliferation with a Minimum Effect on Fluoride and Aluminum Release" Polymers 15, no. 17: 3511. https://doi.org/10.3390/polym15173511
APA StyleHengtrakool, C., Wanichpakorn, S., & Kedjarune-Leggat, U. (2023). Chitosan Resin-Modified Glass Ionomer Cement Containing Epidermal Growth Factor Promotes Pulp Cell Proliferation with a Minimum Effect on Fluoride and Aluminum Release. Polymers, 15(17), 3511. https://doi.org/10.3390/polym15173511