Study of the Self-Polymerization of Epoxy/Phthalonitrile Copolymers and Their High-Performance Fiber-Reinforced Laminates
Abstract
:1. Introduction
2. Experiments
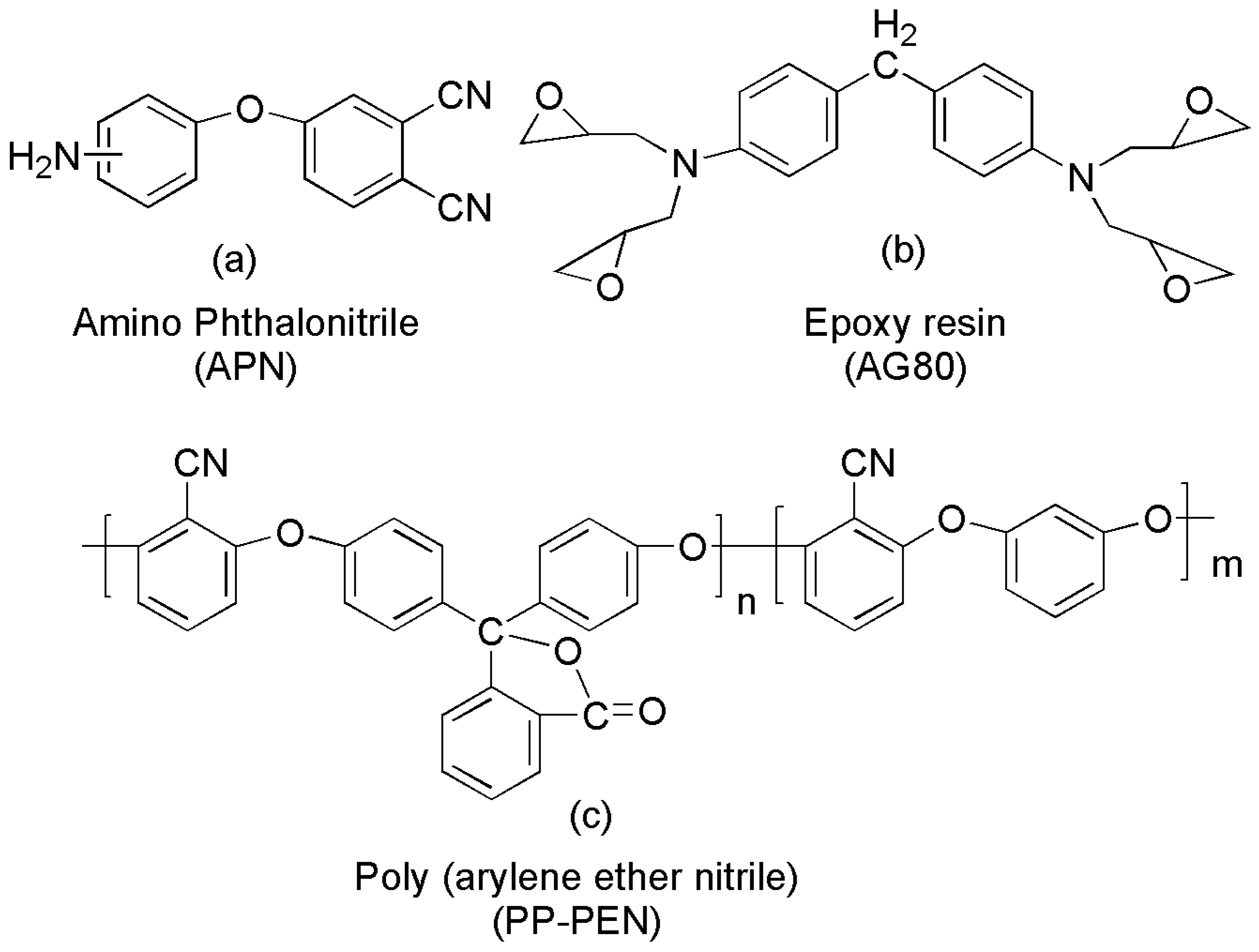
2.1. Materials
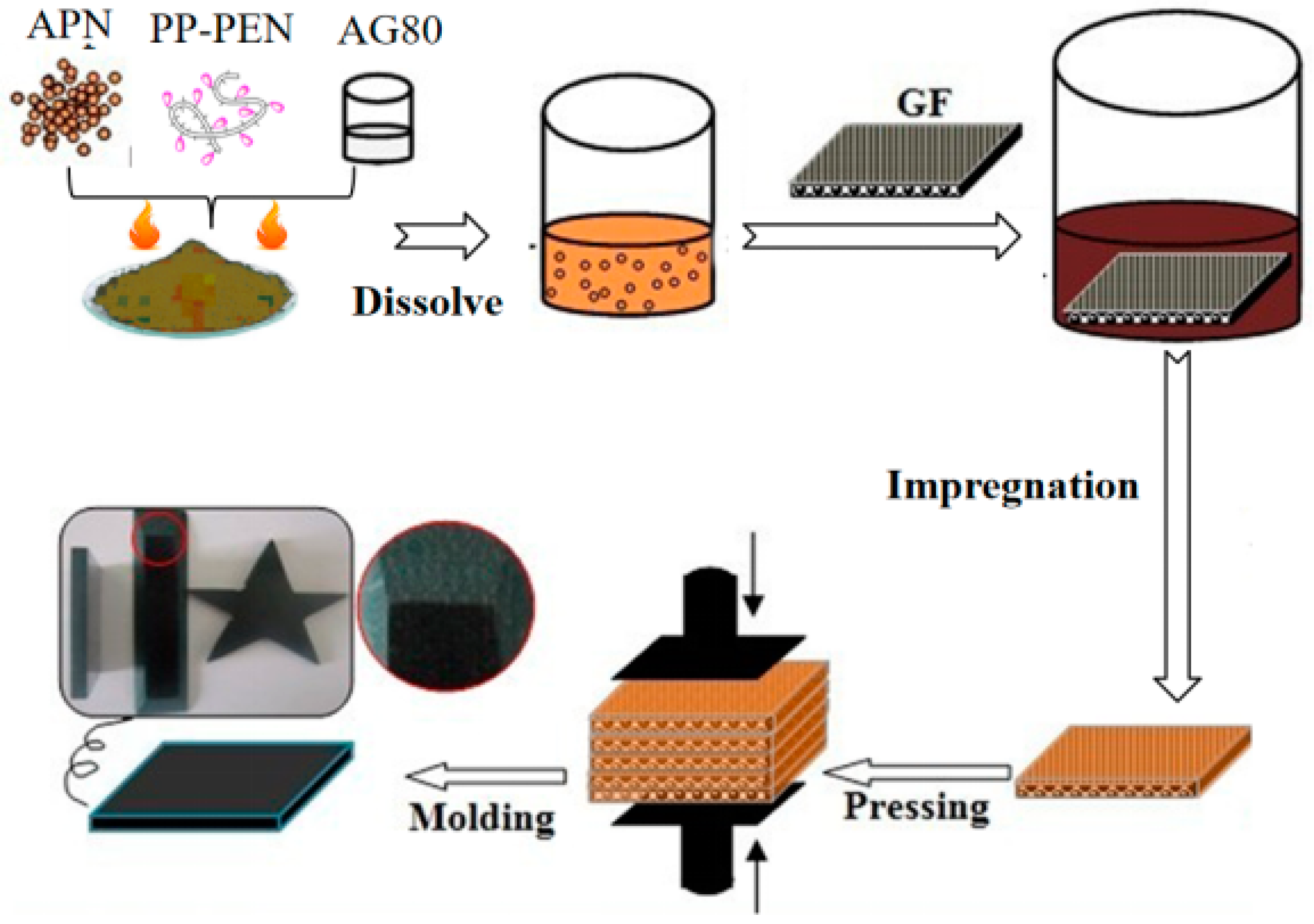
2.2. Preparation of APPEN Prepolymers
2.3. Preparation of Glass-Fiber-Reinforced APPEN Composite Laminates (APPEN/GF)
2.4. Characterizations
3. Results and Discussion
3.1. Polymerization Behaviors and Possible Polymerization Mechanisms of APPEN Prepolymers
3.1.1. Gelation Properties of APPEN Prepolymers
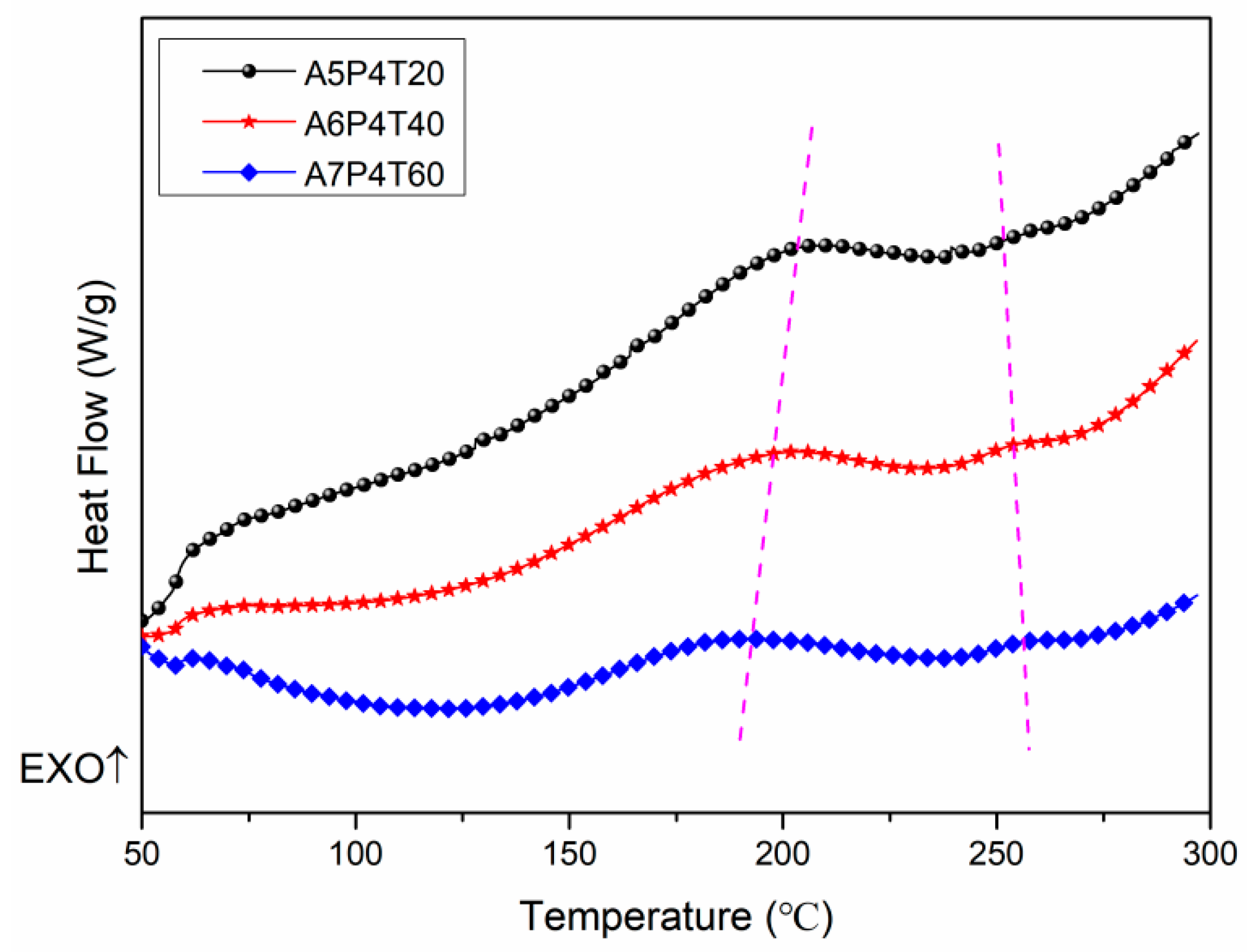
3.1.2. Curing Processes of APPEN Prepolymers
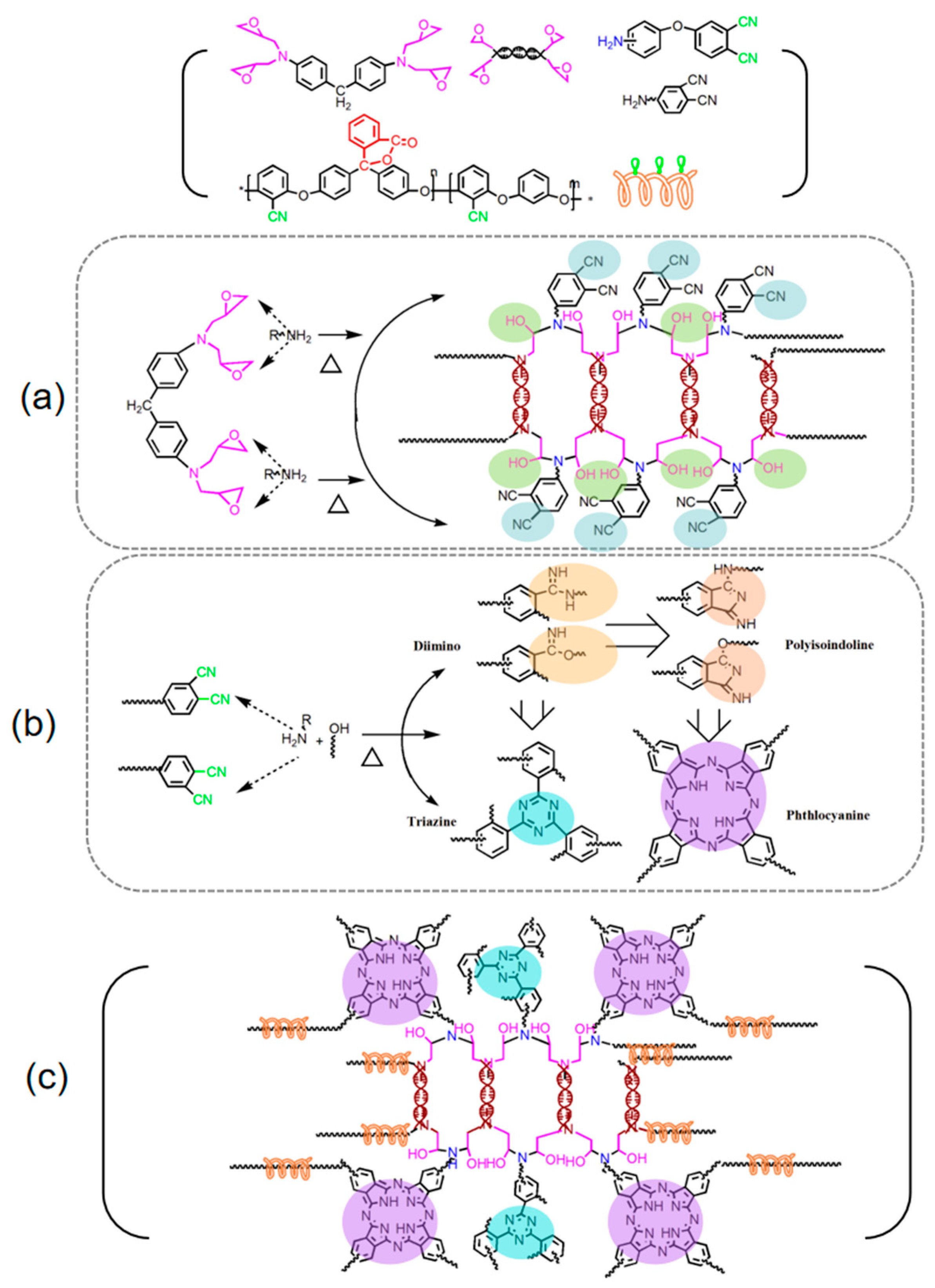
3.1.3. Possible Polymerization Mechanism of APPEN Prepolymers
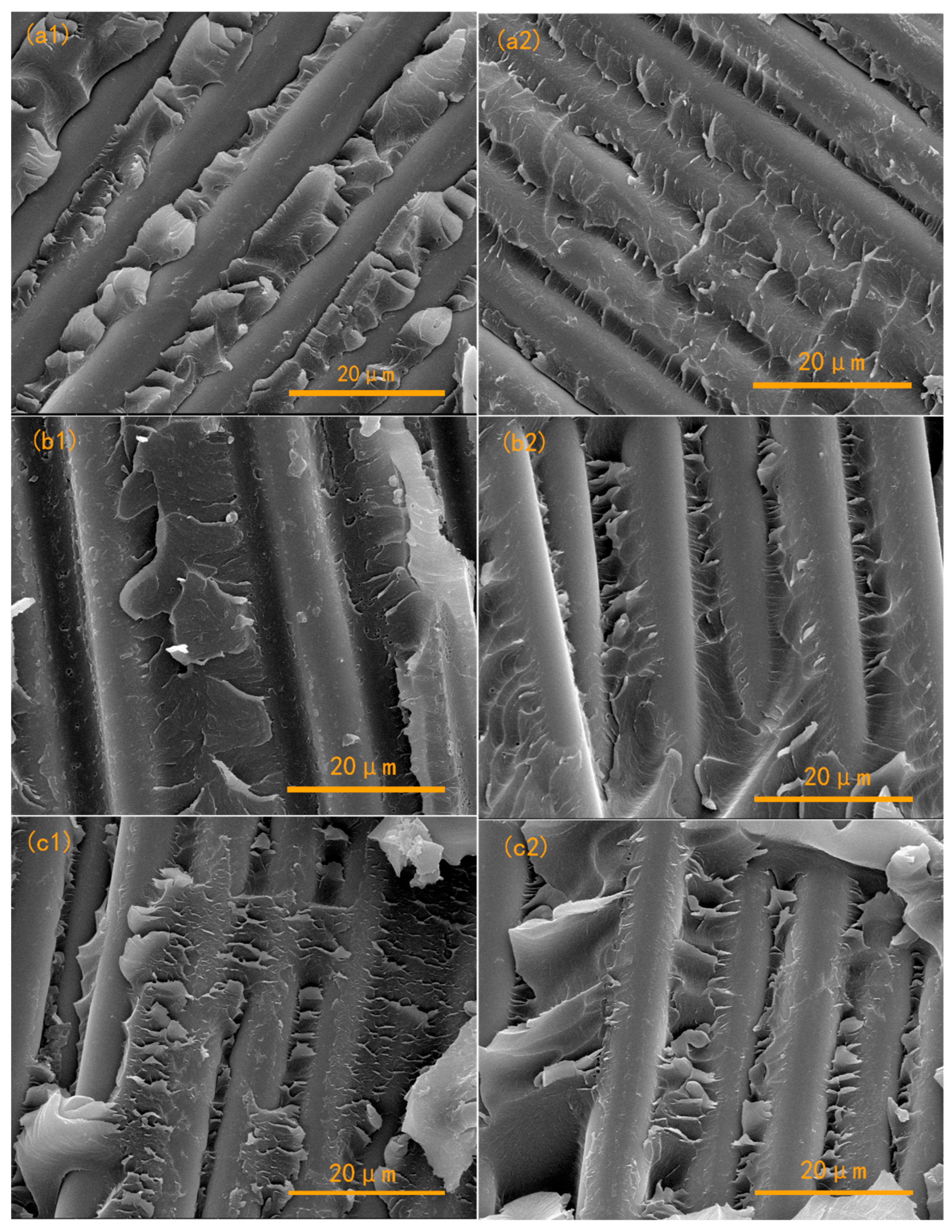
3.2. Mechanical Properties of APPEN/GF Composite Laminates
3.3. Thermomechanical Properties of APPEN/GF Composites
3.4. Thermal Stability of APPEN/GF Composite Laminates
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, F.L.; Li, X.; Park, S.J. Synthesis and application of epoxy resins: A review. J. Ind. Eng. Chem. 2015, 29, 1–11. [Google Scholar] [CrossRef]
- Chrúsciel, J.J.; Lésniak, E. Modifification of epoxy resins with functional silanes, polysiloxanes, silsesquioxanes, silica and silicates. Prog. Polym. Sci. 2015, 41, 67–121. [Google Scholar] [CrossRef]
- Gu, J.W.; Liang, C.B.; Zhao, X.M.; Gan, B.; Qiu, H.; Guo, Y.; Yang, X.; Zhang, Q.; Wang, D.-Y. Highly thermally conductive flame-retardant epoxy nanocomposites with reduced ignitability and excellent electrical conductivities. Compos. Sci. Technol. 2017, 139, 83–89. [Google Scholar] [CrossRef]
- Takahashi, A.; Ohishi, T.; Goseki, R.; Otsuka, H. Degradable epoxy resins prepared from diepoxide monomer with dynamic covalent disulfifide linkage. Polymer 2016, 82, 319–326. [Google Scholar] [CrossRef]
- Gu, J.; Yang, X.; Li, C.; Kou, K. Synthesis of Cyanate Ester Microcapsules via Solvent Evaporation Technique and Its Application in Epoxy Resins as a Healing Agent. Ind. Eng. Chem. Res. 2016, 55, 10941–10946. [Google Scholar] [CrossRef]
- Seo, H.Y.; Im, D.; Kwon, Y.J.; Nam, C.Y.; Kim, S.H.; Nam, T.; Kim, C.; Vivek, E.; Baek, K.Y.; Cho, K.Y.; et al. A strategy for dual-networked epoxy composite systems toward high cross-linking density and solid interfacial adhesion. Compos. Part B 2023, 254, 110524. [Google Scholar] [CrossRef]
- Wang, Z.L.; Hou, D.J.; Wang, F.; Zhou, J.J.; Cai, N.; Guo, J. Facile and Scalable Strategy for Fabricating Highly Thermally Conductive Epoxy Composites Utilizing 3D Graphitic Carbon Nitride Nanosheet Skeleton. ACS Appl. Mater. Interfaces 2023, 15, 23, 28626–28635. [Google Scholar]
- Ayyanar, C.B.; Marimuthu, K.; Mugilan, T.; Gayathri, B.; Sanjay, M.R.; Khan, A.; Siengchin, S. Novel Polyalthia Longifolia seed fillers loaded and E-glass fiber-reinforced sandwich epoxy composites. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2023. [Google Scholar]
- Zhang, R.; Wang, H.S.; Wang, X.Z.; Guan, J.; Li, M.Q.; Chen, Y.F. Rubber-Composite-Nanoparticle-Modified Epoxy Powder Coatings with Low Curing Temperature and High Toughness. Polymers 2023, 15, 195. [Google Scholar] [CrossRef]
- Taieh, N.K.; Khudhur, S.K.; Fahad, E.A.; Zhou, Z.W.; Hui, D.V. High mechanical performance of 3-aminopropyl triethoxy silane/epoxy cured in a sandwich construction of 3D carbon felts foam and woven basalt fibers. Nanotechnol. Rev. 2023, 12, 519. [Google Scholar] [CrossRef]
- Guo, H.; Zou, Y.K.; Chen, Z.R.; Zhang, J.D.; Zhan, Y.Q.; Yang, J.; Liu, X.B. Effects of self-promoted curing behaviors on properties of phthalonitrile/epoxy copolymer. High Perform. Polym. 2013, 24, 571–579. [Google Scholar] [CrossRef]
- Laskoski, M.; Neal, A.; Keller, T.M.; Dominguez, D.; Klug, C.A.; Saab, A.P. Improved Synthesis of Oligomeric Phthalonitriles and Studies Designed for Low Temperature Cure. J. Polym. Sci. Polym. Chem. 2014, 52, 1662–1668. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, B.; Hu, J.; Wang, D.; Zou, Y.; Yang, Y. Curing behavior studies of phenol-containing phthalonitrile monomer for advanced composite materials. Thermochim. Acta 2021, 696, 178837. [Google Scholar] [CrossRef]
- Wu, Z.Q.; Han, J.H.; Li, N.; Weng, Z.H.; Wang, J.Y.; Jian, X.G. Improving the curing process and thermal stability of phthalonitrile resin via novel mixed curing agents. Polym. Int. 2017, 66, 876–881. [Google Scholar] [CrossRef]
- Xu, X.Q.; Xu, M.Z.; Liu, T.; Ren, D.X.; Liu, X.B. Understanding the curing behaviors and properties of phthalonitrile containing benzoxazine with a new type of aniline curing agent. Polym. Test. 2022, 107, 107487. [Google Scholar] [CrossRef]
- Keller, T.M. Phthalonitrile-based high temperature resin. J. Polym. Sci. Part A Polym. Chem. 1988, 26, 3199–3212. [Google Scholar] [CrossRef]
- Keller, T.M. Imide-containing phthalonitrile resin. Polymer 1993, 34, 952–955. [Google Scholar] [CrossRef]
- Hergenrother, P.M. The use, design, synthesis, and properties of high performance/high temperature polymers: An overview. High Perform. Polym. 2003, 15, 3–45. [Google Scholar] [CrossRef]
- Yang, X.L.; Liu, X.B. Study on curing reaction of 4-aminophenoxyphthalonitrile/bisphthalonitrile. Chin. Chem. Lett. 2010, 21, 743–747. [Google Scholar] [CrossRef]
- Ren, D.X.; Lei, Y.X.; Pan, H.; Xu, M.Z.; Liu, X.B. Design of the phthalonitrile-based composite laminates by improving the interfacial compatibility and their enhanced properties. J. Appl. Polym. Sci. 2018, 135, 45881–45889. [Google Scholar] [CrossRef]
- Korokhin, R.A.; Solodilov, V.I.; Zvereva, U.G.; Solomatin, D.V.; Gorbatkina, Y.A.; Shapagin, A.V.; Lebedeva, O.V.; Bamborin, M.Y. Epoxy polymers modified with polyetherimide. Part II: Physicomechanical properties of modified epoxy oligomers and carbon fiber reinforced plastics based on them. Polym. Bull. 2020, 77, 2039–2057. [Google Scholar] [CrossRef]
- Shan, S.Y.; Chen, X.G.; Xi, Z.J.; Yu, X.Y.; Qu, X.W.; Zhang, Q.X. The effect of nitrile-functionalized nano-aluminum oxide on the thermomechanical properties and toughness of phthalonitrile resin. High Perform. Polym. 2017, 29, 113–123. [Google Scholar] [CrossRef]
- Sprenger, S. Nanosilica-Toughened Epoxy Resins. Polymers 2020, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- Rimdusit, S.; Jongvisuttisun, P.; Jubsilp, C.; Tanthapanichakoon, W. Highly Processable Ternary Systems Based on Benzoxazine, Epoxy, and Phenolic Resins for Carbon Fiber Composite Processing. J. Appl. Polym. Sci. 2009, 111, 1225–1234. [Google Scholar] [CrossRef]
- Unnikrishnan, K.S.; Jose, T.S.; Lal, S.D.; Arun, K.J. Glass fiber reinforced bismaleimide/epoxy BaTiO3 nano composites for high voltage applications. Polym. Test. 2020, 87, 106505. [Google Scholar] [CrossRef]
- Tao, L.; Sun, Z.Y.; Min, W.; Ou, H.W.; Qi, L.L.; Yu, M.H. Improving the toughness of thermosetting epoxy resins via blending triblock copolymers. RSC Adv. 2020, 149, 81–84. [Google Scholar] [CrossRef]
- Dong, L.N.; Zhou, W.Y.; Sui, X.Z.; Wang, Z.J.; Cai, H.W.; Wu, P.; Zuo, J.; Liu, X.R. A Carboxyl-Terminated Polybutadiene Liquid Rubber Modified Epoxy Resin with Enhanced Toughness and Excellent Electrical Properties. J. Electron. Mater. 2016, 45, 3776–3785. [Google Scholar] [CrossRef]
- Lei, X.T.; Tong, L.F.; Pan, H.; Yang, G.Y.; Liu, X.B. Preparation of polyarylene ether nitriles/fullerene composites with low dielectric constant by cosolvent evaporation. J. Mater. Sci. Mater. Electron. 2019, 30, 18297–18305. [Google Scholar] [CrossRef]
- Xu, M.Z.; Yang, X.L.; Zhao, R.; Liu, X.B. Copolymerizing Behavior and Processability of Benzoxazine/Epoxy Systems and Their Applications for Glass Fiber Composite Laminates. J. Appl. Polym. Sci. 2013, 128, 1176–1184. [Google Scholar] [CrossRef]
- Xu, M.; Ren, D.; Chen, L.; Li, K.; Liu, X. Understanding of the polymerization mechanism of the phthalonitrile-based resins containing benzoxazine and their thermal stability. Polymer 2018, 143, 28–39. [Google Scholar] [CrossRef]
- Chen, L.; Ren, D.X.; Chen, S.J.; Li, K.; Xu, M.Z.; Liu, X.B. Improved thermal stability and mechanical properties of benzoxazine-based composites with the enchantment of nitrile. Polym. Test. 2019, 74, 127–137. [Google Scholar] [CrossRef]
- Li, Z.; Guo, Y.; Wang, G.; Xu, S.; Han, Y.; Liu, X.; Luo, Z.; Ye, L.; Zhou, H.; Zhao, T. Preparation and characterization of a self-catalyzed fluorinated novolac-phthalonitrile resin. Polym. Adv. Technol. 2018, 29, 2936–2942. [Google Scholar] [CrossRef]
- Ren, D.X.; Li, K.; Chen, L.; Chen, S.J.; Han, M.G.; Xu, M.Z.; Liu, X.B. Modification on glass fiber surface and their improved properties of fiber-reinforced composites via enhanced interfacial properties. Compos. Part B 2019, 177, 107419–107430. [Google Scholar] [CrossRef]
- Gao, C.; Gu, H.B.; Du, A.; Zhou, H.; Pan, D.; Naik, N.; Guo, Z.H. Polyaniline facilitated curing of phthalonitrile resin with enhanced processibility and mechanical property. Polymer 2021, 219, 7–18. [Google Scholar] [CrossRef]
- Wang, G.X.; Guo, Y.; Han, Y.; Li, Z.; Ding, J.N.; Jiang, H.; Zhou, H.; Zhao, T. Enhanced properties of phthalonitrile resins reinforced by novel phthalonitrile-terminated polyaryl ether nitrile containing fluorene group. High Perform. Polym. 2020, 32, 3–11. [Google Scholar] [CrossRef]
- Chen, L.; Ren, D.X.; Chen, S.J.; Pan, H.; Xu, M.Z.; Liu, X.B. Copolymerization of phthalonitrile-based resin containing benzoxazine and cyanate ester: Curing behaviors, fiber-reinforced composite laminates and improved properties. Express Polym. Lett. 2019, 13, 456–468. [Google Scholar] [CrossRef]
- Xu, M.Z.; Lei, Y.X.; Ren, D.X.; Chen, L.; Li, K.; Liu, X.B. Thermal Stability of Allyl-Functional Phthalonitriles-Containing Benzoxazine/Bismaleimide Copolymers and Their Improved Mechanical Properties. Polymers 2018, 10, 596. [Google Scholar] [CrossRef]
- Xiong, X.H.; Zhou, L.; Ren, R.; Liu, S.Y.; Chen, P. The thermal decomposition behavior and kinetics of epoxy resins cured with a novel phthalide-containing aromatic diamine. Polym. Test. 2018, 68, 46–52. [Google Scholar] [CrossRef]
- Zabihi, O.; Khodabandeh, A.; Mostafavi, S.M. Preparation, optimization and thermal characterization of a novel conductive thermoset nanocomposite containing polythiophene nanoparticles using dynamic thermal analysis. Polym. Degrad. Stab. 2012, 97, 3–13. [Google Scholar] [CrossRef]
- Huang, W.; He, W.; Long, L.; Yan, W.; He, M.; Qin, S.; Yu, J. Highly efficient flame-retardant glass-fiber-reinforced polyamide 6T system based on a novel DOPO-based derivative: Flame retardancy, thermal decomposition, and pyrolysis behavior. Polym. Degrad. Stab. 2018, 148, 26–41. [Google Scholar] [CrossRef]
- Chen, S.J.; Ren, D.X.; Li, B.; Li, K.; Chen, L.; Xu, M.Z.; Liu, X.B. Benzoxazine Containing Fluorinated Aromatic Ether Nitrile Linkage: Preparation, Curing Kinetics and Dielectric Properties. Polymers 2019, 11, 1036. [Google Scholar] [CrossRef] [PubMed]






| Samples | A Weight of AG80 (g) | B Weight of APN (g) | C Prepolymerization Time (min) |
|---|---|---|---|
| 1 | 5 | 4 | 20 |
| 2 | 5 | 3 | 40 |
| 3 | 5 | 2 | 60 |
| 4 | 6 | 4 | 40 |
| 5 | 6 | 3 | 60 |
| 6 | 6 | 2 | 20 |
| 7 | 7 | 4 | 60 |
| 8 | 7 | 3 | 20 |
| 9 | 7 | 2 | 40 |
| Samples | A Weight of AG80 (g) | B Weight of APN (g) | C Prepolymerization Time (min) | Gelation Time (s) |
|---|---|---|---|---|
| 1 | 5 | 4 | 20 | 300 |
| 2 | 5 | 3 | 40 | 300 |
| 3 | 5 | 2 | 60 | 300 |
| 4 | 6 | 4 | 40 | 300 |
| 5 | 6 | 3 | 60 | 360 |
| 6 | 6 | 2 | 20 | 600 |
| 7 | 7 | 4 | 60 | 180 |
| 8 | 7 | 3 | 20 | 480 |
| 9 | 7 | 2 | 40 | 510 |
| K1 | 900 | 780 | 1380 | RB > RC > RA |
| K2 | 1260 | 1140 | 1110 | |
| K3 | 1170 | 1410 | 840 | |
| k1 | 300 | 260 | 460 | |
| k2 | 420 | 380 | 370 | |
| k3 | 390 | 470 | 280 | |
| k2 > k3 > k1 | k3 > k2 > k1 | k1 > k2 > k3 | ||
| R | 120 | 210 | 180 |
| Samples | Ttop1 (°C) | Ttop2 (°C) |
|---|---|---|
| A5P4T20 | 202.04 | 252.11 |
| A6P4T40 | 198.65 | 253.71 |
| A7P4T60 | 190.87 | 257.00 |
| Sample | T5% | T10% | Yc | IPDT | ||
|---|---|---|---|---|---|---|
| (°C) | (°C) | (%, 600 °C) | A | K | T (°C) | |
| A5P4T20 | 348.62 | 363.56 | 50.94 | 0.8390 | 2.538 | 1199.98 |
| A6P4T40 | 334.89 | 360.08 | 51.89 | 0.8380 | 2.603 | 1227.90 |
| A7P4T60 | 346.67 | 370.14 | 58.28 | 0.8638 | 3.070 | 1482.18 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Li, B.; Li, X.; Fan, Z.; Ren, D. Study of the Self-Polymerization of Epoxy/Phthalonitrile Copolymers and Their High-Performance Fiber-Reinforced Laminates. Polymers 2023, 15, 3516. https://doi.org/10.3390/polym15173516
Xu M, Li B, Li X, Fan Z, Ren D. Study of the Self-Polymerization of Epoxy/Phthalonitrile Copolymers and Their High-Performance Fiber-Reinforced Laminates. Polymers. 2023; 15(17):3516. https://doi.org/10.3390/polym15173516
Chicago/Turabian StyleXu, Mingzhen, Bo Li, Xiongyao Li, Zexu Fan, and Dengxun Ren. 2023. "Study of the Self-Polymerization of Epoxy/Phthalonitrile Copolymers and Their High-Performance Fiber-Reinforced Laminates" Polymers 15, no. 17: 3516. https://doi.org/10.3390/polym15173516
APA StyleXu, M., Li, B., Li, X., Fan, Z., & Ren, D. (2023). Study of the Self-Polymerization of Epoxy/Phthalonitrile Copolymers and Their High-Performance Fiber-Reinforced Laminates. Polymers, 15(17), 3516. https://doi.org/10.3390/polym15173516







