An Integrated Multi-Functional Thermal Conductive and Flame Retardant Epoxy Composite with Functionalized Carbon Nitride Nanosheets
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
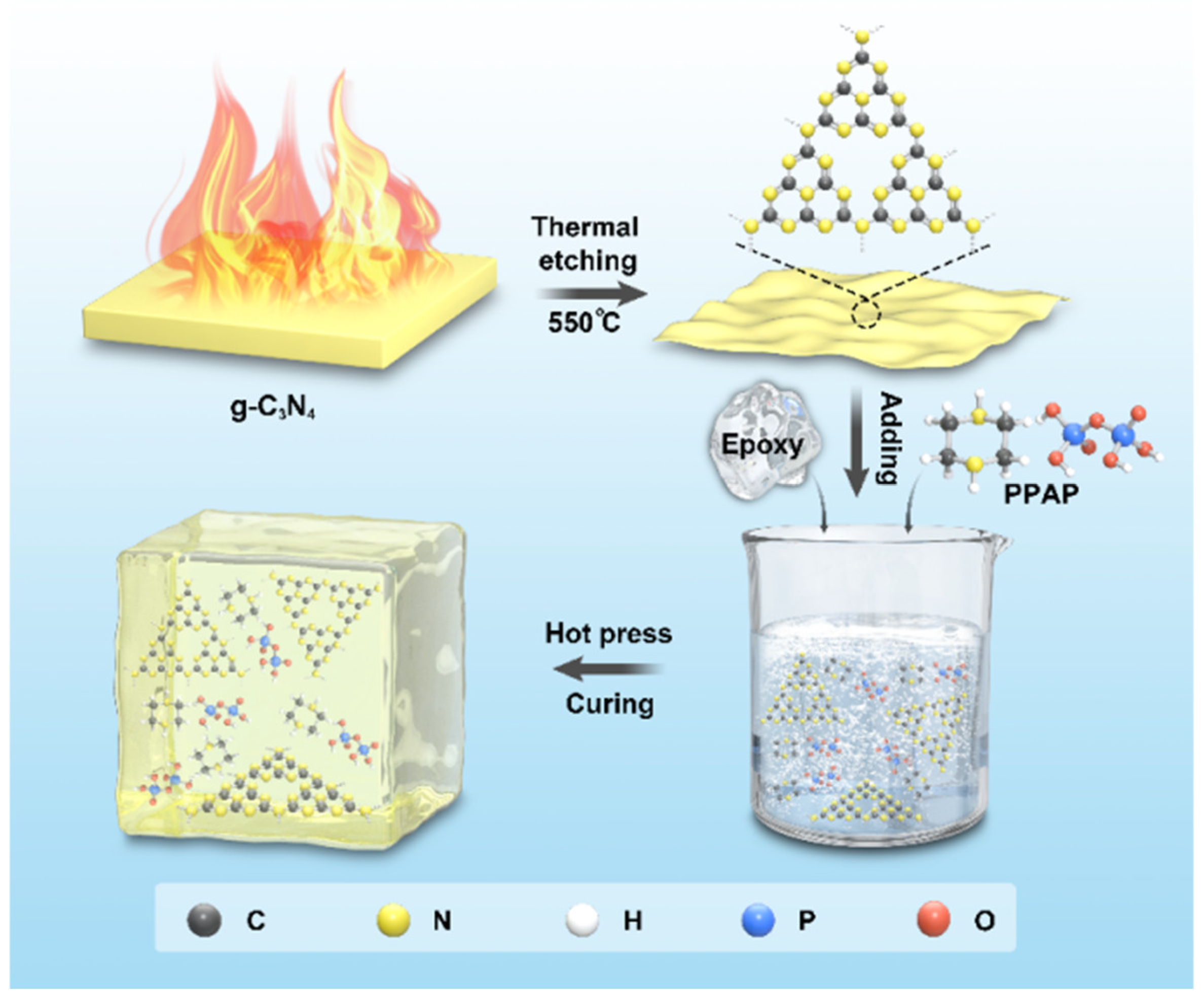
2.2. Preparation of Carbon Nitride Nanosheets
2.3. Preparation of CNNS/Epoxy Composites, PPAP/Epoxy Composites, and CNNS/PPAP/Epoxy Composites
2.4. Characterization
3. Results
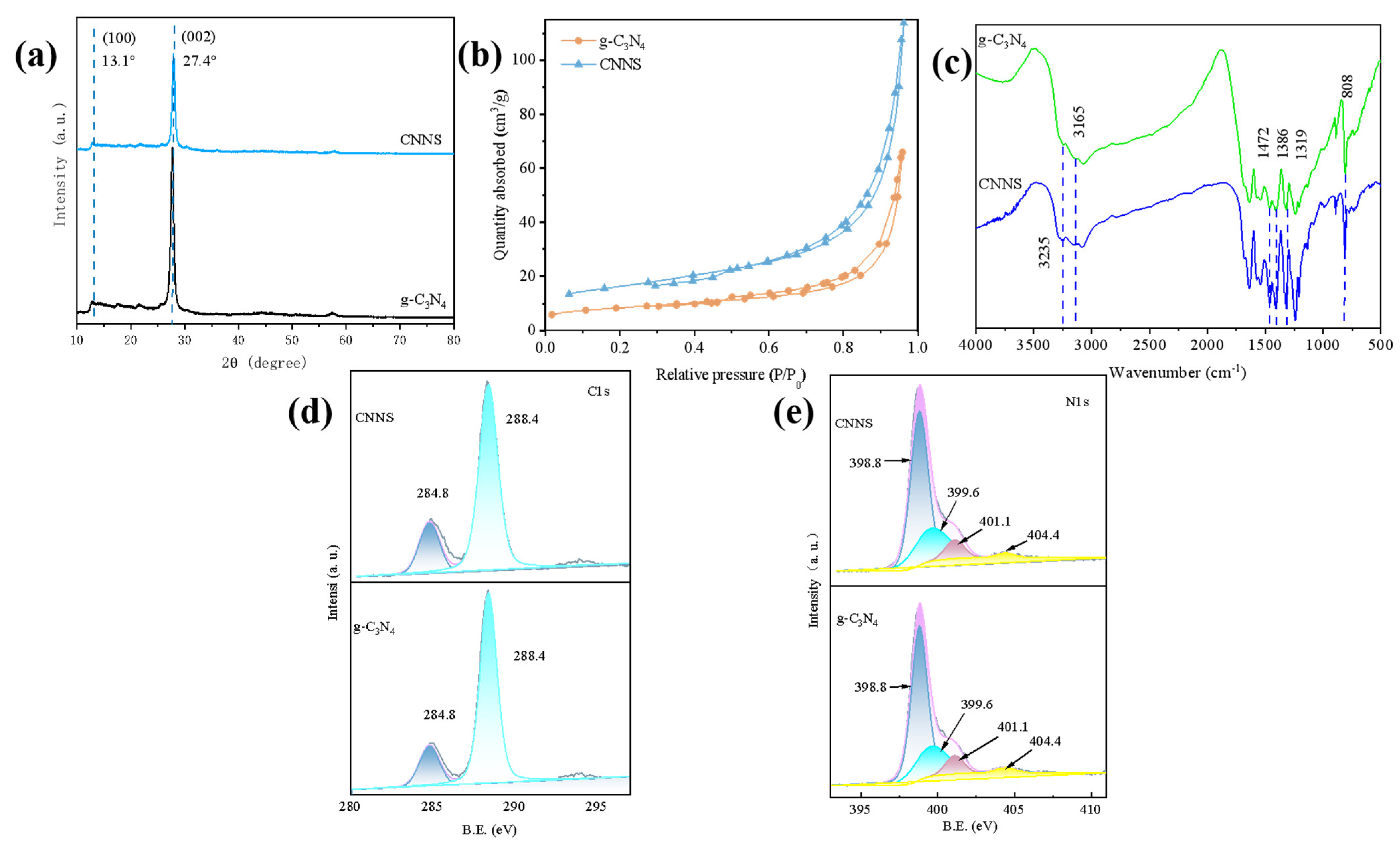
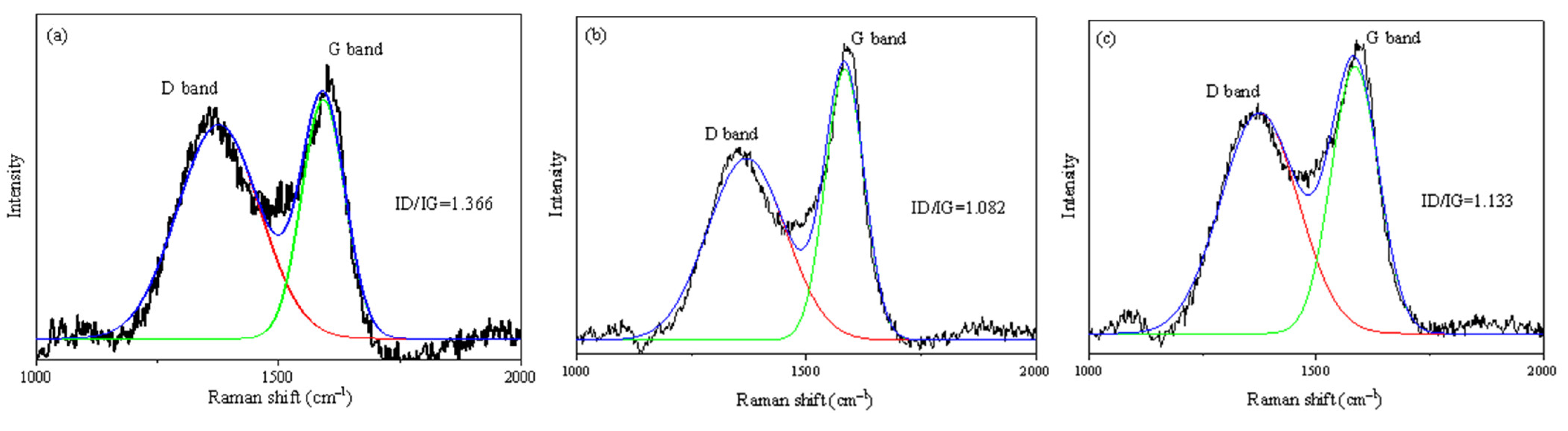
3.1. Characterizations of CNNS
3.2. Thermal Conductivity of EP Composites
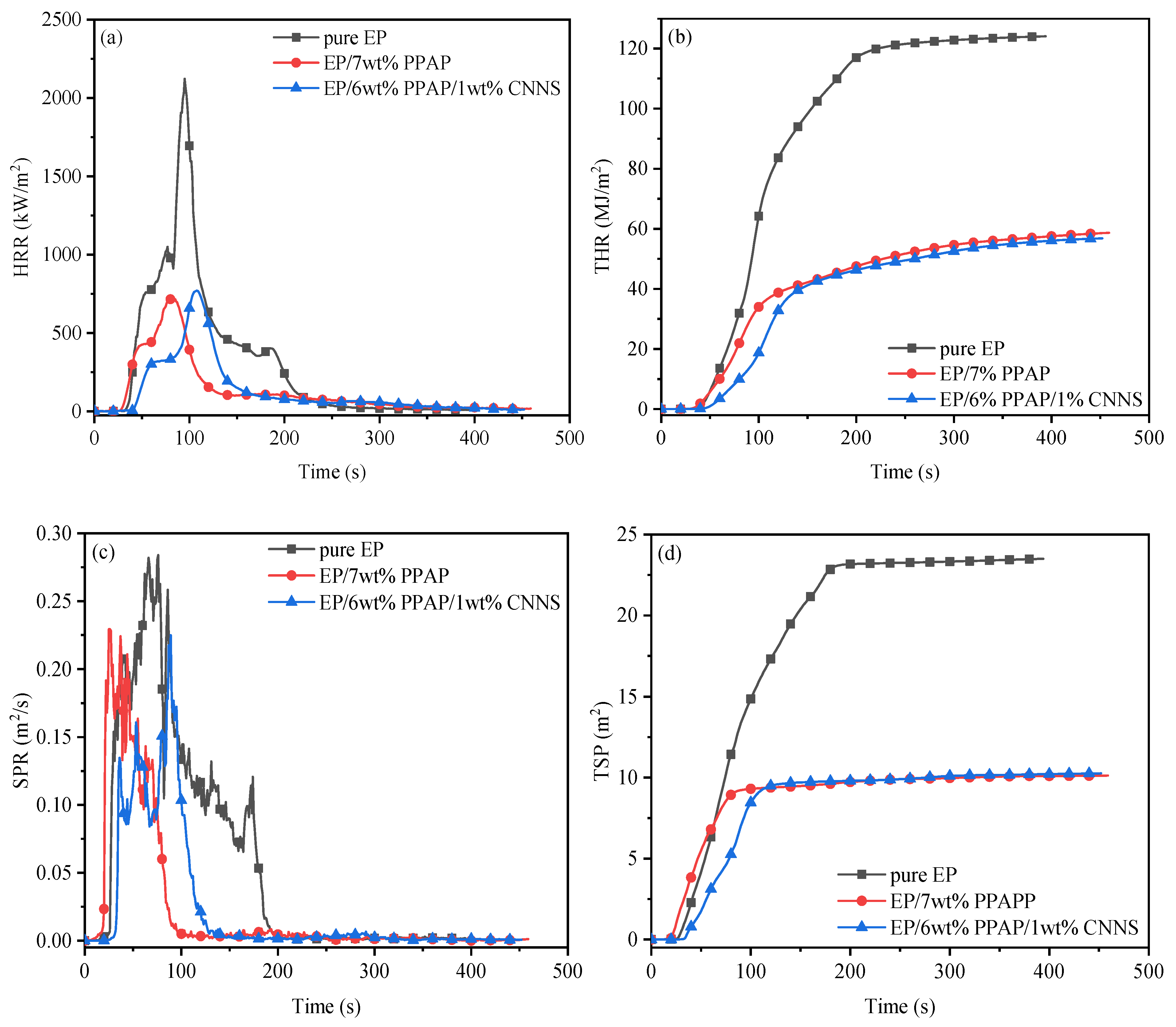
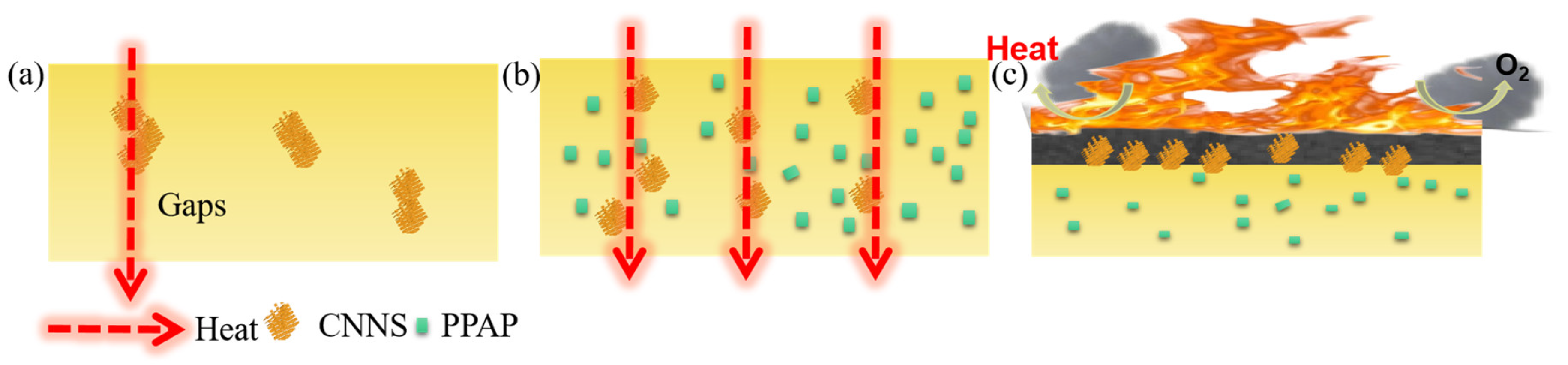
3.3. Thermal Stability and Flame Retardancy of EP/PPAP/CNNS Composites
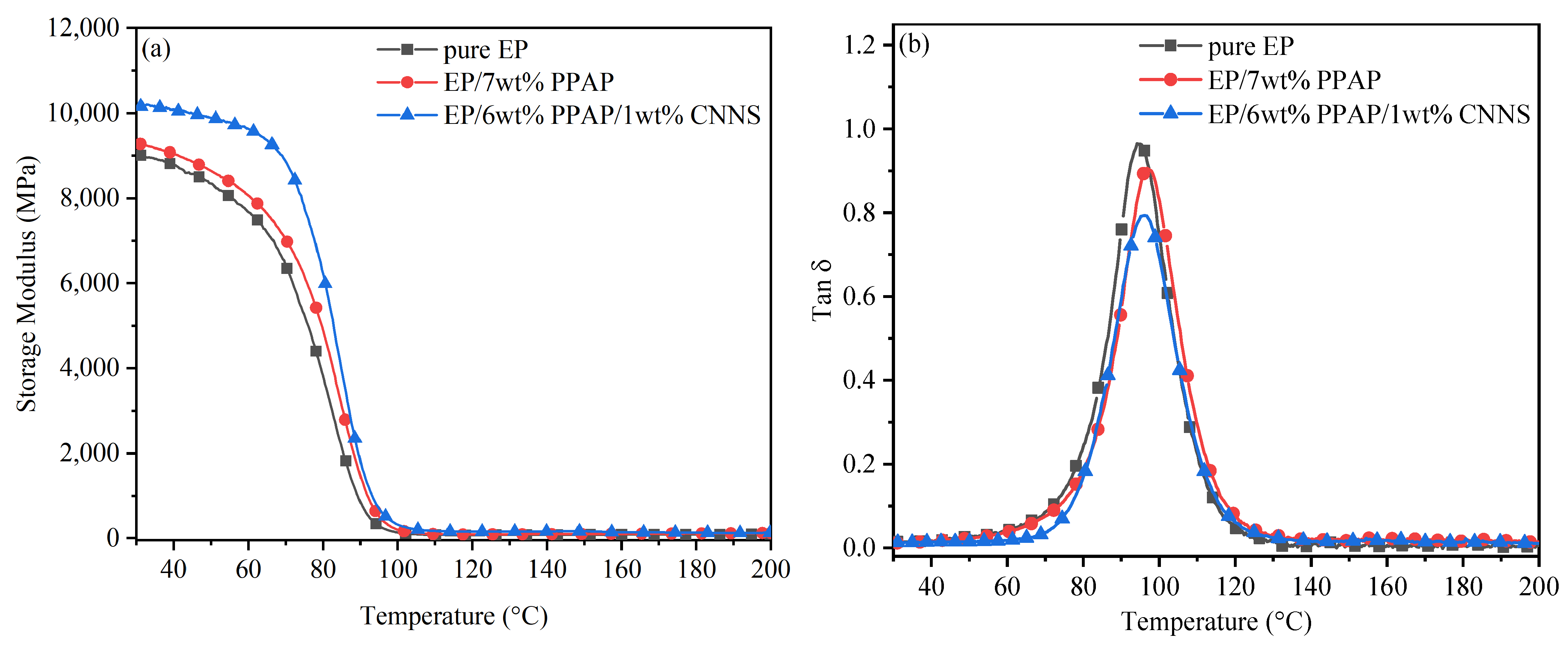
3.4. DMA Analysis of EP Composites
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, W.; Hu, Y.; Tai, Q.; Lu, H.; Song, L.; Yuen, R.K.K. Fire and mechanical performance of nanoclay reinforced glass-fiber/PBT composites containing aluminum hypophosphite particles. Compos. Part A-Appl. Sci. Manuf. 2011, 42, 794–800. [Google Scholar] [CrossRef]
- Yang, W.; Song, L.; Hu, Y.; Lu, H.; Yuen, R.K. Enhancement offire retardancy performance of glass-fibre reinforced poly (ethylene terephthalate) composites with the incorporation of aluminum hypophosphite and melamine cyanurate. Compos. B Eng. 2011, 42, 1057–1065. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Zhang, S.; Lin, M. Investigation on effects of aluminum and magnesium hypophosphites onflame retardancy and thermal degradation of polyamide 6. J. Appl. Polym. Sci. 2012, 125, 1782–1789. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, L.; Long, J.W.; Chen, H.B.; Wang, Y.Z. Aluminum hypophosphite versus alkyl-substituted phosphinate in polyamide 6: Flame retardance, thermal degradation, and pyrolysis behavior. Ind. Eng. Chem. Res. 2013, 52, 2875–2886. [Google Scholar] [CrossRef]
- Tang, G.; Wang, X.; Xing, W.; Zhang, P.; Wang, B.; Hong, N.; Yang, W.; Hu, Y.; Song, L. Thermal degradation and flame retardance of biobased polylactide composites based on aluminum hypophosphite. Ind. Eng. Chem. Res. 2012, 51, 12009–12016. [Google Scholar] [CrossRef]
- Braun, U.; Schartel, B.; Fichera, M.A.; Jäger, C. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine polyphosphate and zinc borate in glass-fibre reinforced polyamide 6, 6. Polym. Degrad. Stab. 2007, 92, 1528–1545. [Google Scholar] [CrossRef]
- Schmitt, E. Phosphorus-based flame retardants for thermoplastics. Plas. Addit. Compd. 2007, 9, 26–30. [Google Scholar] [CrossRef]
- Hill, M.; Hoerold, S.; Krause, W.; Sicken, M. Method for Producing Mixtures of Alkylphosphonous Acid Salts and Dialkylphosphinic Acid Salts. U.S. Patent No. 8,889,772, 18 November 2014. Available online: http://europepmc.org/patents/PAT/WO2011134620 (accessed on 22 October 2021).
- Yang, Q.; Wang, J.; Chen, X.; Yang, S.; Huo, S.; Chen, Q.; Guo, P.; Wang, X.; Liu, F.; Chen, W.; et al. A phosphorus-containing tertiary amine hardener enabled flame retardant, heat resistant and mechanically strong yet tough epoxy resins. Chem. Eng. J. 2023, 468, 143811. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, J.; Liu, L.; Dai, J.; Lu, X.; Huo, S.; Hong, M.; Liu, X.; Lynch, M.; Zeng, X.; et al. Engineering phosphorus-containing lignin for epoxy biocomposites with enhanced thermal stability, fire retardancy and mechanical properties. J. Mater. Sci. Technol. 2023, 167, 82–93. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, L.; Zhang, A.; Wang, W.; Wang, Z.; Zhang, J.; Feng, J.; Huo, S.; Zeng, X.; Song, P. An iron phenylphosphinate@graphene oxide nanohybrid enabled flame-retardant, mechanically reinforced, and thermally conductive epoxy nanocomposites. Chem. Eng. J. 2023, 454, 140424. [Google Scholar] [CrossRef]
- Hu, Z.; Lin, G.P.; Chen, L.; Wang, Y.Z. Flame retardation of glass-fiber-reinforced polyamide 6 by combination of aluminum phenylphosphinate with melamine pyrophosphate. Polym. Adv. Technol. 2011, 22, 1166–1173. [Google Scholar] [CrossRef]
- Isitman, N.A.; Dogan, M.; Bayramli, E.; Kaynak, C. The role of nanoparticle geometry inflame retardancy of polylactide nanocomposites containing aluminium phosphinate. Polym. Degrad. Stab. 2012, 97, 1285–1296. [Google Scholar] [CrossRef]
- Ge, H.; Tang, G.; Hu, W.-Z.; Wang, B.-B.; Pan, Y.; Song, L.; Hu, Y. Aluminum hypophosphite microencapsulated to improve its safety and application to flame retardant polyamide 6. J. Hazard. Mater. 2015, 294, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.Y.; Hamerton, I. Recent developments in the chemistry of halogen-free flame retardant polymers. Prog. Polym. Sci. 2002, 27, 1661–1712. [Google Scholar] [CrossRef]
- Chattopadhyay, D.K.; Webster, D.C. Thermal stability andflame retardancy of polyurethanes. Prog. Polym. Sci. 2009, 34, 1068–1133. [Google Scholar] [CrossRef]
- Martin, C.; Hunt, B.J.; Ebdon, J.R.; Ronda, J.C.; Cadiz, V. Synthesis, polymerization, and effects on the flame retardancy of boron-containing styrenic monomers. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 6419–6430. [Google Scholar] [CrossRef]
- Martin, C.; Hunt, B.J.; Ebdon, J.R.; Ronda, J.C.; Cadiz, V. Synthesis, crosslinking and flame retardance of polymers of boron-containing difunctional styrenic monomers. React. Funct. Polym. 2006, 66, 1047–1054. [Google Scholar] [CrossRef]
- Tai, Q.; Chen, L.; Song, L.; Nie, S.; Hu, Y.; Yuen, R.K. Preparation and thermal properties of a novel flame retardant copolymer. Polym. Degrad. Stab. 2010, 95, 830–836. [Google Scholar] [CrossRef]
- Tai, Q.; Song, L.; Hu, Y.; Yuen, R.K.; Feng, H.; Tao, Y. Novel styrene polymers functionalized with phosphorus-nitrogen containing molecules: Synthesis and properties. Mater. Chem. Phys. 2012, 134, 163–169. [Google Scholar] [CrossRef]
- Tai, Q.; Song, L.; Lv, X.; Lu, H.; Hu, Y.; Yuen, R.K. Flame-retarded polystyrene with phosphorus-and nitrogen-containing oligomer: Preparation and thermal properties. J. Appl. Polym. Sci. 2012, 123, 770–778. [Google Scholar] [CrossRef]
- Costa, L.; Montelera, D.; Rossi, L.; Camino, G.; Weil, E.; Pearce, E. Flame-retardant properties of phenol-formaldehyde-type resins and triphenyl phosphate in styrene-acrylonitrile copolymers. J. Appl. Polym. Sci. 1998, 68, 1067–1076. [Google Scholar] [CrossRef]
- Cao, S.W.; Yu, J.G. G-C3N4-based photocatalysts for hydrogen generation. J. Phys. Chem. Lett. 2014, 5, 2101–2107. [Google Scholar] [CrossRef]
- Xu, M.; Han, L.; Dong, S.J. Facile fabrication of highly efficient g-C3N4/Ag2O hetero structured photocatalysts with enhanced visible-light photocatalytic activity. ACS Appl. Mater. Interfaces 2013, 5, 12533–12540. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yu, B.; Zhou, K.; Yuen, R.K.K.; Gui, Z.; Hu, Y.; Jiang, S. Novel CuCo2O4/graphitic carbon nitride nanohybrids: Highly effective catalysts for reducing CO generation and fire hazards of thermoplastic polyurethane nanocomposites. J. Hazard. Mater. 2015, 293, 87–96. [Google Scholar] [CrossRef]
- Bojdys, M.J.; Müller, J.O.; Antonietti, M.; Thomas, A. Ionothermal synthesis of crystalline, condensed, graphitic carbon nitride. Chem-A Eur. J. 2008, 14, 8177–8182. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Long, Z.; Yu, B.; Zhou, K.; Gui, Z.; Yuen, R.K.K.; Hu, Y. Tunable thermal, flame retardant and toxic effluent suppression properties of polystyrene based on alternating graphitic carbon nitride and multi-walled carbon nanotubes. J. Mater. Chem. A 2015, 3, 17064–17073. [Google Scholar] [CrossRef]
- Hu, W.; Yu, B.; Jiang, S.D.; Song, L.; Hu, Y.; Wang, B. Hyper-branched polymer grafting graphene oxide as an effective flame retardant and smoke suppressant for polystyrene. J. Hazard. Mater. 2015, 300, 58–66. [Google Scholar] [CrossRef]
- Qiu, S.; Hu, W.; Yu, B.; Yuan, B.; Zhu, Y.; Jiang, S.; Wang, B.; Song, L.; Hu, Y. Effect of functionalized graphene oxide with organophosphorus oligomer on the thermal and mechanical properties and fire safety of polystyrene. Ind. Eng. Chem. Res. 2015, 54, 3309–3319. [Google Scholar] [CrossRef]
- Han, Y.; Wu, Y.; Shen, M.; Huang, X.; Zhu, J.; Zhang, X. Preparation and properties of polystyrene nanocomposites with graphite oxide and graphene as flame retardants. J. Mater. Sci. 2013, 48, 4214–4222. [Google Scholar] [CrossRef]
- Yang, L.; Han, X.Y.; Tang, X.J.; Han, C.X.; Zhou, Y.X.; Zhang, B.G. A novel photo-initiated approach for preparing aluminum diethylphosphinate under atmospheric pressure. Chin. Chem. Lett. 2011, 22, 385–388. [Google Scholar] [CrossRef]
- Qiu, Y.; Gao, L. Chemical synthesis of turbostratic carbon nitride, containing CAN crystallites, at atmospheric pressure. Chem. Commun. 2003, 18, 2378–2379. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.C. Interpretation of the Infrared Spectra of Organophosphorus Compounds [by] LC Thomas. 1974. Available online: https://lib.ugent.be/catalog/rug01:000747621 (accessed on 14 November 2021).
- Mostovoy, A.S.; Yakovlev, A.V.; Lopukhova, M.I. Directional control of physico-chemical and mechanical properties of epoxide composites by the addition of graphite-graphene structures. Polym. Technol. Mater. 2020, 59, 874–883. [Google Scholar] [CrossRef]
- Bao, X.; Wu, F.; Wang, J. Thermal Degradation Behavior of Epoxy Resin Containing Modified Carbon Nanotubes. Polymers 2021, 13, 3332. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Tao, R.; Dai, P.; Luo, Z.; He, M. Two-step fabrication of lignin-based flame retardant for enhancing the thermal and fire retardancy properties of epoxy resin composites. Polym. Compos. 2020, 41, 2025–2035. [Google Scholar] [CrossRef]
- Yuan, B.; Bao, C.; Guo, Y.; Song, L.; Liew, K.M.; Hu, Y. Preparation and characterization of flame-retardant aluminum hypophosphite/poly (vinyl alcohol) composite. Ind. Eng. Chem. Res. 2012, 51, 14065–14075. [Google Scholar] [CrossRef]
- Pan, C.; Kou, K.; Jia, Q.; Zhang, Y.; Wu, G.; Ji, T. Improved thermal conductivity and dielectric properties of hBN/PTFE composites via surface treatment by silane coupling agent. Compos. Part B-Eng. 2017, 111, 83–90. [Google Scholar] [CrossRef]

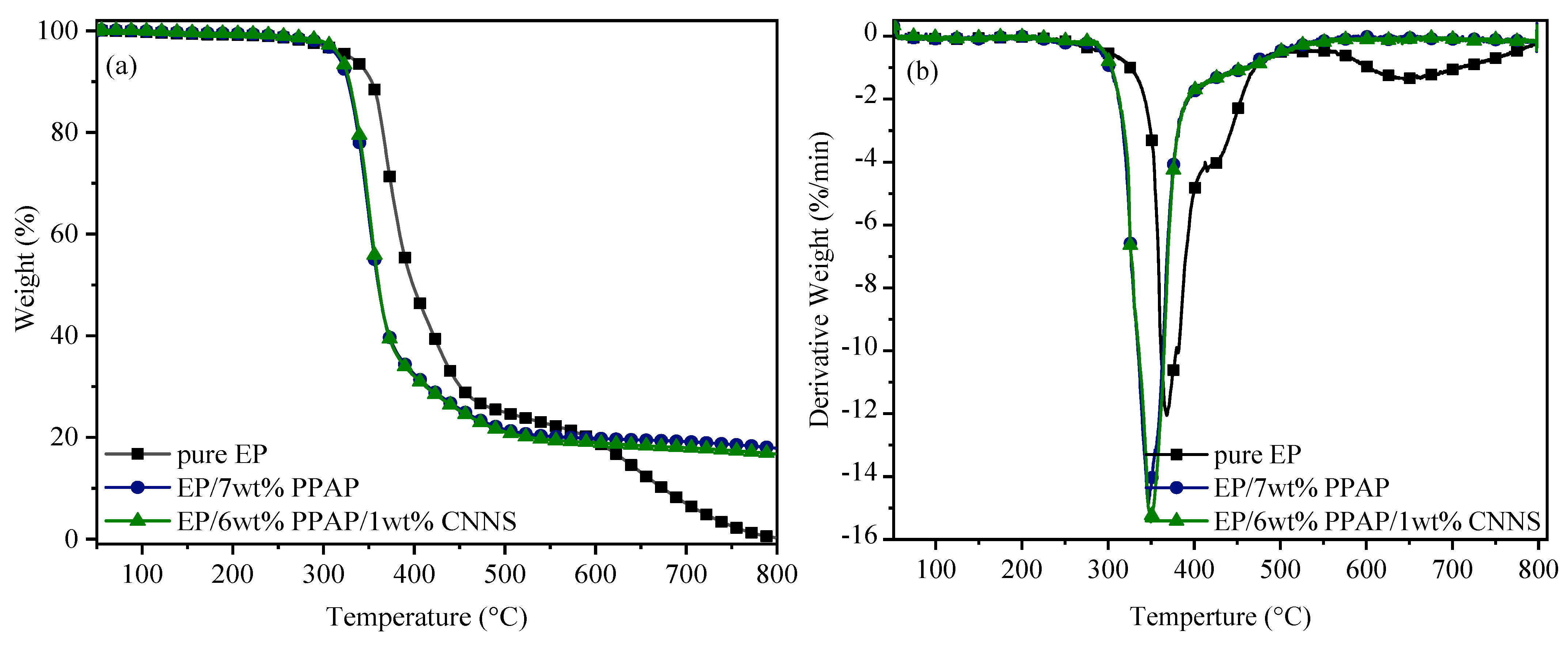



| Sample | Tinitial (°C) | Rpeak1/Tpeak1 (%/min/°C) | Rpeak2/Tpeak2 (%/min/°C) | Char Residue (wt%) 800 °C |
|---|---|---|---|---|
| Pure EP | 327.9 | 12.1/367.9 | 1.4/644.6 | 0.3 |
| EP/7 wt% PPAP | 315.7 | 14.9/347.0 | - | 17.9 |
| EP/6 wt%PPAP/1 wt%CNNS | 317.9 | 15.4/349.7 | - | 16.7 |
| E-51 (g) | CNNS (wt %) | PPAP (wt %) | Thermal Conductivity (W·m−1k−1) | UL-94 | LOI (%) |
|---|---|---|---|---|---|
| 100 | - | 5 | - | NR | 27.2 |
| 100 | - | 6 | - | V-1 | 28.4 |
| 100 | - | 7 | - | V-0 | 29.7 |
| 100 | 0.5 | 6.5 | 0.844 | V-0 | 29.5 |
| 100 | 1 | 6 | 1.118 | V-0 | 28.8 |
| 100 | 1.5 | 5.5 | 1.164 | V-1 | 27.6 |
| Properties | Pure EP | EP/7%PPAP | EP/1%CNNS/6%PPAP |
|---|---|---|---|
| TTI (s) | 43 | 29 | 36 |
| PHRR (kW·m−2) | 2123 | 723 | 771 |
| tp (s) | 95 | 81 | 108 |
| THR (MJ·m−2) | 124.1 | 58.7 | 56.8 |
| PSPR (m2·s−1) | 0.28 | 0.23 | 0.22 |
| tp (s) | 76 | 37 | 89 |
| TSP (m2) | 23.5 | 10.1 | 10.3 |
| Av-EHC (MJ·kg−1) | 25.2 | 7.5 | 7.8 |
| Char residue (%) | 4.1 | 39.3 | 37.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, R.; Leng, Y.; Wang, J.; Xu, M. An Integrated Multi-Functional Thermal Conductive and Flame Retardant Epoxy Composite with Functionalized Carbon Nitride Nanosheets. Polymers 2023, 15, 3143. https://doi.org/10.3390/polym15143143
Yang Y, Wang R, Leng Y, Wang J, Xu M. An Integrated Multi-Functional Thermal Conductive and Flame Retardant Epoxy Composite with Functionalized Carbon Nitride Nanosheets. Polymers. 2023; 15(14):3143. https://doi.org/10.3390/polym15143143
Chicago/Turabian StyleYang, Yuxin, Ruiping Wang, Yang Leng, Jingchun Wang, and Miaojun Xu. 2023. "An Integrated Multi-Functional Thermal Conductive and Flame Retardant Epoxy Composite with Functionalized Carbon Nitride Nanosheets" Polymers 15, no. 14: 3143. https://doi.org/10.3390/polym15143143




