Facile Synthesis of Polymer-Reinforced Silica Aerogel Microspheres as Robust, Hydrophobic and Recyclable Sorbents for Oil Removal from Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of the SAMs
2.3. Characterization
3. Results and Discussion
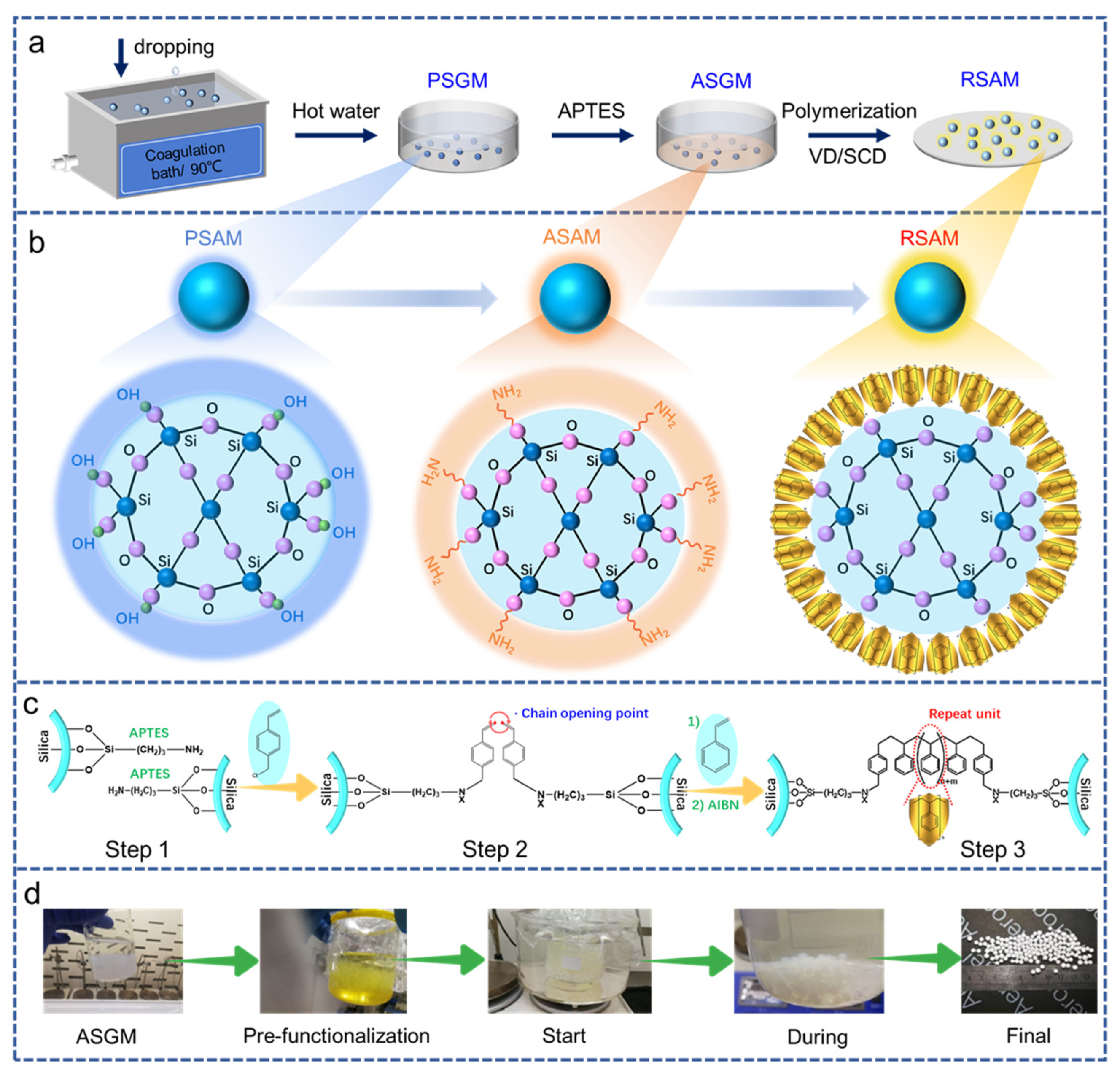
3.1. Synthesis Mechanism
3.2. Physical Properties
3.3. Chemical Structure
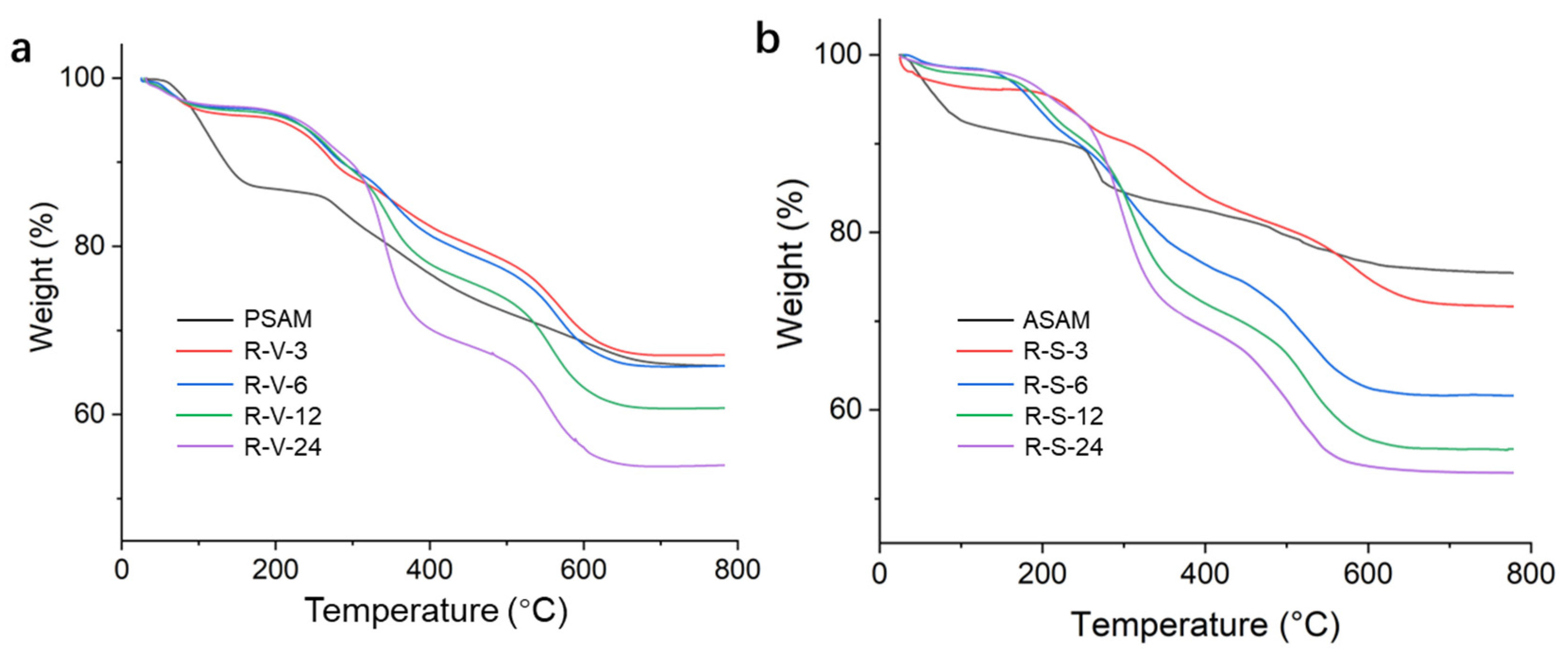
3.4. Thermal Performances
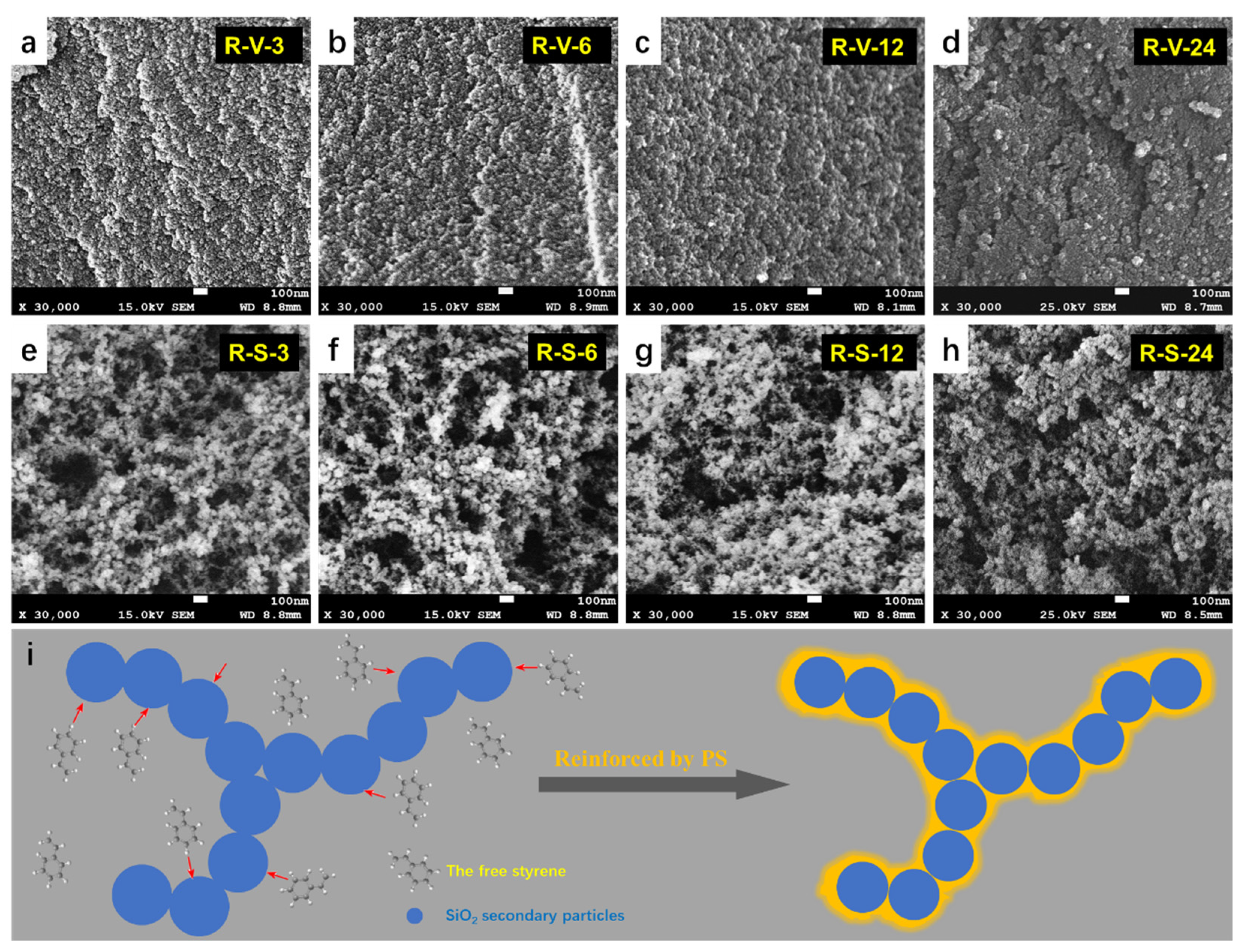
3.5. Microstructure and Morphology
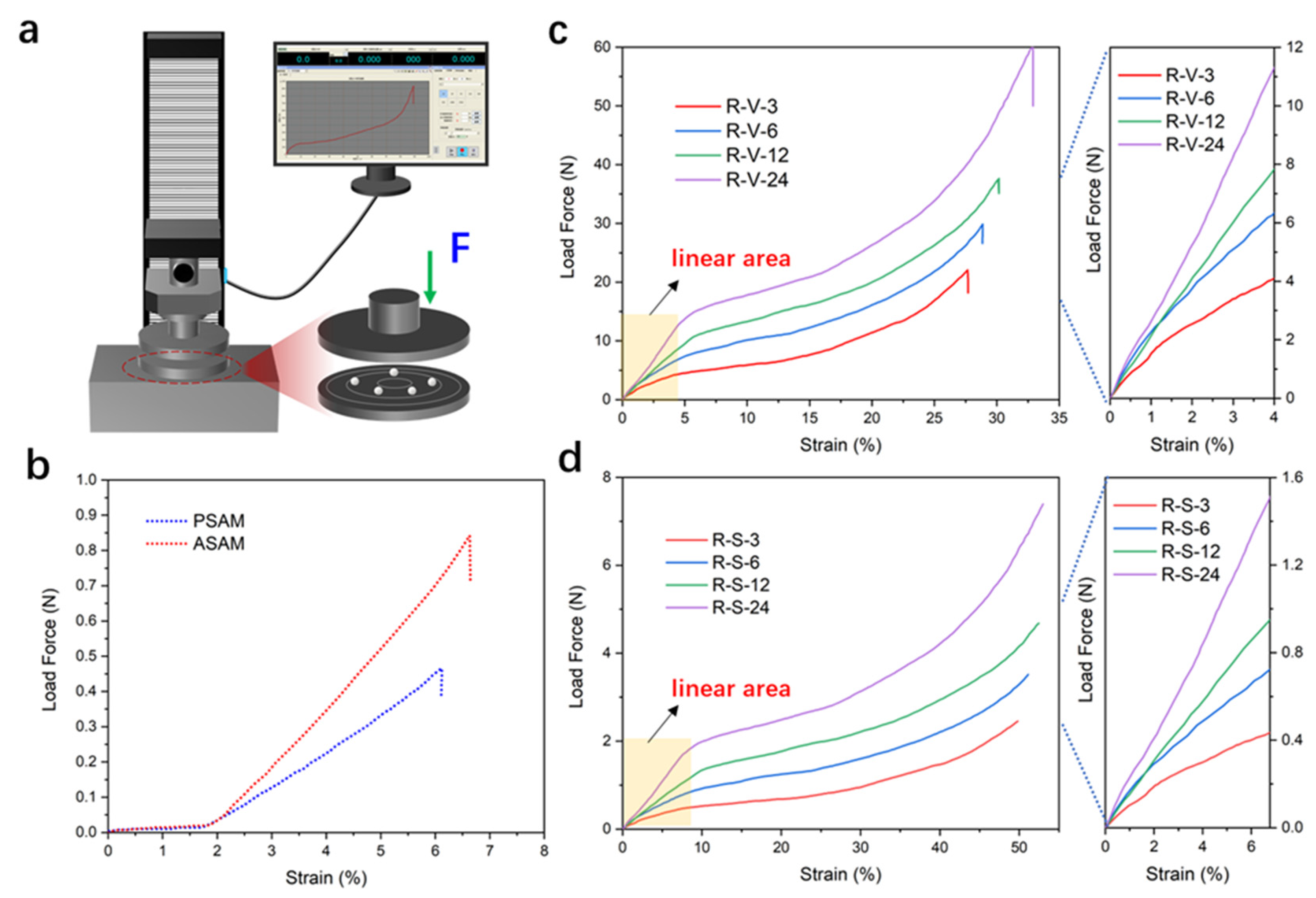
3.6. Mechanical Performance
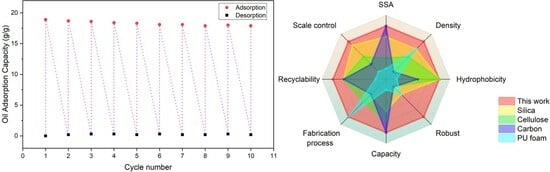
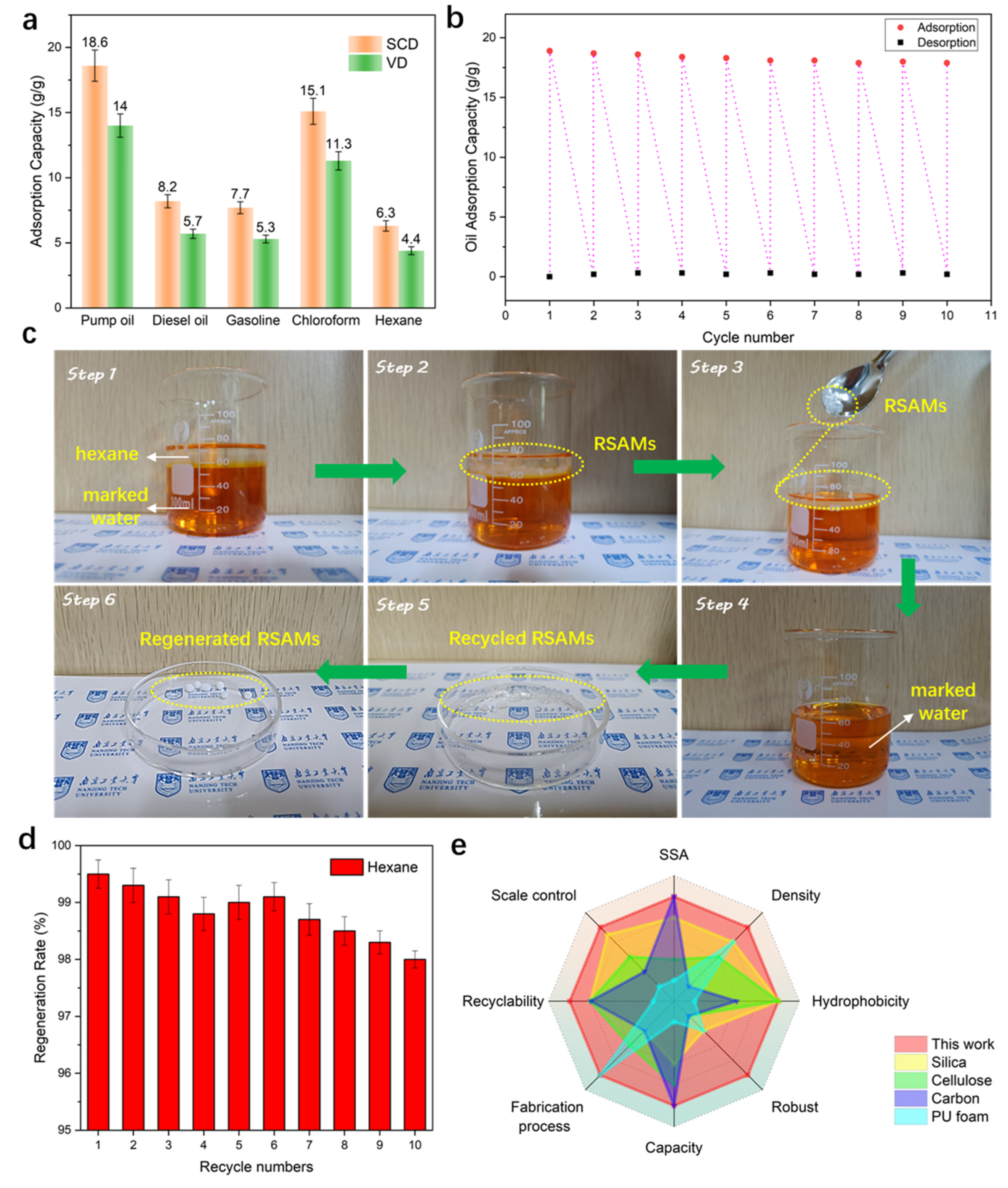
3.7. Adsorption and Recyclability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, W.; Portehault, D.; Liu, D.; Qin, S.; Chen, Y. Porous boron nitride nanosheets for effective water cleaning. Nat. Commun. 2013, 4, 1777. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wu, W.; Huang, Z.; Lei, W.; Li, S.; Zhang, H.; Jia, Q.; Zhang, S. Preparation and characterization of a novel fluorine-free and ph-sensitive hydrophobic porous diatomite ceramic as highly efficient sorbent for oil-water separation. Sep. Purif. Technol. 2021, 254, 117620. [Google Scholar] [CrossRef]
- Wu, X.; Zhong, K.; Ding, J.; Shen, X.; Cui, S.; Zhong, Y.; Ma, J.; Chen, X. Facile synthesis of flexible and hydrophobic polymethylsilsesquioxane based silica aerogel via the co-precursor method and ambient pressure drying technique. J. Non-Cryst. Solids 2020, 530, 119826. [Google Scholar] [CrossRef]
- Zhao, S.; Siqueira, G.; Drdova, S.; Norris, D.; Ubert, C.; Bonnin, A.; Galmarini, S.; Ganobjak, M.; Pan, Z.; Brunner, S.; et al. Additive manufacturing of silica aerogels. Nature 2020, 584, 387–392. [Google Scholar] [CrossRef]
- Ding, J.; Zhong, K.; Liu, S.; Wu, X.; Shen, X.; Cui, S.; Chen, X. Flexible and super hydrophobic polymethylsilsesquioxane based silica aerogel for organic solvent adsorption via ambient pressure drying technique. Powder Technol. 2020, 373, 716–726. [Google Scholar] [CrossRef]
- Liu, S.; Wu, X.; Li, Y.; Cui, S.; Shen, X.; Tan, G. Hydrophobic in-situ SiO2-TiO2 composite aerogel for heavy oil thermal recovery: Synthesis and high temperature performance. Appl. Therm. Eng. 2021, 190, 116745. [Google Scholar] [CrossRef]
- Malfait, W.J.; Zhao, S.; Verel, R.; Iswar, S.; Rentsch, D.; Fener, R.; Zhang, Y.; Milow, B.; Koebel, M.M. Surface chemistry of hydrophobic silica aerogels. Chem. Mater. 2015, 27, 6737–6745. [Google Scholar] [CrossRef]
- Qin, G.; Yao, Y.; Wei, W.; Zhang, T. Preparation of hydrophobic granular silica aerogels and adsorption of phenol from water. Appl. Surf. Sci. 2013, 280, 806–811. [Google Scholar] [CrossRef]
- Cok, S.S.; Koc, F.; Gizli, N. Lightweight and highly hydrophobic silica aerogels dried in ambient pressure for an efficient oil/organic solvent adsorption. J. Hazard. Mater. 2020, 408, 124858. [Google Scholar]
- Minju, N.; Nair, B.N.; Savithri, S. Sodium silicate-derived aerogels: Effect of processing parameters on their applications. RSC Adv. 2021, 11, 15301–15322. [Google Scholar]
- He, S.; Li, Z.; Shi, X.; Yang, H.; Gong, L.; Cheng, X. Rapid synthesis of sodium silicate based hydrophobic silica aerogel granules with large surface area. Adv. Powder Technol. 2015, 26, 537–541. [Google Scholar] [CrossRef]
- Nah, H.-Y.; Parale, V.G.; Jung, H.-N.-R.; Lee, K.-Y.; Lim, C.-H.; Ku, Y.S.; Park, H.-H. Role of oxalic acid in structural formation of sodium silicate-based silica aerogel by ambient pressure drying. J. Sol.-Gel. Sci. Technol. 2018, 85, 302–310. [Google Scholar] [CrossRef]
- Khedkar, M.V.; Somvanshi, S.B.; Humbe, A.V.; Jadhav, K.M. Surface modified sodium silicate based superhydrophobic silica aerogels prepared via ambient pressure drying process. J. Non-Cryst. Solids 2019, 511, 140–146. [Google Scholar] [CrossRef]
- Mazrouei-Sebdani, Z.; Salimian, S.; Khoddami, A.; Shams-Ghahfarokhi, F. Sodium silicate based aerogel for absorbing oil from water: The impact of surface energy on the oil/water separation. Mater. Res. Express 2019, 6, 085059. [Google Scholar] [CrossRef]
- Cho, Y.-S.; Sung, S.; Woo, S.H.; Kim, Y.S. Optimization of oil adsorption capacity by aerogel powder synthesized using emulsion droplets as micro-reactors in ambient conditions from sodium silicate as precursor. Korean J. Met. Mater. 2021, 59, 99–112. [Google Scholar] [CrossRef]
- Wang, D.; McLaughlin, E.; Pfeffer, R.; Lin, Y.S. Aqueous phase adsorption of toluene in a packed and fluidized bed of hydrophobic aerogels. Chem. Eng. J. 2011, 168, 1201–1208. [Google Scholar] [CrossRef]
- Jiang, X.; Kong, Y.; Zhao, Z.; Shen, X. Spherical amine grafted silica aerogels for CO2 capture. RSC Adv. 2020, 10, 25911–25917. [Google Scholar] [CrossRef]
- Jiang, X.; Ren, J.; Kong, Y.; Zhao, Z.; Shen, X.; Fan, M. Shape-tailorable amine grafted silica aerogel microsphere for CO2 capture. Green Chem. Eng. 2020, 1, 140–146. [Google Scholar] [CrossRef]
- Cheng, Y.; Cai, Y.; Wang, Z.; Lu, X.; Xia, H. Ultralight nico@rGO aerogel microspheres with magnetic response for oil/water separation. Chem. Eng. J. 2022, 430, 132894. [Google Scholar] [CrossRef]
- Li, J.; Cheng, R.; Chen, J.; Lan, J.; Li, S.; Zhou, M.; Zeng, T.; Hou, H. Microscopic mechanism about the selective adsorption of Cr(VI) from salt solution on nitrogen-doped carbon aerogel microsphere pyrolysis products. Sci. Total Environ. 2021, 798, 149331. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Zhang, G.; Rawashdeh, A.M.M. Nanoengineering strong silica aerogels. Nano Lett. 2002, 2, 957–960. [Google Scholar] [CrossRef]
- Leventis, N.; Sotiriou-Leventis, C.; Chandrasekaran, N.; Mulik, S.; Larimore, Z.J.; Lu, H.; Churu, G.; Mang, J.T. Multifunctional polyurea aerogels from isocyanates and water. A structure-property case study. Chem. Mater. 2010, 22, 6692–6710. [Google Scholar] [CrossRef]
- Chidambareswarapattar, C.; McCarver, P.M.; Luo, H.; Lu, H.; Sotiriou-Leventis, C.; Leventis, N. Fractal multiscale nanoporous polyurethanes: Flexible to extremely rigid aerogels from multifunctional small molecules. Chem. Mater. 2013, 25, 3205–3224. [Google Scholar] [CrossRef]
- Meador, M.A.B.; Fabrizio, E.F.; Ilhan, F.; Dass, A.; Zhang, G.; Vassilaras, P.; Johnston, J.C.; Leventis, N. Cross-linking amine-modified silica aerogels with epoxies: Mechanically strong lightweight porous materials. Chem. Mater. 2005, 17, 1085–1098. [Google Scholar] [CrossRef]
- Nguyen, B.N.; Meador, M.A.B.; Tousley, M.E.; Shonkwiler, B.; McCorkle, L.; Scheiman, D.A.; Palczer, A. Tailoring elastic properties of silica aerogels cross-linked with polystyrene. ACS Appl. Mater. Interfaces 2009, 1, 621–630. [Google Scholar] [CrossRef]
- Ilhan, F.U.; Fabrizio, E.F.; McCorkle, L.; Scheiman, D.A.; Dass, A.; Palczer, A.; Meador, M.B.; Johnston, J.C.; Leventis, N. Hydrophobic monolithic aerogels by nanocasting polystyrene on amine-modified silica. J. Mater. Chem. 2006, 16, 3046–3054. [Google Scholar] [CrossRef]
- Ren, J.; Zhou, J.T.; Kong, Y.; Jiang, X.; Shen, X.D. Development of regular hydrophobic silica aerogel microspheres for efficient oil adsorption. Langmuir 2023, 39, 478–486. [Google Scholar] [CrossRef]
- Hu, D.; Shao, G.; Wang, J.; Gurlo, A.; Bekheet, M.F. Revealing nanodomain structures of bottom-up-fabricated graphene-embedded silicon oxycarbide ceramics. Polymers 2022, 14, 3675. [Google Scholar] [CrossRef]
- Cai, G.; Ni, H.; Li, X.; Wang, Y.; Zhao, H. Eco-friendly fabrication of highly stable silica aerogel microspheres with core-shell structure. Polymers 2023, 15, 1882. [Google Scholar] [CrossRef]
- Iswar, S.; Galmarini, S.; Bonanomi, L.; Wernery, J.; Roumeli, E.; Nimalshantha, S.; Ben Ishai, A.M.; Lattuada, M.; Koebel, M.M.; Malfait, W.J. Dense and strong, but superinsulating silica aerogel. Acta Mater. 2021, 213, 116959. [Google Scholar] [CrossRef]
- Li, Z.; Cao, M.; Li, P.; Zhao, Y.; Bai, H.; Wu, Y.; Jiang, L. Surface-embedding of functional micro-/nanoparticles for achieving versatile superhydrophobic interfaces. Matter 2019, 1, 661–673. [Google Scholar]
- Al-Oweini, R.; El-Rassy, H. Synthesis and characterization by ftir spectroscopy of silica aerogels prepared using several Si(OR)4 and R′′Si(OR′)3 precursors. J. Mol. Struct. 2009, 919, 140–145. [Google Scholar]
- Zhao, Z.; Cui, Y.; Kong, Y.; Ren, J.; Jiang, X.; Yan, W.; Li, M.; Tang, J.; Liu, X.; Shen, X. Thermal and mechanical performances of the superflexible, hydrophobic, silica-based aerogel for thermal insulation at ultralow temperature. ACS Appl. Mater. Interfaces 2021, 13, 21286–21298. [Google Scholar] [PubMed]
- Ren, J.; Zhang, T.; Kong, Y.; Zhao, Z.; Zhu, K.; Zhang, X.; Shen, X. Facile synthesis of phenolic-reinforced silica aerogel composites for thermal insulation under thermal-force coupling conditions. Ceram. Int. 2023, 49, 29820–29828. [Google Scholar]
- Zhang, Y.; Shen, Q.; Li, X.; Wang, L.; Nie, C. Facile preparation of a phenyl-reinforced flexible silica aerogel with excellent thermal stability and fire resistance. Mater. Chem. Front. 2021, 5, 4214–4224. [Google Scholar]
- Chen, L.; Zheng, J.; Fu, R. Hybrid nanospheres with metastable silica-nanonetwork and confined phenyl for simple fabrication of high-surface-area microporous carbon materials. Carbon 2020, 170, 658–665. [Google Scholar]
- Lin, W.; Zheng, J.; Zhuo, J.; Chen, H.; Zhang, X. Characterization of sol-gel ormosil antireflective coatings from phenyltriethoxysilane and tetraethoxysilane: Microstructure control and application. Surf. Coat. Technol. 2018, 345, 177–182. [Google Scholar]
- Iswar, S.; Griffa, M.; Kaufmann, R.; Beltran, M.; Huber, L.; Brunner, S.; Lattuada, M.; Koebel, M.M.; Malfait, W.J. Effect of aging on thermal conductivity of fiber-reinforced aerogel composites: An x-ray tomography study. Microporous Mesoporous Mater. 2019, 278, 289–296. [Google Scholar]
- Leventis, N. Three-dimensional core-shell superstructures: Mechanically strong aerogels. Acc. Chem. Res. 2007, 40, 874–884. [Google Scholar] [CrossRef] [PubMed]
- Meador, M.A.B.; Capadona, L.A.; McCorkle, L.; Papadopoulos, D.S.; Leventis, N. Structure-property relationships in porous 3d nanostructures as a function of preparation conditions: Isocyanate cross-linked silica aerogels. Chem. Mater. 2007, 19, 2247–2260. [Google Scholar] [CrossRef]
- Katti, A.; Shimpi, N.; Roy, S.; Lu, H.; Fabrizio, E.F.; Dass, A.; Capadona, L.A.; Leventis, N. Chemical, physical, and mechanical characterization of isocyanate cross-linked amine-modified silica aerogels. Chem. Mater. 2006, 18, 285–296. [Google Scholar] [CrossRef]
- Wu, X.; Man, J.; Liu, S.; Huang, S.; Lu, J.; Tai, J.; Zhong, Y.; Shen, X.; Cui, S.; Chen, X. Isocyanate-crosslinked silica aerogel monolith with low thermal conductivity and much enhanced mechanical properties: Fabrication and analysis of forming mechanisms. Ceram. Int. 2021, 47, 26668–26677. [Google Scholar] [CrossRef]
- Meador, M.A.; Weber, A.S.; Hindi, A.; Naumenko, M.; McCorkle, L.; Quade, D.; Vivod, S.L.; Gould, G.L.; White, S.; Deshpande, K. Structure-property relationships in porous 3d nanostructures: Epoxy-cross-linked silica aerogels produced using ethanol as the solvent. ACS Appl. Mater. Interfaces 2009, 1, 894–906. [Google Scholar] [CrossRef] [PubMed]
- Malakooti, S.; Qin, G.; Mandal, C.; Soni, R.; Taghvaee, T.; Ren, Y.; Chen, H.; Tsao, N.; Shiao, J.; Kulkarni, S.S.; et al. Low-cost, ambient-dried, superhydrophobic, high strength, thermally insulating, and thermally resilient polybenzoxazine aerogels. ACS Appl. Mater. Interfaces 2019, 1, 2322–2333. [Google Scholar] [CrossRef]
- Yao, W.Q.; Mao, R.W.; Gao, W.W.; Chen, W.Q.; Xu, Z.; Gao, C. Piezoresistive effect of superelastic graphene aerogel spheres. Carbon 2020, 158, 418–425. [Google Scholar] [CrossRef]
- Gross, J.; Reichenauer, G.; Fricke, J. Mechanical properties of sio2 aerogels. J. Phys. D Appl. Phys. 1988, 21, 1447–1451. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, Z.; Kong, Y.; Ren, J.; Jiang, X.; Shen, X. Auto-continuous synthesis of robust and hydrophobic silica aerogel microspheres from low-cost aqueous sodium silicate for fast dynamic organics removal. Gels 2022, 8, 778. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhong, K.; Liu, W.; Cui, S.; Zhong, Y.; Jiang, S. Preparation and oil adsorption properties of hydrophobic microcrystalline cellulose aerogel. Cellulose 2020, 27, 7663–7675. [Google Scholar] [CrossRef]
- Li, Z.; Lei, S.; Xi, J.; Ye, D.; Hu, W.; Song, L.; Hu, Y.; Cai, W.; Gui, Z. Bio-based multifunctional carbon aerogels from sugarcane residue for organic solvents adsorption and solar-thermal-driven oil removal. Chem. Eng. J. 2021, 426, 129580. [Google Scholar] [CrossRef]
- Dat, N.H.; Tuan, V.M.; Huynh, M.D.; Trung, T.H.; Trang, N.T.T.; Thang, D.X.; Hoa, K.T.Q.; Giang, N.V. Novel method for producing oleophilic polyurethane foam to remove oil from open water. J. Polym. Environ. 2022, 30, 5012–5023. [Google Scholar] [CrossRef]



| Sample | Apparent Density (g/cm3) | Porosity (%) | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Drying Method | Hydrophobic Properties |
|---|---|---|---|---|---|---|
| PSAM | 0.080 | 96.4 | 203.6 | 0.49 | VD a | no |
| ASAM | 0.137 | 93.8 | 230.4 | 2.78 | SCD b | no |
| R-V-3 | 0.158 | 92.8 | 261.1 | 1.39 | VD | no |
| R-V-6 | 0.166 | 92.5 | 256.1 | 1.23 | VD | no |
| R-V-12 | 0.191 | 91.3 | 240.6 | 1.16 | VD | no |
| R-V-24 | 0.210 | 90.5 | 218.2 | 1.07 | VD | yes |
| R-S-3 | 0.141 | 93.6 | 289.8 | 3.81 | SCD | no |
| R-S-6 | 0.148 | 93.3 | 285.7 | 3.77 | SCD | no |
| R-S-12 | 0.155 | 93.0 | 280.5 | 3.68 | SCD | no |
| R-S-24 | 0.163 | 92.6 | 277.9 | 3.52 | SCD | yes |
| Sample | Adsorption Capacity (g/g) | Specific Surface Area (m2/g) | Apparent Density (g/cm3) | Hydrophobicity (°) | Fabrication Process | Robust Level | Ref. |
|---|---|---|---|---|---|---|---|
| Silica aerogel | 6.0 | 296 | 0.08 | 128 | SCD | medium | Sun et al. [47] |
| Cellulose aerogel | 12.0 | 154 | 0.05 | 140 | SCD | low | Zhao et al. [48] |
| Carbon aerogel | 17.1 | 309 | 0.03 | 99 | FD | low | Li et al. [49] |
| PU foam | 7.3 | not reported | 0.06 | 95 | VD | low | Dat et al. [50] |
| RSAMs | 18.6 | 281 | 0.16 | 126 | VD/SCD | high | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Z.; Ren, J.; Liu, W.; Yan, W.; Zhu, K.; Kong, Y.; Jiang, X.; Shen, X. Facile Synthesis of Polymer-Reinforced Silica Aerogel Microspheres as Robust, Hydrophobic and Recyclable Sorbents for Oil Removal from Water. Polymers 2023, 15, 3526. https://doi.org/10.3390/polym15173526
Zhao Z, Ren J, Liu W, Yan W, Zhu K, Kong Y, Jiang X, Shen X. Facile Synthesis of Polymer-Reinforced Silica Aerogel Microspheres as Robust, Hydrophobic and Recyclable Sorbents for Oil Removal from Water. Polymers. 2023; 15(17):3526. https://doi.org/10.3390/polym15173526
Chicago/Turabian StyleZhao, Zhiyang, Jian Ren, Wei Liu, Wenqian Yan, Kunmeng Zhu, Yong Kong, Xing Jiang, and Xiaodong Shen. 2023. "Facile Synthesis of Polymer-Reinforced Silica Aerogel Microspheres as Robust, Hydrophobic and Recyclable Sorbents for Oil Removal from Water" Polymers 15, no. 17: 3526. https://doi.org/10.3390/polym15173526