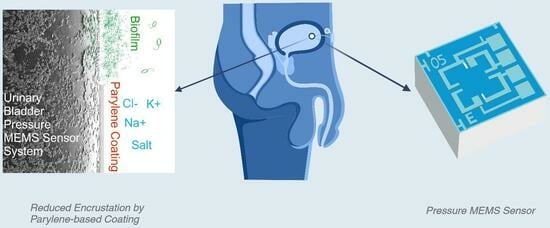
An Evaluation of Parylene Thin Films to Prevent Encrustation for a Urinary Bladder Pressure MEMS Sensor System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thin Film Deposition
2.1.1. Parylene Deposition
2.1.2. SiOx Finish Coatings
2.2. In Vitro Encrustation Test
Fourier Transform Infrared Spectroscopy
2.3. Contact Angle
2.4. Surface Charge
3. Results
3.1. In Vitro Encrustation Test
Fourier Transform Infrared Spectroscopy
3.2. Contact Angle
3.3. Surface Charge
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Stickler, D.J. Clinical Complications of Urinary Catheters Caused by Crystalline Biofilms: Something Needs to Be Done. J. Intern. Med. 2014, 276, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Soria, F. Present and Future of Urinary Stents. In Urinary Stents: Current State and Future Perspectives; Springer International Publishing: Cham, Switzerland, 2022; pp. 1–4. ISBN 978-3-031-04483-0. [Google Scholar]
- Kram, W.; Rebl, H.; de la Cruz, J.E.; Haag, A.; Renner, J.; Epting, T.; Springer, A.; Soria, F.; Wienecke, M.; Hakenberg, O.W. Interactive Effects of Copper-Doped Urological Implants with Tissue in the Urinary Tract for the Inhibition of Cell Adhesion and Encrustation in the Animal Model Rat. Polymers 2022, 14, 3324. [Google Scholar] [CrossRef] [PubMed]
- Forbes, C.; Scotland, K.B.; Lange, D.; Chew, B.H. Innovations in Ureteral Stent Technology. Urol. Clin. N. Am. 2019, 46, 245–255. [Google Scholar] [CrossRef]
- Xia, K.; Shen, X.; Ang, X.; Hou, B.; Chen, Y.; Zhang, K.; Hao, Z. Surface Modification of Ureteral Stents: Development History, Classification, Function, and Future Developments. Expert Rev. Med. Devices 2023, 20, 401–416. [Google Scholar] [CrossRef]
- Laube, N.; Desai, C.; Bernsmann, F. Hydrophobic Forces as a Key Factor in Crystalline Biofilm Formation on Ureteral Stents. Biomed. Eng./Biomed. Tech. 2016, 61, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, Y.; Li, Y.; Xu, M.; Sun, G.; Zou, T.; Wang, F.; Xu, S.; Da, J.; Wang, L. Biomimetic Biodegradable Ag@ Au Nanoparticle-Embedded Ureteral Stent with a Constantly Renewable Contact-Killing Antimicrobial Surface and Antibiofilm and Extraction-Free Properties. Acta Biomater. 2020, 114, 117–132. [Google Scholar] [CrossRef]
- Franz, G.; Schamberger, F.; Zare, H.H.; Bröskamp, S.F.; Jocham, D. Bi-Layer Sandwich Film for Antibacterial Catheters. Beilstein J. Nanotechnol. 2017, 8, 1982–2001. [Google Scholar] [CrossRef] [PubMed]
- Cirioni, O.; Ghiselli, R.; Silvestri, C.; Minardi, D.; Gabrielli, E.; Orlando, F.; Rimini, M.; Brescini, L.; Muzzonigro, G.; Guerrieri, M. Effect of the Combination of Clarithromycin and Amikacin on Pseudomonas Aeruginosa Biofilm in an Animal Model of Ureteral Stent Infection. J. Antimicrob. Chemother. 2011, 66, 1318–1323. [Google Scholar] [CrossRef]
- Minardi, D.; Cirioni, O.; Ghiselli, R.; Silvestri, C.; Mocchegiani, F.; Gabrielli, E.; d’Anzeo, G.; Conti, A.; Orlando, F.; Rimini, M. Efficacy of Tigecycline and Rifampin Alone and in Combination against Enterococcus Faecalis Biofilm Infection in a Rat Model of Ureteral Stent. J. Surg. Res. 2012, 176, 1–6. [Google Scholar] [CrossRef]
- Senturia, S.D. Microsystem Design; Kluwer Academic Publishers: Boston, MA, USA, 2002. [Google Scholar]
- Wisniewski, N.; Moussy, F.; Reichert, W.M. Characterization of Implantable Biosensor Membrane Biofouling. Fresenius J. Anal. Chem. 2000, 366, 611–621. [Google Scholar] [CrossRef]
- Clausen, I.; Tvedt, L.G.W.; Hellandsvik, A.; Rognlien, D.K.W.; Glott, T. An in Vivo MEMS Sensor System for Percutaneous Measurement of Urinary Bladder. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Korea, 11–15 July 2017; pp. 1857–1860. [Google Scholar] [CrossRef]
- Clausen, I.; Tvedt, L.G.W.; Moe, S.; Vogl, A. The Effect of True Human Synovial Fluid on the Functionality of an in Vivo Pressure Sensor Element. In Proceedings of the SENSORS, 2013 IEEE, Baltimore, MD, USA, 3–6 November 2013. [Google Scholar] [CrossRef]
- Clausen, I.; Seeberg, T.M.; Gheorghe, C.; Wang, D.T. Biofouling on Protective Coatings for Implantable MEMS. In Proceedings of the SENSORS, 2010 IEEE, Waikoloa, HI, USA, 1–4 November 2010; pp. 751–754. [Google Scholar] [CrossRef]
- Clausen, I.; Moe, S.T.; Tvedt, L.G.W.; Vogl, A.; Wang, D.T. A Miniaturized Pressure Sensor with Inherent Biofouling Protection Designed for in Vivo Applications. In Proceedings of the 2011 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Boston, MA, USA, 30 August–3 September 2011; pp. 1880–1883. [Google Scholar] [CrossRef]
- Bröskamp, S.F.; Franz, G.; Jocham, D. Internal Coating of Ureteral Stents with Chemical Vapor Deposition of Parylene. Coatings 2021, 11, 739. [Google Scholar] [CrossRef]
- Bernsmann, F.; Laube, N.; Baldsiefen, G.; Castellucci, M. Hydrogenated Amorphous Carbon Coatings on Implants Drastically Reduce Biofilm Formation and Water Permeation. J. Phys. Conf. Ser. 2014, 564, 12001. [Google Scholar] [CrossRef]
- Schoenleber, M.; Vaghela, J.; Ismail, F.; Grahn, M.; Popa, C.; Rehman, I.; Vadgama, P. Complex Electronic Implants and Polymer Packaging Needs BT—World Congress on Medical Physics and Biomedical Engineering 2006; Magjarevic, R., Nagel, J.H., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 703–706. [Google Scholar]
- Kumar, R. New Developments in Parylene Technology for Medical Electronics Advancement. In Proceedings of the SMTA Medical Electronics Symposium, Minneapolis, MN, USA, 15–17 May 2006; pp. 1–9. [Google Scholar]
- Hassler, C.; Von Metzen, R.P.; Ruther, P.; Stieglitz, T. Characterization of Parylene C as an Encapsulation Material for Implanted Neural Prostheses. J. Biomed. Mater. Res.—Part B Appl. Biomater. 2010, 93, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Buchwalder, S.; Borzì, A.; Diaz Leon, J.J.; Bourgeois, F.; Nicolier, C.; Nicolay, S.; Neels, A.; Zywitzki, O.; Hogg, A.; Burger, J. Thermal Analysis of Parylene Thin Films for Barrier Layer Applications. Polymers 2022, 14, 3677. [Google Scholar] [CrossRef] [PubMed]
- Golda-Cepa, M.; Engvall, K.; Hakkarainen, M.; Kotarba, A. Recent Progress on Parylene C Polymer for Biomedical Applications: A Review. Prog. Org. Coatings 2020, 140, 105493. [Google Scholar] [CrossRef]
- Wuu, D.S.; Chen, T.N.; Wu, C.C.; Chiang, C.C.; Chen, Y.P.; Horng, R.H.; Juang, F.S. Transparent Barrier Coatings for Flexible Organic Light-Emitting Diode Applications. Chem. Vap. Depos. 2006, 12, 220–224. [Google Scholar] [CrossRef]
- Chen, T.-N.; Wuu, D.-S.; Wu, C.-C.; Chiang, C.-C.; Chen, Y.-P.; Horng, R.-H. Improvements of Permeation Barrier Coatings Using Encapsulated Parylene Interlayers for Flexible Electronic Applications. Plasma Process. Polym. 2007, 4, 180–185. [Google Scholar] [CrossRef]
- Chen, S.-W.; Wang, Y.-S.; Hu, S.-Y.; Lee, W.-H.; Chi, C.-C.; Wang, Y.-L. A Study of Trimethylsilane (3MS) and Tetramethylsilane (4MS) Based α-SiCN:H/α-SiCO:H Diffusion Barrier Films. Materials 2012, 5, 377–384. [Google Scholar] [CrossRef]
- Mitschker, F.; Schücke, L.; Hoppe, C.; Jaritz, M.; Dahlmann, R.; De Los Arcos, T.; Hopmann, C.; Grundmeier, G.; Awakowicz, P. Comparative Study on the Deposition of Silicon Oxide Permeation Barrier Coatings for Polymers Using Hexamethyldisilazane (HMDSN) and Hexamethyldisiloxane (HMDSO). J. Phys. D Appl. Phys. 2018, 51, 235201. [Google Scholar] [CrossRef]
- Kim, N. Recent Progress of Functional Coating Materials and Technologies for Polycarbonate. J. Coat. Technol. Res. 2017, 14, 21–34. [Google Scholar] [CrossRef]
- Bose, M.; Bose, D.N.; Basa, D.K. Plasma Enhanced Growth, Composition and Refractive Index of Silicon Oxynitride Films. Mater. Lett. 2002, 52, 417–422. [Google Scholar] [CrossRef]
- Zajíčková, L.; Buršíková, V.; Peřina, V.; Macková, A.; Subedi, D.; Janča, J.; Smirnov, S. Plasma Modification of Polycarbonates. Surf. Coatings Technol. 2001, 142, 449–454. [Google Scholar] [CrossRef]
- Yang, M.-R.; Chen, K.-S.; Hsu, S.-T.; Wu, T.-Z. Fabrication and Characteristics of SiOx Films by Plasma Chemical Vapor Deposition of Tetramethylorthosilicate. Surf. Coatings Technol. 2000, 123, 204–209. [Google Scholar] [CrossRef]
- Pavlovic, E.; Quist, A.P.; Gelius, U.; Oscarsson, S. Surface Functionalization of Silicon Oxide at Room Temperature and Atmospheric Pressure. J. Colloid Interface Sci. 2002, 254, 200–203. [Google Scholar] [CrossRef] [PubMed]
- Pujari, S.P.; Scheres, L.; Marcelis, A.T.M.; Zuilhof, H. Covalent Surface Modification of Oxide Surfaces. Angew. Chemie Int. Ed. 2014, 53, 6322–6356. [Google Scholar] [CrossRef] [PubMed]
- Gorham, W.F. A New, General Synthetic Method for the Preparation of Linear Poly-p-Xylylenes. J. Polym. Sci. Part A-1 Polym. Chem. 1966, 4, 3027–3039. [Google Scholar] [CrossRef]
- Menon, P.R.; Li, W.; Tooker, A.; Tai, Y.C. Characterization of Water Vapor Permeation through Thin Film Parylene C. In Proceedings of the TRANSDUCERS 2009—15th International Conference Solid-State Sensors, Actuators Microsystems, Denver, CO, USA, 21–25 June 2009; pp. 1892–1895. [Google Scholar] [CrossRef]
- Kahouli, A.; Sylvestre, A.; Laithier, J.F.; Lutsen, L.; Pairis, S.; André, E.; Garden, J.L. Structural and Dielectric Properties of Parylene-VT4 Thin Films. Mater. Chem. Phys. 2014, 143, 908–914. [Google Scholar] [CrossRef]
- Zanini, S.; Riccardi, C.; Orlandi, M.; Grimoldi, E. Characterisation of SiOxCyHz Thin Films Deposited by Low-Temperature PECVD. Vacuum 2007, 82, 290–293. [Google Scholar] [CrossRef]
- Hogg, A.; Uhl, S.; Feuvrier, F.; Girardet, Y.; Graf, B.; Aellen, T.; Keppner, H.; Tardy, Y.; Burger, J. Protective Multilayer Packaging for Long-Term Implantable Medical Devices. Surf. Coatings Technol. 2014, 255, 124–129. [Google Scholar] [CrossRef]
- Griffith, D.P.; Musher, D.M.; Itin, C. Urease. The Primary Cause of Infection-Induced Urinary Stones. Invest. Urol. 1976, 13, 346–350. [Google Scholar]
- Rosier, P.F.W.M.; Gammie, A.; Valdevenito, J.P.; Speich, J.; Smith, P.; Sinha, S. ICS-SUFU Standard: Theory, Terms, and Recommendations for Pressure-Flow Studies Performance, Analysis, and Reporting, Part 2: Analysis of PFS, Reporting, and Diagnosis. Continence 2023, 100709. [Google Scholar] [CrossRef]
- Yao, M.; Simoes, A. Urodynamic Testing and Interpretation; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Buchholz, N.; Budia, A.; de la Cruz, J.; Kram, W.; Humphreys, O.; Reches, M.; Valero Boix, R.; Soria, F. Urinary Stent Development and Evaluation Models: In Vitro, Ex Vivo and In Vivo—A European Network of Multidisciplinary Research to Improve Urinary Stents (ENIUS) Initiative. Polymers 2022, 14, 1641. [Google Scholar] [CrossRef] [PubMed]
- Staehlke, S.; Rebl, H.; Finke, B.; Mueller, P.; Gruening, M.; Nebe, J.B. Enhanced Calcium Ion Mobilization in Osteoblasts on Amino Group Containing Plasma Polymer Nanolayer. Cell Biosci. 2018, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, B.R.; Sterne, J.A.C. Essential Medical Statistics; John Wiley & Sons: Hoboken, NJ, USA, 2010; ISBN 1444392840. [Google Scholar]
- Kassambara, A. Rstatix: Pipe-Friendly Framework for Basic Statistical Tests. 2023. Available online: https://CRAN.R-project.org/package=rstatix (accessed on 25 July 2023).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022. [Google Scholar]
- Siener, R.; Herwig, H.; Rüdy, J.; Schaefer, R.M.; Lossin, P.; Hesse, A. Urinary Stone Composition in Germany: Results from 45,783 Stone Analyses. World J. Urol. 2022, 40, 1813–1820. [Google Scholar] [CrossRef]
- Cloutier, J.; Villa, L.; Traxer, O.; Daudon, M. Kidney Stone Analysis: “Give Me Your Stone, I Will Tell You Who You Are!”. World J. Urol. 2015, 33, 157–169. [Google Scholar] [CrossRef]
- Rouprêt, M.; Daudon, M.; Hupertan, V.; Gattegno, B.; Thibault, P.; Traxer, O. Can Ureteral Stent Encrustation Analysis Predict Urinary Stone Composition? Urology 2005, 66, 246–251. [Google Scholar] [CrossRef]
- Gomes, D.J.C.; de Souza, N.C.; Silva, J.R. Using a Monocular Optical Microscope to Assemble a Wetting Contact Angle Analyser. Measurement 2013, 46, 3623–3627. [Google Scholar] [CrossRef]
- Kim, B.J.; Meng, E. Micromachining of Parylene C for BioMEMS. Polym. Adv. Technol. 2016, 27, 564–576. [Google Scholar] [CrossRef]
- Homsy, A.; Laux, E.; Jeandupeux, L.; Charmet, J.; Bitterli, R.; Botta, C.; Rebetez, Y.; Banakh, O.; Keppner, H. Solid on Liquid Deposition, a Review of Technological Solutions. Microelectron. Eng. 2015, 141, 267–279. [Google Scholar] [CrossRef]
- Mandel, N. Mechanism of Stone Formation. Semin. Nephrol. 1996, 16, 364–374. [Google Scholar]
- Stamatelou, K.; Goldfarb, D.S. Epidemiology of Kidney Stones. Healthcare 2023, 11, 424. [Google Scholar] [CrossRef] [PubMed]
- Flannigan, R.; Choy, W.H.; Chew, B.; Lange, D. Renal Struvite Stones—Pathogenesis, Microbiology, and Management Strategies. Nat. Rev. Urol. 2014, 11, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Gaunt, R.A.; Prochazka, A. Control of Urinary Bladder Function with Devices: Successes and Failures. In Autonomic Dysfunction After Spinal Cord Injury; Weaver, L.C., Polosa, C.B.T.-P., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; Volume 152, pp. 163–194. ISBN 0079-6123. [Google Scholar]
- Kleinen, L.; Syring, I.; Laube, N. Reduction of Biofilm Formation on A-C: H Coated Implants: Investigation of Biofilm-surface Interactions by Variation of Thin Film Properties. Plasma Process. Polym. 2009, 6, S41–S45. [Google Scholar] [CrossRef]
- Rebl, H.; Renner, J.; Kram, W.; Springer, A.; Fritsch, N.; Hansmann, H.; Hakenberg, O.W.; Nebe, J.B. Prevention of Encrustation on Ureteral Stents: Which Surface Parameters Provide Guidance for the Development of Novel Stent Materials? Polymers 2020, 12, 558. [Google Scholar] [CrossRef] [PubMed]
- Buchwalder, S.; Nicolier, C.; Hersberger, M.; Bourgeois, F.; Hogg, A.; Burger, J. Development of a Water Transmission Rate (WTR) Measurement System for Implantable Barrier Coatings. Polymers 2023, 15, 2557. [Google Scholar] [CrossRef]
- Wu, J.; Fei, F.; Wei, C.; Chen, X.; Nie, S.; Zhang, D.; Su, W.; Cui, Z. Efficient Multi-Barrier Thin Film Encapsulation of OLED Using Alternating Al2O3 and Polymer Layers. RSC Adv. 2018, 8, 5721–5727. [Google Scholar] [CrossRef]
- Xie, X.; Rieth, L.; Caldwell, R.; Diwekar, M.; Tathireddy, P.; Sharma, R.; Solzbacher, F. Long-Term Bilayer Encapsulation Performance of Atomic Layer Deposited Al2O3 and Parylene c for Biomedical Implantable Devices. IEEE Trans. Biomed. Eng. 2013, 60, 2943–2951. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, M.; Lei, Y.; Li, Z.; Jin, Y.; Wang, W. Parylene-MEMS Technique-Based Flexible Electronics. Sci. China Inf. Sci. 2018, 61, 60419. [Google Scholar] [CrossRef]
- Von Metzen, R.P.; Stieglitz, T. The Effects of Annealing on Mechanical, Chemical, and Physical Properties and Structural Stability of Parylene C. Biomed. Microdevices 2013, 15, 727–735. [Google Scholar] [CrossRef]




| Solution A | Solution B |
|---|---|
| calcium chloride-dihydrate | urea |
| magnesium chloride-hexahydrate | potassium-dihydrogenphosphate |
| sodium citrate | creatinine |
| sodium chloride | ammonium chloride |
| sodium sulfate | albumin |
| potassium chloride | sodium oxalate |
| urease, type IX |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchwalder, S.; Hersberger, M.; Rebl, H.; Seemann, S.; Kram, W.; Hogg, A.; Tvedt, L.G.W.; Clausen, I.; Burger, J. An Evaluation of Parylene Thin Films to Prevent Encrustation for a Urinary Bladder Pressure MEMS Sensor System. Polymers 2023, 15, 3559. https://doi.org/10.3390/polym15173559
Buchwalder S, Hersberger M, Rebl H, Seemann S, Kram W, Hogg A, Tvedt LGW, Clausen I, Burger J. An Evaluation of Parylene Thin Films to Prevent Encrustation for a Urinary Bladder Pressure MEMS Sensor System. Polymers. 2023; 15(17):3559. https://doi.org/10.3390/polym15173559
Chicago/Turabian StyleBuchwalder, Sébastien, Mario Hersberger, Henrike Rebl, Susanne Seemann, Wolfgang Kram, Andreas Hogg, Lars G. W. Tvedt, Ingelin Clausen, and Jürgen Burger. 2023. "An Evaluation of Parylene Thin Films to Prevent Encrustation for a Urinary Bladder Pressure MEMS Sensor System" Polymers 15, no. 17: 3559. https://doi.org/10.3390/polym15173559