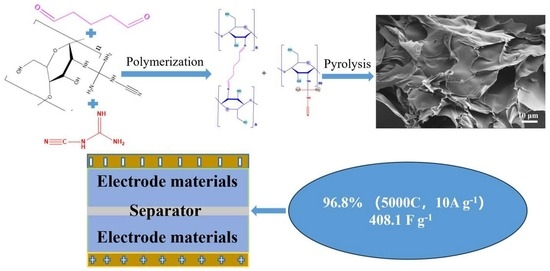
3D Porous VOx/N-Doped Carbon Nanosheet Hybrids Derived from Cross-Linked Dicyandiamide–Chitosan Hydrogels for Superior Supercapacitor Electrode Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 3D VNCN
2.2. Materials Characterization
2.3. Electrochemical Measurement
3. Results
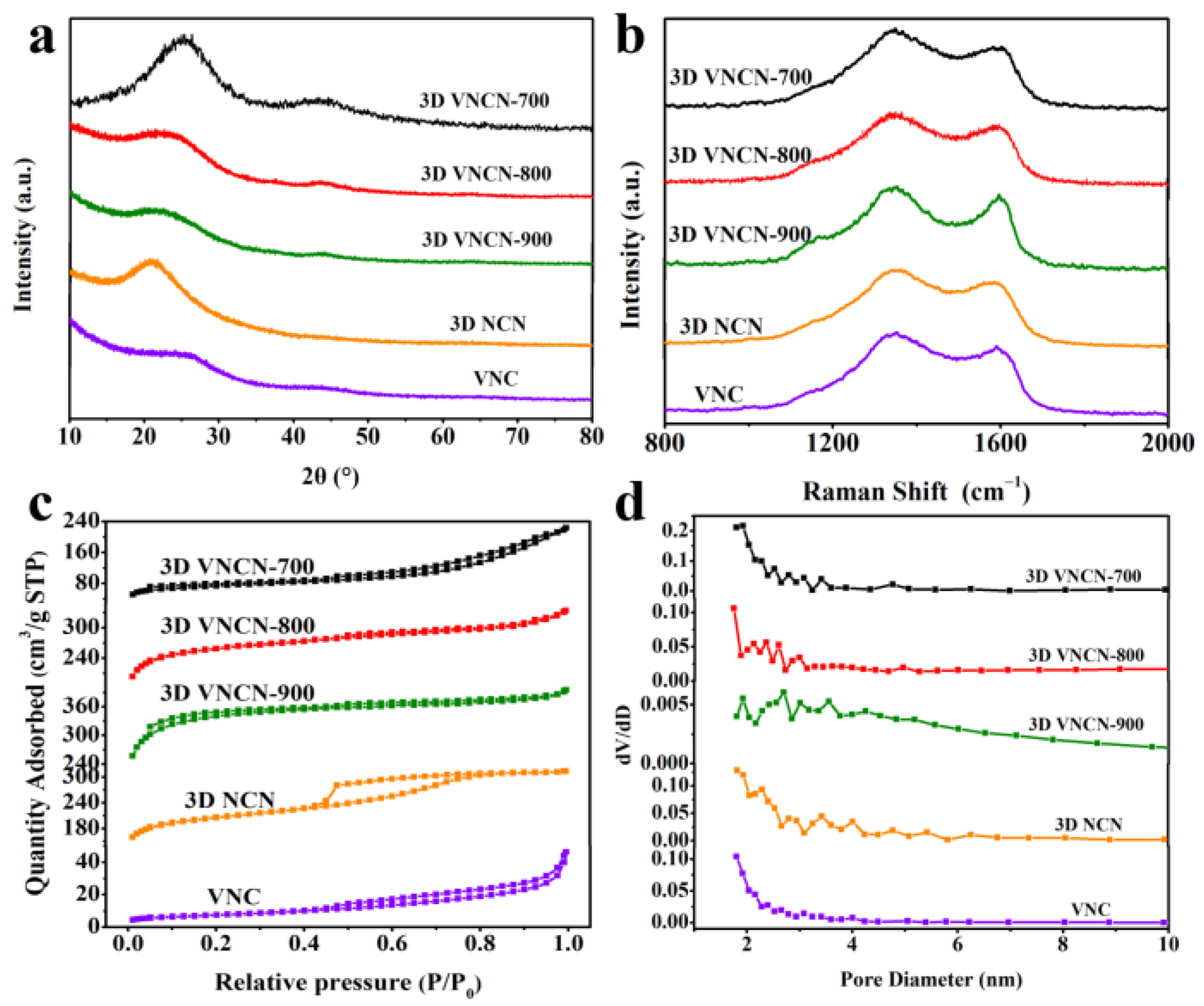
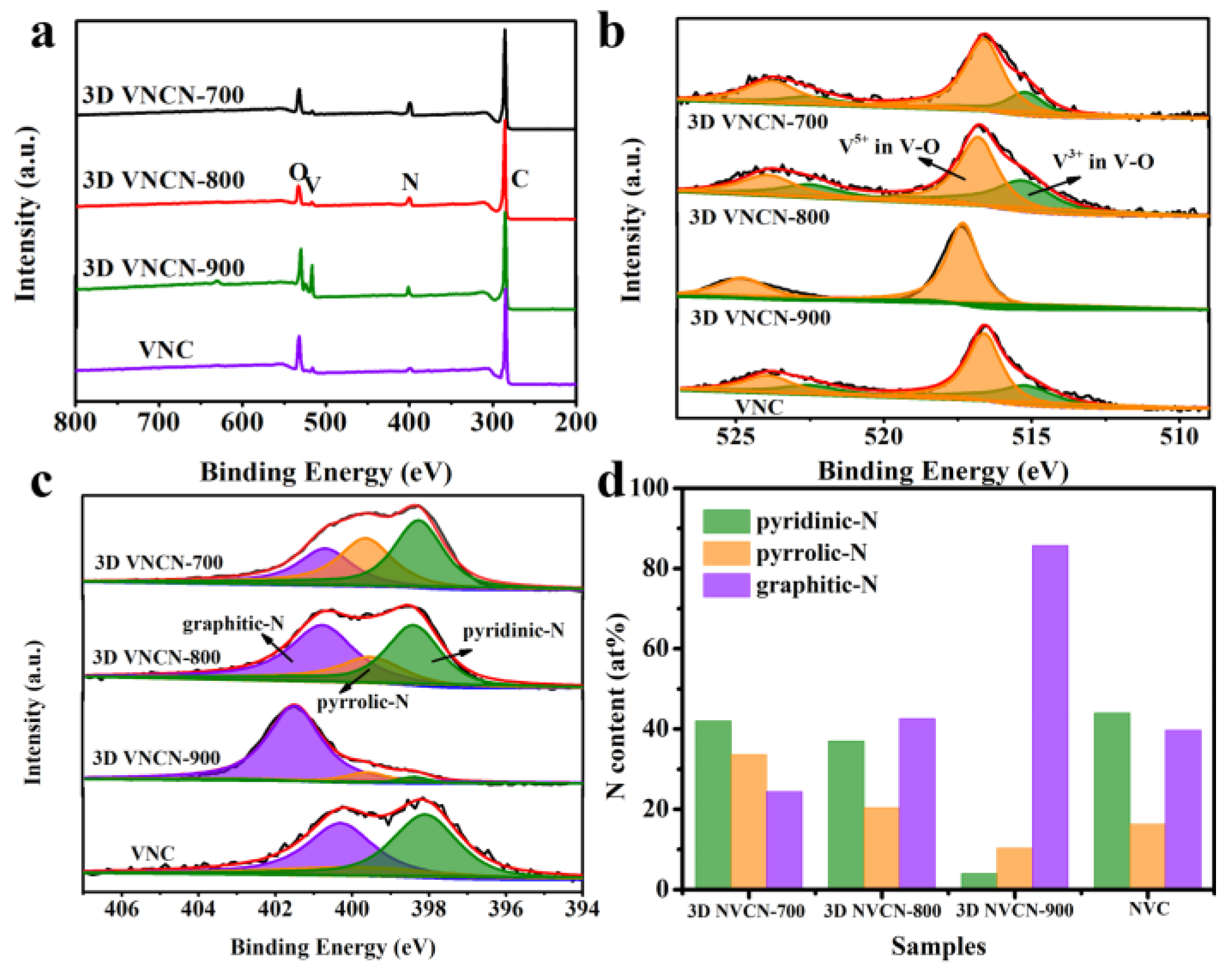
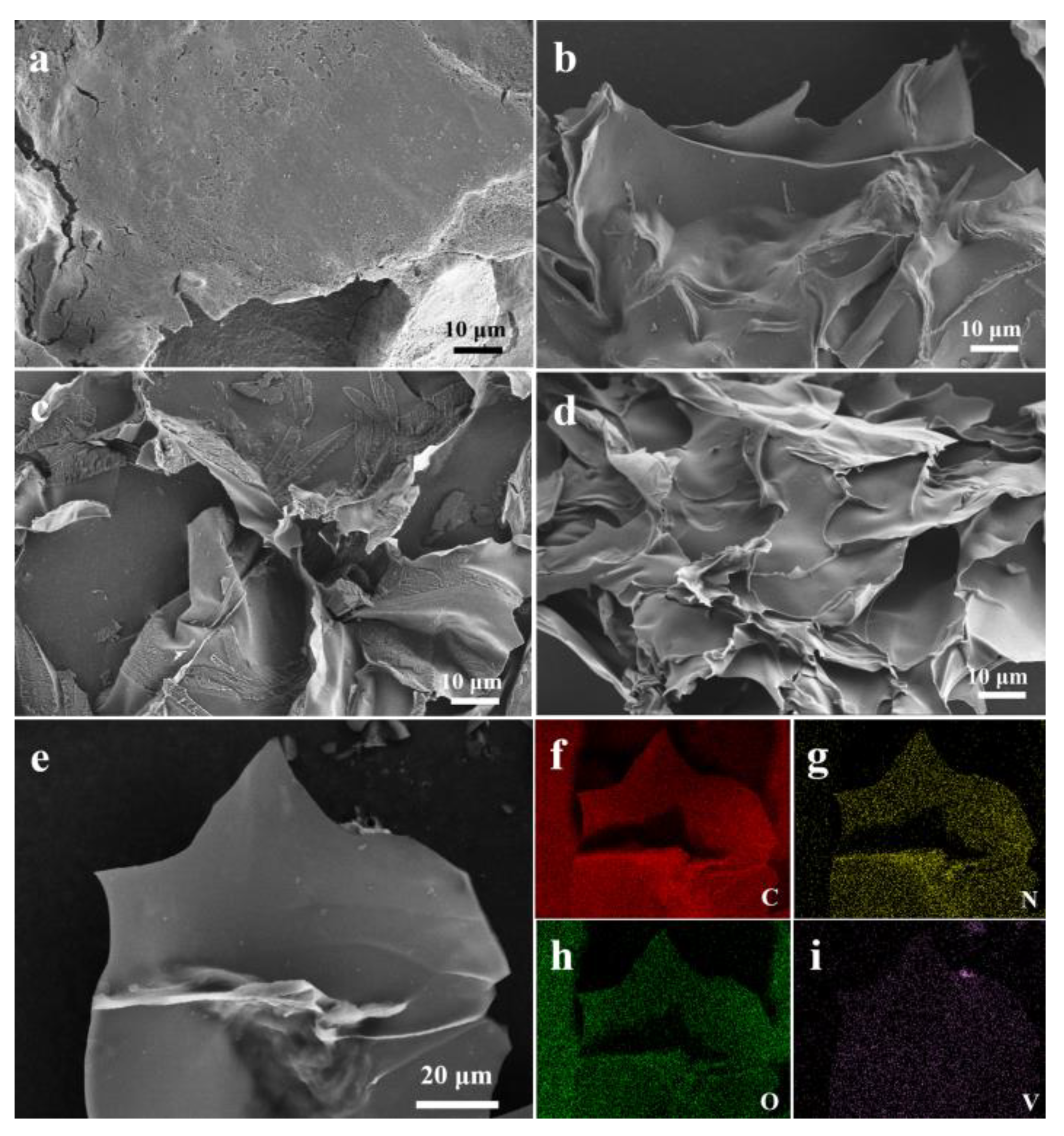
3.1. Characterization of Prepared Materials
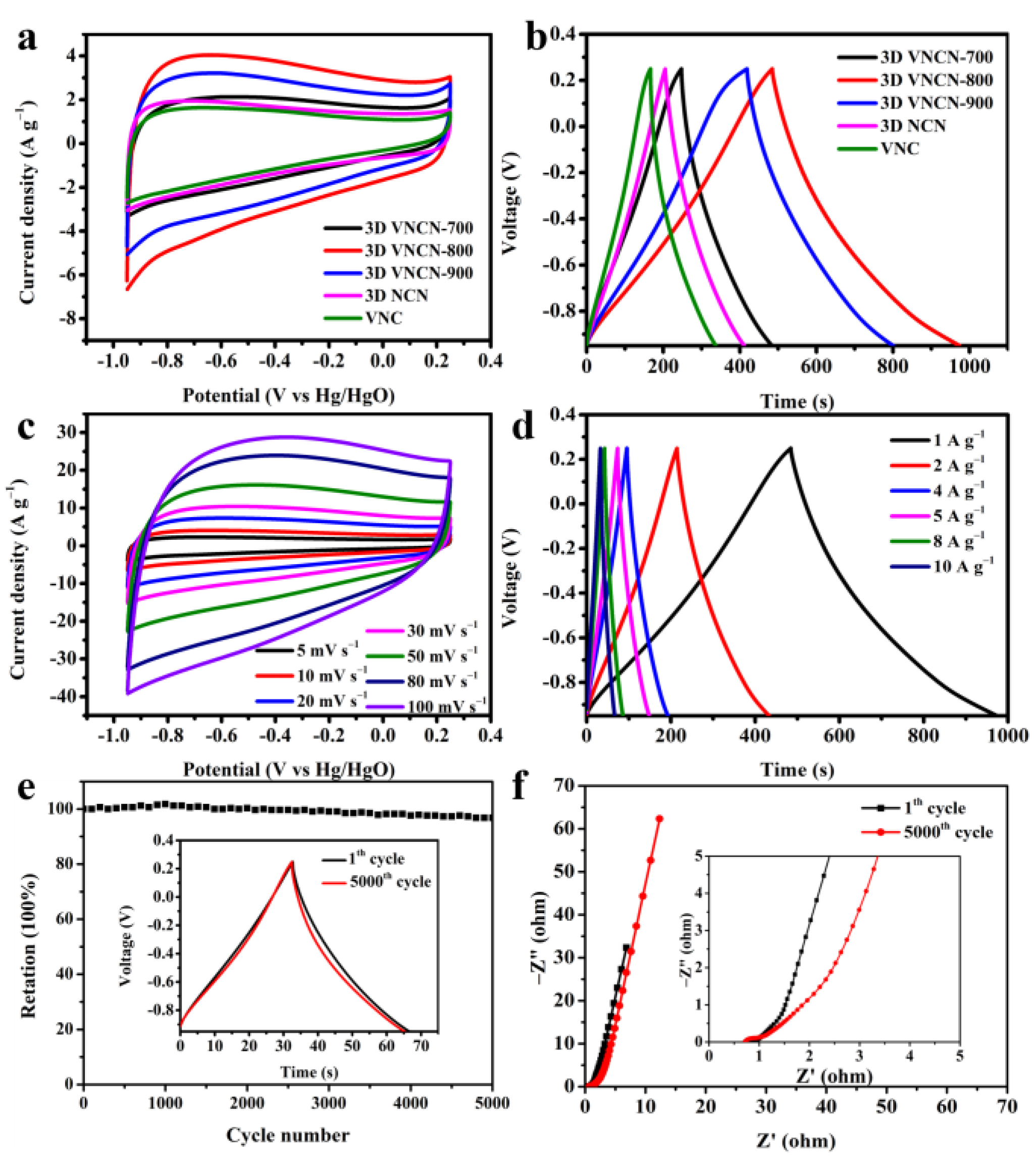
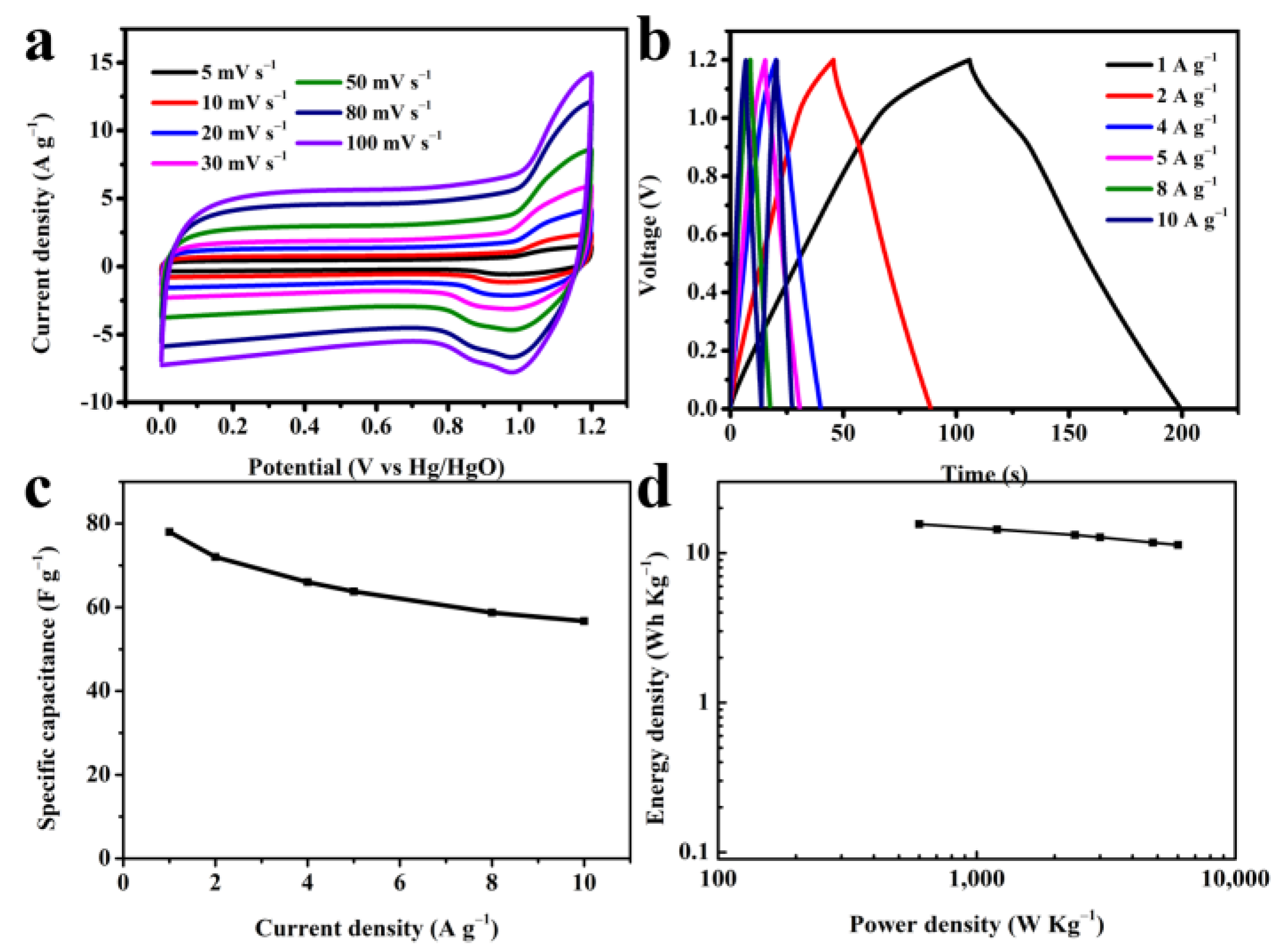
3.2. Electrochemical Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roy, B.K.; Tahmid, I.; Rashid, T.U. Chitosan-based materials for supercapacitor applications: A review. J. Mater. Chem. A 2021, 9, 17592–17642. [Google Scholar] [CrossRef]
- Hamsan, M.H.; Nofal, M.M.; Aziz, S.B.; Brza, M.; Dannoun, E.M.; Murad, A.R.; Kadir, M.; Muzakir, S. Plasticized polymer blend electrolyte based on chitosan for energy storage application: Structural, circuit modeling, morphological and electrochemical properties. Polymers 2021, 13, 1233. [Google Scholar] [CrossRef]
- Şentürk, S.B.; Kahraman, D.; Alkan, C.; Gökçe, İ. Biodegradable PEG/cellulose, PEG/agarose and PEG/chitosan blends as shape stabilized phase change materials for latent heat energy storage. Carbohydr. Polym. 2011, 84, 141–144. [Google Scholar] [CrossRef]
- Sun, G.; Li, B.; Ran, J.; Shen, X.; Tong, H. Three-dimensional hierarchical porous carbon/graphene composites derived from graphene oxide-chitosan hydrogels for high performance supercapacitors. Electrochim. Acta 2015, 171, 13–22. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Fan, Z. Carbon materials for high volumetric performance supercapacitors: Design, progress, challenges and opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- Li, Q.; Dai, Z.; Wu, J.; Liu, W.; Di, T.; Jiang, R.; Zheng, X.; Wang, W.; Ji, X.; Li, P.; et al. Fabrication of ordered macro-microporous single-crystalline MOF and its derivative carbon material for supercapacitor. Adv. Energy Mater. 2020, 10, 1903750. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, S.; Zhou, Y.-N.; Tang, W.; Yang, J.; Peng, C.; Guo, Z. Biomass-derived carbon materials for high-performance supercapacitors: Current status and perspective. Electrochem. Energy Rev. 2021, 4, 219–248. [Google Scholar] [CrossRef]
- Dong, Y.; Zhu, J.; Li, Q.; Zhang, S.; Song, H.; Jia, D. Carbon materials for high mass-loading supercapacitors: Filling the gap between new materials and practical applications. J. Mater. Chem. A 2020, 8, 21930–21946. [Google Scholar] [CrossRef]
- Liu, M.; Huo, S.; Xu, M.; Wu, L.; Liu, M.; Xue, Y.; Yan, Y.-M. Structural engineering of N/S co-doped carbon material as high-performance electrode for supercapacitors. Electrochim. Acta 2018, 274, 389–399. [Google Scholar] [CrossRef]
- Cheng, J.; Liu, Y.; Zhang, X.; Miao, X.; Chen, Y.; Chen, S.; Lin, J.; Zhang, Y. Structure engineering in interconnected porous hollow carbon spheres with superior rate capability for supercapacitors and lithium-sulfur batteries. Chem. Eng. J. 2021, 419, 129649. [Google Scholar] [CrossRef]
- Borchardt, L.; Oschatz, M.; Kaskel, S. Tailoring porosity in carbon materials for supercapacitor applications. Mater. Horiz. 2014, 1, 157–168. [Google Scholar] [CrossRef]
- Shao, J.; Song, M.; Wu, G.; Zhou, Y.; Wan, J.; Ren, X.; Ma, F. 3D carbon nanocage networks with multiscale pores for high-rate supercapacitors by flower-like template and in-situ coating. Energy Storage Mater. 2018, 13, 57–65. [Google Scholar] [CrossRef]
- Kumar, S.; Saeed, G.; Zhu, L.; Hui, K.N.; Kim, N.H.; Lee, J.H. 0D to 3D carbon-based networks combined with pseudocapacitive electrode material for high energy density supercapacitor: A review. Chem. Eng. J. 2021, 403, 126352. [Google Scholar] [CrossRef]
- Li, Y.; Qu, J.; Li, F.; Qu, Z.; Tang, H.; Liu, L.; Zhu, M.; Schmidt, O.G. Advanced architecture designs towards high-performance 3D microbatteries. Nano Mater. Sci. 2021, 3, 140–153. [Google Scholar] [CrossRef]
- Hou, J.; Jiang, K.; Wei, R.; Tahir, M.; Wu, X.; Shen, M.; Wang, X.; Cao, C. Popcorn-derived porous carbon flakes with an ultrahigh specific surface area for superior performance supercapacitors. ACS Appl. Mater. Interfaces 2017, 9, 30626–30634. [Google Scholar] [CrossRef]
- Gao, Y.; Li, L.; Jin, Y.; Wang, Y.; Yuan, C.; Wei, Y.; Chen, G.; Ge, J.; Lu, H. Porous carbon made from rice husk as electrode material for electrochemical double layer capacitor. Appl. Energy 2015, 153, 41–47. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, X.; Xue, B.; Li, W.; Ding, Z.; Yang, X.; Qiu, J.; Wang, Z. Rice husk-based hierarchical porous carbon for high performance supercapacitors: The structure-performance relationship. Carbon 2020, 161, 432–444. [Google Scholar] [CrossRef]
- Luo, M.; Wang, X.; Meng, T.; Yang, P.; Zhu, Z.; Min, H.; Chen, M.; Chen, W.; Zhou, X. Rapid one-step preparation of hierarchical porous carbon from chitosan-based hydrogel for high-rate supercapacitors: The effect of gelling agent concentration. Int. J. Biol. Macromol. 2020, 146, 453–461. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Q.; Xia, K.; Han, B.; Zhou, C.; Gao, Q.; Wang, H.; Pu, S.; Wu, J. Facile Synthesis of Hierarchically Porous N/P Codoped Carbon with Simultaneously High-Level Heteroatom-Doping and Moderate Porosity for High-Performance Supercapacitor Electrodes. ACS Sustain. Chem. Eng. 2019, 7, 5717–5726. [Google Scholar] [CrossRef]
- Wang, X.; Sun, G.; Routh, P.; Kim, D.-H.; Huang, W.; Chen, P. Heteroatom-doped graphene materials: Syntheses, properties and applications. Chem. Soc. Rev. 2014, 43, 7067–7098. [Google Scholar] [CrossRef]
- Deng, Y.; Xie, Y.; Zou, K.; Ji, X. Review on recent advances in nitrogen-doped carbons: Preparations and applications in supercapacitors. J. Mater. Chem. A 2016, 4, 1144–1173. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Q.; Sun, L.; Li, N.; Wang, X.; Wang, Q.; Zhang, D.; Wang, B. Hydrothermal synthesis of 3D hierarchical ordered porous carbon from yam biowastes for enhanced supercapacitor performance. Chem. Eng. Sci. 2022, 252, 117514. [Google Scholar] [CrossRef]
- Shang, Z.; An, X.; Zhang, H.; Shen, M.; Baker, F.; Liu, Y.; Liu, L.; Yang, J.; Cao, H.; Xu, Q.; et al. Houttuynia-derived nitrogen-doped hierarchically porous carbon for high-performance supercapacitor. Carbon 2020, 161, 62–70. [Google Scholar] [CrossRef]
- Fu, Q.; Zhao, H.; Sarapulova, A.; Dsoke, S. V2O5 as a versatile electrode material for postlithium energy storage systems. Appl. Res. 2023, 2, e202200070. [Google Scholar] [CrossRef]
- Saravanakumar, B.; Purushothaman, K.K.; Muralidharan, G. V2O5/nitrogen enriched mesoporous carbon spheres nanocomposite as supercapacitor electrode. Microporous Mesoporous Mater. 2018, 258, 83–94. [Google Scholar] [CrossRef]
- Pei, Y.R.; Zhao, M.; Zhu, Y.P.; Yang, C.C.; Jiang, Q. VN nanoparticle-assembled hollow microspheres/N-doped carbon nanofibers: An anode material for superior potassium storage. Nano Mater. Sci. 2022, 4, 104–112. [Google Scholar] [CrossRef]
- Wu, X.-M.; Liu, M.-M.; Guo, H.-X.; Ying, S.-M.; Chen, Z.-X. Polyoxovanadate-based MOFs microsphere constructed from 3-D discrete nano-sheets as supercapacitor. Chin. J. Struct. Chem. 2021, 40, 994–998. [Google Scholar]
- Khalil, E.S.; Saad, B.; Negim, E.-S.M.; Saleh, M.I. Novel water-soluble chitosan derivative prepared by graft polymerization of dicyandiamide: Synthesis, characterisation, and its antibacterial property. J. Polym. Res. 2015, 22, 116. [Google Scholar] [CrossRef]
- Aziz, B.S.; Hamsan, M.H.; Nofal, M.M.; San, S.; Abdulwahid, R.T.; Raza Saeed, S.; Brza, M.A.; Kadir, M.F.; Mohammed, S.J.; Al-Zangana, S. From cellulose, shrimp and crab shells to energy storage EDLC cells: The study of structural and electrochemical properties of proton conducting chitosan-based biopolymer blend electrolytes. Polymers 2020, 12, 1526. [Google Scholar] [CrossRef]
- Xu, D.; Jin, J.; Chen, C.; Wen, Z. From Nature to Energy Storage: A novel sustainable 3D cross-linked chitosan–PEGGE-based gel polymer electrolyte with excellent Lithium-ion transport properties for Lithium batteries. ACS Appl. Mater. Interfaces 2018, 10, 38526–38537. [Google Scholar] [CrossRef]
- Boorboor Azimi, E.; Badiei, A.; Hossaini Sadr, M.; Amiri, A. A template-free method to synthesize porous g-C3N4 with efficient visible light photodegradation of organic pollutants in water. Adv. Powder Technol. 2018, 29, 2785–2791. [Google Scholar] [CrossRef]
- Wang, Q.F.; Li, S.M.; Wang, Z.Y.; Liu, H.Z.; Li, C.J. Preparation and characterization of a positive thermoresponsive hydrogel for drug loading and release. J. Appl. Polym. Sci. 2009, 111, 1417–1425. [Google Scholar] [CrossRef]
- Partheeban, T.; Kesavan, T.; Vivekanantha, M.; Sasidharan, M. One-pot solvothermal synthesis of V2O5/MWCNT composite cathode for Li ion batteries. Appl. Surf. Sci. 2019, 493, 1106–1114. [Google Scholar] [CrossRef]
- Wei, Q.; Liu, J.; Feng, W.; Sheng, J.; Tian, X.; He, L.; An, Q.; Mai, L. Hydrated vanadium pentoxide with superior sodium storage capacity. J. Mater. Chem. A 2015, 3, 8070–8075. [Google Scholar] [CrossRef]
- Zhang, X.; Han, R.; Liu, Y.; Li, H.; Shi, W.; Yan, X.; Zhao, X.; Li, Y.; Liu, B. Porous and graphitic structure optimization of biomass-based carbon materials from 0D to 3D for supercapacitors: A review. Chem. Eng. J. 2023, 460, 141607. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Y.; Ju, Z.; Luo, J.; Sheng, O.; Nai, J.; Liu, T.; Zhou, Y.; Wang, Y.; Tao, X. Synthesis of diverse green carbon nanomaterials through fully utilizing biomass carbon source assisted by KOH. ACS Appl. Mater. Interfaces 2019, 11, 24205–24211. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tang, P.; Zhu, Y.; Xie, W.; Yang, P.; Zhang, Z.; Liu, B. Green aerogel adsorbent for removal of organic compounds in shale gas wastewater: High-performance tuning and adsorption mechanism. Chem. Eng. J. 2021, 416, 129100. [Google Scholar] [CrossRef]
- Hou, L.; Hu, Z.; Wang, X.; Qiang, L.; Zhou, Y.; Lv, L.; Li, S. Hierarchically porous and heteroatom self-doped graphitic biomass carbon for supercapacitors. J. Colloid Interface Sci. 2019, 540, 88–96. [Google Scholar] [CrossRef]
- Lu, X.; Yu, M.; Zhai, T.; Wang, G.; Xie, S.; Liu, T.; Liang, C.; Tong, Y.; Li, Y. High energy density asymmetric quasi-solid-state supercapacitor based on porous vanadium nitride nanowire anode. Nano Lett. 2013, 13, 2628–2633. [Google Scholar] [CrossRef]
- Long, B.; Balogun, M.-S.; Luo, L.; Luo, Y.; Qiu, W.; Song, S.; Zhang, L.; Tong, Y. Encapsulated vanadium-based hybrids in amorphous N-doped carbon matrix as anode materials for Lithium-ion batteries. Small 2017, 13, 1702081. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhao, Y.; Zhang, B.; Xia, H.; Zhou, J.; Xie, W.; Li, H. Nano-micro carbon spheres anchored on porous carbon derived from dual-biomass as high rate performance supercapacitor electrodes. J. Power Sources 2018, 381, 116–126. [Google Scholar] [CrossRef]
- Yang, H.; Ning, P.; Cao, H.; Yuan, M.; Feng, J.; Yue, J.; Liu, Z.; Xu, G.; Li, Y. Selectively anchored vanadate host for self-boosting catalytic synthesis of ultra-fine vanadium nitride/nitrogen-doped hierarchical carbon hybrids as superior electrode materials. Electrochim. Acta 2020, 332, 135387. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhao, X.; Tan, Y.; Liu, Y.; Wang, Z.; Ran, F. A novel hierarchical porous 3D structured vanadium nitride/carbon membranes for high-performance supercapacitor negative electrodes. Nano-Micro Lett. 2018, 10, 63. [Google Scholar]
- Sun, W.; Gao, G.; Zhang, K.; Liu, Y.; Wu, G. Self-assembled 3D N-CNFs/V2O5 aerogels with core/shell nanostructures through vacancies control and seeds growth as an outstanding supercapacitor electrode material. Carbon 2018, 132, 667–677. [Google Scholar] [CrossRef]
- Ping, Y.; Yang, S.; Han, J.; Li, X.; Zhang, H.; Xiong, B.; Fang, P.; He, C. N-self-doped graphitic carbon aerogels derived from metal–organic frameworks as supercapacitor electrode materials with high-performance. Electrochim. Acta 2021, 380, 138237. [Google Scholar] [CrossRef]
- Shi, C.; Li, S.; Pan, Y.; Guo, L.; Wang, Y. Self-standing porous N doped carbon/carbon foam for high-performance supercarpacitor. Diam. Relat. Mater. 2020, 110, 108138. [Google Scholar] [CrossRef]
- Zhang, L.F.; Tang, J.; Liu, S.Y.; Peng, O.W.; Shi, R.; Chandrashekar, B.N.; Li, Y.; Li, X.; Li, X.N.; Xu, B.M.; et al. A laser irradiation synthesis of strongly-coupled VOx-reduced graphene oxide composites as enhanced performance supercapacitor electrodes. Mater. Today Energy 2017, 5, 222–229. [Google Scholar] [CrossRef]
- Zhang, G.; Ren, L.; Hu, D.; Zhang, S.; Gu, H. Fabrication of mesoporous carbon hollow spheres intercalated three-dimensional network structure V2O5 nanosheets with enhanced electrochemical performance. J. Alloys Compd. 2019, 781, 407–414. [Google Scholar] [CrossRef]
- Geng, D.; Zhang, S.; Jiang, Y.; Jiang, Z.; Shi, M.; Chang, J.; Liang, S.; Zhang, M.; Feng, J.; Wei, T. 3D interconnected porous carbon derived from spontaneous merging of the nano-sized ZIF-8 polyhedrons for high-mass-loading supercapacitor electrodes. J. Mater. Chem. A 2022, 10, 2027–2034. [Google Scholar] [CrossRef]
- Liu, H.; Huang, X.; Zhou, M.; Gu, J.; Xu, M.; Jiang, L.; Zheng, M.; Li, S.; Miao, Z. Efficient conversion of biomass waste to N/O co-doped hierarchical porous carbon for high performance supercapacitors. J. Anal. Appl. Pyrolysis 2023, 169, 105844. [Google Scholar] [CrossRef]
- Zuo, S.; Chen, J.; Liu, W.; Li, X.; Kong, Y.; Yao, C.; Fu, Y. Preparation of 3D interconnected hierarchical porous N-doped carbon nanotubes. Carbon 2018, 129, 199–206. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, L.; Wu, D.; Li, X.; Li, J.; Huo, P.; Wang, H.; Yan, Y. Preparation of 3D porous g-C3N4@V2O5 composite electrode via simple calcination and chemical precipitation for supercapacitors. J. Alloys Compd. 2020, 817, 152707. [Google Scholar] [CrossRef]

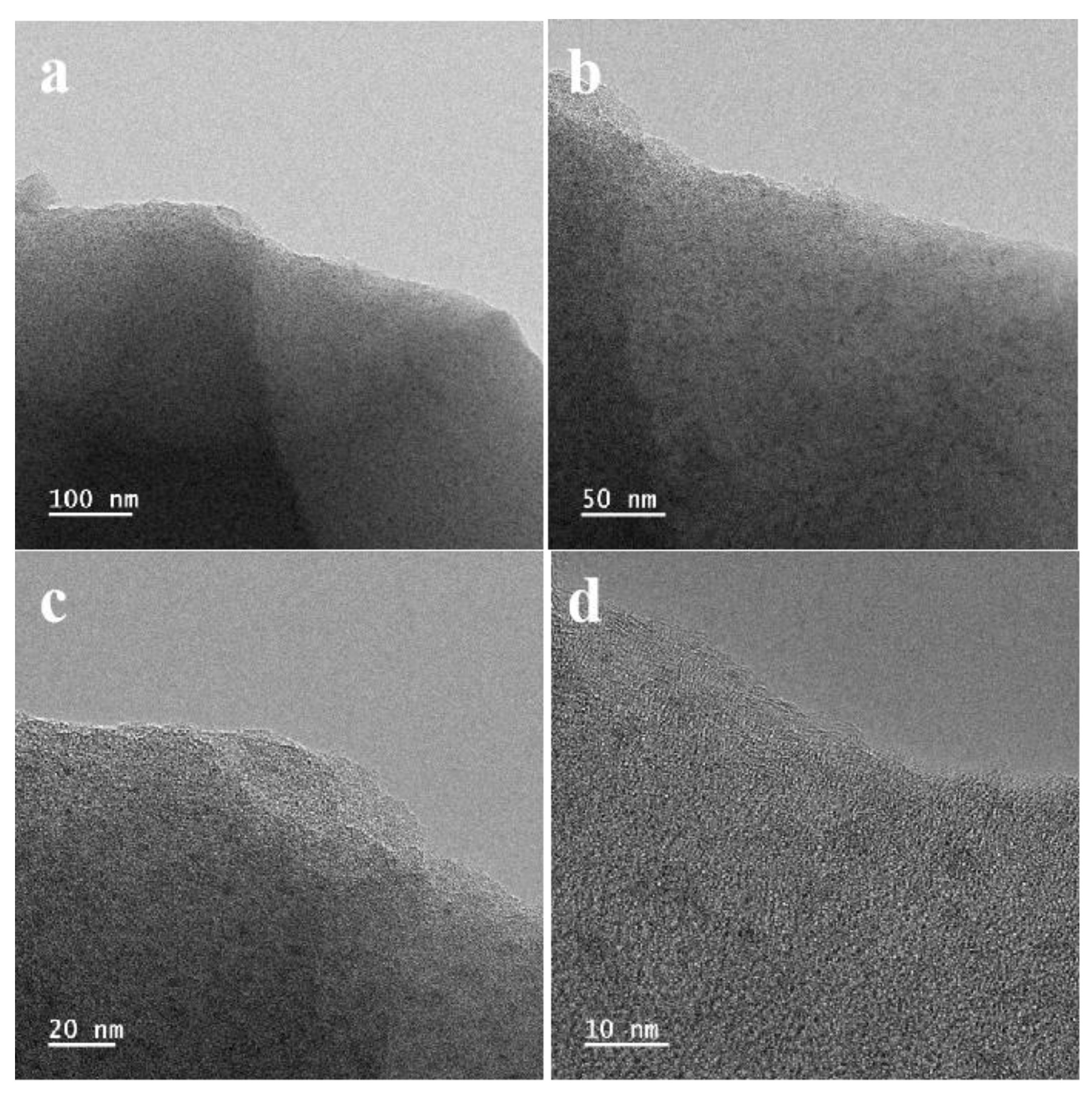
| Sample | C (at%) | N (at%) | O (at%) | V (at%) |
|---|---|---|---|---|
| 3D VNCN-700 | 76.23 | 9.05 | 10.72 | 4.00 |
| 3D VNCN-800 | 80.37 | 6.95 | 9.03 | 3.65 |
| 3D VNCN-900 | 73.97 | 4.61 | 13.77 | 7.65 |
| VNC | 77.19 | 2.96 | 14.15 | 5.70 |
| Materials | Cs (1 A·g−1, F·g−1) | Cs (10 Ag−1, F·g−1) | Retention (1–10 A·g−1) |
|---|---|---|---|
| VNC | 142.4 | 78.3 | 55.0% |
| 3D NCN | 172.3 | 125 | 72.5% |
| 3D VNCN-700 | 197.5 | 115.8 | 58.6% |
| 3D VNCN-800 | 408.1 | 282.5 | 69.2% |
| 3D VNCN-900 | 318.6 | 210.8 | 66.2% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; He, X.; Cai, J.; Zhou, J.; Liu, B.; Zhang, S.; Sun, Z.; Su, P.; Qu, D.; Li, Y. 3D Porous VOx/N-Doped Carbon Nanosheet Hybrids Derived from Cross-Linked Dicyandiamide–Chitosan Hydrogels for Superior Supercapacitor Electrode Materials. Polymers 2023, 15, 3565. https://doi.org/10.3390/polym15173565
Liu J, He X, Cai J, Zhou J, Liu B, Zhang S, Sun Z, Su P, Qu D, Li Y. 3D Porous VOx/N-Doped Carbon Nanosheet Hybrids Derived from Cross-Linked Dicyandiamide–Chitosan Hydrogels for Superior Supercapacitor Electrode Materials. Polymers. 2023; 15(17):3565. https://doi.org/10.3390/polym15173565
Chicago/Turabian StyleLiu, Jinghua, Xiong He, Jiayang Cai, Jie Zhou, Baosheng Liu, Shaohui Zhang, Zijun Sun, Pingping Su, Dezhi Qu, and Yudong Li. 2023. "3D Porous VOx/N-Doped Carbon Nanosheet Hybrids Derived from Cross-Linked Dicyandiamide–Chitosan Hydrogels for Superior Supercapacitor Electrode Materials" Polymers 15, no. 17: 3565. https://doi.org/10.3390/polym15173565