Extraction and Characterization of Acidolysis Lignin from Turkey Oak (Quercus cerris L.) and Eucalypt (Eucalyptus camaldulensis Dehnh.) Wood from Population Stands in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Lignin Isolation
2.3. High Performance Size Exclusion Chromatography (HP-SEC)
2.4. Fourier Transform Infrared (FTIR) Spectroscopy
2.5. Pyrolysis–Gas Chromatography–Mass Spectrometry (Py–GCMS)
2.6. Two-Dimensional (2D) HSQC NMR Spectroscopy
3. Results and Discussion
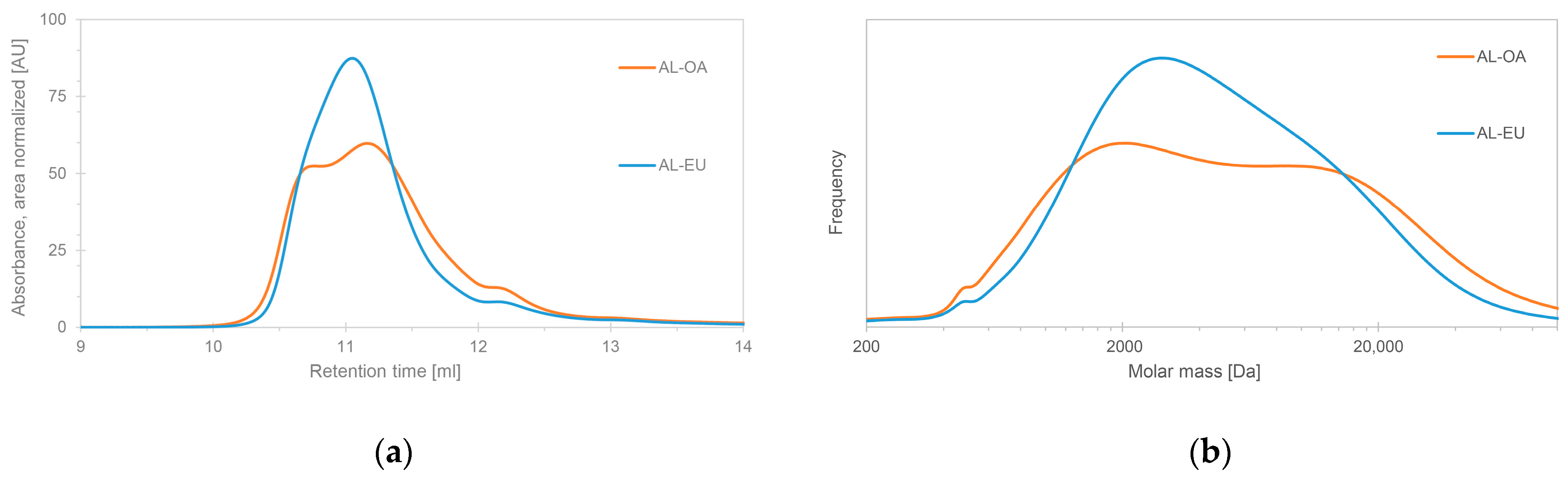
3.1. Molar Mass Distribution (MMD)
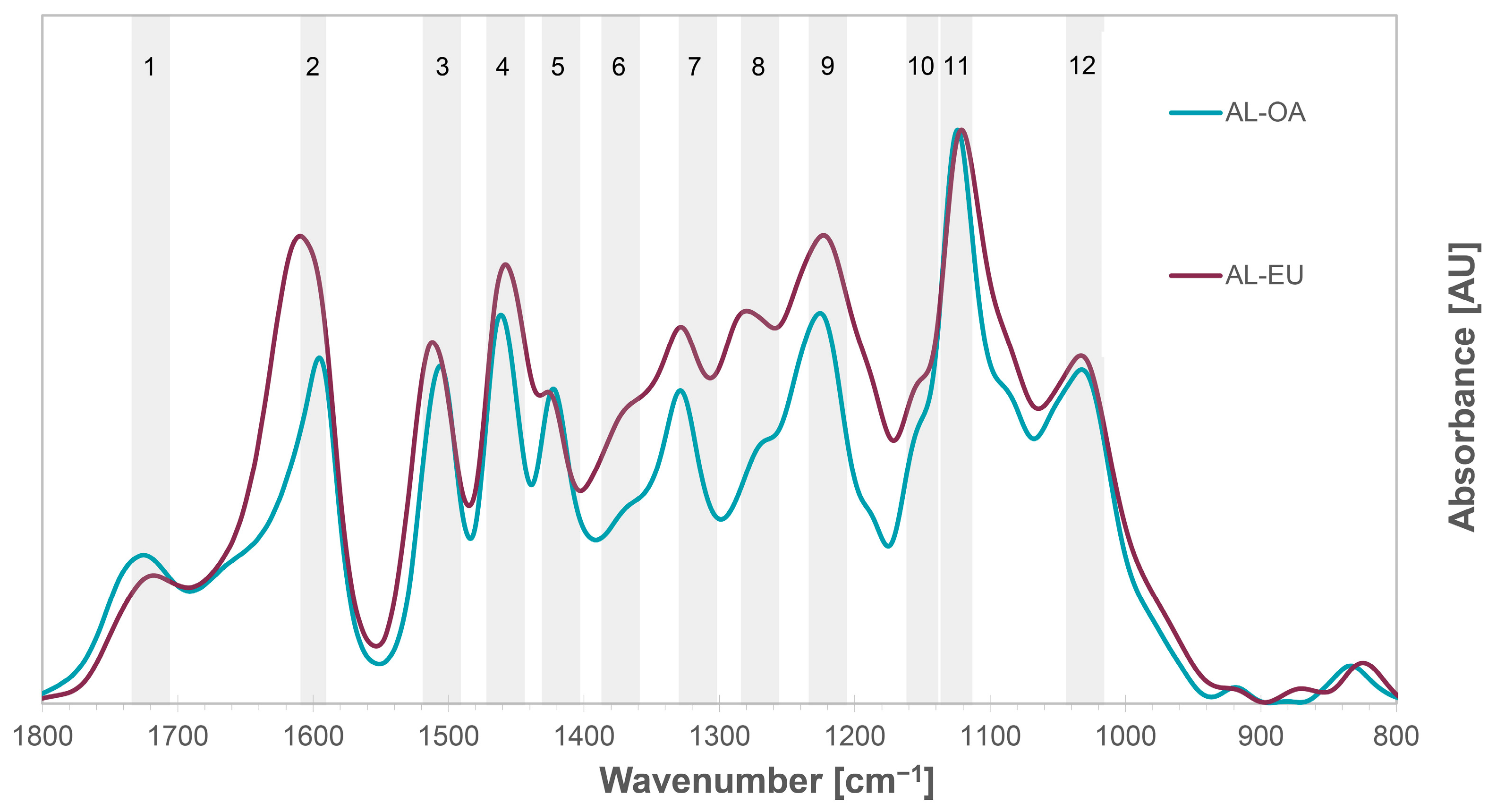
3.2. FTIR Spectroscopy
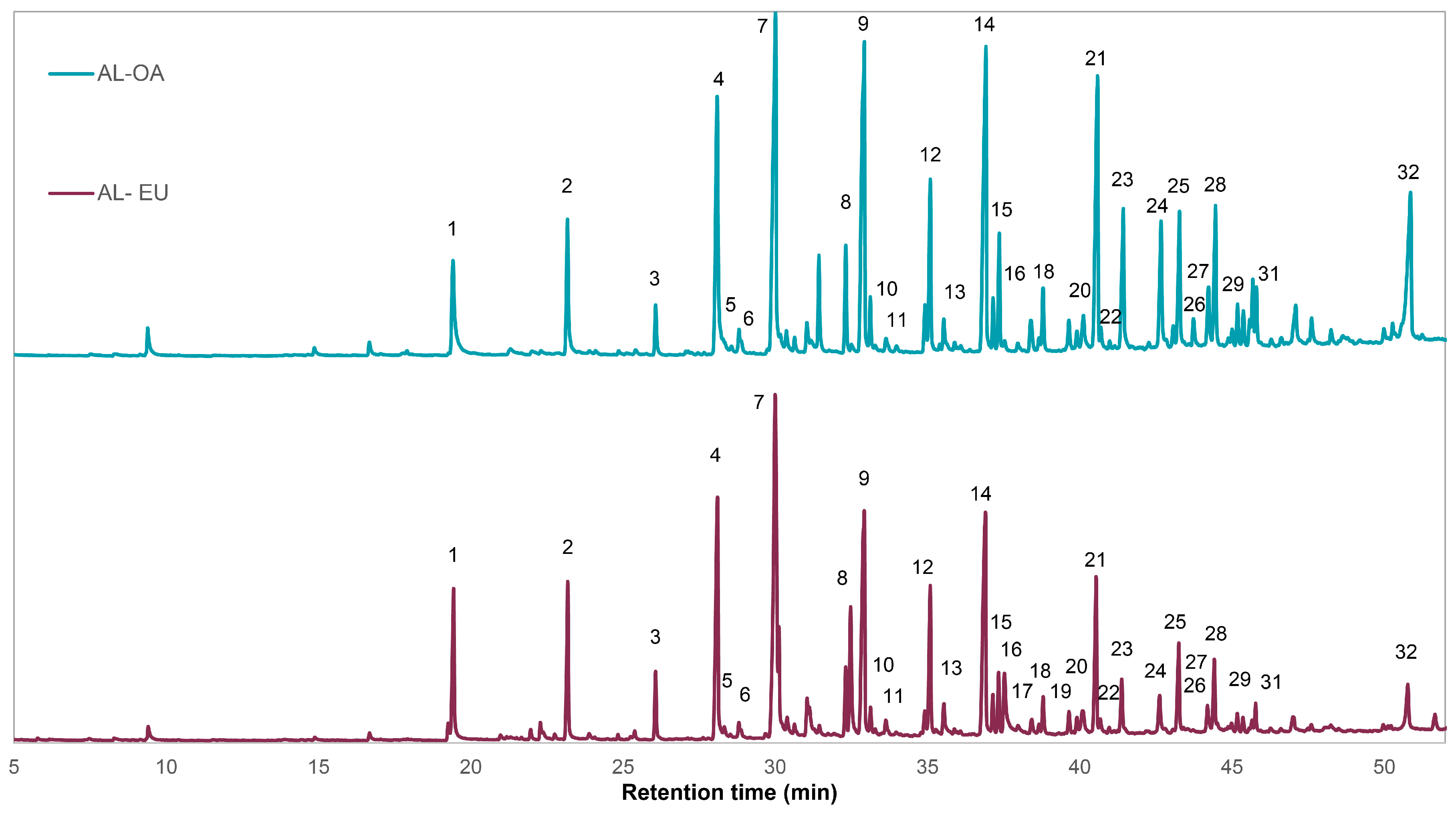
3.3. Analytical Py-GCMS
3.4. 2D NMR Spectroscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Vis, M.; Mantau, U.; Allen, B. CASCADES: Study on the Optimised Cascading Use of Wood. 2016. Available online: https://data.europa.eu/doi/10.2873/827106 (accessed on 13 July 2023).
- Stafford, W.; De Lange, W.; Nahman, A.; Chunilall, V.; Lekha, P.; Andrew, J.; Johakimu, J.; Sithole, B.; Trotter, D. Forestry biorefineries. Renew. Energy 2020, 154, 461–475. [Google Scholar] [CrossRef]
- Navare, K.; Arts, W.; Faraca, G.; Van den Bossche, G.; Sels, B.; Van Acker, K. Environmental impact assessment of cascading use of wood in bio-fuels and bio-chemicals. Resour. Conserv. Recycl. 2022, 186, 106588. [Google Scholar] [CrossRef]
- Tamantini, S.; Del Lungo, A.; Romagnoli, M.; Paletto, A.; Keller, M.; Bersier, J.; Zikeli, F. Basic steps to promote biorefinery value chains in forestry in Italy. Sustainability 2021, 13, 11731. [Google Scholar] [CrossRef]
- Tavares, D.; Cavali, M.; Tanobe, V.d.O.A.; Torres, L.A.Z.; Rozendo, A.S.; Zandoná Filho, A.; Soccol, C.R.; Woiciechowski, A.L. Lignin from Residual Sawdust of Eucalyptus spp.—Isolation, Characterization, and Evaluation of the Antioxidant Properties. Biomass 2022, 2, 195–208. [Google Scholar] [CrossRef]
- Tofani, G.; Cornet, I.; Tavernier, S. Estimation of hydrogen peroxide effectivity during bleaching using the Kappa number. Chem. Pap. 2021, 75, 5749–5758. [Google Scholar] [CrossRef]
- Gan, M.J.; Niu, Y.Q.; Qu, X.J.; Zhou, C.H. Lignin to value-added chemicals and advanced materials: Extraction, degradation, and functionalization. Green Chem. 2022, 24, 7705–7750. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A.; Sulaeva, I.; Schlee, P.; Huang, Z.; Feng, M.; Borghei, M.; Rojas, O.J.; Potthast, A.; Rosenau, T. New opportunities in the valorization of technical lignins. ChemSusChem 2021, 14, 1016–1036. [Google Scholar] [CrossRef]
- Tardy, B.L.; Lizundia, E.; Guizani, C.; Hakkarainen, M.; Sipponen, M.H. Prospects for the integration of lignin materials into the circular economy. Mater. Today 2023, 65, 122–132. [Google Scholar] [CrossRef]
- Kai, D.; Tan, M.J.; Chee, P.L.; Chua, Y.K.; Yap, Y.L.; Loh, X.J. Towards lignin-based functional materials in a sustainable world. Green Chem. 2016, 18, 1175–1200. [Google Scholar] [CrossRef]
- Tian, D.; Hu, J.; Bao, J.; Chandra, R.P.; Saddler, J.N.; Lu, C. Lignin valorization: Lignin nanoparticles as high-value bio-additive for multifunctional nanocomposites. Biotechnol. Biofuels 2017, 10, 1–11. [Google Scholar] [CrossRef]
- Laurichesse, S.; Avérous, L. Chemical modification of lignins: Towards biobased polymers. Prog. Polym. Sci. 2014, 39, 1266–1290. [Google Scholar] [CrossRef]
- Chung, H.; Washburn, N.R. Chemistry of lignin-based materials. Green Mater. 2013, 1, 137–160. [Google Scholar] [CrossRef]
- Bergamasco, S.; Tamantini, S.; Zikeli, F.; Vinciguerra, V.; Scarascia Mugnozza, G.; Romagnoli, M. Synthesis and characterizations of eco-friendly organosolv lignin-based polyurethane coating films for the coating industry. Polymers 2022, 14, 416. [Google Scholar] [CrossRef]
- Scarica, C.; Suriano, R.; Levi, M.; Turri, S.; Griffini, G. Lignin functionalized with succinic anhydride as building block for biobased thermosetting polyester coatings. ACS Sustain. Chem. Eng. 2018, 6, 3392–3401. [Google Scholar] [CrossRef]
- Hao, C.; Liu, T.; Zhang, S.; Brown, L.; Li, R.; Xin, J.; Zhong, T.; Jiang, L.; Zhang, J. A high-lignin-content, removable, and glycol-assisted repairable coating based on dynamic covalent bonds. ChemSusChem 2019, 12, 1049–1058. [Google Scholar] [CrossRef]
- Tofani, G.; Cornet, I.; Tavernier, S. Multiple linear regression to predict the brightness of waste fibres mixtures before bleaching. Chem. Pap. 2022, 76, 4351–4365. [Google Scholar] [CrossRef]
- Bajwa, D.; Pourhashem, G.; Ullah, A.H.; Bajwa, S. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crops Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Romagnoli, M.; Vinciguerra, V.; Silvestri, A. Heat treatment effect on lignin and carbohydrates in Corsican pine earlywood and latewood studied by PY–GC–MS technique. J. Wood Chem. Technol. 2018, 38, 57–70. [Google Scholar] [CrossRef]
- Vivas, N.; Nonier, M.-F.; Pianet, I.; de Gaulejac, N.V.; Fouquet, É. Structure of extracted lignins from oak heartwood (Quercus petraea Liebl., Q. Robur L.). Comptes Rendus Chim. 2006, 9, 1221–1233. [Google Scholar] [CrossRef]
- Sabo, V.A.; Knezevic, P. Antimicrobial activity of Eucalyptus camaldulensis Dehn. plant extracts and essential oils: A review. Ind. Crops Prod. 2019, 132, 413–429. [Google Scholar] [CrossRef]
- Deidda, A.; Buffa, F.; Linaldeddu, B.T.; Pinna, C.; Scanu, B.; Deiana, V.; Satta, A.; Franceschini, A.; Floris, I. Emerging pests and diseases threaten Eucalyptus camaldulensis plantations in Sardinia, Italy. Iforest-Biogeosci. For. 2016, 9, 883. [Google Scholar] [CrossRef]
- Nocetti, M.; Pröller, M.; Brunetti, M.; Dowse, G.P.; Wessels, C.B. Investigating the potential of strength grading green Eucalyptus grandis lumber using multi-sensor technology. BioResources 2017, 12, 9273–9286. [Google Scholar] [CrossRef]
- Palmieri, N.; Suardi, A.; Pari, L. Italian consumers’ willingness to pay for eucalyptus firewood. Sustainability 2020, 12, 2629. [Google Scholar] [CrossRef]
- Lin, S.Y.; Dence, C.W. Methods in Lignin Chemistry; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar] [CrossRef]
- Vinciguerra, V.; Spina, S.; Luna, M.; Petrucci, G.; Romagnoli, M. Structural analysis of lignin in chestnut wood by pyrolysis-gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2011, 92, 273–279. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; Taddei, A.R.; D’Annibale, A.; Romagnoli, M.; Scarascia Mugnozza, G. Isolation and characterization of lignin from beech wood and chestnut sawdust for the preparation of lignin nanoparticles (LNPs) from wood industry side-streams. Holzforschung 2018, 72, 961–972. [Google Scholar] [CrossRef]
- Zikeli, F.; Vinciguerra, V.; D’Annibale, A.; Capitani, D.; Romagnoli, M.; Scarascia Mugnozza, G. Preparation of lignin nanoparticles from wood waste for wood surface treatment. Nanomaterials 2019, 9, 281. [Google Scholar] [CrossRef]
- Gellerstedt, G.; Pranda, J.; Lindfors, E.-L. Structural and molecular properties of residual birch kraft lignins. J. Wood Chem. Technol. 1994, 14, 467–482. [Google Scholar] [CrossRef]
- Faix, O.; Meier, D.; Fortmann, I. Thermal degradation products of wood. Gas chromatographic separation and mass spectrometric characterization of monomeric lignin-derived products. Holz Als Roh-Und Werkst. 1990, 48, 281–285. [Google Scholar] [CrossRef]
- Lourenco, A.; Rencoret, J.; Chemetova, C.; Gominho, J.; Gutierrez, A.; Del Rio, J.C.; Pereira, H. Lignin composition and structure differs between xylem, phloem and phellem in Quercus suber L. Front. Plant Sci. 2016, 7, 1612. [Google Scholar] [CrossRef]
- Penín, L.; Lange, H.; Santos, V.; Crestini, C.; Parajó, J.C. Characterization of Eucalyptus nitens lignins obtained by biorefinery methods based on ionic liquids. Molecules 2020, 25, 425. [Google Scholar] [CrossRef]
- Marques António, V.; Rencoret, J.; Gutiérrez, A.; del Río José, C.; Pereira, H. Ferulates and lignin structural composition in cork. Holzforschung 2016, 70, 275–289. [Google Scholar] [CrossRef]
- Xiao, M.-Z.; Chen, W.-J.; Hong, S.; Pang, B.; Cao, X.-F.; Wang, Y.-Y.; Yuan, T.-Q.; Sun, R.-C. Structural characterization of lignin in heartwood, sapwood, and bark of eucalyptus. Int. J. Biol. Macromol. 2019, 138, 519–527. [Google Scholar] [CrossRef]
- Li, H.-Y.; Wang, C.-Z.; Chen, X.; Cao, X.-F.; Sun, S.-N.; Sun, R.-C. Structural elucidation of Eucalyptus lignin and its dynamic changes in the cell walls during an integrated process of ionic liquids and successive alkali treatments. Bioresour. Technol. 2016, 222, 175–181. [Google Scholar] [CrossRef]
- Rico, A.; Rencoret, J.; Del Río, J.C.; Martínez, A.T.; Gutiérrez, A. In-depth 2D NMR study of lignin modification during pretreatment of Eucalyptus wood with laccase and mediators. BioEnergy Res. 2015, 8, 211–230. [Google Scholar] [CrossRef]
- Ralph, S.; Ralph, J.; Landucci, L. NMR Database of Lignin and Cell Wall Model Compounds. 2009. Available online: https://www.glbrc.org/databases_and_software/nmrdatabase/ (accessed on 15 July 2022).
- Bajraktari, A.; Nunes, L.; Knapic, S.; Pimenta, R.; Pinto, T.; Duarte, S.; Miranda, I.; Pereira, H. Chemical characterization, hardness and termite resistance of Quercus cerris heartwood from Kosovo. Maderas. Cienc. Y Tecnol. 2018, 20, 305–314. [Google Scholar] [CrossRef]
- Todaro, L.; Dichicco, P.; Moretti, N.; D’Auria, M. Effect of combined steam and heat treatments on extractives and lignin in sapwood and heartwood of Turkey oak (Quercus cerris L.) wood. BioResources 2013, 8, 1718–1730. [Google Scholar] [CrossRef]
- Wang, H.-M.; Wang, B.; Wen, J.-L.; Yuan, T.-Q.; Sun, R.-C. Structural characteristics of lignin macromolecules from different Eucalyptus species. ACS Sustain. Chem. Eng. 2017, 5, 11618–11627. [Google Scholar] [CrossRef]
- Vieira, T.A.S.; Arriel, T.G.; Zanuncio, A.J.V.; Carvalho, A.G.; Branco-Vieira, M.; Carabineiro, S.A.C.; Trugilho, P.F. Determination of the chemical composition of Eucalyptus spp. for cellulosic pulp production. Forests 2021, 12, 1649. [Google Scholar] [CrossRef]
- Kawamura, I.; Bland, D. The Lignins of Eucalyptus Wood from Tropical and Temperate Zones. Holzforschung 1967, 21, 65–74. [Google Scholar] [CrossRef]
- Baumberger, S.; Abaecherli, A.; Fasching, M.; Gellerstedt, G.; Gosselink, R.; Hortling, B.; Li, J.; Saake, B.; de Jong, E. Molar mass determination of lignins by size-exclusion chromatography: Towards standardisation of the method. Holzforschung 2007, 61, 459–468. [Google Scholar] [CrossRef]
- Zheng, L.; Lu, G.; Pei, W.; Yan, W.; Li, Y.; Zhang, L.; Huang, C.; Jiang, Q. Understanding the relationship between the structural properties of lignin and their biological activities. Int. J. Biol. Macromol. 2021, 190, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Awal, A.; Sain, M. Spectroscopic studies and evaluation of thermorheological properties of softwood and hardwood lignin. J. Appl. Polym. Sci. 2011, 122, 956–963. [Google Scholar] [CrossRef]
- Rana, R.; Langenfeld-Heyser, R.; Finkeldey, R.; Polle, A. FTIR spectroscopy, chemical and histochemical characterisation of wood and lignin of five tropical timber wood species of the family of Dipterocarpaceae. Wood Sci. Technol. 2010, 44, 225–242. [Google Scholar] [CrossRef]
- Yan, C.; Yin, M.; Zhang, N.; Jin, Q.; Fang, Z.; Lin, Y.; Cai, Y. Stone cell distribution and lignin structure in various pear varieties. Sci. Hortic. 2014, 174, 142–150. [Google Scholar] [CrossRef]
- Faix, O. Classification of lignins from different botanical origins by FT-IR spectroscopy. Holzforschung 1991, 45, 21–28. [Google Scholar] [CrossRef]
- Reyes-Rivera, J.; Terrazas, T. Lignin analysis by HPLC and FTIR. Xylem Methods Protoc. 2017, 1544, 193–211. [Google Scholar] [CrossRef]
- Pandey, K.; Pitman, A. FTIR studies of the changes in wood chemistry following decay by brown-rot and white-rot fungi. Int. Biodeterior. Biodegrad. 2003, 52, 151–160. [Google Scholar] [CrossRef]
- Dörrstein, J.; Scholz, R.; Schwarz, D.; Schieder, D.; Sieber, V.; Walther, F.; Zollfrank, C. Dataset on the structural characterization of organosolv lignin obtained from ensiled Poaceae grass and load-dependent molecular weight changes during thermoplastic processing. Data Brief 2018, 17, 647–652. [Google Scholar] [CrossRef]
- Moghaddam, L.; Rencoret, J.; Maliger, V.R.; Rackemann, D.W.; Harrison, M.D.; Gutierrez, A.; del Río, J.C.; Doherty, W.O. Structural characteristics of bagasse furfural residue and its lignin component. An NMR, Py-GC/MS, and FTIR study. ACS Sustain. Chem. Eng. 2017, 5, 4846–4855. [Google Scholar] [CrossRef]
- Rodrigues, J.; Meier, D.; Faix, O.; Pereira, H. Determination of tree to tree variation in syringyl/guaiacyl ratio of Eucalyptus globulus wood lignin by analytical pyrolysis. J. Anal. Appl. Pyrolysis 1999, 48, 121–128. [Google Scholar] [CrossRef]
- Happs, R.M.; Addison, B.; Doeppke, C.; Donohoe, B.S.; Davis, M.F.; Harman-Ware, A.E. Comparison of methodologies used to determine aromatic lignin unit ratios in lignocellulosic biomass. Biotechnol. Biofuels 2021, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]


| Wood Species | Extractives (%) | AL (%) | Klason Lignin (%) |
|---|---|---|---|
| Q. cerris | 2.0 | 7.3 | 23.2–25.5 |
| E. camaldulensis | 6.1 | 7.2 | 21.4–27.7 |
| LIGNIN SAMPLE | Mw (Da) | Mn (Da) | Đ |
|---|---|---|---|
| AL-OA | 5170 | 1227 | 4.21 |
| AL-EU | 4841 | 1472 | 3.29 |
| AL-B | 4510 | 1032 | 4.37 |
| AL-Ch | 3583 | 1099 | 3.26 |
| AL-IR | 3270 | 1084 | 3.02 |
| AL-IRNS | 3895 | 1126 | 3.46 |
| IR Band | Wavenumber (cm−1) | Assignment | Reference | |
|---|---|---|---|---|
| Oak | Eucalypt | |||
| 1 | 1725 | 1719 | C=O stretch in unconjugated ketones, in carbonyl and ester group | [45,46] |
| 2 | 1593 | 1611 | Aromatic skeletal vibrations plus C=O stretching, C=C aromatic ring vibration (S > G), G condensed > G etherified | [45,47,48] |
| 3 | 1505 | 1512 | C=C aromatic ring vibration (G> S) | [49] |
| 4 | 1461 | 1459 | C-H deformation in methyl and methylene groups | [48] |
| 5 | 1422 | 1425 | C=C aromatic ring vibration and C-H in plane deformation | [45] |
| 6 | 1367 | 1372 | Aliphatic C-H stretching in CH3 (not in OCH3) and phenolic O-H stretching vibration, C-H bending vibration in cellulose and hemicelluloses | [47,50] |
| 7 | 1328 | 1327 | Syringyl ring breathing with C-O stretching | [49] |
| 8 | 1265 | 1282 | Guaiacyl ring breathing, C=O stretching in lignin and C-O linkage in guaiacyl aromatic methoxyl groups | [49] |
| 9 | 1224 | 1223 | C-C plus C-O stretching and C=O stretching; G condensed > G etherified, syringyl and guaiacyl ring breathing | [51,52] |
| 10 | 1148 | 1150 | C-H stretching in aromatic ring (guaiacyl) | [49] |
| 11 | 1125 | 1122 | Aromatic skeletal and C-O stretching (typical for S units) | [48,50] |
| 12 | 1031 | 1031 | Aromatic C-H in-plane deformation in guaiacyl and C–O deformation in primary alcohol | [48,50] |
| Peak Number | Pyrolysis Product | Origin | RT (min) | AL–OA | AL–EU |
|---|---|---|---|---|---|
| Area (%) | Area (%) | ||||
| 1 | Guaiacol | G | 19.42 | 2.39 | 3.50 |
| 2 | 4-Methylguaiacol | G | 23.17 | 2.43 | 3.30 |
| 3 | 4-Ethylguaiacol | G | 26.09 | 0.80 | 1.34 |
| 4 | 4-Vinylguaiacol | G | 28.09 | 6.24 | 7.08 |
| 5 | Eugenol | G | 28.85 | 0.34 | 0.28 |
| 6 | 4-Propylguaiacol | G | 28.86 | 0.15 | 0.15 |
| 7 | Syringol | S | 29.99 | 9.47 | 13.06 |
| 8 | Isoeugenol (trans) | G | 32.30 | 1.61 | 1.35 |
| 9 | 4-Methylsyringol | S | 32.91 | 8.00 | 7.84 |
| 10 | Vanillin | G | 33.11 | 0.77 | 0.57 |
| 11 | Homovanillin | G | 34.90 | 0.82 | 0.59 |
| 12 | 4-Ethylsyringol | S | 35.08 | 2.85 | 3.46 |
| 13 | Acetoguaiacol | G | 35.52 | 0.70 | 0.91 |
| 14 | 4-Vinylsyringol | S | 36.91 | 7.67 | 7.67 |
| 15 | Guaiayl acetone | G | 37.15 | 0.70 | 0.79 |
| 16 | 4-Allylsyringol | S | 37.34 | 1.69 | 1.21 |
| 17 | Propioguaiacone | G | 37.90 | 0.17 | 0.44 |
| 18 | Coniferyl alcohol (structure isomer) | G | 38.39 | 0.69 | 0.41 |
| 19 | 4-Propenylsyringol (cis) | S | 38.78 | 0.98 | 0.77 |
| 20 | Levoglucosan | PS | 40.11 | 0.97 | 1.00 |
| 21 | 4-Propenylsyringol (trans) | S | 40.55 | 6.29 | 3.85 |
| 22 | Dihydroconiferyl alcohol | G | 40.67 | 0.44 | 0.40 |
| 23 | Syringaldehyde | S | 41.39 | 2.86 | 1.32 |
| 24 | Homosyringaldehyde | S | 42.66 | 2.50 | 1.05 |
| 25 | Acetosyringone | S | 43.26 | 2.43 | 2.20 |
| 26 | Coniferyl alcohol (cis) | G | 43.74 | 0.50 | 0.04 |
| 27 | Coniferaldehyde | G | 44.21 | 1.15 | 0.70 |
| 28 | Syringyl acetone | S | 44.43 | 2.51 | 1.66 |
| 29 | Propiosyringone | S | 54.17 | 0.60 | 0.37 |
| 30 | 4-Oxy-allylsyringol | S | 45.57 | 0.41 | --- |
| 31 | Sinapyl alcohol (structure isomer) | S | 45.66 | 0.98 | 0.25 |
| 32 | Sinapaldehyde | S | 50.81 | 5.36 | 1.43 |
| S/G Ratio | 2.73 | 2.11 |
| Lignin Sample | Monomers (%) | Interunit Linkages (%) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S Units | Sox Units | G Units | Gox Units | H Units | SA | CA | FA | S/G Ratio | β-O-4′ | α-oxid. β-O-4′ | β-5′ | β-β′ | BE | Open β-1 | Stilbenes | |
| AL-OA | 62 | 5 | 24 | 5 | 1 | 1 | 1 | 1 | 2.31 | 79 | 3 | 4 | 7 | 3 | 2 | 2 |
| AL-EU | 57 | 3 | 33 | 3 | 1 | 2 | 0 | 1 | 1.69 | 73 | 2 | 6 | 8 | 5 | 4 | 2 |
| δC/δH (ppm) | Assignment (Label) |
|---|---|
| 52.7/3.43 | Cβ-Hβ in phenylcoumaran β-5′ substructures (Bβ) |
| 53.3/3.05 | Cβ-Hβ in resinol substructures β-β′ (Cβ) |
| 54.2/2.82 | Cβ-Hβ in open β-1′ substructures (Eβ) |
| 59.6/3.05–3.93 | Cγ-Hγ in γ-hydroxylated β-O-4′ substructures (Aγ) |
| 62.1/3.17 | C5-H5 in β-D-xylopyranoside (X5) |
| 62.5/3.76 | Cγ-Hγ in phenylcoumaran β-5′ substructures (Bγ) |
| 62.5/4.29 | Cγ-Hγ in γ-acylated β-O-4′ substructures (Aγ′) |
| 66.9/4.14 | Cγ-Hγ in Hibbert’s ketone (HKγ) |
| 70.8/3.80 + 4.15 | Cγ-Hγ in resinol substructures β-β′ (Cγ) |
| 71.5/4.84 | Cα-Hα in β-O-4′ substructures (Aα) linked to a G unit |
| 74.1/3.02 | C2-H2 in β-D-xylopyranoside (X2) |
| 72.6/4.49 | C2-H2 in 2-O-acetyl-β-D-xylopyranoside (X′2) |
| 74.7/3.23 | C3-H3 in β-D-xylopyranoside (X3) |
| 75.5/4.86 | C3-H3 in 3-O-acetyl-β-D-xylopyranoside (X′3) |
| 76.0/3.48 | C4-H4 in β-D-xylopyranoside (X4) |
| 81.5/4.73 | Cα-Hα in benzyl ether substructures (BEα) |
| 81.6/4.53 | Cβ-Hβ in β-O-4′ substructures (Aβ(H)) linked to a H unit |
| 83.1/5.20 | Cβ-Hβ in α-oxidized (Cα=O) β-O-4′ substructures (Aoxβ) |
| 83.4/4.28 | Cβ-Hβ in β-O-4′ substructures (Aβ(G)) linked to a G unit |
| 85.0/4.67 | Cα-Hα in resinol β-β′ substructures (Cα) |
| 86.2/4.01 | Cβ-Hβ in β-O-4′ substructures (Aβ(S)) linked to a S unit |
| 87.1/5.50 | Cα-Hα in phenylcoumaran β-5′ substructures (Bα) |
| 103.4/6.64 | C2,6-H2,6 in syringyl units (S2,6) |
| 106.2/7.06 | C2,6-H2,6 in sinapaldehyde units (SA2,6) |
| 106.2/7.30 | C2,6-H2,6 in α-oxidized (Cα=O) syringyl units (S′2,6) |
| 110.6/7.32 | C2-H2 in ferulic acid (FA2) |
| 110.6/7.51 | C2-H2 in oxidized guaiacyl units (G′2) |
| 110.8/6.97 | C2-H2 in guaiacyl units (G2) |
| 111.8/6.66 | C3,5-H3,5 in p-hydroxyphenyl units (H3,5) |
| 112.9/7.33 | C2-H2 in coniferaldehyde (CA2) |
| 115.2/6.80 | C5-H5 in guaiacyl units (G5) |
| 119.3/6.88 | C6-H6 in guaiacyl units (G6) |
| 118.4/7.32 | C6-H6 in coniferaldehyde (CA6) |
| 123.0/7.11 | C6-H6 in ferulic acid (FA6) |
| 122.8/7.62 | C6-H6 in oxidized guaiacyl units (G′6) |
| 125.6/7.01 | Cα,β-Hα,β in stilbene units (Iα,β) |
| 126.0/6.77 | Cβ-Hβ in cinnamyl aldehyde end groups (CAβ) |
| 128.1/7.14 | C2,6-H2,6 in p-hydroxyphenyl units (H2,6) |
| 153.6/7.60 | Cα-Hα in cinnamyl aldehyde end groups (CAα, SAα) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bergamasco, S.; Zikeli, F.; Vinciguerra, V.; Sobolev, A.P.; Scarnati, L.; Tofani, G.; Scarascia Mugnozza, G.; Romagnoli, M. Extraction and Characterization of Acidolysis Lignin from Turkey Oak (Quercus cerris L.) and Eucalypt (Eucalyptus camaldulensis Dehnh.) Wood from Population Stands in Italy. Polymers 2023, 15, 3591. https://doi.org/10.3390/polym15173591
Bergamasco S, Zikeli F, Vinciguerra V, Sobolev AP, Scarnati L, Tofani G, Scarascia Mugnozza G, Romagnoli M. Extraction and Characterization of Acidolysis Lignin from Turkey Oak (Quercus cerris L.) and Eucalypt (Eucalyptus camaldulensis Dehnh.) Wood from Population Stands in Italy. Polymers. 2023; 15(17):3591. https://doi.org/10.3390/polym15173591
Chicago/Turabian StyleBergamasco, Sara, Florian Zikeli, Vittorio Vinciguerra, Anatoly Petrovich Sobolev, Luca Scarnati, Giorgio Tofani, Giuseppe Scarascia Mugnozza, and Manuela Romagnoli. 2023. "Extraction and Characterization of Acidolysis Lignin from Turkey Oak (Quercus cerris L.) and Eucalypt (Eucalyptus camaldulensis Dehnh.) Wood from Population Stands in Italy" Polymers 15, no. 17: 3591. https://doi.org/10.3390/polym15173591









