A Critical Review of Palladium Nanoparticles Decorated in Smart Microgels
Abstract
:1. Introduction
2. Classifications
2.1. Pd Nanoparticles Decorated Uniformly in Microgels
2.1.1. Monometallic Nanoparticles Encapsulated in Microgels
2.1.2. Bimetallic Nanoparticles Encapsulated in Microgels
2.1.3. Bimetallic Alloy Nanoparticles Decorated in Microgels
2.1.4. Bimetallic Core-Shell Nanoparticles Decorated in Microgels
2.2. Palladium Nanoparticles Decorated Core Surrounded by Crosslinked Organic Polymers
2.2.1. Palladium Nanoparticles Loaded in Inorganic Core Surrounded by Crosslinked Organic Polymers
2.2.2. Palladium Nanoparticles Loaded in a Crosslinked Organic Core Surrounded by Another Crosslinked Organic Polymer
2.3. Core Surrounded by Metal Nanoparticles Loaded Crosslinked Organic Shell
2.3.1. Inorganic Core Surrounded by Palladium Nanoparticles and a Decorated Organic Shell
2.3.2. Organic Core Surrounded by Metal Nanoparticles and Decorated Organic Shell
Organic Core Encapsulated with Palladium Nanoparticles and Decorated Crosslinked Organic Polymers
Organic Core Encapsulated with Palladium Nanoparticles and Decorated Crosslinked Organic Polymers
2.4. Crosslinked Organic Polymer Encapsulated with Metal Nanoparticles
2.4.1. Crosslinked Organic Polymer Surrounded by Palladium Nanoparticles
2.4.2. Crosslinked Organic Polymer Covered with Bimetallic Nanoparticles
2.5. Organic Polymer Crosslinked with Inorganic Materials Decorated with Palladium Nanoparticles
3. Synthesis
3.1. Formation of Pd Nanoparticles in Already Synthesized Microgels
3.2. Palladium Nanoparticles in Microgels by Mixing Both Palladium Nanoparticles and Microgels
4. Characterization Techniques
5. Properties of Microgels Decorated with Palladium Nanoparticles
5.1. pH Effect
5.1.1. Anionic Microgels and Hybrid Microgels
5.1.2. Cationic Microgels and Hybrid Microgels
5.1.3. Both Cationic and Anionic Microgels and Hybrid Microgels
5.2. Temperature Effect
5.3. Nature of Solvent
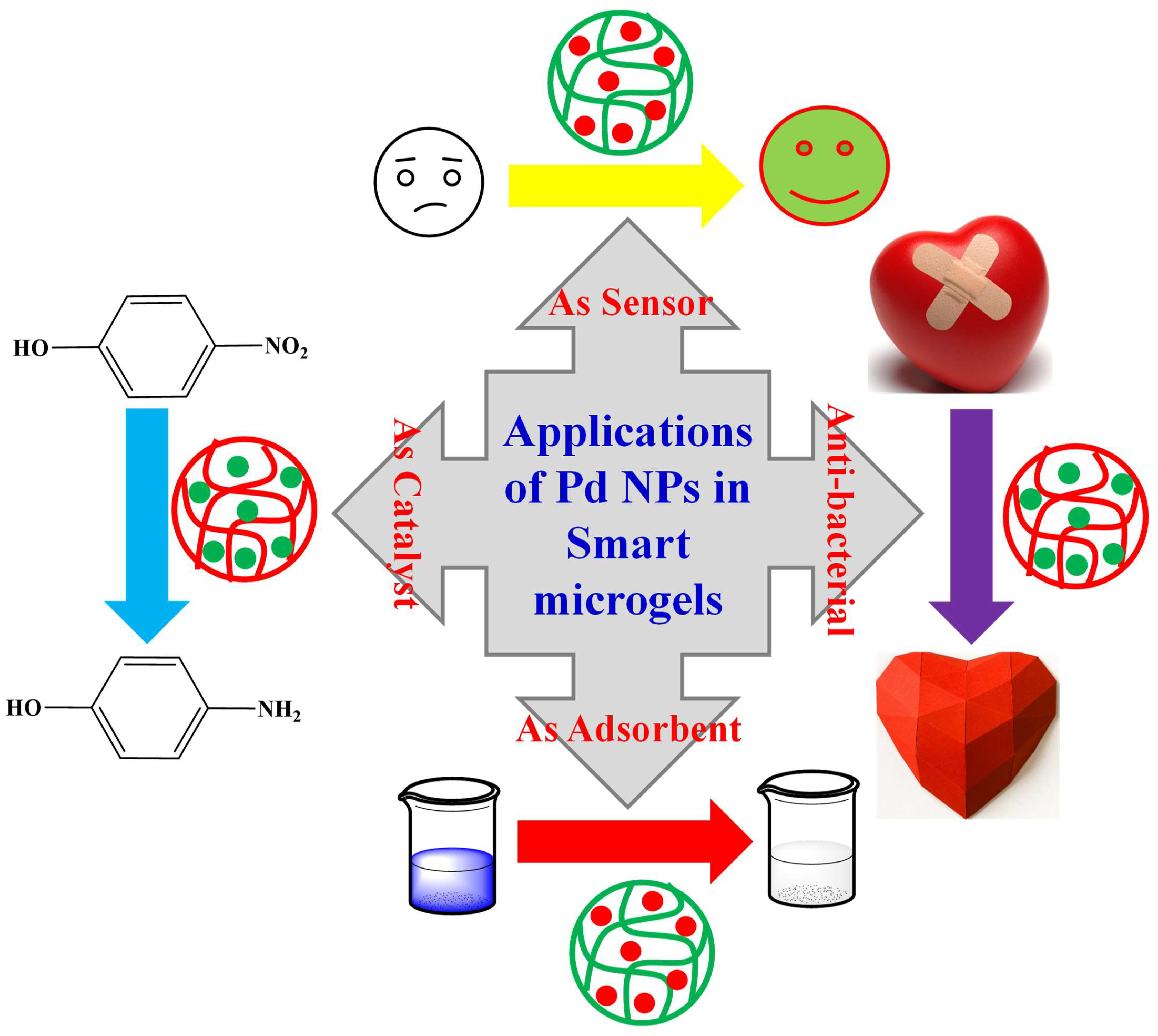
6. Applications of Palladium Nanoparticles Decorated in Microgels
6.1. As Sensor
6.2. As Anti-Bacterial
6.3. As Adsorbent
6.4. As a Catalyst
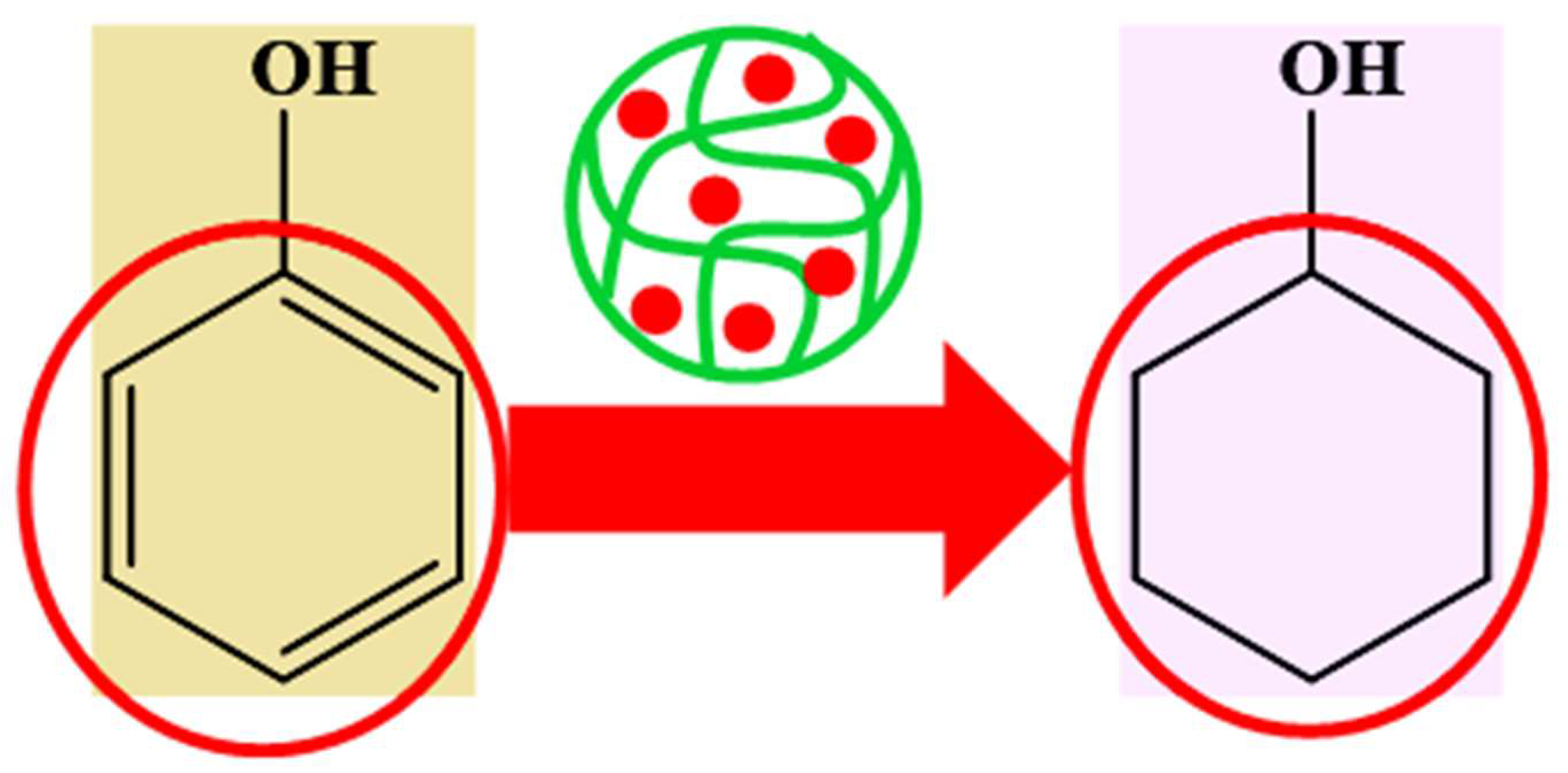
6.4.1. Catalytic Reduction of Nitroarenes and Organic Dyes
6.4.2. Hydrogenation of Different Organic Compounds
6.4.3. Suzuki Coupling and Heck Reactions
6.4.4. Some Other Chemical Reactions
7. Conclusions and Future Directions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NIA | N-isopropylacrylamide |
| 3NiP | 3-Nitrophenol |
| 4NiP | 4-Nitrophenol |
| AA | Acrylic acid |
| AMS | 2-Acrylamido-2-methylpropane |
| RhAB | Rhodamine-B |
| ChS | Chitosan |
| HDD | Hydrodynamic diameter |
| DLS | Dynamic light scattering |
| SoDS | Sodium dodecyl sulfate |
| EDX | Energy dispersive X-rays |
| FTIR | Fourier Transformed Infrared Spectroscopy |
| P(SR) | Polystyrene |
| LCST | lower critical solution temperature |
| MeBA | N,N′-Methylene-bisacrylamide |
| AmPS | Ammonium persulfate |
| AcA | Acrylamide |
| SEM | Scanning electron microscopy |
| TGA | Thermo gravimetric analysis |
| UV-Vis | UV/Visible spectroscopy |
| VPTT | Volume phase transition temperature |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
| 4ViP | 4-vinylpyridine |
| DeW | Deionized water |
| A-P(EtI) | Alginate-poly(ethyleneimine) |
| IO-P(2ViP) | Fe3O4-poly(2-vinylpyridine) |
| MeAA | Methacrylic acid |
| NNA | N-n-propylacrylamide |
| P(DA) | Polydopamine |
| BaNC | Bacterial nanocellulose |
| HyMBP | 2-Hydroxy-4-(methacryloyloxy)-benzophenone |
| P(DiVB-DCIIL) | Poly(divinylbenzene-co-dication imidazole ionic liquid) |
| GO | Graphene Oxide |
| P(ImZ/ImZM) | Poly(Imidazol/Imidazolium) |
| GA | Glutaraldehyde |
| CeL | Cellulose |
| CyD | Β-Cyclodextrin |
| DMMB | Dimethylaminoethylmethacrylate bromododecane |
| P(UNP) | Poly(undecylenic acid-co-N-isopropylacrylamide-co-potassium-4-acryloxyoylpyridine-2,6-dicarboxylate) |
| ViBIC | 1-vinyl-3-butylimidazolium chloride |
References
- Wróblewska-Krepsztul, J.; Rydzkowski, T.; Borowski, G.; Szczypiński, M.; Klepka, T.; Thakur, V.K. Recent progress in biodegradable polymers and nanocomposite-based packaging materials for sustainable environment. Int. J. Polym. Anal. Charact. 2018, 23, 383–395. [Google Scholar] [CrossRef]
- Trzepieciński, T.; Najm, S.M.; Sbayti, M.; Belhadjsalah, H.; Szpunar, M.; Lemu, H.G. New Advances and Future Possibilities in Forming Technology of Hybrid Metal–Polymer Composites Used in Aerospace Applications. J. Compos. Sci. 2021, 5, 217. [Google Scholar] [CrossRef]
- Ouyang, J. Application of intrinsically conducting polymers in flexible electronics. SmartMat 2021, 2, 263–285. [Google Scholar] [CrossRef]
- Chaterji, S.; Kwon, I.K.; Park, K. Smart polymeric gels: Redefining the limits of biomedical devices. Prog. Polym. Sci. 2007, 32, 1083–1122. [Google Scholar] [CrossRef]
- Patil, A.; Patel, A.; Purohit, R. An overview of Polymeric Materials for Automotive Applications. Mater. Today Proc. 2017, 4, 3807–3815. [Google Scholar] [CrossRef]
- Hirao, A.; Hayashi, M.; Loykulnant, S.; Sugiyama, K.; Ryu, S.; Haraguchi, N.; Matsuo, A.; Higashihara, T. Precise syntheses of chain-multi-functionalized polymers, star-branched polymers, star-linear block polymers, densely branched polymers, and dendritic branched polymers based on iterative approach using functionalized 1,1-diphenylethylene derivatives. Prog. Polym. Sci. 2005, 30, 111–182. [Google Scholar] [CrossRef]
- Nasongkia, N.; Chen, B.; Macaraeg, N.; Fox, M.E.; Fréchet, J.M.J.; Szoka, F.C. Dependence of pharmacokinetics and biodistribution on polymer architecture: Effect of cyclic versus Linear polymers. J. Am. Chem. Soc. 2009, 131, 3842–3843. [Google Scholar] [CrossRef]
- Shahid, M.; Farooqi, Z.H.; Begum, R.; Arif, M.; Azam, M.; Irfan, A.; Farooq, U. Multi-functional organic-inorganic hydrogel microspheres as efficient catalytic system for reduction of toxic dyes in aqueous medium. Z. fur Phys. Chem. 2022, 236, 87–105. [Google Scholar] [CrossRef]
- Van Zee, N.J.; Nicolaÿ, R. Vitrimers: Permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020, 104, 101233. [Google Scholar] [CrossRef]
- Arif, M. A review on copper nanoparticles loaded in smart microgels. Mater. Today Commun. 2023, 36, 106580. [Google Scholar] [CrossRef]
- Siddhanta, S.K.; Gangopadhyay, R. Conducting polymer gel: Formation of a novel semi-IPN from polyaniline and crosslinked poly(2-acrylamido-2-methyl propanesulphonicacid). Polymer 2005, 46, 2993–3000. [Google Scholar] [CrossRef]
- Arif, M. A tutorial review on bimetallic nanoparticles loaded in smart organic polymer microgels/hydrogels. J. Mol. Liq. 2023, 375, 121346. [Google Scholar] [CrossRef]
- Arif, M. Extraction of iron (III) ions by core-shell microgel for in situ formation of iron nanoparticles to reduce harmful pollutants from water. J. Environ. Chem. Eng. 2023, 11, 109270. [Google Scholar] [CrossRef]
- Velasco, D.; Tumarkin, E.; Kumacheva, E. Microfluidic Encapsulation of Cells in Polymer Microgels. Small 2012, 8, 1633–1642. [Google Scholar] [CrossRef] [PubMed]
- Arif, M. Catalytic degradation of azo dyes by bimetallic nanoparticles loaded in smart polymer microgels. RSC Adv. 2023, 13, 3008–3019. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Eckert, F.; Boyko, V.; Pich, A. Temperature-, pH-, and Magnetic-Field-Sensitive Hybrid Microgels. Small 2007, 3, 650–657. [Google Scholar] [CrossRef]
- Karg, M.; Pastoriza-Santos, I.; Rodriguez-González, B.; Von Klitzing, R.; Wellert, S.; Hellweg, T. Temperature, pH, and ionic strength induced changes of the swelling behavior of PNIPAM-poly(allylacetic acid) copolymer microgels. Langmuir 2008, 24, 6300–6306. [Google Scholar] [CrossRef]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Arif, M.; Shahid, M.; Irfan, A.; Nisar, J.; Wu, W.; Farooqi, Z.H.; Begum, R. Polymer microgels for the stabilization of gold nanoparticles and their application in the catalytic reduction of nitroarenes in aqueous media. RSC Adv. 2022, 12, 5105–5117. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Naseem, K.; Ali, F.; Batool, M.; Xiao, J.; Irfan, A. Applications of UV/Vis Spectroscopy in Characterization and Catalytic Activity of Noble Metal Nanoparticles Fabricated in Responsive Polymer Microgels: A Review. Crit. Rev. Anal. Chem. 2018, 48, 503–516. [Google Scholar] [CrossRef]
- Haleem, A.; Syaal, S.B.; Ajmal, M.; Ambreen, J.; Rauf, S.; Ali, N.; Muhammad, S.; Shah, A.; Zia, M.A.; Siddiq, M. Silver and palladium nanoparticle embedded poly(n-isopropylacrylamide-co-2-acrylamido-2-methylpropane sulfonic acid) hybrid microgel catalyst with pH and temperature dependent catalytic activity. Korean J. Chem. Eng. 2020, 37, 614–622. [Google Scholar] [CrossRef]
- Dhanavel, S.; Manivannan, N.; Mathivanan, N.; Gupta, V.K.; Narayanan, V.; Stephen, A. Preparation and characterization of cross-linked chitosan/palladium nanocomposites for catalytic and antibacterial activity. J. Mol. Liq. 2018, 257, 32–41. [Google Scholar] [CrossRef]
- Gholami Derami, H.; Gupta, P.; Gupta, R.; Rathi, P.; Morrissey, J.J.; Singamaneni, S. Palladium Nanoparticle-Decorated Mesoporous Polydopamine/Bacterial Nanocellulose as a Catalytically Active Universal Dye Removal Ultrafiltration Membrane. ACS Appl. Nano Mater. 2020, 3, 5437–5448. [Google Scholar] [CrossRef]
- Tang, Y.; Cao, Y.; Wang, S.; Shen, G.; Yu, R. Surface attached-poly(acrylic acid) network as nanoreactor to in-situ synthesize palladium nanoparticles for H2O2 sensing. Sens. Actuators B Chem. 2009, 137, 736–740. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, X.; Qin, Z.; Zhang, L.; Ye, Y.; Cao, M.; Gao, L.; Jiao, T. Preparation of PdNPs doped chitosan-based composite hydrogels as highly efficient catalysts for reduction of 4-nitrophenol. Colloids Surf. A Physicochem. Eng. Asp. 2021, 611, 125889. [Google Scholar] [CrossRef]
- Thomas, A.M.; Mohan, A.; Rout, L.; Nagappan, S.; Park, S.S.; Ha, C.-S. Pd nanoparticle incorporated mesoporous silicas with excellent catalytic activity and dual responsivity. Colloids Surf. A Physicochem. Eng. Asp. 2020, 585, 124074. [Google Scholar] [CrossRef]
- Peng, W.; Yan, Y.; Zhang, D.; Zhou, Y.; Na, D.; Xiao, C.; Yang, C.; Wen, G.; Zhang, J. Preparation of thermal stable supported metal (Cu, Au, Pd) nanoparticles via cross-linking cellulose gel confinement strategy. Colloids Surf. A Physicochem. Eng. Asp. 2021, 624, 126809. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, T.; Yu, C.; Liu, Q.; Wang, G.; Yang, H.; Yang, M.; Jiang, L.; Liu, M. Amphiphilic Pd@micro-organohydrogels with controlled wettability for enhancing gas-liquid-solid triphasic catalytic performance. Nano Res. 2022, 15, 557–563. [Google Scholar] [CrossRef]
- Biffis, A. Functionalised microgels: Novel stabilisers for catalytically active metal colloids. J. Mol. Catal. A Chem. 2001, 165, 303–307. [Google Scholar] [CrossRef]
- Begum, R.; Farooqi, Z.H.; Xiao, J.; Ahmed, E.; Sharif, A.; Irfan, A. Crosslinked polymer encapsulated palladium nanoparticles for catalytic reduction and Suzuki reactions in aqueous medium. J. Mol. Liq. 2021, 338, 116780. [Google Scholar] [CrossRef]
- Wang, Z.; Chen, X.; Li, K.; Bi, S.; Wu, C.; Chen, L. Preparation and catalytic property of PVDF composite membrane with polymeric spheres decorated by Pd nanoparticles in membrane pores. J. Memb. Sci. 2015, 496, 95–107. [Google Scholar] [CrossRef]
- Contin, A.; Biffis, A.; Sterchele, S.; Dörmbach, K.; Schipmann, S.; Pich, A. Metal nanoparticles inside microgel/clay nanohybrids: Synthesis, characterization and catalytic efficiency in cross-coupling reactions. J. Colloid Interface Sci. 2014, 414, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Fang, Q.; Zhou, L.; Xu, Z.; Qiu, J.; Wang, M.; Wang, J. Comparative study of cross-linked and linear thermo-responsive carriers supported palladium nanoparticles in the reduction of 4-nitrophenol: Structure, catalytic activity and responsive catalysis property. React. Funct. Polym. 2019, 142, 104–111. [Google Scholar] [CrossRef]
- Vescovo, R.; Becker, M.; Natile, M.M.; Canton, P.; Evangelisti, C.; Biffis, A. Microgels as Soluble Scaffolds for the Preparation of Noble Metal Nanoparticles Supported on Nanostructured Metal Oxides. ACS Appl. Nano Mater. 2021, 4, 8343–8351. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F. Bimetallic (AuAg, AuPd and AgPd) nanoparticles supported on cellulose-based hydrogel for reusable catalysis. Carbohydr. Polym. 2023, 310, 120726. [Google Scholar] [CrossRef]
- Ullah, K.; Khan, K.; Ullah, A. Synthesis and structural characterization of energy crop peelu methyl esters, using hybrid metallic nano-particles. A step forward to bioenergy industry. Fuel 2021, 300, 119241. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Li, K.; Zhang, C.; He, Y.; Du, R.; Wang, J.; Chen, L. Coupling membrane and Fe–Pd bimetallic nanoparticles for trichloroethene removing from water. J. Ind. Eng. Chem. 2019, 78, 198–209. [Google Scholar] [CrossRef]
- Nadagouda, M.N.; Varma, R.S. Preparation of Novel Metallic and Bimetallic Cross-Linked Poly(vinyl alcohol) Nanocomposites under Microwave Irradiation. Macromol. Rapid Commun. 2007, 28, 465–472. [Google Scholar] [CrossRef]
- Kakar, M.U.; Khan, K.; Akram, M.; Sami, R.; Khojah, E.; Iqbal, I.; Helal, M.; Hakeem, A.; Deng, Y.; Dai, R. Synthesis of bimetallic nanoparticles loaded on to PNIPAM hybrid microgel and their catalytic activity. Sci. Rep. 2021, 11, 14759. [Google Scholar] [CrossRef]
- Nemygina, N.A.; Nikoshvili, L.Z.; Tiamina, I.Y.; Bykov, A.V.; Smirnov, I.S.; Lagrange, T.; Kaszkur, Z.; Matveeva, V.G.; Sulman, E.M.; Kiwi-Minsker, L. Au Core-Pd Shell Bimetallic Nanoparticles Immobilized within Hyper-Cross-Linked Polystyrene for Mechanistic Study of Suzuki Cross-Coupling: Homogeneous or Heterogeneous Catalysis? Org. Process Res. Dev. 2018, 22, 1606–1613. [Google Scholar] [CrossRef]
- Sabadasch, V.; Wiehemeier, L.; Kottke, T.; Hellweg, T. Core–shell microgels as thermoresponsive carriers for catalytic palladium nanoparticles. Soft Matter 2020, 16, 5422–5430. [Google Scholar] [CrossRef]
- Sabadasch, V.; Dirksen, M.; Fandrich, P.; Hellweg, T. Multifunctional Core-Shell Microgels as Pd-Nanoparticle Containing Nanoreactors With Enhanced Catalytic Turnover. Front. Chem. 2022, 10, 889521. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Liu, W.; Zhang, X.; Bian, F.; Yu, W. Palladium supported on a magnetic microgel: An efficient and recyclable catalyst for Suzuki and Heck reactions in water. Green Chem. 2013, 15, 3429–3437. [Google Scholar] [CrossRef]
- Mei, Y.; Lu, Y.; Polzer, F.; Ballauff, M.; Drechsler, M. Catalytic activity of palladium nanoparticles encapsulated in spherical poly electrolyte brushes and core-shell microgels. Chem. Mater. 2007, 19, 1062–1069. [Google Scholar] [CrossRef]
- Wen, F.; Zhang, W.; Wei, G.; Wang, Y.; Zhang, J.; Zhang, M.; Shi, L. Synthesis of noble metal nanoparticles embedded in the shell layer of core-shell poly(styrene-co-4-vinylpyridine) micospheres and their application in catalysis. Chem. Mater. 2008, 20, 2144–2150. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, W. Pd Nanoparticles Immobilized on pH-Responsive and Chelating Nanospheres as an Efficient and Recyclable Catalyst for Suzuki Reaction in Water. J. Phys. Chem. C 2008, 112, 6245–6252. [Google Scholar] [CrossRef]
- Zhang, T.; Li, L.; Ye, Z.; Yang, Q.; Tian, Y.; Guo, X. Preparation and characterization of Ag–Pd bimetallic nano-catalysts in thermosensitive microgel nano-reactor. RSC Adv. 2018, 8, 18252–18259. [Google Scholar] [CrossRef]
- Mutharani, B.; Ranganathan, P.; Chen, S.-M.; Karuppiah, C. Enzyme-free electrochemical detection of nanomolar levels of the organophosphorus pesticide paraoxon-ethyl by using a poly(N-isopropyl acrylamide)-chitosan microgel decorated with palladium nanoparticles. Microchim. Acta 2019, 186, 167. [Google Scholar] [CrossRef]
- Hu, B.; Wu, T.; Ding, K.; Zhou, X.; Jiang, T.; Han, B. Seeding growth of Pd/Au bimetallic nanoparticles on highly cross-linked polymer microspheres with ionic liquid and solvent-free hydrogenation. J. Phys. Chem. C 2010, 114, 3396–3400. [Google Scholar] [CrossRef]
- Nabid, M.R.; Bide, Y.; Aghaghafari, E.; Rezaei, S.J.T. PdNPs@P2VP-Fe3O4 organic-inorganic hybrid microgels as a nanoreactor for selective aerobic oxidation of alcohols. Catal. Lett. 2014, 144, 355–363. [Google Scholar] [CrossRef]
- Liu, W.; Cai, H.; Lu, P.; Xu, Q.; Zhongfu, Y.; Dong, J. Polymer hydrogel supported Pd–Ni–B nanoclusters as robust catalysts for hydrogen production from hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2013, 38, 9206–9216. [Google Scholar] [CrossRef]
- Ge, L.; Zhang, M.; Wang, R.; Li, N.; Zhang, L.; Liu, S.; Jiao, T. Fabrication of CS/GA/RGO/Pd composite hydrogels for highly efficient catalytic reduction of organic pollutants. RSC Adv. 2020, 10, 15091–15097. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Mills, R.; Wan, H.; Ormsbee, L.; Bhattacharyya, D. Thermoresponsive PNIPAm-PMMA-functionalized PVDF membranes with reactive Fe-Pd nanoparticles for PCB degradation. Ind. Eng. Chem. Res. 2020, 59, 16614–16625. [Google Scholar] [CrossRef]
- Sabadasch, V.; Dirksen, M.; Fandrich, P.; Cremer, J.; Biere, N.; Anselmetti, D.; Hellweg, T. Pd Nanoparticle-Loaded Smart Microgel-Based Membranes as Reusable Catalysts. ACS Appl. Mater. Interfaces 2022, 14, 49181–49188. [Google Scholar] [CrossRef]
- Jiao, N.; Li, Z.; Xia, C.; Liu, J. Palladium Nanoparticles Immobilized on Cross-Linked Polymeric Ionic Liquid Material: Application as Efficient and Recoverable Catalyst for the Hydrogenation of Nitroarenes. ChemistrySelect 2017, 730000, 4545–4556. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Safaie, N.; Hosseini, S.H.; Bennett, C. Graphene oxide/poly(imidazole/imidazolium) nanocomposite: An effective support for immobilization of large amounts of Pd nanoparticles. J. Ind. Eng. Chem. 2016, 38, 82–92. [Google Scholar] [CrossRef]
- Sabadasch, V.; Dachwitz, S.; Hannappel, Y.; Hellweg, T.; Sewald, N. Acrylamide-Based Pd-Nanoparticle Carriers as Smart Catalysts for the Suzuki–Miyaura Cross-Coupling of Amino Acids. Synthesis 2022, 54, 3180–3192. [Google Scholar] [CrossRef]
- Feng, Y.; Yin, J.; Liu, S.; Wang, Y.; Li, B.; Jiao, T. Facile Synthesis of Ag/Pd Nanoparticle-Loaded Poly(ethylene imine) Composite Hydrogels with Highly Efficient Catalytic Reduction of 4-Nitrophenol. ACS Omega 2020, 5, 3725–3733. [Google Scholar] [CrossRef]
- Chen, S.; Liu, H. Self-reductive palladium nanoparticles loaded on polydopamine-modified MXene for highly efficient and quickly catalytic reduction of nitroaromatics and dyes. Colloids Surf. A Physicochem. Eng. Asp. 2022, 635, 128038. [Google Scholar] [CrossRef]
- Li, B.; Chen, X.; Ma, Y.; Wang, J.; Zhai, X.; He, Y.; Li, Y.; Ma, R.; Zhang, W. Catalytic behavior of a thermo-responsive PVDF/microgel@Pd membrane for 2- nitroaniline degradation. J. Environ. Chem. Eng. 2021, 9, 104757. [Google Scholar] [CrossRef]
- Johnson, J.A.; Makis, J.J.; Marvin, K.A.; Rodenbusch, S.E.; Stevenson, K.J. Size-dependent hydrogenation of p-nitrophenol with Pd nanoparticles synthesized with poly(amido)amine dendrimer templates. J. Phys. Chem. C 2013, 117, 22644–22651. [Google Scholar] [CrossRef]
- Ebadi, M.; Asikin-Mijan, N.; Md. Jamil, M.S.; Iqbal, A.; Yousif, E.; Md Zain, A.R.; Aziz, T.H.T.; Rahimi Yusop, M. Palladium Nanoparticles on Chitosan-Coated Superparamagnetic Manganese Ferrite: A Biocompatible Heterogeneous Catalyst for Nitroarene Reduction and Allyl Carbamate Deprotection. Polymers 2023, 15, 232. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, L.N.; Malatjie, K.I.; Donga, C.; Mishra, A.K.; Nxumalo, E.N.; Mishra, S.B. Catalytic degradation of methyl orange using beta cyclodextrin modified polyvinylidene fluoride mixed matrix membranes imbedded with in-situ generated palladium nanoparticles. J. Appl. Polym. Sci. 2023, 140, e53270. [Google Scholar] [CrossRef]
- Lv, X.; Lv, A.; Tian, S.; Xie, T.; Sun, S. A tough and highly active catalyst carrier tailored by nanoparticles-encapsulation poly(ionic liquid) hydrogel: Synthesis and catalytic applications. Eur. Polym. J. 2023, 182, 111713. [Google Scholar] [CrossRef]
- Sabadasch, V.; Fandrich, P.; Annegarn, M.; Hellweg, T. Effect of Methacrylic Acid in PNNPAM Microgels on the Catalytic Activity of Embedded Palladium Nanoparticles. Macromol. Chem. Phys. 2022, 223, 2200045. [Google Scholar] [CrossRef]
- Dong, Y.; Jin, Y.; Wang, J.; Shu, J.; Zhang, M. Pd nanoparticles stabilized by a simple pH-sensitive P(acrylamide-co-acrylic acid) copolymer: A recyclable and highly active catalyst system in aqueous medium. Chem. Eng. J. 2017, 324, 303–312. [Google Scholar] [CrossRef]
- Wang, S.; Mo, Y.; Vincent, T.; Roux, J.C.; Rodríguez-Castellón, E.; Faur, C.; Guibal, E. Palladium nanoparticles supported on amine-functionalized alginate foams for hydrogenation of 3-nitrophenol. J. Mater. Sci. 2020, 55, 2032–2051. [Google Scholar] [CrossRef]
- Molla, R.A.; Bhanja, P.; Ghosh, K.; Islam, S.S.; Bhaumik, A.; Islam, S.M. Pd Nanoparticles Decorated on Hypercrosslinked Microporous Polymer: A Highly Efficient Catalyst for the Formylation of Amines through Carbon Dioxide Fixation. ChemCatChem 2017, 9, 1939–1946. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y.; Havener, K.; Zhang, M.; Zhang, L.; Wu, W.; Huang, K. Temperature-Controlled Selectivity of Hydrogenation and Hydrodeoxygenation of Biomass by Superhydrophilic Nitrogen/Oxygen Co-Doped Porous Carbon Nanosphere Supported Pd Nanoparticles. Small 2022, 18, 2106893. [Google Scholar] [CrossRef]
- Yu, H.; Xu, Y.; Zhang, M.; Zhang, L.; Wu, W.; Huang, K. Magnetically-separable cobalt catalyst embedded in metal nitrate-promoted hierarchically porous N-doped carbon nanospheres for hydrodeoxygenation of lignin-derived species. Fuel 2023, 331, 125917. [Google Scholar] [CrossRef]
- Seku, K.; Bhagavanth Reddy, G.; Hussaini, S.S.; Pejjai, B.; Hussain, M.; Reddy, D.M.; Khazaleh, M.A.K.; Mangatayaru, G. An efficient biosynthesis of palladium nanoparticles using Bael gum and evaluation of their catalytic and antibacterial activity. Int. J. Biol. Macromol. 2022, 209, 912–922. [Google Scholar] [CrossRef]
- Mizuno, S.; Asoh, T.-A.; Takashima, Y.; Harada, A.; Uyama, H. Palladium nanoparticle loaded β-cyclodextrin monolith as a flow reactor for concentration enrichment and conversion of pollutants based on molecular recognition. Chem. Commun. 2020, 56, 14408–14411. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, L.; Gao, S.; Wang, H.; He, Z.; Xu, Y.; Huang, K. In situ encapsulated ultrafine Pd nanoparticles in nitrogen-doped porous carbon derived from hyper-crosslinked polymers effectively catalyse hydrogenation. J. Catal. 2021, 396, 342–350. [Google Scholar] [CrossRef]
- Zhang, Y.; Quek, X.-Y.; Wu, L.; Guan, Y.; Hensen, E.J. Palladium nanoparticles entrapped in polymeric ionic liquid microgels as recyclable hydrogenation catalysts. J. Mol. Catal. A Chem. 2013, 379, 53–58. [Google Scholar] [CrossRef]
- Albino, M.; Burden, T.J.; Piras, C.C.; Whitwood, A.C.; Fairlamb, I.J.S.; Smith, D.K. Mechanically Robust Hybrid Gel Beads Loaded with “Naked” Palladium Nanoparticles as Efficient, Reusable, and Sustainable Catalysts for the Suzuki-Miyaura Reaction. ACS Sustain. Chem. Eng. 2023, 11, 1678–1689. [Google Scholar] [CrossRef]
- Wang, B.; Dai, L.; Yang, G.; Bendrich, G.; Ni, Y.; Fang, G. A highly efficient thermo responsive palladium nanoparticles incorporated guar gum hydrogel for effective catalytic reactions. Carbohydr. Polym. 2019, 226, 115289. [Google Scholar] [CrossRef]
- Hong, M.C.; Ahn, H.; Choi, M.C.; Lee, Y.; Kim, J.; Rhee, H. Pd nanoparticles immobilized on PNIPAM-halloysite: Highly active and reusable catalyst for Suzuki-Miyaura coupling reactions in water. Appl. Organomet. Chem. 2014, 28, 156–161. [Google Scholar] [CrossRef]
- Biffis, A.; Minati, L. Efficient aerobic oxidation of alcohols in water catalysed by microgel-stabilised metal nanoclusters. J. Catal. 2005, 236, 405–409. [Google Scholar] [CrossRef]
- Chappa, S.; Mhatre, A.M.; Adya, V.C.; Pandey, A.K. Egg-shell membrane mimicking synthetic polymer membrane supported palladium nanoparticles for catalyzing reduction of uranyl(VI) ions. Appl. Catal. B Environ. 2017, 203, 53–64. [Google Scholar] [CrossRef]

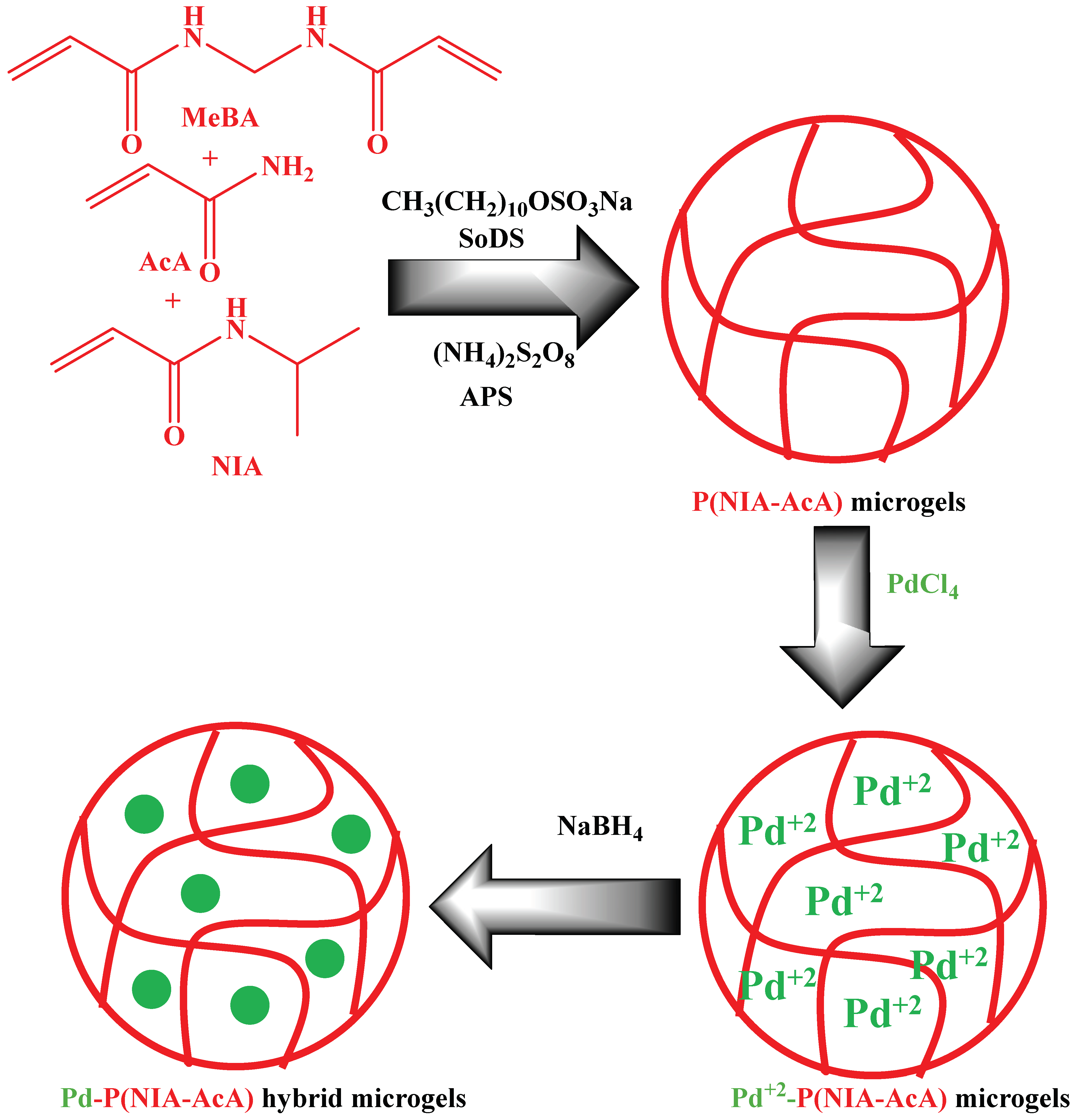
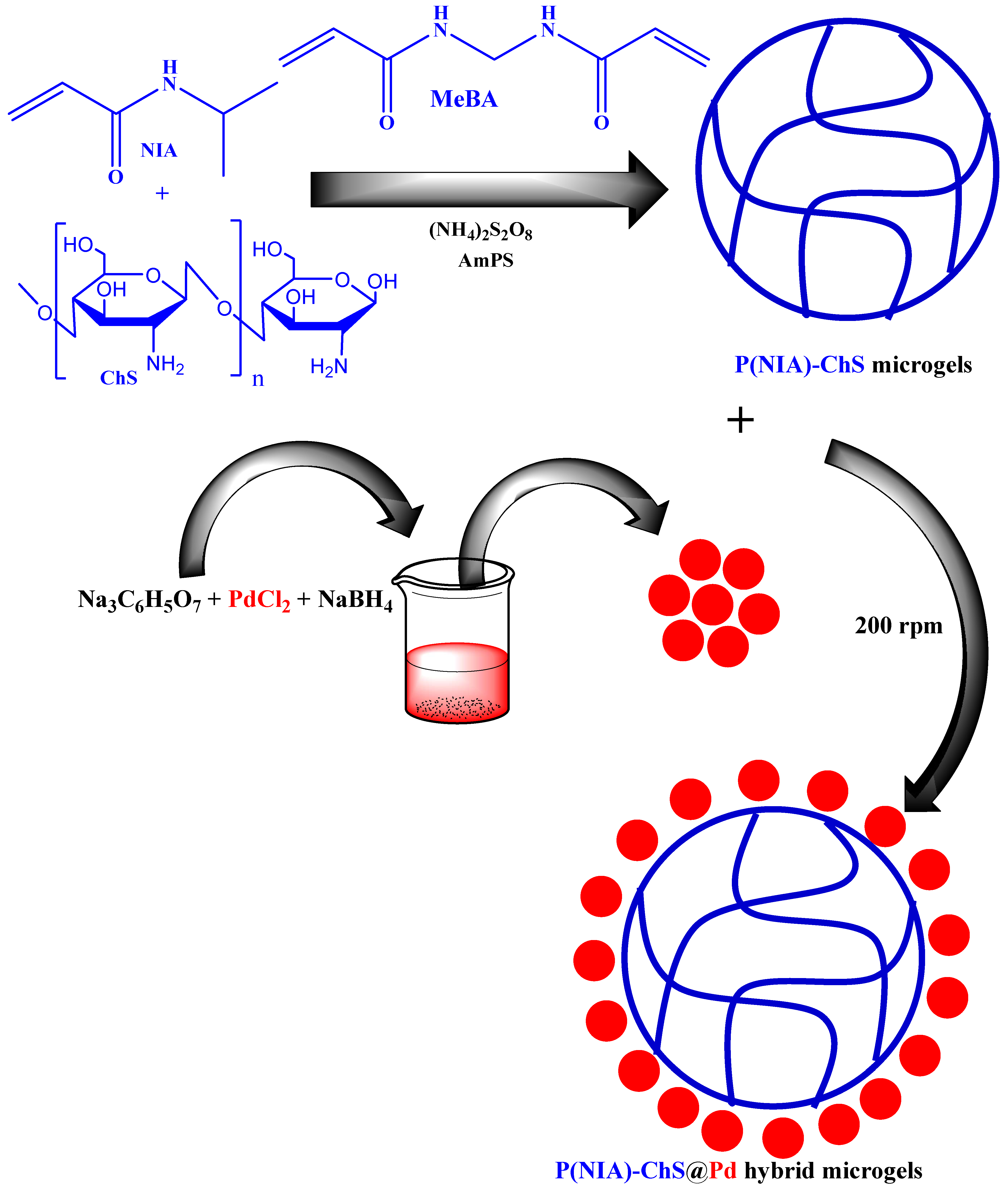


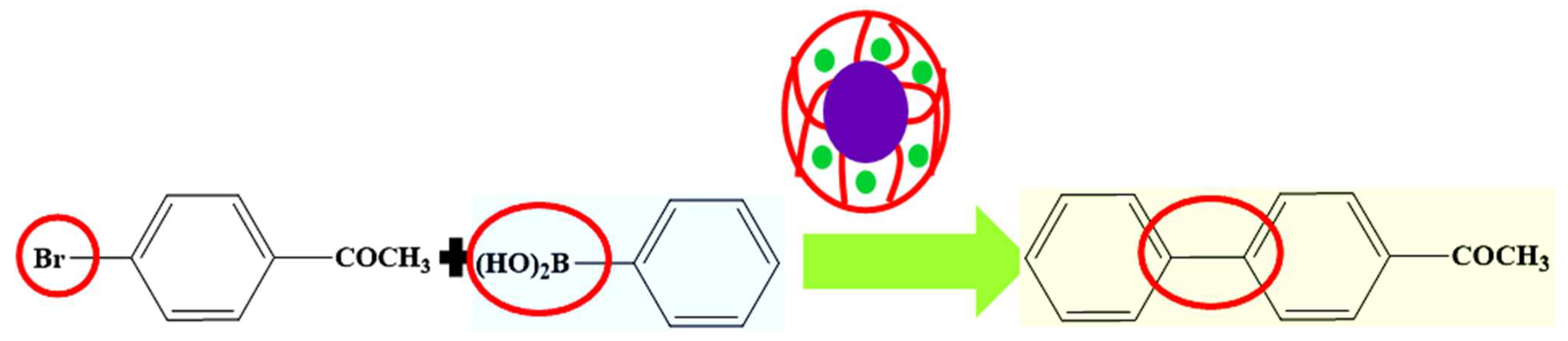
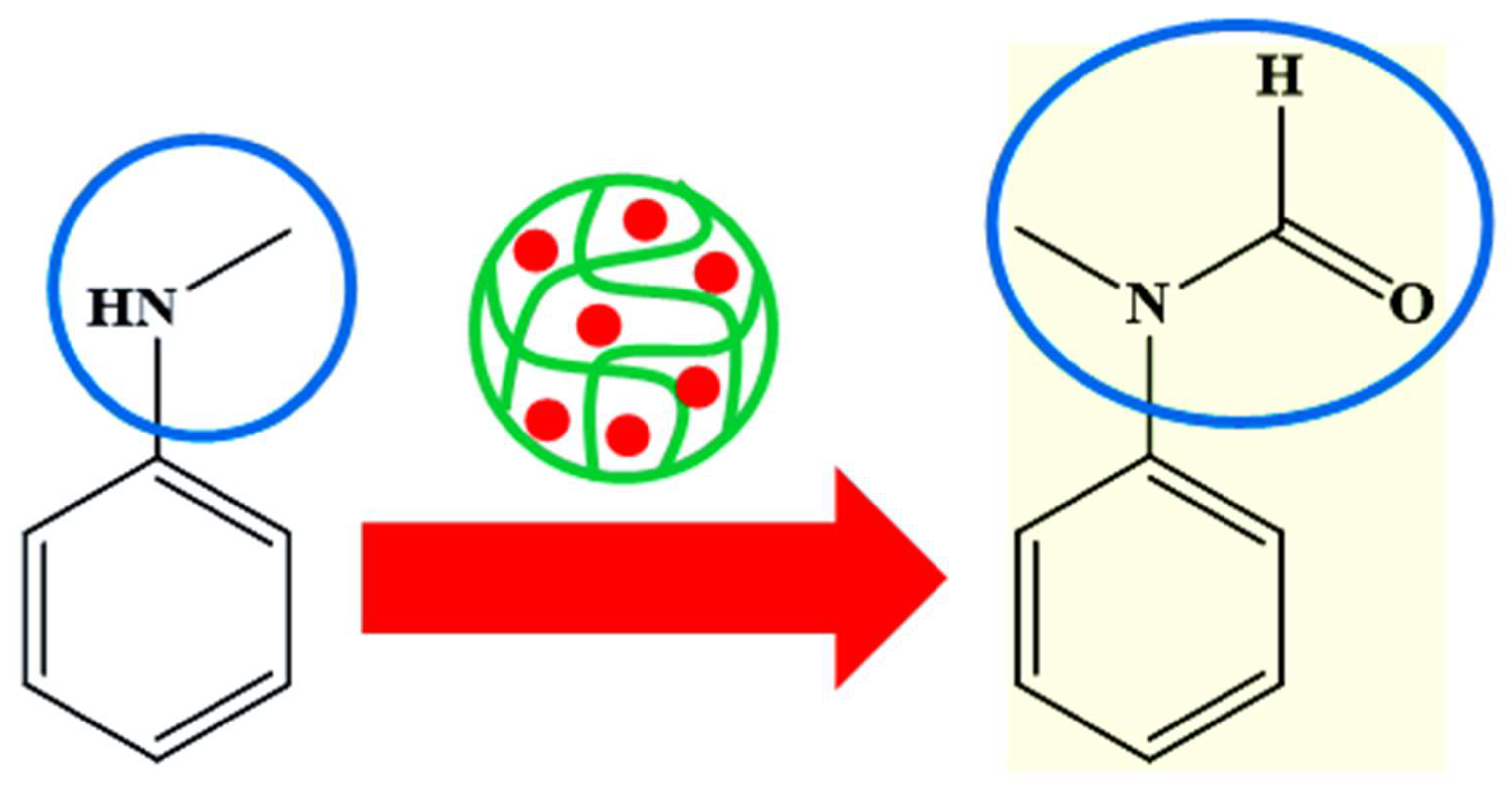
| Pictorial Diagram | Morphology of Hybrid Microgels | Abbreviations of Hybrid Microgels | Class of Pd NPs in Smart Microgels | References |
|---|---|---|---|---|
 | Homogenous microsphere | Pd-P(NIA-AcA) | Pd NPs in P(NIA-AcA) | [32] |
 | Homogenous microsphere | Fe/Pd-P(AA) | Fe/Pd NPs in P(AA) | [39] |
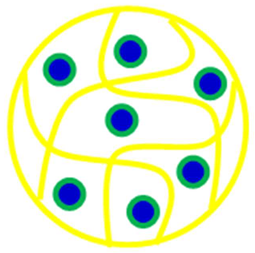 | Homogenous microsphere | Au@Pd-P(SR) | Au@Pd NPs in P(SR) | [42] |
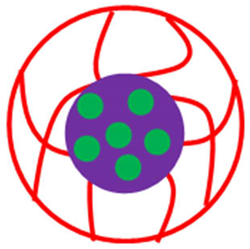 | Core shell system (Core = Pd-SiO2 Shell = P(DMM)) | Pd-SiO2@P(DMM) | Pd NPs in SiO2 encapsulated with P(DMM) | [28] |
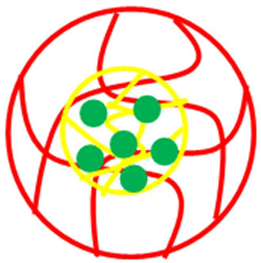 | Core shell system (Core = Pd-P(NIA-MeAA Shell = P(NIA-HyMBP)) | Pd-P(NIA-MeAA)@P(NIA-HyMBP) | Pd NPs in P(NIA-MeAA) encapsulated with P(NIA-HyMBP) | [44] |
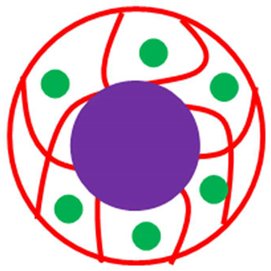 | Core Shell system (Core = Fe3O4 Shell = Pd-P(UNP)) | Fe3O4@Pd-P(UNP) | Fe3O4 encapsulated with Pd NPs decorated in P(UNP) | [45] |
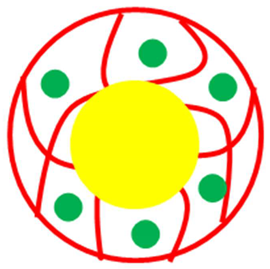 | Core shell system (Core = P(SR) Shell = Pd-P(NIA)) | P(SR)@Pd-P(NIA) | P(SR) encapsulated with Pd NPs decorated in P(NIA) | [46] |
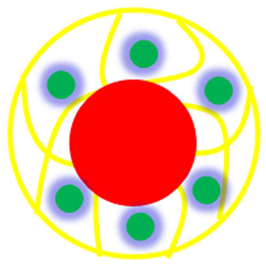 | Core shell system (Core = P(SR) Shell = Ag/Pd-P(NIA) | P(SR)@Ag/Pd-P(NIA) | P(SR) encapsulated with Ag/Pd NPs decorated in P(NIA) | [49] |
 | Core shell system (Core = P(NIA-ChS)@Pd NPs) | P(NIA-ChS)@Pd | P(NIA-ChS) encapsulate with Pd NPs | [50] |
 | Core shell system (Core = P(ViBIC-IL) Shell = Pd@Au) | P(ViBIC-IL)@(Pd@Au) | P(ViBIC-IL encapsulate with Pd NPs surrounded Au NPs | [51] |
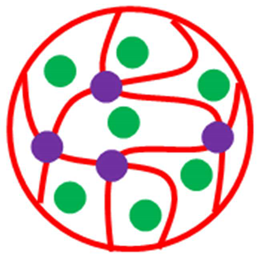 | Homogenous microsphere with inorganic crosslinker | Pd-P(ViP) | P(ViP) decorated with Pd NPs (crosslinker = Fe3O4) | [52] |
| System | Monomers | Metal Nanoparticles | Characterization Techniques | References |
|---|---|---|---|---|
| Pd-BaNC-P(DA) | DA | Pd | SEM, XPS, TEM, XRD, TGA, UV-Vis, ICP-MS | [23] |
| Pd-P(MeAA)@HyMBP | MeAA, HyMBP | Pd | UV-Vis, SEM, AFM, AAS | [54] |
| Pd-P(DiVB-DCIIL) | DiVB, DCIIL | Pd | TEM, XRD, XPS, TGA | [55] |
| Pd-GO/P(ImZ/ImZI) | GO, ImZ, ImZI | Pd | FTIR, NMR, TGA, TEM, SEM, XRD, UV-Vis, AAS, ICP-AES | [56] |
| Pd-ChS-GA-RGO | ChS, GA, RGO | Pd | SEM, XRD, TEM, TGA, BET, UV-Vis | [52] |
| Pd-P(EtI), Ag-P(EtI) | EtI | Pd or Ag | SEM, TEM, XRD, XPS, TGA, BET | [58] |
| Pd-Ti3C2-P(DoA) | DoA, Ti3C2 | Pd | XRD, FT-IR, TGA, SEM, TEM, BET, and XPS | [59] |
| Au/Pd-CeL-CD, Ag/Pd-CeL-CD | CeL, CD | Au and Pd, Ag and Pd | TEM, EDS, SEM, XRD, XPS, NMR | [35] |
| Pd-ChS | ChS | Pd | XRD, HR-TEM, FTIR, FE-SEM, EDX, UV-Vis | [22] |
| Pd-P(NIA-AA) | NIA, AA | Pd | FE-SSEM, AFM, XRD, FTIR, ICP-AES | [60] |
| P(SR)@Ag/Pd-P(NIA) | SR, NIA | Ag and Pd | ICP-AES, DLS, TEM, UV-Vis., SAXS | [47] |
| Pd@Cu-P(NIA-AA) | NIA, AA | Pd and Cu | XPS, TGA, UV-Vis, XRD, TEM, SEM | [39] |
| Pd/Ni-P(AcA) | AcA | Pd and Ni | XRD, TEM, ICP-AES, FTIR | [51] |
| Fe@Pd-P(NIA-MMA) | NIA, MMA | Pd and Fe | ICP-OES, FIB-SEM, ED-XRD | [53] |
| Pd-P(MMA-4ViP) | MMA, 4ViP | Pd | XRD, TGA/DSC, TEM, XPS, EDX | [34] |
| Pd-P(NIA-MeAA) | NIA, MeAA | Pd | PCS, HP-LC, LC-MS, NMR | [57] |
| Pd-P(NIA-AcA) | NIA, AcA | Pd | STEM, EDX, TEM, XRD, TLC, HR-TEM, NMR, UV-Vis | [30] |
| Cu/Pd-P(ViP), Pt/Pd-P(ViP), Pd/Fe-P(ViP) | ViP | Cu and Pd, Pt and Pd, Pd, and Fe | SEM, FTIR, TEM, UV-Vis, Digital Camera | [38] |
| Pd-P(AmAm) | AmAm | Pd | UV-Vis, TEM, HR-TEM, FTIR | [61] |
| Fe/Pd-P(AA) | AA | Fe and Pd | ATR-FTIR, FE-SEM, EDS, XPS, ICP-AES, BET | [37] |
| Pd-ChS@MnFe2O4 | ChS | Pd, Mn, Fe2O4 | FTIR, TEM, FE-SEM, XPS, XRD, ICP-MS, UV-Vis | [62] |
| Pd-CD-P(ViDF) | CD, ViDF | Pd | SEM, EDS, TEM, ATR-FTIR, XPS, AFM | [63] |
| Pd-P(AcA-DMMB-IL) | AcA, DMMB, IL | Pd | FTIR, NMR, UV-Vis, TEM, SEM, EDS, XRD, | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arif, M. A Critical Review of Palladium Nanoparticles Decorated in Smart Microgels. Polymers 2023, 15, 3600. https://doi.org/10.3390/polym15173600
Arif M. A Critical Review of Palladium Nanoparticles Decorated in Smart Microgels. Polymers. 2023; 15(17):3600. https://doi.org/10.3390/polym15173600
Chicago/Turabian StyleArif, Muhammad. 2023. "A Critical Review of Palladium Nanoparticles Decorated in Smart Microgels" Polymers 15, no. 17: 3600. https://doi.org/10.3390/polym15173600
APA StyleArif, M. (2023). A Critical Review of Palladium Nanoparticles Decorated in Smart Microgels. Polymers, 15(17), 3600. https://doi.org/10.3390/polym15173600






