Vinylene-Linked Emissive Covalent Organic Frameworks for White-Light-Emitting Diodes
Abstract
:1. Introduction
2. Materials and Methods
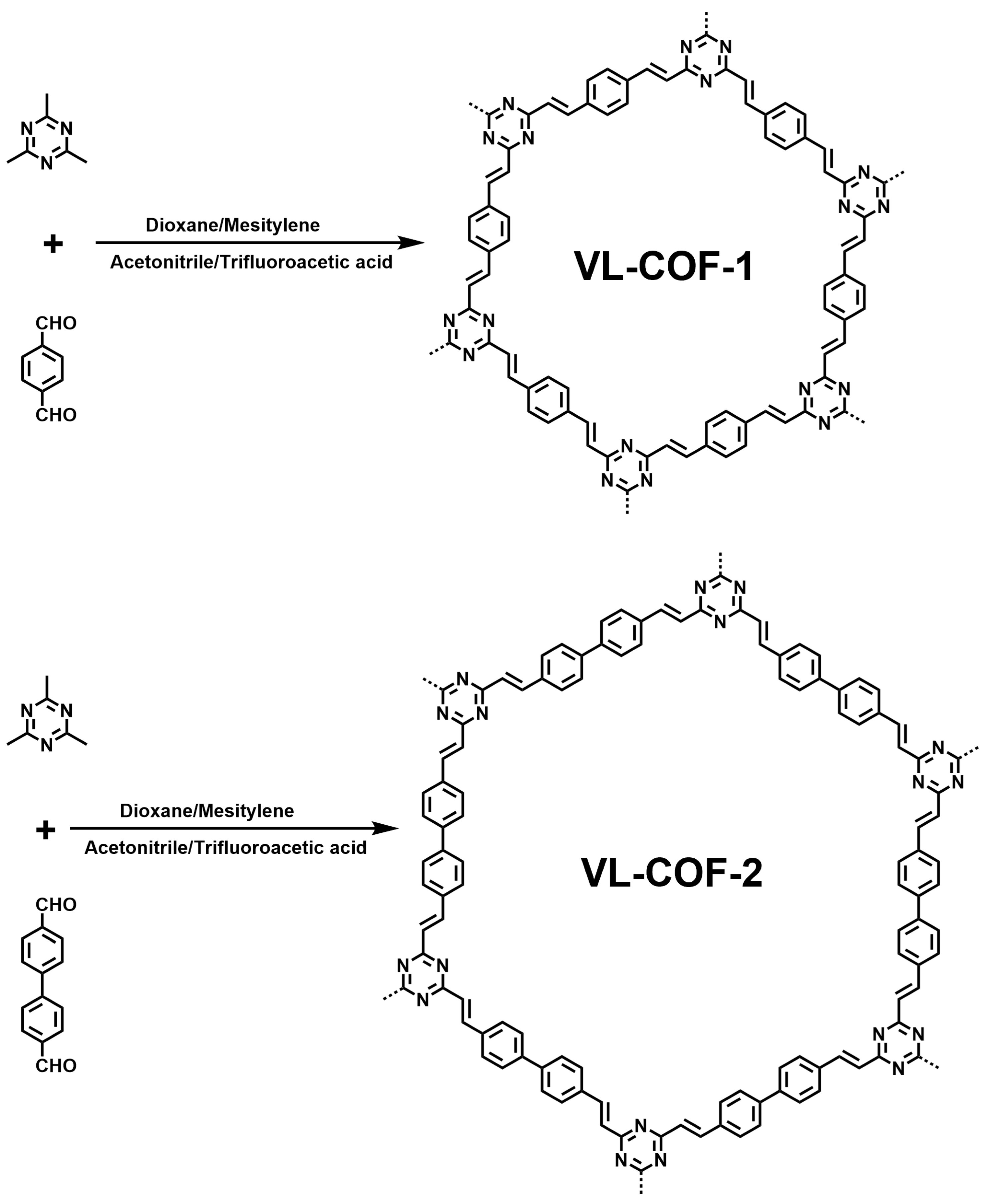
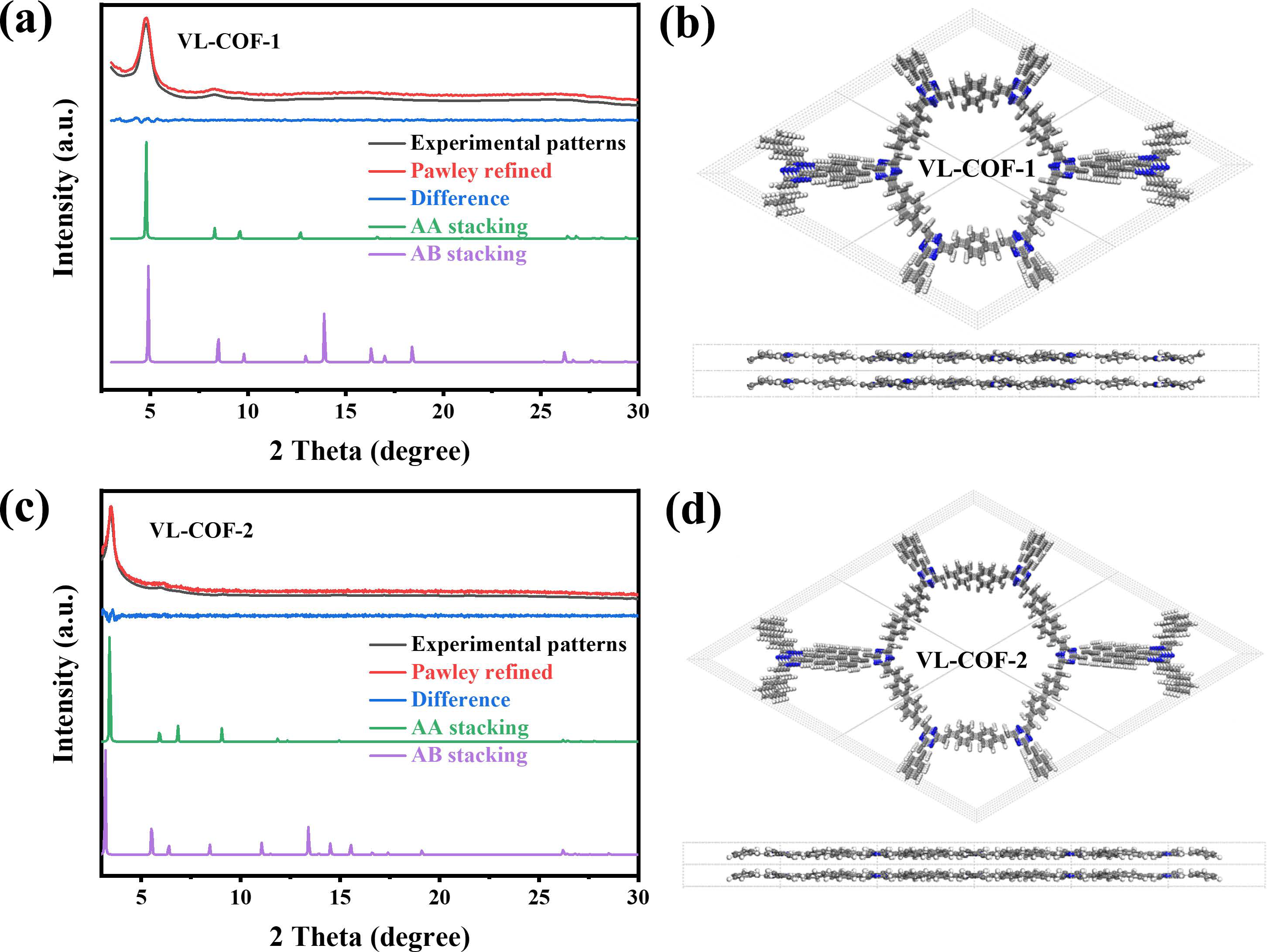
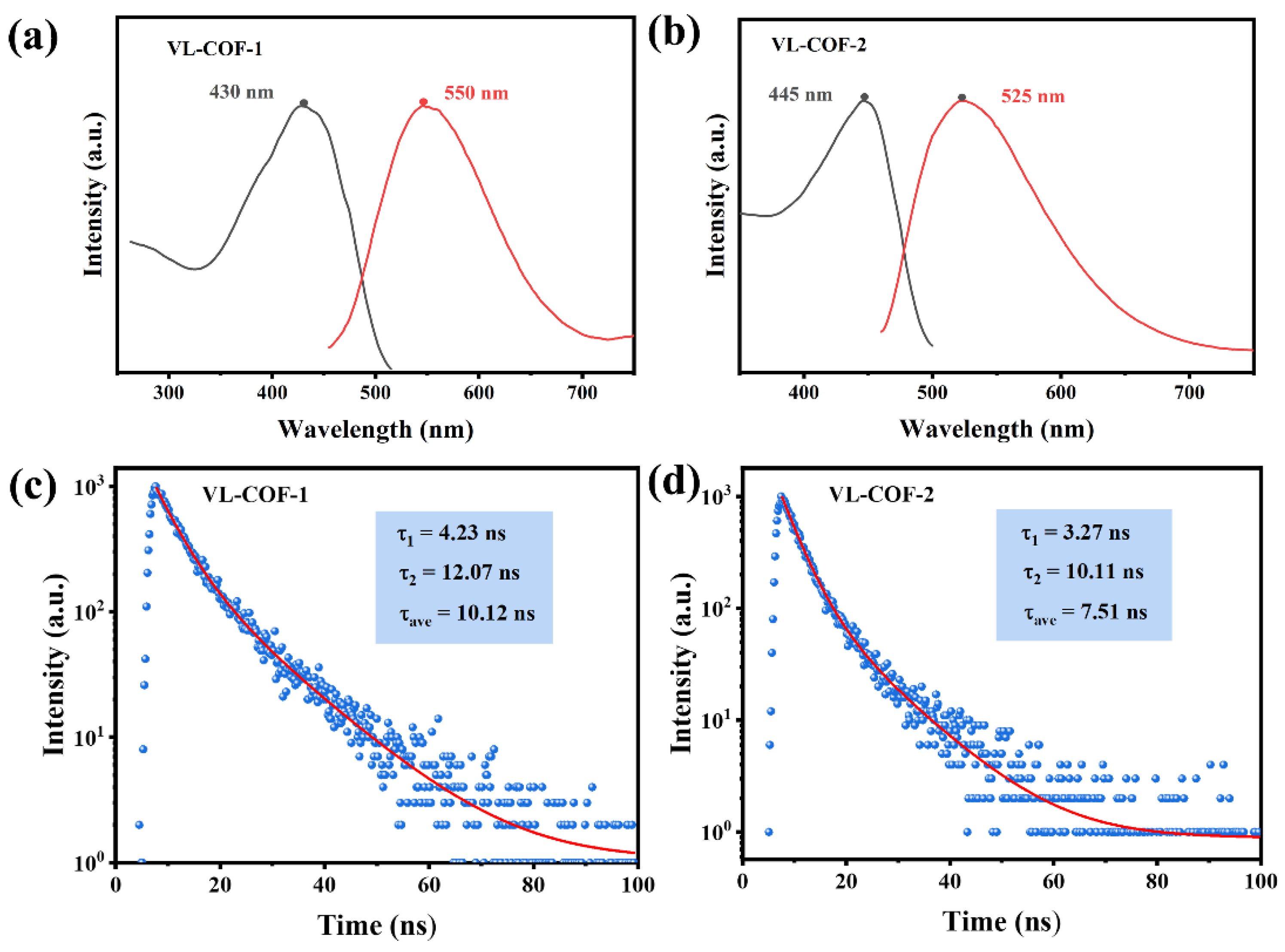
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Xie, R.-J.; Suehiro, T.; Takeda, T.; Hirosaki, N. Down-Conversion Nitride Materials for Solid State Lighting: Recent Advances and Perspectives. Chem. Rev. 2018, 118, 1951–2009. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Xiao, F.; Pan, Y.X.; Ma, Y.Y.; Zhang, Q.Y. Phosphors in phosphor-converted white light-emitting diodes: Recent advances in materials, techniques and properties. Mater. Sci. Eng. R Rep. 2010, 71, 1–34. [Google Scholar] [CrossRef]
- Nair, G.B.; Swart, H.C.; Dhoble, S.J. A review on the advancements in phosphor-converted light emitting diodes (pc-LEDs): Phosphor synthesis, device fabrication and characterization. Prog. Mater. Sci. 2020, 109, 100622. [Google Scholar] [CrossRef]
- Tian, Y. Development of phosphors with high thermal stability and efficiency for phosphor-converted LEDs. J. Solid State Light. 2014, 1, 11. [Google Scholar] [CrossRef]
- Fang, M.-H.; Bao, Z.; Huang, W.-T.; Liu, R.-S. Evolutionary Generation of Phosphor Materials and Their Progress in Future Applications for Light-Emitting Diodes. Chem. Rev. 2022, 122, 11474–11513. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Xin, S.; Wang, Q.; Li, Y.; Wu, Q.; Wang, C.; Wang, X.; Ding, X.; Geng, W. Recent development in rare earth doped phosphors for white light emitting diodes. J. Rare Earths 2015, 33, 1–12. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, Y.; Zhong, J. A review on Mn4+ activators in solids for warm white light-emitting diodes. RSC Adv. 2016, 6, 86285–86296. [Google Scholar] [CrossRef]
- Guner, T.; Demir, M.M. A Review on Halide Perovskites as Color Conversion Layers in White Light Emitting Diode Applications. Phys. Status Solidi A 2018, 215, 1800120. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, H.; Cao, W.; Cui, Y.; Qian, G. Luminescent Metal–Organic Frameworks for White LEDs. Adv. Opt. Mater. 2021, 9, 2001817. [Google Scholar] [CrossRef]
- Guan, X.; Chen, F.; Fang, Q.; Qiu, S. Design and applications of three dimensional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 1357–1384. [Google Scholar] [CrossRef]
- Gui, B.; Lin, G.; Ding, H.; Gao, C.; Mal, A.; Wang, C. Three-Dimensional Covalent Organic Frameworks: From Topology Design to Applications. Acc. Chem. Res. 2020, 53, 2225–2234. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Li, J.; Xie, G.; Lin, G.; Chen, R.; Peng, Z.; Yang, C.; Wang, B.; Sun, J.; Wang, C. An AIEgen-based 3D covalent organic framework for white light-emitting diodes. Nat. Commun. 2018, 9, 5234. [Google Scholar] [CrossRef] [PubMed]
- Waller, P.J.; Gándara, F.; Yaghi, O.M. Chemistry of Covalent Organic Frameworks. Acc. Chem. Res. 2015, 48, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Tan, K.T.; Gong, Y.; Chen, Y.; Li, Z.; Xie, S.; He, T.; Lu, Z.; Yang, H.; Jiang, D. Covalent organic frameworks: An ideal platform for designing ordered materials and advanced applications. Chem. Soc. Rev. 2021, 50, 120–242. [Google Scholar] [CrossRef]
- Haase, F.; Lotsch, B.V. Solving the COF trilemma: Towards crystalline, stable and functional covalent organic frameworks. Chem. Soc. Rev. 2020, 49, 8469–8500. [Google Scholar] [CrossRef]
- Biswal, B.P.; Kandambeth, S.; Chandra, S.; Shinde, D.B.; Bera, S.; Karak, S.; Garai, B.; Kharul, U.K.; Banerjee, R. Pore surface engineering in porous, chemically stable covalent organic frameworks for water adsorption. J. Mater. Chem. A 2015, 3, 23664–23669. [Google Scholar] [CrossRef]
- Xu, S.; Richter, M.; Feng, X. Vinylene-Linked Two-Dimensional Covalent Organic Frameworks: Synthesis and Functions. Acc. Mater. Res. 2021, 2, 252–265. [Google Scholar] [CrossRef]
- Ding, S.-Y.; Wang, W. Covalent organic frameworks (COFs): From design to applications. Chem. Soc. Rev. 2013, 42, 548–568. [Google Scholar] [CrossRef]
- Liu, X.; Huang, D.; Lai, C.; Zeng, G.; Qin, L.; Wang, H.; Yi, H.; Li, B.; Liu, S.; Zhang, M.; et al. Recent advances in covalent organic frameworks (COFs) as a smart sensing material. Chem. Soc. Rev. 2019, 48, 5266–5302. [Google Scholar] [CrossRef]
- Segura, J.L.; Mancheño, M.J.; Zamora, F. Covalent organic frameworks based on Schiff-base chemistry: Synthesis, properties and potential applications. Chem. Soc. Rev. 2016, 45, 5635–5671. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Yang, S.; Lu, C.; Cui, C.-X.; Xu, Q.; Liu, J.; Yang, X.; Meng, X.; Lu, S.; Zhuang, X.; et al. CoN5 Sites Constructed by Anchoring Co Porphyrins on Vinylene-Linked Covalent Organic Frameworks for Electroreduction of Carbon Dioxide. Small 2022, 18, 2200736. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Cai, K.; Zhou, W.; Tao, F.; Li, Z.; Lin, Q.; Wang, L.; Yu, Z.; Zhang, J.; Zhou, H. Nanoporous Vinylene-Linked 2D Covalent Organic Frameworks for Visible-Light-Driven Aerobic Oxidation. ACS Appl. Nano Mater. 2023, 6, 8396–8403. [Google Scholar] [CrossRef]
- EL-Mahdy, A.F.M.; Young, C.; Kim, J.; You, J.; Yamauchi, Y.; Kuo, S.-W. Hollow Microspherical and Microtubular [3 + 3] Carbazole-Based Covalent Organic Frameworks and Their Gas and Energy Storage Applications. ACS Appl. Mater. Interfaces 2019, 11, 9343–9354. [Google Scholar] [CrossRef]
- She, P.; Qin, Y.; Wang, X.; Zhang, Q. Recent Progress in External-Stimulus-Responsive 2D Covalent Organic Frameworks. Adv. Mater. 2022, 34, 2101175. [Google Scholar] [CrossRef]
- Li, Z.; Moon, H.; Cho, S.-K.; Li, C.; Seo, J.-M.; Baek, J.-B.; Xu, H.; Lee, S.-Y. An Anionic Covalent Organic Framework as an Electrostatic Molecular Rectifier for Stabilizing Mg Metal Electrodes. CCS Chem. 2023, 1–9. [Google Scholar] [CrossRef]
- Nie, R.; Chu, W.; Li, Z.; Li, H.; Chen, S.; Chen, Y.; Zhang, Z.; Liu, X.; Guo, W.; Seok, S.I. Simultaneously Suppressing Charge Recombination and Decomposition of Perovskite Solar Cells by Conjugated Covalent Organic Frameworks. Adv. Energy Mater. 2022, 12, 2200480. [Google Scholar] [CrossRef]
- Zhai, L.; Yao, Y.; Ma, B.; Hasan, M.M.; Han, Y.; Mi, L.; Nagao, Y.; Li, Z. Accumulation of Sulfonic Acid Groups Anchored in Covalent Organic Frameworks as an Intrinsic Proton-Conducting Electrolyte. Macromol. Rapid Commun. 2022, 43, 2100590. [Google Scholar] [CrossRef]
- Yin, H.-Q.; Yin, F.; Yin, X.-B. Strong dual emission in covalent organic frameworks induced by ESIPT. Chem. Sci. 2019, 10, 11103–11109. [Google Scholar] [CrossRef]
- Zeng, J.-Y.; Wang, X.-S.; Zhang, X.-Z. Research Progress in Covalent Organic Frameworks for Photoluminescent Materials. Chem. Eur. J. 2020, 26, 16568–16581. [Google Scholar] [CrossRef]
- Li, Z.; Geng, K.; He, T.; Tan, K.T.; Huang, N.; Jiang, Q.; Nagao, Y.; Jiang, D. Editing Light Emission with Stable Crystalline Covalent Organic Frameworks via Wall Surface Perturbation. Angew. Chem. Int. Ed. 2021, 60, 19419–19427. [Google Scholar] [CrossRef] [PubMed]
- Dalapati, S.; Jin, S.; Gao, J.; Xu, Y.; Nagai, A.; Jiang, D. An Azine-Linked Covalent Organic Framework. J. Am. Chem. Soc. 2013, 135, 17310–17313. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Xia, H.; Mu, Y.; Liu, X. A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu2+ ions. Chem. Commun. 2016, 52, 6613–6616. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Shi, W.; Li, Y.; Yu, Y.; Wu, X.; Li, Z.; Lee, S.Y.; Lee, K.H. Excellent Crystallinity and Stability Covalent–Organic Frameworks with High Emission and Anions Sensing. Macromol. Rapid Commun. 2022, 43, 2200393. [Google Scholar] [CrossRef]
- Yang, S.; Streater, D.; Fiankor, C.; Zhang, J.; Huang, J. Conjugation- and Aggregation-Directed Design of Covalent Organic Frameworks as White-Light-Emitting Diodes. J. Am. Chem. Soc. 2021, 143, 1061–1068. [Google Scholar] [CrossRef]
- Bu, R.; Zhang, L.; Liu, X.-Y.; Yang, S.-L.; Li, G.; Gao, E.-Q. Synthesis and Acid-Responsive Properties of a Highly Porous Vinylene-Linked Covalent Organic Framework. ACS Appl. Mater. Interfaces 2021, 13, 26431–26440. [Google Scholar] [CrossRef]
- Krishnaraj, C.; Kaczmarek, A.M.; Jena, H.S.; Leus, K.; Chaoui, N.; Schmidt, J.; van Deun, R.; van der Voort, P. Triggering White-Light Emission in a 2D Imine Covalent Organic Framework through Lanthanide Augmentation. ACS Appl. Mater. Interfaces 2019, 11, 27343–27352. [Google Scholar] [CrossRef]
- Li, X.; Tang, H.; Gao, L.; Chen, Z.; Li, H.; Wang, Y.; Yang, K.; Lu, S.; Wang, K.; Zhou, Q.; et al. A sp2-carbon-linked covalent organic framework containing tetraphenylethene units used as yellow phosphors in white light-emitting diodes. Polymer 2022, 241, 124474. [Google Scholar] [CrossRef]
- Gao, L.; Li, W.; Tang, H.; Qin, J.; Lu, S.; Zhang, M.; Yang, K.; Jiao, Y. A fully π-conjugated triazine-based 2D covalent organic framework used as metal-free yellow phosphors in white light-emitting diodes. Eur. Polym. J. 2023, 187, 111887. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, Y.-Z.; Wu, K.-M.; Yang, D.-H.; Liu, X.-F.; Ding, X.; Han, B.-H. Linkages Make a Difference in the Photoluminescence of Covalent Organic Frameworks. Angew. Chem. Int. Ed. 2023, e202310794. [Google Scholar] [CrossRef]
- Jin, E.; Li, J.; Geng, K.; Jiang, Q.; Xu, H.; Xu, Q.; Jiang, D. Designed synthesis of stable light-emitting two-dimensional sp2 carbon-conjugated covalent organic frameworks. Nat. Commun. 2018, 9, 4143. [Google Scholar] [CrossRef] [PubMed]
- Li, X. sp2 carbon-conjugated covalent organic frameworks: Synthesis, properties, and applications. Mater. Chem. Front. 2021, 5, 2931–2949. [Google Scholar] [CrossRef]
- Jadhav, T.; Fang, Y.; Patterson, W.; Liu, C.-H.; Hamzehpoor, E.; Perepichka, D.F. 2D Poly(arylene vinylene) Covalent Organic Frameworks via Aldol Condensation of Trimethyltriazine. Angew. Chem. Int. Ed. 2019, 58, 13753–13757. [Google Scholar] [CrossRef]
- Bi, S.; Meng, F.; Wu, D.; Zhang, F. Synthesis of Vinylene-Linked Covalent Organic Frameworks by Monomer Self-Catalyzed Activation of Knoevenagel Condensation. J. Am. Chem. Soc. 2022, 144, 3653–3659. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Zhang, F.; Zhang, W.; Qiang, P.; Yu, K.; Fu, X.; Wu, D.; Bi, S. Semiconducting 2D Triazine-Cored Covalent Organic Frameworks with Unsubstituted Olefin Linkages. J. Am. Chem. Soc. 2019, 141, 14272–14279. [Google Scholar] [CrossRef]
- Acharjya, A.; Pachfule, P.; Roeser, J.; Schmitt, F.-J.; Thomas, A. Vinylene-Linked Covalent Organic Frameworks by Base-Catalyzed Aldol Condensation. Angew. Chem. Int. Ed. 2019, 58, 14865–14870. [Google Scholar] [CrossRef]
- Wu, L.; Dai, P.; Wen, D. New Structural Design Strategy: Optical Center VO4-Activated Broadband Yellow Phosphate Phosphors for High-Color-Rendering WLEDs. ACS Sustain. Chem. Eng. 2022, 10, 3757–3765. [Google Scholar] [CrossRef]
- Cao, L.; Li, W.; Devakumar, B.; Ma, N.; Huang, X.; Lee, A.F. Full-Spectrum White Light-Emitting Diodes Enabled by an Efficient Broadband Green-Emitting CaY2ZrScAl3O12:Ce3+ Garnet Phosphor. ACS Appl. Mater. Interfaces 2022, 14, 5643–5652. [Google Scholar] [CrossRef]
- Li, X.; Gao, Q.; Wang, J.; Chen, Y.; Chen, Z.-H.; Xu, H.-S.; Tang, W.; Leng, K.; Ning, G.-H.; Wu, J.; et al. Tuneable near white-emissive two-dimensional covalent organic frameworks. Nat. Commun. 2018, 9, 2335. [Google Scholar] [CrossRef]
- Yang, M.; Mo, C.; Fang, L.; Li, J.; Yuan, Z.; Chen, Z.; Jiang, Q.; Chen, X.; Yu, D. Multibranched Octupolar Module Embedded Covalent Organic Frameworks Enable Efficient Two-Photon Fluorescence. Adv. Funct. Mater. 2020, 30, 2000516. [Google Scholar] [CrossRef]
- Albacete, P.; Martínez, J.I.; Li, X.; López-Moreno, A.; Mena-Hernando, S.; Platero-Prats, A.E.; Montoro, C.; Loh, K.P.; Pérez, E.M.; Zamora, F. Layer-Stacking-Driven Fluorescence in a Two-Dimensional Imine-Linked Covalent Organic Framework. J. Am. Chem. Soc. 2018, 140, 12922–12929. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, S.; Yan, Y.; Xia, F.; Huang, A.; Xian, Y. Highly Fluorescent Polyimide Covalent Organic Nanosheets as Sensing Probes for the Detection of 2,4,6-Trinitrophenol. ACS Appl. Mater. Interfaces 2017, 9, 13415–13421. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, N.; Lee, K.H.; Feng, Y.; Tao, S.; Jiang, Q.; Nagao, Y.; Irle, S.; Jiang, D. Light-Emitting Covalent Organic Frameworks: Fluorescence Improving via Pinpoint Surgery and Selective Switch-On Sensing of Anions. J. Am. Chem. Soc. 2018, 140, 12374–12377. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Wu, X.; Zhang, J.; Han, C.; Cao, M.; Li, X.; Wan, J. Vinylene-Linked Emissive Covalent Organic Frameworks for White-Light-Emitting Diodes. Polymers 2023, 15, 3704. https://doi.org/10.3390/polym15183704
Li Y, Wu X, Zhang J, Han C, Cao M, Li X, Wan J. Vinylene-Linked Emissive Covalent Organic Frameworks for White-Light-Emitting Diodes. Polymers. 2023; 15(18):3704. https://doi.org/10.3390/polym15183704
Chicago/Turabian StyleLi, Yan, Xiaohan Wu, Jinyi Zhang, Congcong Han, Mengmeng Cao, Xiangrong Li, and Jieqiong Wan. 2023. "Vinylene-Linked Emissive Covalent Organic Frameworks for White-Light-Emitting Diodes" Polymers 15, no. 18: 3704. https://doi.org/10.3390/polym15183704
APA StyleLi, Y., Wu, X., Zhang, J., Han, C., Cao, M., Li, X., & Wan, J. (2023). Vinylene-Linked Emissive Covalent Organic Frameworks for White-Light-Emitting Diodes. Polymers, 15(18), 3704. https://doi.org/10.3390/polym15183704





