Recent Development of Polymer Nanofibers in the Field of Optical Sensing
Abstract
:1. Introduction
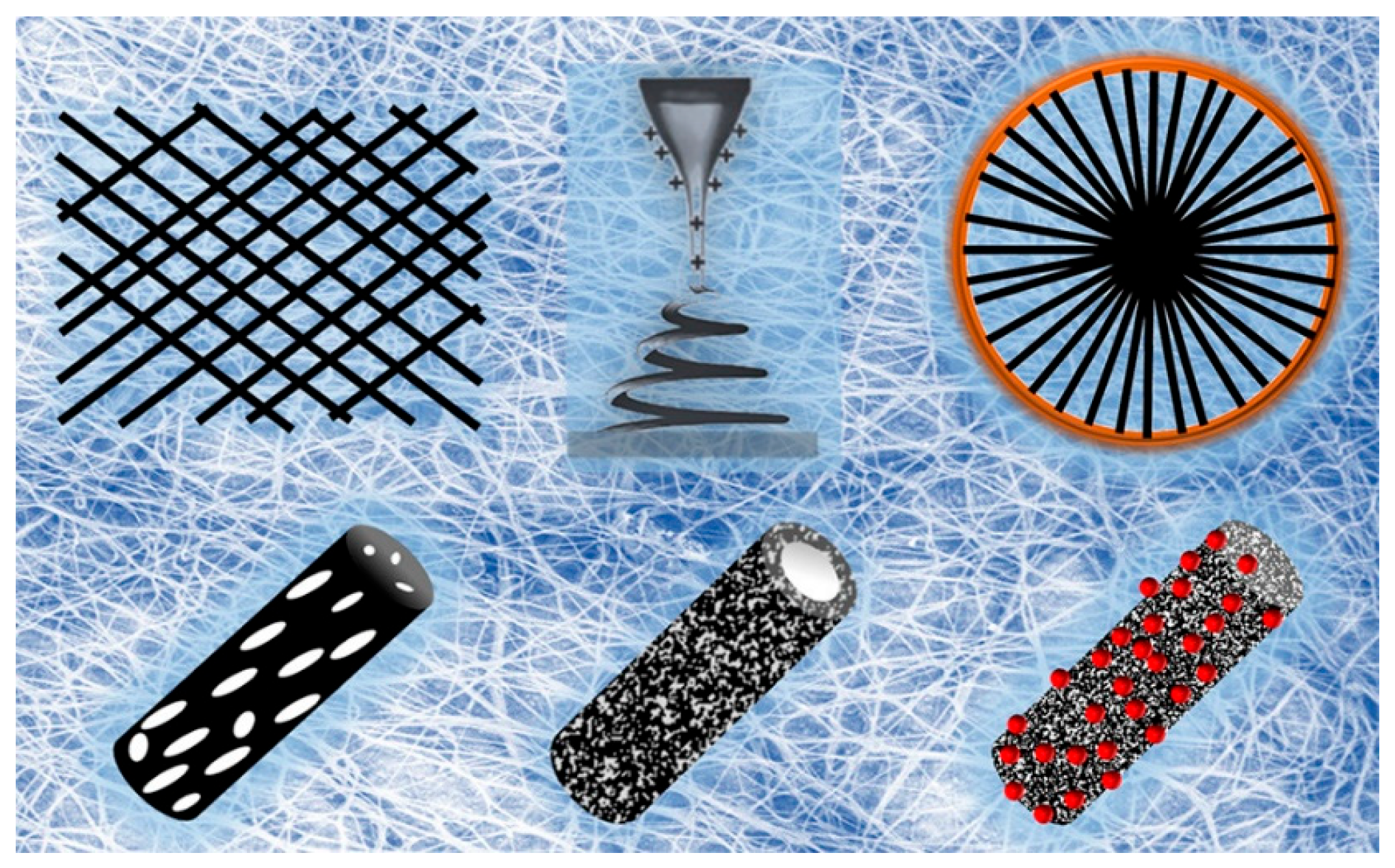
2. Preparation Methods and Properties of Nanofibers
2.1. Preparation Methods of Nanofibers
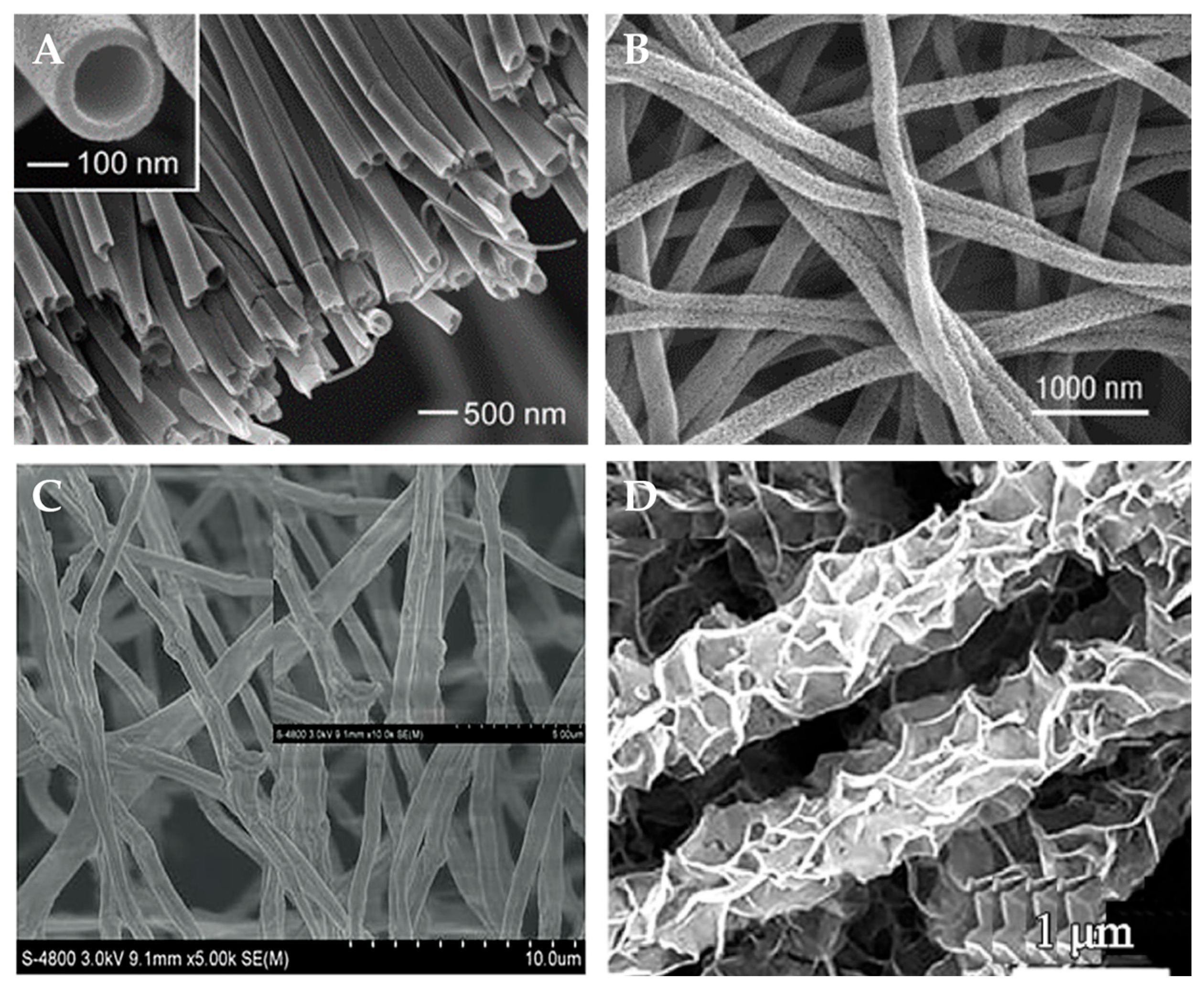
2.1.1. Electrospinning
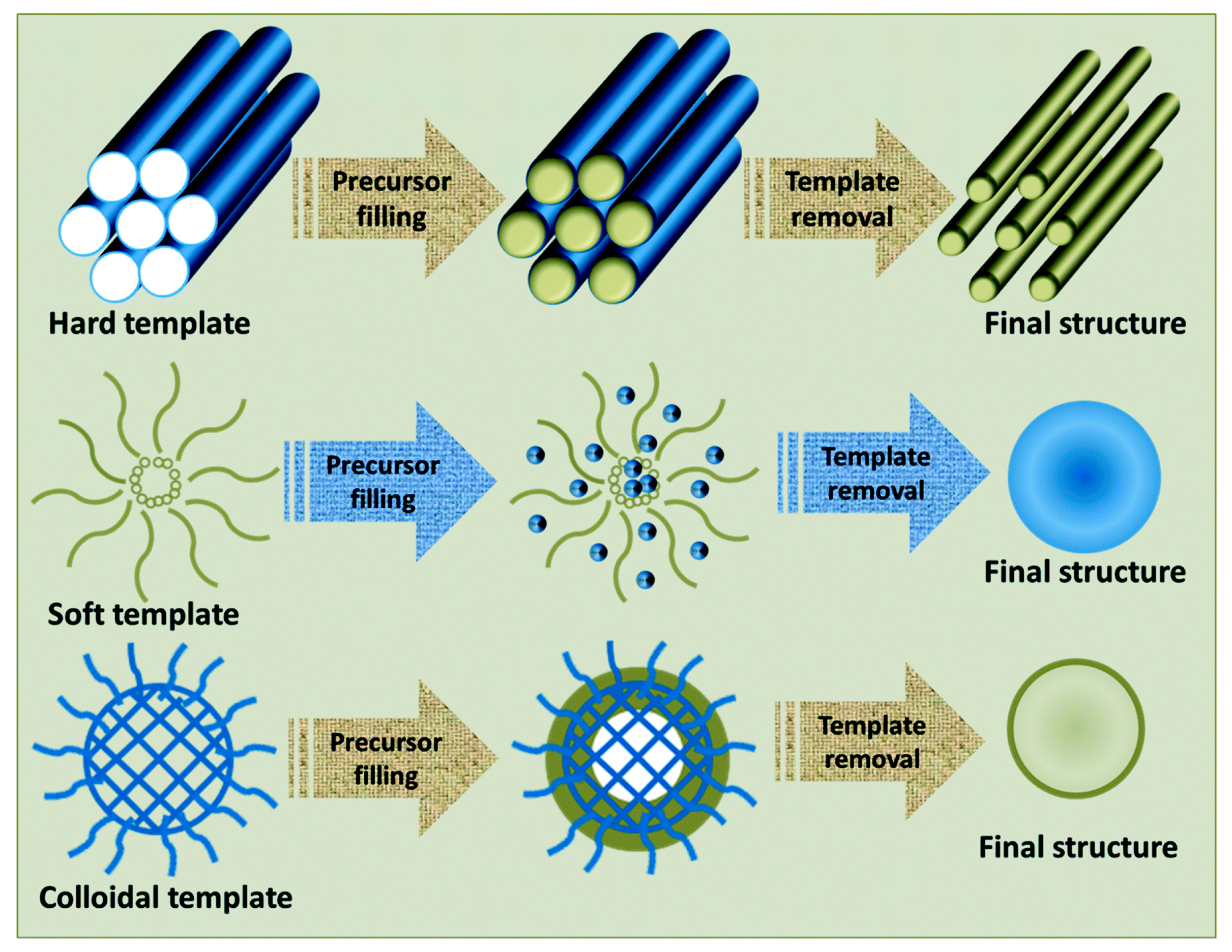
2.1.2. Template Method
2.1.3. Vapor Deposition Method
2.1.4. Self-Assembly Method
2.2. Properties of Nanofibers
2.2.1. Structure Properties
2.2.2. Optical Properties
2.2.3. Electrical Properties
2.2.4. Thermal Properties
3. Nanofiber Optical Sensors
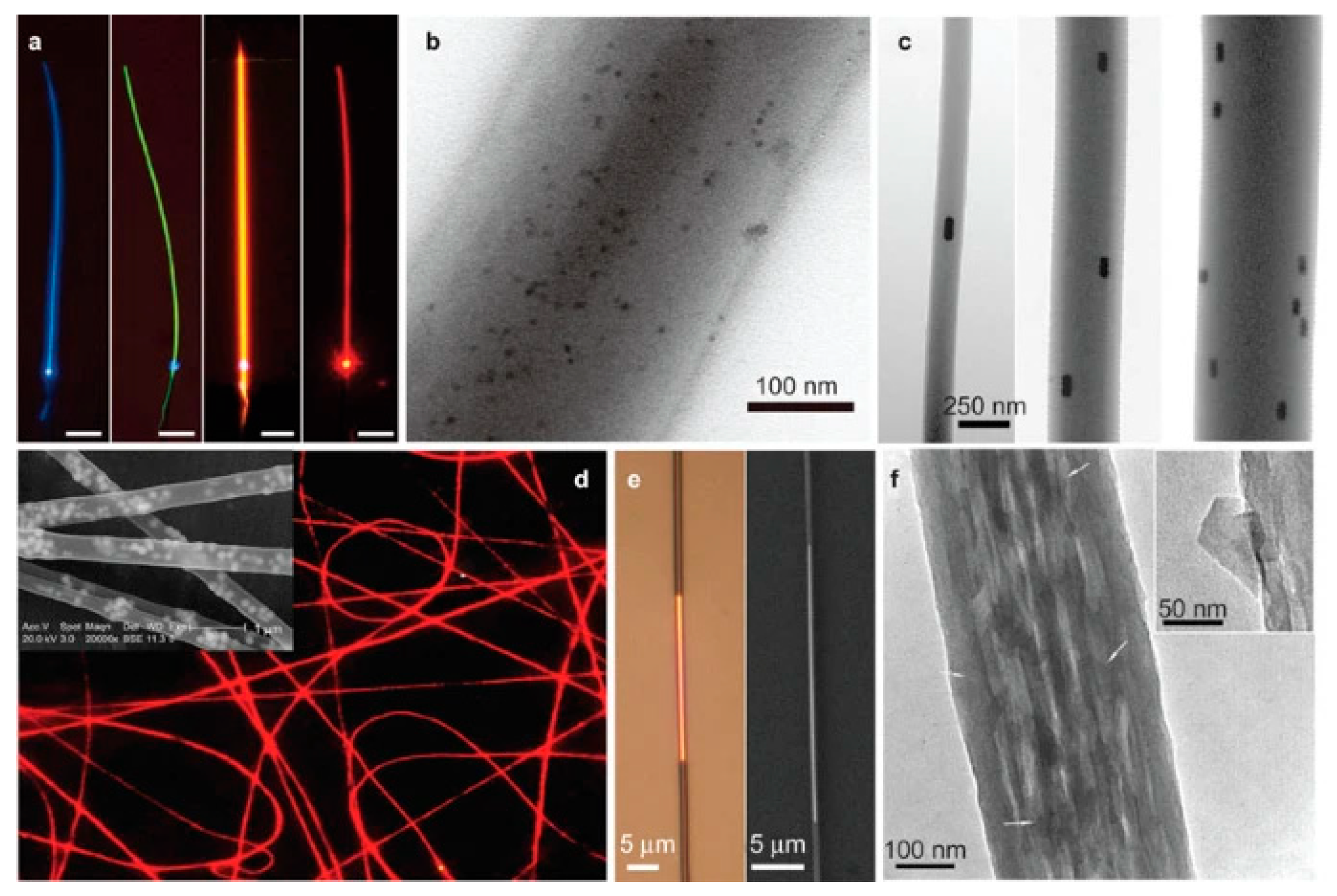
3.1. Fluorescence Sensor
| Material | Application | Performance | Ref. | |
|---|---|---|---|---|
| pH | poly(HEMA-co-NMA-co-RhBN2AM) | highly selective for pH and Hg2+ | for Hg2+ is 10−7 M (LOD) | [86] |
| BC | detect pH and glucose | for glucose is 0.026 mM (LOD) | [73] | |
| PNI | high sensitivity to pH (4–10) | pH (4–10) (detection range) | [74] | |
| PHEM-PNMA-PNBD | high sensitivity in sensing Fe3+ and pH | - | [88] | |
| PAN | for the pH of alkaline vapors and aqueous media | 4.08 ppb (LOD) | [89] | |
| Gas | PEO | tested as phosgene chemosensors | 0.7–2.8 ppb (detection range) | [72] |
| NFM | volatile organic compounds | 3–40 μM (detection range) | [78] | |
| PMI | detection of various inert VOCs | 1160 ppm to 116 ppm (detection range) | [75] | |
| TOCN, CD, TN | effectively detect and remove Cr (VI) | 228.2 mg/g (maximum adsorption capacity) | [76] | |
| Phos-3 | detection of phosgene in the gas phase | 25 ppb (LOD) | [77] | |
| PMMA/PFO | monitor volatile organic compounds | 47.9 and 15.4 ppm (LOD) | [90] | |
| PEO/MePyCz | fast response and high quenching efficiency towards DNT vapor | - | [91] | |
| Biomolecules | PVA | detect prostate specific antigen (PSA) | 0.46 pg·mL−1 (LOD) | [79] |
| CA/Fe/CDs | detection of mercury (II) and lead (II) ions | - | [80] | |
| PVP | detect the nerve agent stimulant DECP | 1.3 nM (LOD) | [81] | |
| Explosives | Oligotriphenylene | detection of nitro-based explosives | 1 nM (LOD) | [82] |
| PVP/BaWO4 | detection 2-nitrotoluene and H2O2 | for 2-nitrotoluene is 1–400 ppb (detection range) | [83] | |
| PS/PyCz | detect nitro explosive vapors | 91%, 90% and 94% (fluorescence quenching efficiencies) | [92] | |
| Biogenic amines | TO-CNFs | detection limit for BAs was as low as 1 ppm | 1 ppm (LOD) | [84] |
| ethanol/chloroform | solid-state DNA based nanofibers can be an efficient matrix for FRET | - | [18] | |
| Environment monitoring | NIPAAm | detection Hg2+, temperature, and magnetism | for Hg2+ is 10−3 M (LOD) | [93] |
| Polyaniline | detection of As (III) in contaminated water | 0.001 ppb (LOD) | [94] | |
| FNFM/PAN | the detection of mercuric ions (II) | 1 ppb (LOD) | [95] | |
| CA/PCL | detection of Mercury ion | 0.309 ppb (LOD) | [96] | |
| cellulose acetate | detecting Pb2+ | 0.16 ppb (LOD) | [97] | |
| PAN | sensors of Fe (III) | 3.95 μM (LOD) | [98] | |
| Poly(NIPAAm- co -NMA- co -RHPMA) | sense Cu2+ both in solution and in solid state | 1 × 10−6 to 1 × 10−5 M (detection range) | [99] |
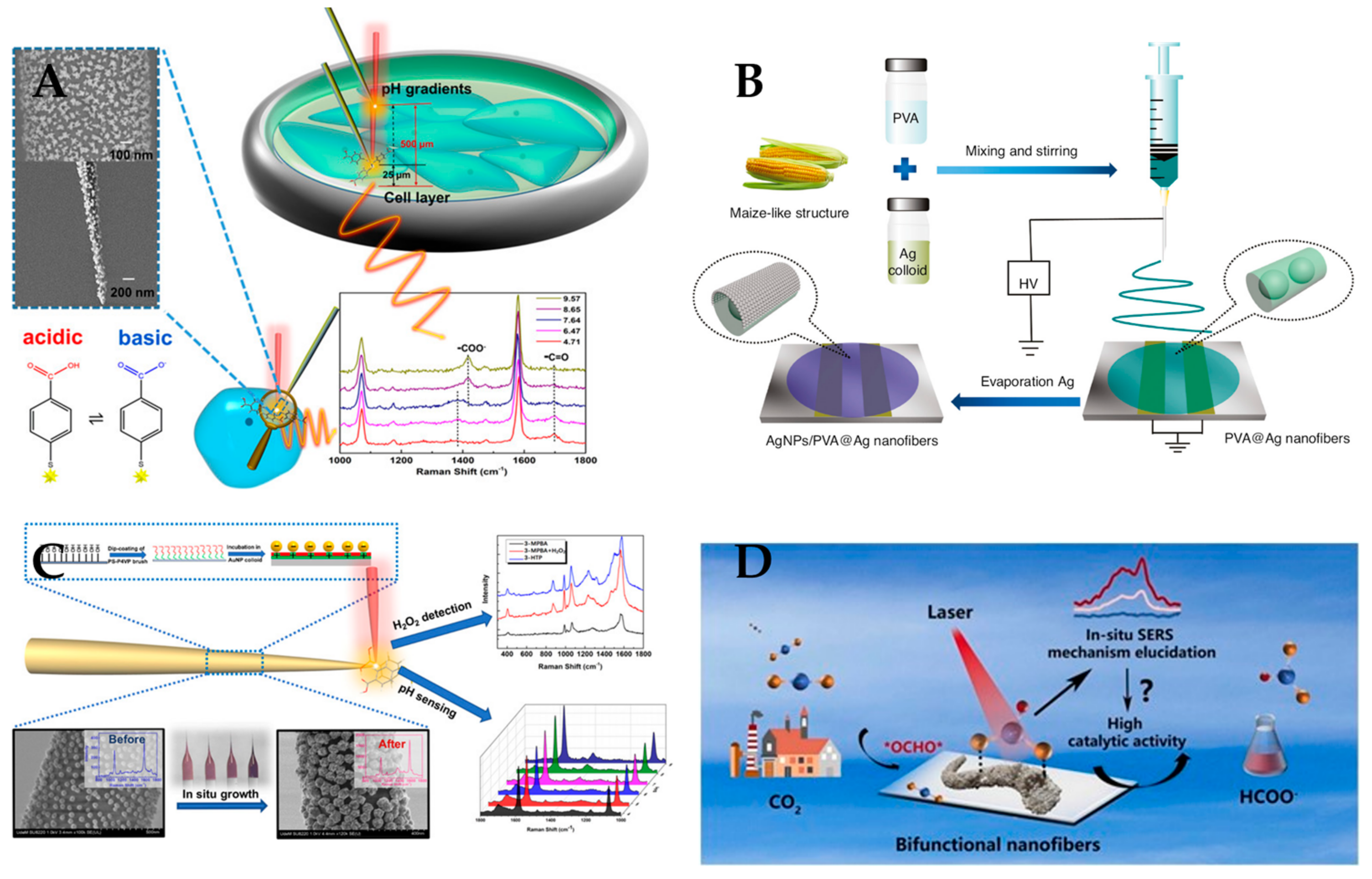
3.2. Raman Sensor
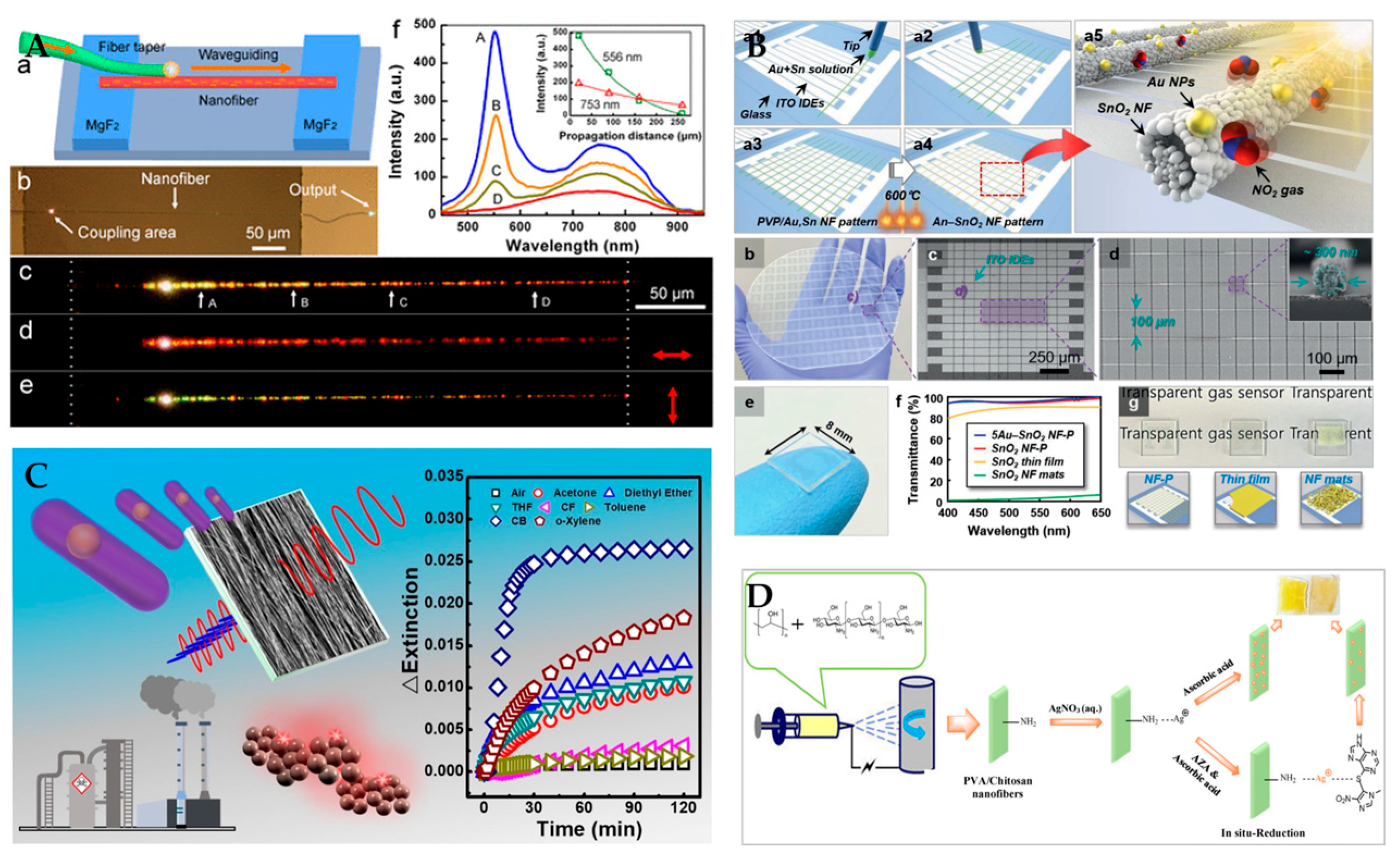
3.3. Surface Plasmon Resonance Sensor
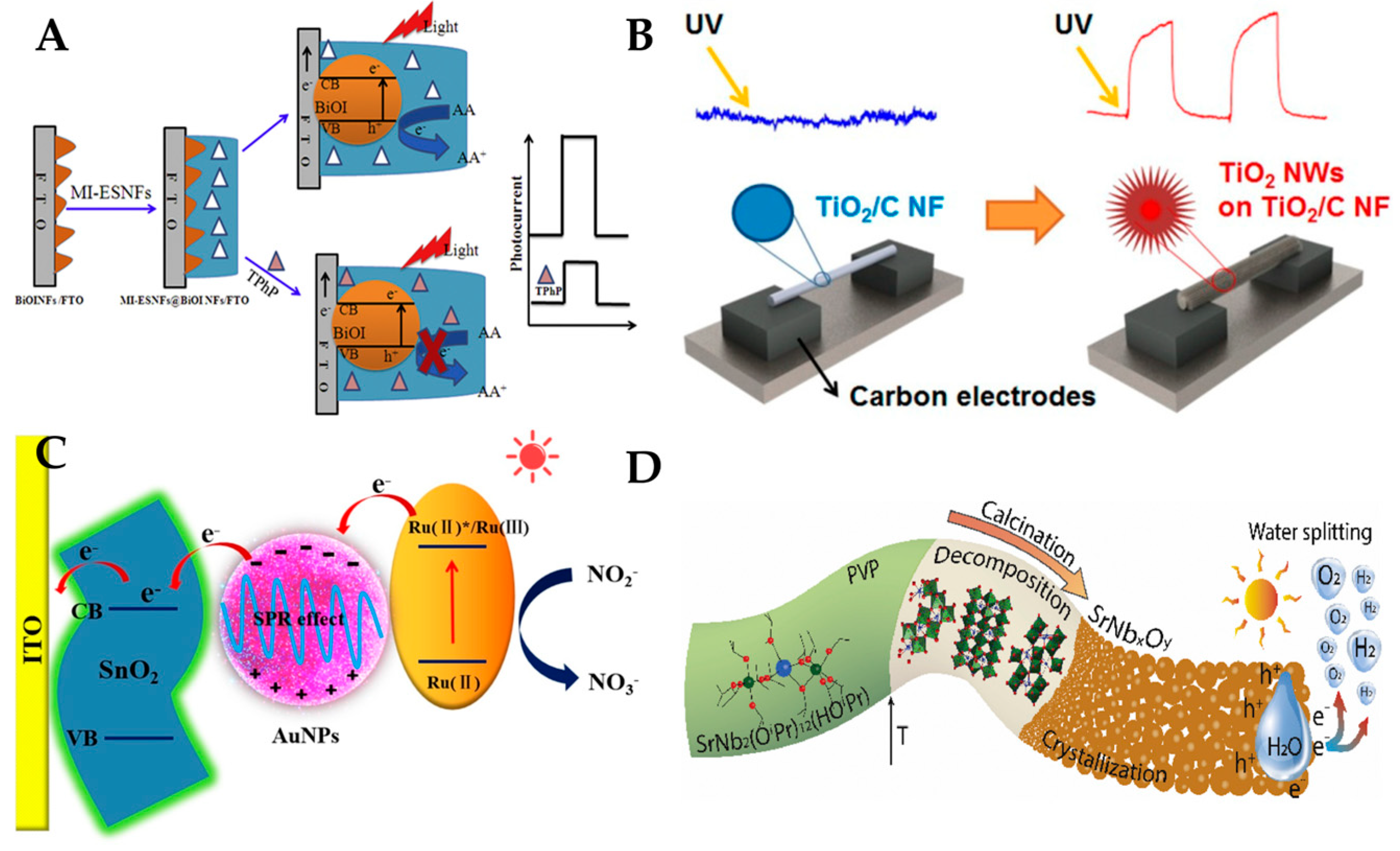
3.4. Photoelectrochemical Sensor
3.5. Other Types of One-Dimensional Nanofiber Optical Sensors
4. Applications and Prospects of Nanofiber Optical Sensors
4.1. Biomedicine
4.2. Environmental Monitoring
4.3. Food Safety
4.4. Development and Prospect
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PEO | Poly (ethylene oxide) |
| TO-CNFs | TEMPO- oxidized cellulose nanofibers |
| PMI | Poly(α-methyleneindane) |
| CD | Carbon dot |
| RhBN2AM | Rhodamine derivative |
| PVA | Poly(vinyl alcohol) |
| CA | Cellulose acetate |
| PMMA_PFO | Poly(methyl methacrylate)_polyfluorene |
| NIPAAm | N-isopropylacrylamide |
| PVP | Polyvinyl pyrrolidone |
| BaWO4 | Barium tungsten oxide |
| TN | Titanate nanofibers |
| PAN | Polyacrylonitrile |
| PS/PyCz | PS/PyCz polystyrene/9-(pyren-1-yl)-9H-carbazole |
| BC | Bacterial cellulose |
| FNFM | Fluorescent nanofibrous membrane |
| NFM | Nanofibrous membrane |
| PNI | Poly-1,8-naphthimide |
| PCL | Polycaprolactone |
| MePyCz | 4-(2-(2-(2-methoxyethoxy)ethoxy)ethoxy)-9-(pyren-1-yl)-9H-carbazole |
| Poly(NIPAAm- co -NMA- co -RHPMA) | Poly[(N-isopropylacrylamide)-co-(N-hydroxymethyl acrylamide)-co-(4-rhodamine hydrazonomethyl-3-hydroxy-phenyl methacrylate)] |
| PHEM-PNMA-PNBD | Poly(2-hydroxyethyl methacrylate-co-N-methylolacrylamide-co-nitrobenzoxadiazolyl derivative) |
| 4-Mpy | 4-mercaptopyridine |
| PS-P4VP | Polystyrene-b-poly(4-vinyl pyridine) |
| PVDF | Poly(vinylidene fluoride) |
| TPU | Thermoplastic polyurethane |
| TEA | Triethanolamine |
| SbQ | N-methyl-4(4′-formylstyryl) pyridinium methosulfate acetal |
| CS | Chitosan |
| PAA | Poly(acrylic acid) |
| Poly(NIPAAm- co -NMA- co -RHPMA) | Poly[(N-isopropylacrylamide)-co-(N-hydroxymethyl acrylamide)-co-(4-rhodamine hydrazonomethyl-3-hydroxy-phenyl methacrylate)] |
References
- Steinegger, A.; Wolfbeis, O.S.; Borisov, S.M. Optical sensing and imaging of pH values: Spectroscopies, materials, and applications. Chem. Rev. 2020, 120, 12357–12489. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Albero Blanquer, L.; Bonefacino, J.; Logan, E.R.; Alves Dalla Corte, D.; Delacourt, C.; Gallant, B.M.; Boles, S.T.; Dahn, J.; Tam, H.-Y. Operando decoding of chemical and thermal events in commercial Na (Li)-ion cells via optical sensors. Nat. Energy 2020, 5, 674–683. [Google Scholar] [CrossRef]
- Xu, K.; Chen, Y.; Okhai, T.A.; Snyman, L.W. Micro optical sensors based on avalanching silicon light-emitting devices monolithically integrated on chips. Opt. Mater. Express 2019, 9, 3985–3997. [Google Scholar] [CrossRef]
- Shin, J.; Liu, Z.; Bai, W.; Liu, Y.; Yan, Y.; Xue, Y.; Kandela, I.; Pezhouh, M.; MacEwan, M.R.; Huang, Y. Bioresorbable optical sensor systems for monitoring of intracranial pressure and temperature. Sci. Adv. 2019, 5, eaaw1899. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Y.; Gao, Y.; Qin, S.; Wang, Z.; Zhou, D.; Li, Z.; Yu, Y.; Shao, M.; Zhang, X. Broadband multi-wavelength optical sensing based on photothermal effect of 2D MXene films. Nanophotonics 2019, 9, 123–131. [Google Scholar] [CrossRef]
- Al-Dhahebi, A.M.; Gopinath, S.C.B.; Saheed, M.S.M. Graphene impregnated electrospun nanofiber sensing materials: A comprehensive overview on bridging laboratory set-up to industry. Nano Converg. 2020, 7, 27. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, S.; Wang, J.; Yu, A.; Wei, G. Carbon nanofiber-based functional nanomaterials for sensor applications. Nanomaterials 2019, 9, 1045. [Google Scholar] [CrossRef]
- Alam, M.M.; Lee, S.; Kim, M.; Han, K.S.; Cao, V.A.; Nah, J. Ultra-flexible nanofiber-based multifunctional motion sensor. Nano Energy 2020, 72, 104672. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Z.; Qi, D.; Zhao, C. High sensitive and fast response humidity sensor based on polymer composite nanofibers for breath monitoring and non-contact sensing. Sens. Actuators B Chem. 2021, 330, 129239. [Google Scholar] [CrossRef]
- Horne, J.; McLoughlin, L.; Bridgers, B.; Wujcik, E.K. Recent developments in nanofiber-based sensors for disease detection, immunosensing, and monitoring. Sens. Actuators Rep. 2020, 2, 100005. [Google Scholar] [CrossRef]
- Li, T.; Qu, M.; Carlos, C.; Gu, L.; Jin, F.; Yuan, T.; Wu, X.; Xiao, J.; Wang, T.; Dong, W. High-performance poly (vinylidene difluoride)/dopamine core/shell piezoelectric nanofiber and its application for biomedical sensors. Adv. Mater. 2021, 33, 2006093. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lin, Y.; Mohamed, A.; Ji, Q.; Jia, H. High strength and flexible aramid nanofiber conductive hydrogels for wearable strain sensors. J. Mater. Chem. C 2021, 9, 575–583. [Google Scholar] [CrossRef]
- Terra, I.A.; Mercante, L.A.; Andre, R.S.; Correa, D.S. Fluorescent and colorimetric electrospun nanofibers for heavy-metal sensing. Biosensors 2017, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, M.; Veeramuthu, L.; Liang, F.-C.; Chen, W.-C.; Cho, C.-J.; Chen, C.-W.; Chen, J.-Y.; Yan, Y.; Chang, S.-H.; Kuo, C.-C. Evolution of electrospun nanofibers fluorescent and colorimetric sensors for environmental toxicants, pH, temperature, and cancer cells–A review with insights on applications. Chem. Eng. J. 2020, 397, 125431. [Google Scholar] [CrossRef]
- Zhao, X.; Campbell, S.; Wallace, G.Q.; Claing, A.; Bazuin, C.G.; Masson, J.-F. Branched Au nanoparticles on nanofibers for surface-enhanced Raman scattering sensing of intracellular pH and extracellular pH gradients. ACS Sens. 2020, 5, 2155–2167. [Google Scholar] [CrossRef]
- Gupta, B.D.; Kant, R. Recent advances in surface plasmon resonance based fiber optic chemical and biosensors utilizing bulk and nanostructures. Opt. Laser Technol. 2018, 101, 144–161. [Google Scholar] [CrossRef]
- Zhang, X.; Li, L.; Peng, X.; Chen, R.; Huo, K.; Chu, P.K. Non-enzymatic hydrogen peroxide photoelectrochemical sensor based on WO3 decorated core–shell TiC/C nanofibers electrode. Electrochim. Acta 2013, 108, 491–496. [Google Scholar] [CrossRef]
- Ner, Y.; Grote, J.G.; Stuart, J.A.; Sotzing, G.A. White luminescence from multiple-dye-doped electrospun DNA nanofibers by fluorescence resonance energy transfer. Angew. Chem. 2009, 121, 5236–5240. [Google Scholar] [CrossRef]
- Ghosh, T.; Das, T.; Purwar, R. Review of electrospun hydrogel nanofiber system: Synthesis, properties and applications. Polym. Eng. Sci. 2021, 61, 1887–1911. [Google Scholar] [CrossRef]
- Li, N.; Xue, F.; Zhang, H.; Sanyour, H.J.; Rickel, A.P.; Uttecht, A.; Fanta, B.; Hu, J.; Hong, Z. Fabrication and characterization of pectin hydrogel nanofiber scaffolds for differentiation of mesenchymal stem cells into vascular cells. ACS Biomater. Sci. Eng. 2019, 5, 6511–6519. [Google Scholar] [CrossRef]
- Chhetri, K.; Subedi, S.; Muthurasu, A.; Ko, T.H.; Dahal, B.; Kim, H.Y. A review on nanofiber reinforced aerogels for energy storage and conversion applications. J. Energy Storage 2022, 46, 103927. [Google Scholar] [CrossRef]
- Chen, Y.; Shafiq, M.; Liu, M.; Morsi, Y.; Mo, X. Advanced fabrication for electrospun three-dimensional nanofiber aerogels and scaffolds. Bioact. Mater. 2020, 5, 963–979. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, M.H.; Sundararaj, U. Review of the mechanical properties of carbon nanofiber/polymer composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 2126–2142. [Google Scholar] [CrossRef]
- Lim, C.T. Nanofiber technology: Current status and emerging developments. Prog. Polym. Sci. 2017, 70, 1–17. [Google Scholar]
- Huang, X.; Zhang, J.; Xiao, S.; Sang, T.; Chen, G. Unique electromagnetic properties of the zinc ferrite nanofiber. Mater. Lett. 2014, 124, 126–128. [Google Scholar] [CrossRef]
- Esfahani, H.; Jose, R.; Ramakrishna, S. Electrospun ceramic nanofiber mats today: Synthesis, properties, and applications. Materials 2017, 10, 1238. [Google Scholar] [CrossRef]
- Toriello, M.; Afsari, M.; Shon, H.K.; Tijing, L.D. Progress on the fabrication and application of electrospun nanofiber composites. Membranes 2020, 10, 204. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and electrospun nanofibers: Methods, materials, and applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Mailley, D.; Hébraud, A.; Schlatter, G. A review on the impact of humidity during electrospinning: From the nanofiber structure engineering to the applications. Macromol. Mater. Eng. 2021, 306, 2100115. [Google Scholar] [CrossRef]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; Mo, X. Electrospinning nanofiber scaffolds for soft and hard tissue regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Babu, A.; Aazem, I.; Walden, R.; Bairagi, S.; Mulvihill, D.M.; Pillai, S.C. Electrospun nanofiber based TENGs for wearable electronics and self-powered sensing. Chem. Eng. J. 2023, 452, 139060. [Google Scholar] [CrossRef]
- Rathore, P.; Schiffman, J.D. Beyond the single-nozzle: Coaxial electrospinning enables innovative nanofiber chemistries, geometries, and applications. ACS Appl. Mater. Interfaces 2020, 13, 48–66. [Google Scholar] [CrossRef] [PubMed]
- Fadil, F.; Affandi, N.D.N.; Misnon, M.I.; Bonnia, N.N.; Harun, A.M.; Alam, M.K. Review on electrospun nanofiber-applied products. Polymers 2021, 13, 2087. [Google Scholar] [CrossRef]
- Xue, J.; Xie, J.; Liu, W.; Xia, Y. Electrospun nanofibers: New concepts, materials, and applications. Acc. Chem. Res. 2017, 50, 1976–1987. [Google Scholar] [CrossRef] [PubMed]
- Charara, M.; Luo, W.; Saha, M.C.; Liu, Y. Investigation of lightweight and flexible carbon nanofiber/poly dimethylsiloxane nanocomposite sponge for piezoresistive sensor application. Adv. Eng. Mater. 2019, 21, 1801068. [Google Scholar] [CrossRef]
- Lamm, M.E.; Li, K.; Qian, J.; Wang, L.; Lavoine, N.; Newman, R.; Gardner, D.J.; Li, T.; Hu, L.; Ragauskas, A.J. Recent advances in functional materials through cellulose nanofiber templating. Adv. Mater. 2021, 33, 2005538. [Google Scholar] [CrossRef]
- Liu, S.; Shan, H.; Xia, S.; Yan, J.; Yu, J.; Ding, B. Polymer template synthesis of flexible SiO2 nanofibers to upgrade composite electrolytes. ACS Appl. Mater. Interfaces 2020, 12, 31439–31447. [Google Scholar] [CrossRef]
- Yan, J.; Han, Y.; Xia, S.; Wang, X.; Zhang, Y.; Yu, J.; Ding, B. Polymer template synthesis of flexible BaTiO3 crystal nanofibers. Adv. Funct. Mater. 2019, 29, 1907919. [Google Scholar] [CrossRef]
- Poolakkandy, R.R.; Menamparambath, M.M. Soft-template-assisted synthesis: A promising approach for the fabrication of transition metal oxides. Nanoscale Adv. 2020, 2, 5015–5045. [Google Scholar] [CrossRef]
- Lv, X.; Zhang, Y.; Wang, Y.; Zhang, G.; Zhao, Y.; Liu, J. Formation of carbon nanofibers/nanotubes by chemical vapor deposition using Al2O3/KOH. Diam. Relat. Mater. 2021, 113, 108265. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Wu, X.; Wang, T.-Y.; Zhao, L.; Truong, Y.B.; Ng, D.; Zheng, Y.-M.; Xie, Z. Omniphobic surface modification of electrospun nanofiber membrane via vapor deposition for enhanced anti-wetting property in membrane distillation. J. Membr. Sci. 2020, 606, 118075. [Google Scholar] [CrossRef]
- Xue, Z.; Xiong, Q.; Zou, C.; Chi, H.; Hu, X.; Ji, Z. Growth of carbon nanofibers through chemical vapor deposition for enhanced sodium ion storage. Mater. Res. Bull. 2021, 133, 111049. [Google Scholar] [CrossRef]
- Cui, L.; Liu, S.; Wang, F.; Li, J.; Song, Y.; Sheng, Y.; Zou, H. Growth of uniform g-C3N4 shells on 1D TiO2 nanofibers via vapor deposition approach with enhanced visible light photocatalytic activity. J. Alloy. Compd. 2020, 826, 154001. [Google Scholar] [CrossRef]
- Koh, E.; Lee, Y.T. Preparation of an omniphobic nanofiber membrane by the self-assembly of hydrophobic nanoparticles for membrane distillation. Sep. Purif. Technol. 2021, 259, 118134. [Google Scholar] [CrossRef]
- Yang, S.; Choi, T.-L. Rapid formation and real-time observation of micron-sized conjugated nanofibers with tunable lengths and widths in 20 minutes by living crystallization-driven self-assembly. Chem. Sci. 2020, 11, 8416–8424. [Google Scholar] [CrossRef]
- Zhuang, Y.; Liu, Y.; Meng, X. Fabrication of TiO2 nanofibers/MXene Ti3C2 nanocomposites for photocatalytic H2 evolution by electrostatic self-assembly. Appl. Surf. Sci. 2019, 496, 143647. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Qi, W.; Zhang, Q.; Zhu, J.; Zhao, P. Supramolecular self-assembly of nitrogen-deficient Ag/g-C3N4 nanofiber films with enhanced charge transfer dynamics for efficient visible-light photocatalytic activity. ACS Appl. Mater. Interfaces 2021, 13, 49993–50004. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Direct fabrication of composite and ceramic hollow nanofibers by electrospinning. Nano Lett. 2004, 4, 933–938. [Google Scholar] [CrossRef]
- Veverková, I.; Lovětinská-Šlamborová, I. Modified Silica Nanofibers with Antibacterial Activity. J. Nanomater. 2016, 2016, 2837197. [Google Scholar] [CrossRef]
- Wang, L.; Pai, C.-L.; Boyce, M.C.; Rutledge, G.C. Wrinkled surface topographies of electrospun polymer fibers. Appl. Phys. Lett. 2009, 94, 151916. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, X.; Wang, C.; Fu, S.; Liu, Y.; Shao, C. Polyacrylonitrile and carbon nanofibers with controllable nanoporous structures by electrospinning. Macromol. Mater. Eng. 2009, 294, 673–678. [Google Scholar] [CrossRef]
- He, J.-H.; Qian, M.-Y.; Li, Y. The maximal wrinkle angle during the bubble collapse and its application to the bubble electrospinning. Front. Mater. 2022, 8, 626. [Google Scholar] [CrossRef]
- Badmus, M.; Liu, J.; Wang, N.; Radacsi, N.; Zhao, Y. Hierarchically electrospun nanofibers and their applications: A review. Nano Mater. Sci. 2021, 3, 213–232. [Google Scholar] [CrossRef]
- Ahmadpoor, P.; Nateri, A.S.; Motaghitalab, V. The optical properties of PVA/TiO2 composite nanofibers. J. Appl. Polym. Sci. 2013, 130, 78–85. [Google Scholar] [CrossRef]
- Blachowicz, T.; Ehrmann, A. Optical Properties of Electrospun Nanofiber Mats. Membranes 2023, 13, 441. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Chen, J.; Zhang, Q.; Lei, M.; He, J.; Xiao, A.; Ma, C.; Li, S.; Xiong, H. Well-aligned cellulose nanofiber-reinforced polyvinyl alcohol composite film: Mechanical and optical properties. Carbohydr. Polym. 2016, 140, 238–245. [Google Scholar] [CrossRef]
- Sharma, A.; Mandal, T.; Goswami, S. Fabrication of cellulose acetate nanocomposite films with lignocelluosic nanofiber filler for superior effect on thermal, mechanical and optical properties. Nano-Struct. Nano-Objects 2021, 25, 100642. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Y.; Tong, L. Functionalized polymer nanofibers: A versatile platform for manipulating light at the nanoscale. Light Sci. Appl. 2013, 2, e102. [Google Scholar] [CrossRef]
- Bal, S. Experimental study of mechanical and electrical properties of carbon nanofiber/epoxy composites. Mater. Des. 2010, 31, 2406–2413. [Google Scholar] [CrossRef]
- Parit, M.; Du, H.; Zhang, X.; Prather, C.; Adams, M.; Jiang, Z. Polypyrrole and cellulose nanofiber based composite films with improved physical and electrical properties for electromagnetic shielding applications. Carbohydr. Polym. 2020, 240, 116304. [Google Scholar] [CrossRef]
- Bhawal, P.; Ganguly, S.; Das, T.K.; Mondal, S.; Nayak, L.; Das, N.C. A comparative study of physico-mechanical and electrical properties of polymer-carbon nanofiber in wet and melt mixing methods. Mater. Sci. Eng. B 2019, 245, 95–106. [Google Scholar] [CrossRef]
- Said, Z.; Abdelkareem, M.A.; Rezk, H.; Nassef, A.M.; Atwany, H.Z. Stability, thermophysical and electrical properties of synthesized carbon nanofiber and reduced-graphene oxide-based nanofluids and their hybrid along with fuzzy modeling approach. Powder Technol. 2020, 364, 795–809. [Google Scholar] [CrossRef]
- Chen, M.-C.; Sun, Y.-C.; Chen, Y.-H. Electrically conductive nanofibers with highly oriented structures and their potential application in skeletal muscle tissue engineering. Acta Biomater. 2013, 9, 5562–5572. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Kuboki, T.; Wong, A.; Park, C.B.; Sain, M. Rheology, thermal properties, and foaming behavior of high d-content polylactic acid/cellulose nanofiber composites. RSC Adv. 2015, 5, 91544–91557. [Google Scholar] [CrossRef]
- Frone, A.N.; Berlioz, S.; Chailan, J.-F.; Panaitescu, D.M. Morphology and thermal properties of PLA–cellulose nanofibers composites. Carbohydr. Polym. 2013, 91, 377–384. [Google Scholar] [CrossRef]
- Zhang, T.; Wu, X.; Luo, T. Polymer nanofibers with outstanding thermal conductivity and thermal stability: Fundamental linkage between molecular characteristics and macroscopic thermal properties. J. Phys. Chem. C 2014, 118, 21148–21159. [Google Scholar] [CrossRef]
- Zhou, X.; Ding, C.; Cheng, C.; Liu, S.; Duan, G.; Xu, W.; Liu, K.; Hou, H. Mechanical and thermal properties of electrospun polyimide/rGO composite nanofibers via in-situ polymerization and in-situ thermal conversion. Eur. Polym. J. 2020, 141, 110083. [Google Scholar] [CrossRef]
- Uetani, K.; Hatori, K. Thermal conductivity analysis and applications of nanocellulose materials. Sci. Technol. Adv. Mater. 2017, 18, 877–892. [Google Scholar] [CrossRef]
- Zhai, X.; Chen, R.; Shen, W. Aggregation-induced emission active luminescent polymeric nanofibers: From design, synthesis, fluorescent mechanism to applications. TrAC Trends Anal. Chem. 2022, 146, 116502. [Google Scholar] [CrossRef]
- Zhang, B.-T.; Liu, H.; Liu, Y.; Teng, Y. Application trends of nanofibers in analytical chemistry. TrAC Trends Anal. Chem. 2020, 131, 115992. [Google Scholar] [CrossRef]
- Han, S.W.; Koh, W.-G. Hydrogel-framed nanofiber matrix integrated with a microfluidic device for fluorescence detection of matrix metalloproteinases-9. Anal. Chem. 2016, 88, 6247–6253. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Chen, L.; Jung, H.; Zeng, Y.; Lee, S.; Swamy, K.M.K.; Zhou, X.; Kim, M.H.; Yoon, J. Effective strategy for colorimetric and fluorescence sensing of phosgene based on small organic dyes and nanofiber platforms. ACS Appl. Mater. Interfaces 2016, 8, 22246–22252. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Ji, P.; Wang, B.; Wang, H.; Chen, S. Color-tunable luminescent macrofibers based on CdTe QDs-loaded bacterial cellulose nanofibers for pH and glucose sensing. Sens. Actuators B Chem. 2018, 254, 110–119. [Google Scholar] [CrossRef]
- Xu, L.; Liu, X.; Jia, J.; Wu, H.; Xie, J.; Jia, Y. Electrospun nanofiber membranes from 1, 8-naphthimide-based polymer/poly (vinyl alcohol) for pH fluorescence sensing. Molecules 2022, 27, 520. [Google Scholar] [PubMed]
- Zhou, Z.; Xiong, W.; Zhang, Y.; Yang, D.; Wang, T.; Che, Y.; Zhao, J. Internanofiber spacing adjustment in the bundled nanofibers for sensitive fluorescence detection of volatile organic compounds. Anal. Chem. 2017, 89, 3814–3818. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Huang, X.; Luo, Y.; Yuan, H.; Ren, T.; Li, X.; Xu, D.; Guo, X.; Wu, Y. Fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots for adsorption and detection of Cr (VI). Chem. Eng. J. 2021, 407, 127050. [Google Scholar] [CrossRef]
- Wang, S.-L.; Zhang, C.-L.; Song, Q.-H. Selectively instant-response nanofibers with a fluorescent chemosensor toward phosgene in gas phase. J. Mater. Chem. C 2019, 7, 1510–1517. [Google Scholar]
- Zhao, L.; Liang, X.; Ni, Z.; Zhao, H.; Ge, B.; Li, W. Covalent organic framework modified polyacrylamide electrospun nanofiber membrane as a “turn-on” fluorescent sensor for primary aliphatic amine gas. Sens. Actuators B Chem. 2022, 366, 131988. [Google Scholar] [CrossRef]
- Yang, T.; Hou, P.; Zheng, L.L.; Zhan, L.; Gao, P.F.; Li, Y.F.; Huang, C.Z. Surface-engineered quantum dots/electrospun nanofibers as a networked fluorescence aptasensing platform toward biomarkers. Nanoscale 2017, 9, 17020–17028. [Google Scholar] [CrossRef]
- Ahmadian-Fard-Fini, S.; Ghanbari, D.; Amiri, O.; Salavati-Niasari, M. Electro-spinning of cellulose acetate nanofibers/Fe/carbon dot as photoluminescence sensor for mercury (II) and lead (II) ions. Carbohydr. Polym. 2020, 229, 115428. [Google Scholar] [CrossRef]
- Chen, L.; Oh, H.; Wu, D.; Kim, M.H.; Yoon, J. An ESIPT fluorescent probe and a nanofiber platform for selective and sensitive detection of a nerve gas mimic. Chem. Commun. 2018, 54, 2276–2279. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.Z.; Strong, V.; Wang, Y.; Li, X.G.; Wang, X.; Kaner, R.B. Oligotriphenylene Nanofiber Sensors for Detection of Nitro-Based Explosives. Adv. Funct. Mater. 2012, 22, 726–735. [Google Scholar] [CrossRef]
- George, G.; Edwards, C.S.; Hayes, J.I.; Yu, L.; Ede, S.R.; Wen, J.; Luo, Z. A novel reversible fluorescent probe for the highly sensitive detection of nitro and peroxide organic explosives using electrospun BaWO4 nanofibers. J. Mater. Chem. C 2019, 7, 14949–14961. [Google Scholar] [CrossRef]
- Quan, Z.; He, H.; Zhou, H.; Liang, Y.; Wang, L.; Tian, S.; Zhu, H.; Wang, S. Designing an intelligent nanofiber ratiometric fluorescent sensor sensitive to biogenic amines for detecting the freshness of shrimp and pork. Sens. Actuators B Chem. 2021, 333, 129535. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, S.; Fang, W.; Fan, Y. Synthesis of N-doped graphene quantum dots from bulk N-doped carbon nanofiber film for fluorescence detection of Fe3+ and ascorbic acid. Fuller. Nanotub. Carbon Nanostruct. 2021, 29, 218–226. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Kuo, C.-C.; Cho, C.-J.; Liang, F.-C.; Jeng, R.-J. Novel fluorescent chemosensory filter membranes composed of electrospun nanofibers with ultra-selective and reversible pH and Hg2+ sensing characteristics. Dye. Pigment. 2017, 143, 129–142. [Google Scholar] [CrossRef]
- Azzouz, A.; Vikrant, K.; Kim, K.-H.; Ballesteros, E.; Rhadfi, T.; Malik, A.K. Advances in colorimetric and optical sensing for gaseous volatile organic compounds. TrAC Trends Anal. Chem. 2019, 118, 502–516. [Google Scholar] [CrossRef]
- Chen, B.-Y.; Kuo, C.-C.; Huang, Y.-S.; Lu, S.-T.; Liang, F.-C.; Jiang, D.-H. Novel highly selective and reversible chemosensors based on dual-ratiometric fluorescent electrospun nanofibers with pH-and Fe3+-modulated multicolor fluorescence emission. ACS Appl. Mater. Interfaces 2015, 7, 2797–2808. [Google Scholar] [CrossRef]
- Khattab, T.A.; Rehan, M.; Aly, S.A.; Hamouda, T.; Haggag, K.M.; Klapötke, T.M. Fabrication of PAN-TCF-hydrazone nanofibers by solution blowing spinning technique: Naked-eye colorimetric sensor. J. Environ. Chem. Eng. 2017, 5, 2515–2523. [Google Scholar] [CrossRef]
- Terra, I.A.; Sanfelice, R.C.; Valente, G.T.; Correa, D.S. Optical sensor based on fluorescent PMMA/PFO electrospun nanofibers for monitoring volatile organic compounds. J. Appl. Polym. Sci. 2018, 135, 46128. [Google Scholar] [CrossRef]
- Xue, W.; Zhang, Y.; Duan, J.; Liu, D.; Ma, Y.; Shi, N.; Chen, S.; Xie, L.; Qian, Y.; Huang, W. A highly sensitive fluorescent sensor based on small molecules doped in electrospun nanofibers: Detection of explosives as well as color modulation. J. Mater. Chem. C 2015, 3, 8193–8199. [Google Scholar] [CrossRef]
- Wu, W.; Shi, N.; Zhang, J.; Wu, X.; Wang, T.; Yang, L.; Yang, R.; Ou, C.; Xue, W.; Feng, X. Electrospun fluorescent sensors for the selective detection of nitro explosive vapors and trace water. J. Mater. Chem. A 2018, 6, 18543–18550. [Google Scholar] [CrossRef]
- Liang, F.-C.; Luo, Y.-L.; Kuo, C.-C.; Chen, B.-Y.; Cho, C.-J.; Lin, F.-J.; Yu, Y.-Y.; Borsali, R. Novel magnet and thermoresponsive chemosensory electrospinning fluorescent nanofibers and their sensing capability for metal ions. Polymers 2017, 9, 136. [Google Scholar] [CrossRef] [PubMed]
- Saikia, A.; Karak, N. Polyaniline nanofiber/carbon dot nanohybrid as an efficient fluorimetric sensor for As (III) in water and effective antioxidant. Mater. Today Commun. 2018, 14, 82–89. [Google Scholar] [CrossRef]
- Ma, L.; Liu, K.; Yin, M.; Chang, J.; Geng, Y.; Pan, K. Fluorescent nanofibrous membrane (FNFM) for the detection of mercuric ion (II) with high sensitivity and selectivity. Sens. Actuators B Chem. 2017, 238, 120–127. [Google Scholar] [CrossRef]
- Tonsomboon, K.; Noppakuadrittidej, P.; Sutikulsombat, S.; Petdum, A.; Panchan, W.; Wanichacheva, N.; Sooksimuang, T.; Karoonuthaisiri, N. Turn-On fluorescence resonance energy transfer (FRET)-based electrospun fibrous membranes: Rapid and ultrasensitive test strips for on-site detection of Mercury (II) ion. Sens. Actuators B Chem. 2021, 344, 130212. [Google Scholar] [CrossRef]
- Mousa, N.; El-Hosainy, H.; Shoueir, K.; El-Kemary, M. Photoluminescence sensing of Pb2+ using cellulose acetate nanofiber decorated with Au nanoparticles. J. Alloy. Compd. 2023, 931, 167481. [Google Scholar] [CrossRef]
- Li, S.; Zhou, S.; Xu, H.; Xiao, L.; Wang, Y.; Shen, H.; Wang, H.; Yuan, Q. Luminescent properties and sensing performance of a carbon quantum dot encapsulated mesoporous silica/polyacrylonitrile electrospun nanofibrous membrane. J. Mater. Sci. 2016, 51, 6801–6811. [Google Scholar] [CrossRef]
- Wu, W.-C.; Lai, H.-J. Preparation of thermo-responsive electrospun nanofibers containing rhodamine-based fluorescent sensor for Cu 2+ detection. J. Polym. Res. 2016, 23, 223. [Google Scholar] [CrossRef]
- Zhu, H.; Lussier, F.l.; Ducrot, C.; Bourque, M.-J.e.; Spatz, J.P.; Cui, W.; Yu, L.; Peng, W.; Trudeau, L.-E.r.; Bazuin, C.G. Block copolymer brush layer-templated gold nanoparticles on nanofibers for surface-enhanced Raman scattering optophysiology. ACS Appl. Mater. Interfaces 2019, 11, 4373–4384. [Google Scholar] [CrossRef]
- Turasan, H.; Cakmak, M.; Kokini, J. A disposable ultrasensitive surface enhanced Raman spectroscopy biosensor platform fabricated from biodegradable zein nanofibers. J. Appl. Polym. Sci. 2022, 139, e52622. [Google Scholar] [CrossRef]
- Wan, M.; Zhao, H.; Peng, L.; Zhao, Y.; Sun, L. Facile One-Step deposition of Ag nanoparticles on SiO2 electrospun nanofiber surfaces for label-free SERS detection and antibacterial dressing. ACS Appl. Bio Mater. 2021, 4, 6549–6557. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Campbell, S.; El-Khoury, P.Z.; Jia, Y.; Wallace, G.Q.; Claing, A.; Bazuin, C.G.; Masson, J.-F. Surface-enhanced raman scattering optophysiology nanofibers for the detection of heavy metals in single breast cancer cells. ACS Sens. 2021, 6, 1649–1662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, Z.; Zou, Y.; Farooq, S.; Li, Y.; Zhang, H. Novel Ag-coated nanofibers prepared by electrospraying as a SERS platform for ultrasensitive and selective detection of nitrite in food. Food Chem. 2023, 412, 135563. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, L.; Ning, P.; Yuan, H.; Zhang, Y.; Zhang, M.; Fu, T.; He, X. In-situ growth of gold nanoparticles on electrospun flexible multilayered PVDF nanofibers for SERS sensing of molecules and bacteria. Nano Res. 2021, 14, 4885–4893. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, J.; Wei, H.; Wu, Z.; Wei, Y.; Wang, X.; Pei, Y. Fabrication of Ag NPs decorated on electrospun PVA/PEI nanofibers as SERS substrate for detection of enrofloxacin. J. Food Meas. Charact. 2022, 16, 2314–2322. [Google Scholar] [CrossRef]
- Juntaracena, K.; Yuangkaew, T.; Horprathum, M.; Triroj, N.; Jaroenapibal, P. Surface-enhanced Raman scattering activities and recyclability of Ag-incorporated WO3 nanofiber-based substrates. Vib. Spectrosc. 2021, 115, 103276. [Google Scholar] [CrossRef]
- Nasr, O.; Lin, Y.-Y.; Chou, Y.-S.; Huang, C.-W.; Chuang, W.-S.; Lee, S.-W.; Chen, C.-Y. Surface-enhanced Raman scattering of CoTiO3@ Ag nanofibers for high-performance sensing applications. Appl. Surf. Sci. 2022, 573, 151509. [Google Scholar] [CrossRef]
- Sang, Y.; Chen, X.; Zhang, L.; Li, D.; Xu, H. Electrospun polymeric nanofiber decorated with sea urchin-like gold nanoparticles as an efficient and stable SERS platform. J. Colloid Interface Sci. 2021, 590, 125–133. [Google Scholar] [CrossRef]
- Wan, M.; Zhao, H.; Peng, L.; Zou, X.; Zhao, Y.; Sun, L. Loading of Au/Ag bimetallic nanoparticles within and outside of the flexible SiO2 electrospun nanofibers as highly sensitive, stable, repeatable substrates for versatile and trace SERS detection. Polymers 2020, 12, 3008. [Google Scholar] [CrossRef]
- Zhao, X.; Li, C.; Li, Z.; Yu, J.; Pan, J.; Si, H.; Yang, C.; Jiang, S.; Zhang, C.; Man, B. In-situ electrospun aligned and maize-like AgNPs/PVA@ Ag nanofibers for surface-enhanced Raman scattering on arbitrary surface. Nanophotonics 2019, 8, 1719–1729. [Google Scholar] [CrossRef]
- Qi, Y.; Zhao, Y.; Bao, H.; Jin, W.; Ho, H.L. Nanofiber enhanced stimulated Raman spectroscopy for ultra-fast, ultra-sensitive hydrogen detection with ultra-wide dynamic range. Optica 2019, 6, 570–576. [Google Scholar] [CrossRef]
- Chung, M.; Skinner, W.H.; Robert, C.; Campbell, C.J.; Rossi, R.M.; Koutsos, V.; Radacsi, N. Fabrication of a wearable flexible sweat pH sensor based on SERS-active Au/TPU electrospun nanofibers. ACS Appl. Mater. Interfaces 2021, 13, 51504–51518. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Luo, X.; Bazuin, C.G.; Masson, J.-F. In situ growth of AuNPs on glass nanofibers for SERS sensors. ACS Appl. Mater. Interfaces 2020, 12, 55349–55361. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ma, B.; Xie, Z.; Li, W.; Yang, Y.; Mu, M.; Zou, X.; Zhao, B.; Song, W. Bifunctional porous SnO2/Ag nanofibers for efficient electroreduction of carbon dioxide to formate and its mechanism elucidation by in-situ surface-enhanced Raman scattering. Appl. Catal. B Environ. 2023, 325, 122350. [Google Scholar] [CrossRef]
- Ham, J.; Lee, K.M.; Ko, J.; Lee, K.; Koh, W.-G. Suspension Arrays Prepared from Nanofiber-Based Microparticles for a Platform of SERS-Based Multiplex Immunoassay. ACS Appl. Polym. Mater. 2022, 5, 625–634. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, J.; Wei, H.; Wu, Z.; Wang, X.; Pei, Y. Synthesis of polyvinyl alcohol/Ag electrospun nanofibers as highly efficient flexible SERS substrates. Vib. Spectrosc. 2021, 114, 103246. [Google Scholar] [CrossRef]
- Rezaei, Z.B.; Rastegarzadeh, S.; Kiasat, A. In-situ decorated silver nanoparticles on electrospun poly (vinyl alcohol)/chitosan nanofibers as a plasmonic sensor for azathioprine determination. Colloids Surf. A Physicochem. Eng. Asp. 2018, 559, 266–274. [Google Scholar] [CrossRef]
- Lim, K.; Jo, Y.M.; Yoon, J.W.; Kim, J.S.; Lee, D.J.; Moon, Y.K.; Yoon, J.W.; Kim, J.H.; Choi, H.J.; Lee, J.H. A Transparent Nanopatterned Chemiresistor: Visible-Light Plasmonic Sensor for Trace-Level NO2 Detection at Room Temperature. Small 2021, 17, 2100438. [Google Scholar] [CrossRef]
- Wu, M.-C.; Kao, C.-K.; Lin, T.-F.; Chan, S.-H.; Chen, S.-H.; Lin, C.-H.; Huang, Y.-C.; Zhou, Z.; Wang, K.; Lai, C.-S. Surface plasmon resonance amplified efficient polarization-selective volatile organic compounds CdSe-CdS/Ag/PMMA sensing material. Sens. Actuators B Chem. 2020, 309, 127760. [Google Scholar] [CrossRef]
- Marega, C.; Maculan, J.; Rizzi, G.A.; Saini, R.; Cavaliere, E.; Gavioli, L.; Cattelan, M.; Giallongo, G.; Marigo, A.; Granozzi, G. Polyvinyl alcohol electrospun nanofibers containing Ag nanoparticles used as sensors for the detection of biogenic amines. Nanotechnology 2015, 26, 075501. [Google Scholar] [CrossRef] [PubMed]
- Netsuwan, P.; Mimiya, H.; Baba, A.; Sriwichai, S.; Shinbo, K.; Kato, K.; Kaneko, F.; Phanichphant, S. Long-range surface plasmon resonance immunosensor based on water-stable electrospun poly (acrylic acid) fibers. Sens. Actuators B Chem. 2014, 204, 770–776. [Google Scholar] [CrossRef]
- Pule, B.O.; Degni, S.; Torto, N. Electrospun fibre colorimetric probe based on gold nanoparticles for on-site detection of 17β-estradiol associated with dairyfarming effluents. Water SA 2015, 41, 27–32. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Xia, Y.; Tong, L.; Xu, X.; Ying, Y. Polymer nanofibers embedded with aligned gold nanorods: A new platform for plasmonic studies and optical sensing. Nano Lett. 2012, 12, 3145–3150. [Google Scholar] [CrossRef]
- Bao, Y.; Fong, H.; Jiang, C. Manipulating the Collective Surface Plasmon Resonances of Aligned Gold Nanorods in Electrospun Composite Nanofibers. J. Phys. Chem. C 2013, 117, 21490–21497. [Google Scholar] [CrossRef]
- Barakat, N.A.; Woo, K.-D.; Kanjwal, M.A.; Choi, K.E.; Khil, M.S.; Kim, H.Y. Surface plasmon resonances, optical properties, and electrical conductivity thermal hystersis of silver nanofibers produced by the electrospinning technique. Langmuir 2008, 24, 11982–11987. [Google Scholar] [CrossRef]
- Tsuboi, K.; Matsumoto, H.; Minagawa, M.; Tanioka, A. Light scattering assisted surface plasmon resonance at electrospun nanofiber-coated gold surfaces. Appl. Phys. Lett. 2011, 98, 241109. [Google Scholar] [CrossRef]
- Shi, W.; Lu, W.; Jiang, L. The fabrication of photosensitive self-assembly Au nanoparticles embedded in silica nanofibers by electrospinning. J. Colloid Interface Sci. 2009, 340, 291–297. [Google Scholar] [CrossRef]
- Yang, X.; Li, X.; Zhang, L.; Gong, J. Electrospun template directed molecularly imprinted nanofibers incorporated with BiOI nanoflake arrays as photoactive electrode for photoelectrochemical detection of triphenyl phosphate. Biosens. Bioelectron. 2017, 92, 61–67. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, Y.; Guo, X.; Ying, Y.; Wen, Y.; Lin, P.; Sun, Y.; Yang, H.; Wu, Y. SnO2 nanofibers decorated with Au nanoparticles for Ru (bpy) 32+ sensitized photoelectrochemical determination of NO2− in urine. Sens. Actuators B Chem. 2020, 309, 127714. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Vishnuraj, R.; Wang, T.-J.; Pullithadathil, B. Multidimensional nanoarchitectures of TiO2/Au nanofibers with O-doped C3N4 nanosheets for electrochemical detection of nitrofurazone. Appl. Surf. Sci. 2022, 604, 154474. [Google Scholar] [CrossRef]
- Cui, Y.; Shang, Y.; Shi, R.; Wang, Y.; Zhu, Y.; Yang, P. Boosting photoelectrochemical and photocatalytic performances of electronspun WO3 nanofibers by combining with biphase-Ag2WO4. Mater. Sci. Semicond. Process. 2020, 120, 105285. [Google Scholar] [CrossRef]
- Lee, W.S.; Park, Y.-S.; Cho, Y.-K. Hierarchically structured suspended TiO2 nanofibers for use in UV and pH sensor devices. ACS Appl. Mater. Interfaces 2014, 6, 12189–12195. [Google Scholar] [CrossRef] [PubMed]
- Shaibani, P.M.; Etayash, H.; Jiang, K.; Sohrabi, A.; Hassanpourfard, M.; Naicker, S.; Sadrzadeh, M.; Thundat, T. Portable nanofiber-light addressable potentiometric sensor for rapid Escherichia coli detection in orange juice. ACS Sens. 2018, 3, 815–822. [Google Scholar] [CrossRef]
- Farzin, L.; Sadjadi, S.; Shamsipur, M.; Sheibani, S.; Hasan Mousazadeh, M. Employing AgNPs doped amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibers for target induced strand displacement-based electrochemical aptasensing of CA125 in ovarian cancer patients. Mater. Sci. Eng. C 2019, 97, 679–687. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Etayash, H.; Naicker, S.; Kaur, K.; Thundat, T. Metabolic study of cancer cells using a pH sensitive hydrogel nanofiber light addressable potentiometric sensor. ACS Sens. 2017, 2, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Niu, Y.; Deng, Y.; Cheng, H.; Ruan, C.; Li, G.; Sun, W. Electrochemical aptamer sensor for highly sensitive detection of mercury ion with Au/Pt@ carbon nanofiber-modified electrode. J. Chin. Chem. Soc. 2021, 68, 114–120. [Google Scholar] [CrossRef]
- Mei, Q.; Fu, R.; Ding, Y.; Wang, A.; Duan, D.; Ye, D. Electrospinning of highly dispersed Ni/CoO carbon nanofiber and its application in glucose electrochemical sensor. J. Electroanal. Chem. 2019, 847, 113075. [Google Scholar] [CrossRef]
- Graf, D.; Queralto, A.; Lepcha, A.; Appel, L.; Frank, M.; Mathur, S. Electrospun SrNb2O6 photoanodes from single-source precursors for photoelectrochemical water splitting. Sol. Energy Mater. Sol. Cells 2020, 210, 110485. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Sun, H.; Wang, L.; Zhang, J.; Deng, L.; Ma, T. An optical fiber sensor coated with electrospinning polyvinyl alcohol/carbon nanotubes composite film. Sensors 2020, 20, 6996. [Google Scholar] [CrossRef]
- Deng, L.; Li, J.; Xi, J.; Zhang, J.; Huang, X.; Sun, H. A peanut-shaped optical fiber sensor coated with electrospinning polyvinyl alcohol/nano-ZnO film. Sens. Actuators A Phys. 2022, 335, 113370. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Sun, H.; Wang, L.; Zhang, J.; Huang, X.; Deng, L.; Xi, J.; Ma, T. A new type of optical fiber glucose biosensor with enzyme immobilized by electrospinning. IEEE Sens. J. 2021, 21, 16078–16085. [Google Scholar] [CrossRef]
- Feng, D.; Zheng, H.; Sun, H.; Li, J.; Xi, J.; Deng, L.; Guo, Y.; Jiang, B.; Zhao, J. SnO2/polyvinyl alcohol nanofibers wrapped tilted fiber grating for high-sensitive humidity sensing and fast human breath monitoring. Sens. Actuators B Chem. 2023, 388, 133807. [Google Scholar] [CrossRef]
- Davis, B.W.; Niamnont, N.; Hare, C.D.; Sukwattanasinitt, M.; Cheng, Q. Nanofibers doped with dendritic fluorophores for protein detection. ACS Appl. Mater. Interfaces 2010, 2, 1798–1803. [Google Scholar] [CrossRef]
- Lee, S.J.; Tatavarty, R.; Gu, M.B. Electrospun polystyrene–poly (styrene-co-maleic anhydride) nanofiber as a new aptasensor platform. Biosens. Bioelectron. 2012, 38, 302–307. [Google Scholar] [CrossRef]
- Kirbay, F.O.; Yazgan, İ.; Demirkol, D.O. Comparison of direct and sandwich type immunoassays on electrospun nanofibers using of metal organic frameworks as a fluorescence probe. Sens. Actuators B Chem. 2022, 372, 132621. [Google Scholar] [CrossRef]
- Song, N.; Ma, F.; Zhu, Y.; Chen, S.; Wang, C.; Lu, X. Fe3C/nitrogen-doped carbon nanofibers as highly efficient biocatalyst with oxidase-mimicking activity for colorimetric sensing. ACS Sustain. Chem. Eng. 2018, 6, 16766–16776. [Google Scholar] [CrossRef]
- Naghdi, T.; Golmohammadi, H.; Yousefi, H.; Hosseinifard, M.; Kostiv, U.; Horak, D.; Merkoci, A. Chitin nanofiber paper toward optical (bio) sensing applications. ACS Appl. Mater. Interfaces 2020, 12, 15538–15552. [Google Scholar] [CrossRef]
- Chavoshy, H.Z.; Ghasemi, R. Fabrication of a novel fluorescent polyacrylonitrile electrospun nanofiber for DNA-based optical biosensing of microRNA-21. Nano Express 2020, 1, 020031. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, T.; Wang, G.; Gu, T.; Xie, C.; Huang, J.; Li, X.; Best, S.; Han, G. Production of a fluorescence resonance energy transfer (FRET) biosensor membrane for microRNA detection. J. Mater. Chem. B 2017, 5, 7133–7139. [Google Scholar] [CrossRef]
- Pebdeni, A.B.; Hosseini, M.; Barkhordari, A. Smart fluorescence aptasensor using nanofiber functionalized with carbon quantum dot for specific detection of pathogenic bacteria in the wound. Talanta 2022, 246, 123454. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Mirahmadi-zare, S.Z.; Allafchian, A.; Behmanesh, M. Fast fluorescent screening assay and dual electrochemical sensing of bacterial infection agent (Streptococcus agalactiae) based on a fluorescent-immune nanofibers. Sens. Actuators B Chem. 2022, 352, 130968. [Google Scholar] [CrossRef]
- Zhang, R.; Hong, Y.; Reinhard, B.M.; Liu, P.; Wang, R.; Dal Negro, L. Plasmonic nanotrough networks for scalable bacterial Raman biosensing. ACS Appl. Mater. Interfaces 2018, 10, 27928–27935. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Blaney, L.; Cagnetta, G.; Huang, J.; Wang, B.; Wang, Y.; Deng, S.; Yu, G. Degradation of ofloxacin by perylene diimide supramolecular nanofiber sunlight-driven photocatalysis. Environ. Sci. Technol. 2019, 53, 1564–1575. [Google Scholar] [CrossRef]
- Raj, S.; Shankaran, D.R. Curcumin based biocompatible nanofibers for lead ion detection. Sens. Actuators B Chem. 2016, 226, 318–325. [Google Scholar] [CrossRef]
- Li, Y.; Wen, Y.; Wang, L.; He, J.; Al-Deyab, S.S.; El-Newehy, M.; Yu, J.; Ding, B. Simultaneous visual detection and removal of lead (II) ions with pyromellitic dianhydride-grafted cellulose nanofibrous membranes. J. Mater. Chem. A 2015, 3, 18180–18189. [Google Scholar] [CrossRef]
- Wen, H.-Y.; Hsu, H.-C.; Tsai, Y.-T.; Feng, W.-K.; Lin, C.-L.; Chiang, C.-C. U-shaped optical fiber probes coated with electrically doped GQDs for humidity measurements. Polymers 2021, 13, 2696. [Google Scholar] [CrossRef]
- Cai, D.; Tong, T.; Zhang, Z.; Pan, J.; Zhang, L.; Tong, L. Functional film coated optical micro/nanofibers for high-performance gas sensing. IEEE Sens. J. 2019, 19, 9229–9234. [Google Scholar] [CrossRef]
- Zheng, S.; Fang, Y.; Chen, Y.; Kong, Q.; Wang, F.; Chen, X. Benzothiazole derivatives based colorimetric and fluorescent probes for detection of amine/ammonia and monitoring the decomposition of urea by urease. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120616. [Google Scholar] [CrossRef]
- Xue, R.; Ge, C.; Richardson, K.; Palmer, A.; Viapiano, M.; Lannutti, J.J. Microscale sensing of oxygen via encapsulated porphyrin nanofibers: Effect of indicator and polymer “core” permeability. ACS Appl. Mater. Interfaces 2015, 7, 8606–8614. [Google Scholar] [CrossRef]
- Shehata, N.; Samir, E.; Gaballah, S.; Hamed, A.; Elrasheedy, A. Embedded ceria nanoparticles in crosslinked PVA electrospun nanofibers as optical sensors for radicals. Sensors 2016, 16, 1371. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Peng, J.; Ren, T.; Luo, Q.; Luo, Y.; Zhang, N.; Huang, Y.; Guo, X.; Wu, Y. Novel fluorescent lignin-based hydrogel with cellulose nanofibers and carbon dots for highly efficient adsorption and detection of Cr (VI). Sci. Total Environ. 2021, 760, 143395. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Wu, X.; Zhang, F.; Zhao, H.; Zhong, W.; Cao, Y.; Ma, X.; Leng, X.; Zhou, H.; She, M. Cu2+/ATP reversible ratiometric fluorescent probe through strip, hydrogel, and nanofiber, and its application in living cells and edaphic ecological safety assessment. Dye. Pigment. 2020, 182, 108677. [Google Scholar] [CrossRef]
- Lokesh, K.; Kavitha, G.; Manikandan, E.; Mani, G.K.; Kaviyarasu, K.; Rayappan, J.B.B.; Ladchumananandasivam, R.; Aanand, J.S.; Jayachandran, M.; Maaza, M. Effective ammonia detection using n-ZnO/p-NiO heterostructured nanofibers. IEEE Sens. J. 2016, 16, 2477–2483. [Google Scholar] [CrossRef]
- Wei, J.H.; Yi, J.W.; Han, M.L.; Li, B.; Liu, S.; Wu, Y.P.; Ma, L.F.; Li, D.S. A Water-Stable Terbium (III)–Organic Framework as a Chemosensor for Inorganic Ions, Nitro-Containing Compounds and Antibiotics in Aqueous Solutions. Chem. Asian J. 2019, 14, 3694–3701. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Chen, N.; Wang, Y.; Gao, X.; Zhou, X. Simultaneous Detection of Magnetic Field and Temperature Using Micro-Nanofiber Cascaded Fiber Bragg Grating Structure. IEEE Sens. J. 2022, 22, 19267–19272. [Google Scholar] [CrossRef]
- Jia, R.; Tian, W.; Bai, H.; Zhang, J.; Wang, S.; Zhang, J. Amine-responsive cellulose-based ratiometric fluorescent materials for real-time and visual detection of shrimp and crab freshness. Nat. Commun. 2019, 10, 795. [Google Scholar] [CrossRef]
- Guo, M.; Wang, H.; Wang, Q.; Chen, M.; Li, L.; Li, X.; Jiang, S. Intelligent double-layer fiber mats with high colorimetric response sensitivity for food freshness monitoring and preservation. Food Hydrocoll. 2020, 101, 105468. [Google Scholar] [CrossRef]
- Aghaei, Z.; Emadzadeh, B.; Ghorani, B.; Kadkhodaee, R. Cellulose acetate nanofibres containing alizarin as a halochromic sensor for the qualitative assessment of rainbow trout fish spoilage. Food Bioprocess Technol. 2018, 11, 1087–1095. [Google Scholar] [CrossRef]
- Valdez, M.; Gupta, S.K.; Lozano, K.; Mao, Y. ForceSpun polydiacetylene nanofibers as colorimetric sensor for food spoilage detection. Sens. Actuators B Chem. 2019, 297, 126734. [Google Scholar] [CrossRef]
- Yildiz, E.; Sumnu, G.; Kahyaoglu, L.N. Monitoring freshness of chicken breast by using natural halochromic curcumin loaded chitosan/PEO nanofibers as an intelligent package. Int. J. Biol. Macromol. 2021, 170, 437–446. [Google Scholar] [CrossRef]
- Kiryukhin, M.V.; Lau, H.H.; Goh, S.H.; Teh, C.; Korzh, V.; Sadovoy, A. A membrane film sensor with encapsulated fluorescent dyes towards express freshness monitoring of packaged food. Talanta 2018, 182, 187–192. [Google Scholar] [CrossRef]
- He, Y.; Hong, S.; Wang, M.; Wang, J.; Abd El-Aty, A.; Hacimuftuoglu, A.; Khan, M.; She, Y. Development of fluorescent lateral flow test strips based on an electrospun molecularly imprinted membrane for detection of triazophos residues in tap water. New J. Chem. 2020, 44, 6026–6036. [Google Scholar] [CrossRef]
- Nag, P.; Sadani, K.; Mohapatra, S.; Mukherji, S.; Mukherji, S. Evanescent wave optical fiber sensors using enzymatic hydrolysis on nanostructured polyaniline for detection of β-lactam antibiotics in food and environment. Anal. Chem. 2021, 93, 2299–2308. [Google Scholar] [CrossRef]
- Zhang, J.; Hurren, C.; Lu, Z.; Wang, D. Nanofiber-based colorimetric platform for point-of-care detection of E. coli. Chem. Eng. J. 2023, 463, 142357. [Google Scholar] [CrossRef]
- Shaibani, P.M.; Jiang, K.; Haghighat, G.; Hassanpourfard, M.; Etayash, H.; Naicker, S.; Thundat, T. The detection of Escherichia coli (E. coli) with the pH sensitive hydrogel nanofiber-light addressable potentiometric sensor (NF-LAPS). Sens. Actuators B Chem. 2016, 226, 176–183. [Google Scholar] [CrossRef]
- Abedalwafa, M.A.; Tang, Z.; Qiao, Y.; Mei, Q.; Yang, G.; Li, Y.; Wang, L. An aptasensor strip-based colorimetric determination method for kanamycin using cellulose acetate nanofibers decorated DNA–gold nanoparticle bioconjugates. Microchim. Acta 2020, 187, 360. [Google Scholar] [CrossRef]
- Pirsa, S.; Chavoshizadeh, S. Design of an optical sensor for ethylene based on nanofiber bacterial cellulose film and its application for determination of banana storage time. Polym. Adv. Technol. 2018, 29, 1385–1393. [Google Scholar] [CrossRef]
- Teixeira, A.; Paris, J.L.; Roumani, F.; Diéguez, L.; Prado, M.; Espiña, B.; Abalde-Cela, S.; Garrido-Maestu, A.; Rodriguez-Lorenzo, L. Multifuntional gold nanoparticles for the SERS detection of pathogens combined with a LAMP–in–microdroplets approach. Materials 2020, 13, 1934. [Google Scholar] [CrossRef]
- Nguyen, N.L.T.; Baek, S.H.; Akbar, Z.A.; Jang, S.-Y.; Ha, S.; Park, J.P.; Park, T.J. Rapid determination of ethyl alcohol in alcoholic beverages using a fluorescent nanofiber film. BioChip J. 2018, 12, 240–248. [Google Scholar] [CrossRef]
- Luo, M.; Wang, W.; Zhao, Q.; Li, M.; Chen, Y.; Lu, Z.; Liu, K.; Wang, D. Chemiluminescence biosensor for hydrogen peroxide determination by immobilizing horseradish peroxidase onto PVA-co-PE nanofiber membrane. Eur. Polym. J. 2017, 91, 307–314. [Google Scholar] [CrossRef]
- Forghani, S.; Almasi, H.; Moradi, M. Electrospun nanofibers as food freshness and time-temperature indicators: A new approach in food intelligent packaging. Innov. Food Sci. Emerg. Technol. 2021, 73, 102804. [Google Scholar] [CrossRef]





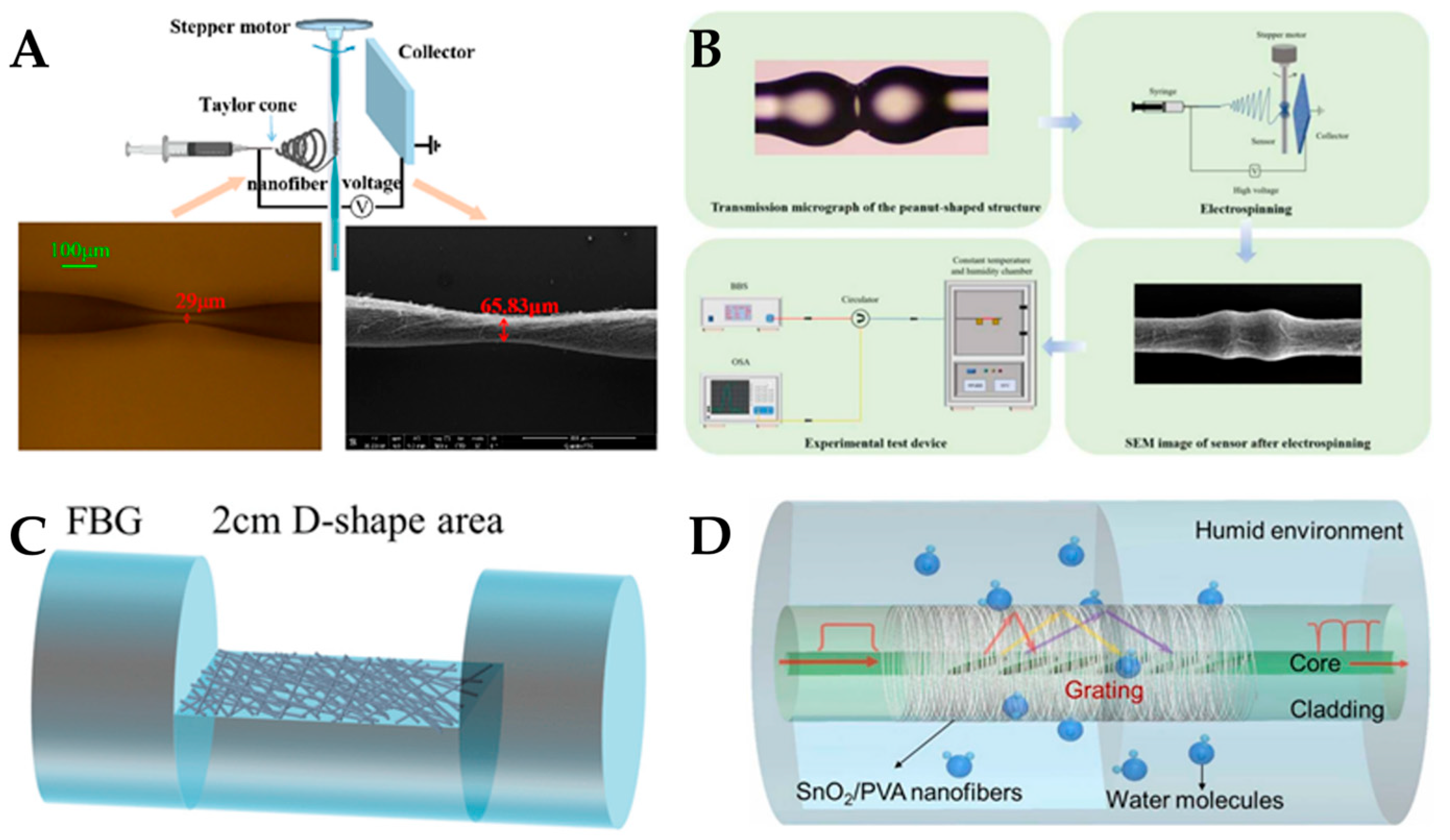
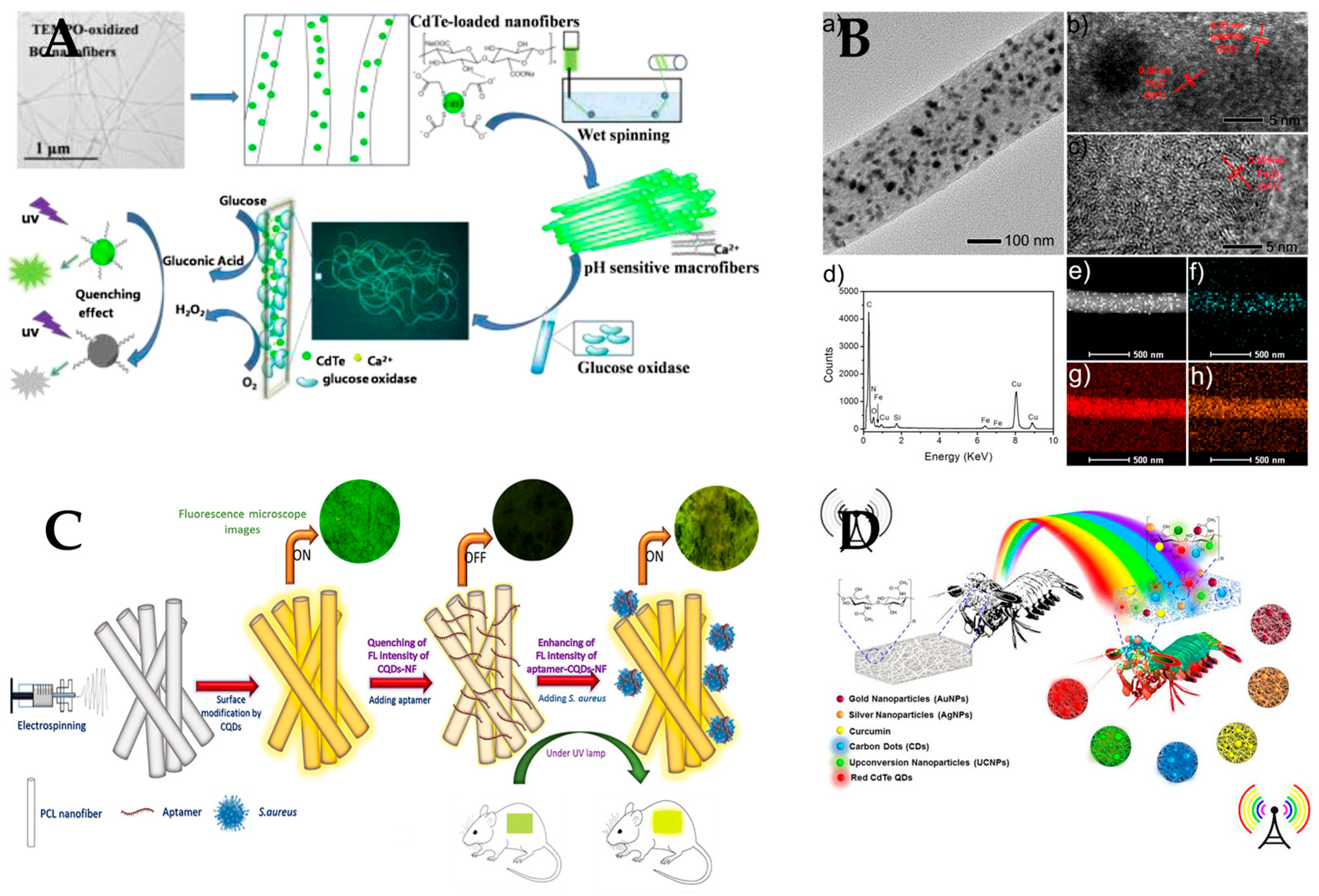
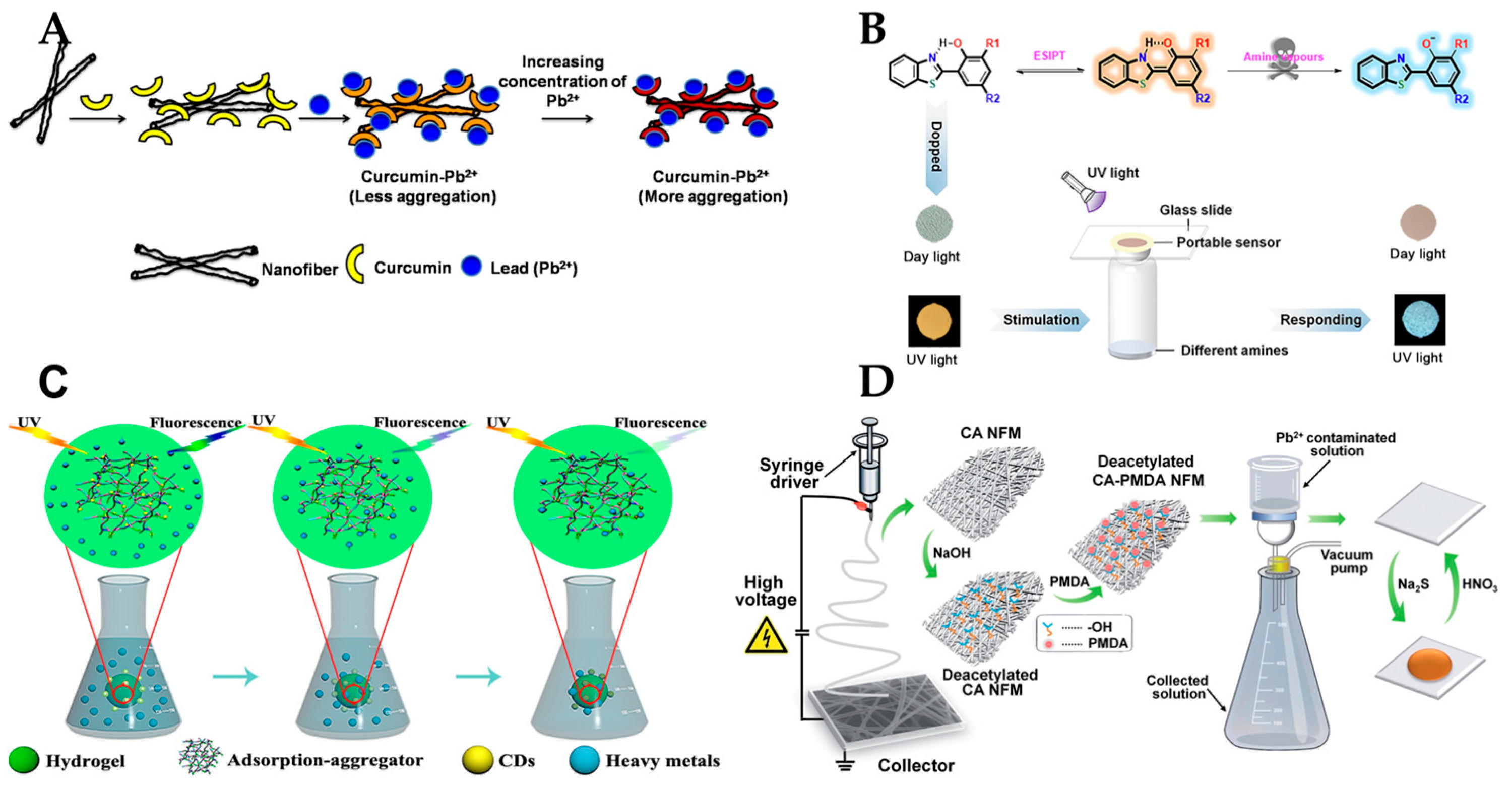
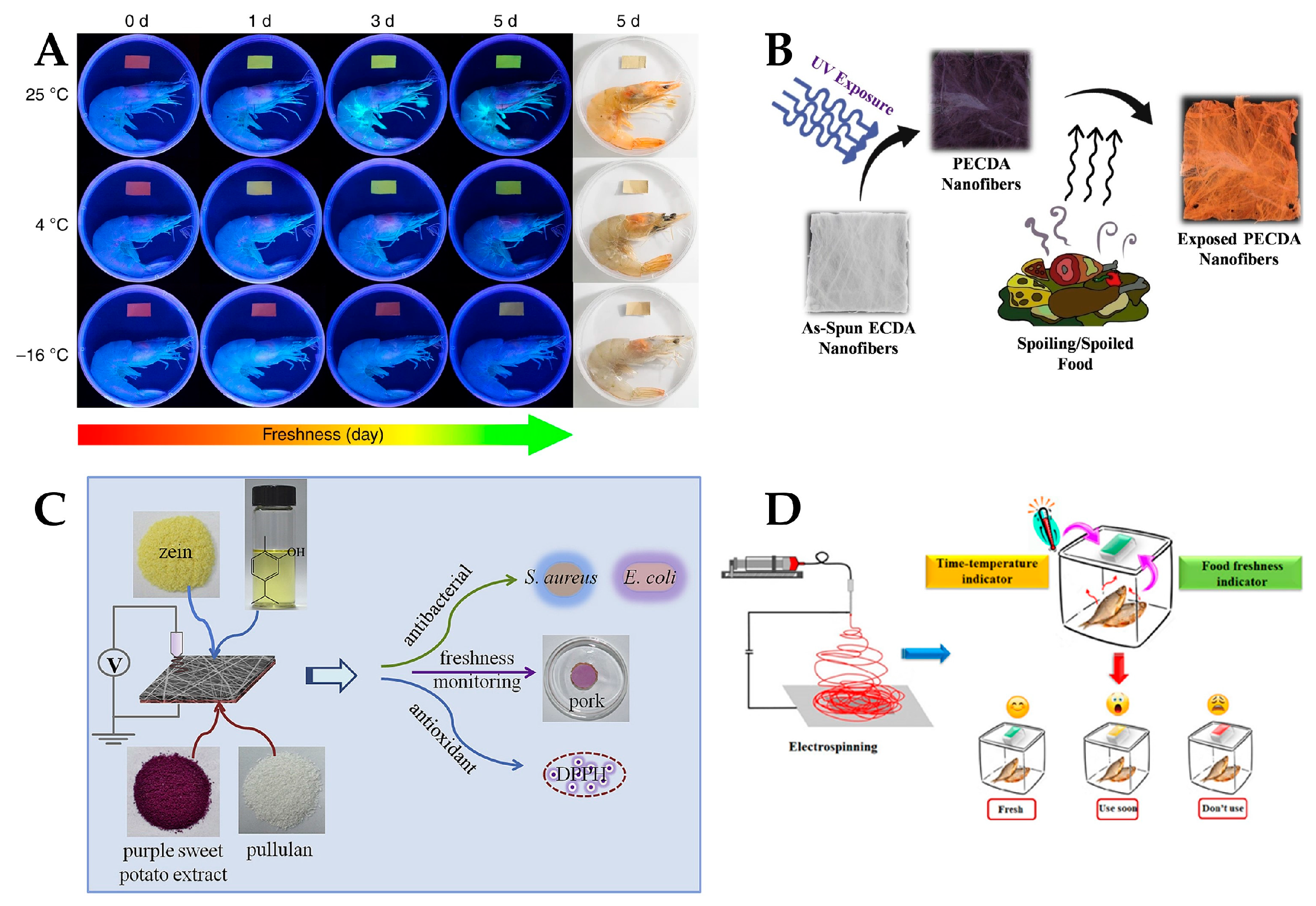
| Material | Application | Performance | Ref. | |
|---|---|---|---|---|
| Biology | AuNRB/AuNS(S) | capturing cell pH heterogeneity | pH 6.5–9.5 (detection range) | [15] |
| Zein | zein nanofiber-based flexible SERS platform | 2.06 ng/mL (LOD) | [101] | |
| Ag-TEA@SiO2 | detection of biomacromolecules of bacteria | 10−10 mol/L (LOD) | [102] | |
| PVA-SbQ | (SERS)-based immunoassay | - | [116] | |
| Ion | 4-Mpy/PS-P4VP | detect Hg2+ and Ag+ | 5 nM–5 μM (detection range) | [103] |
| gluten/zein film | to assay five common nitrite foods | 1 ppm (LOD) | [104] | |
| PVA/Ag | Rhodamine 6 G detecting | 1.3 nM (LOD) | [117] | |
| Dye | AgNPs/PVA@Ag | detection of Crystal Violet | - | [111] |
| PVDF | SERS sensing of molecules and bacteria | 1160 ppm to 116 ppm (detection range) | [105] | |
| PVA/PEI | detection of enrofloxacin | - | [106] | |
| Ag/WO3/PVA | SERS substrates with recyclability | 0.001 ppb (LOD) | [107] | |
| CoTiO3@Ag/PVP | detect R6G | 10−9–10−3 M (detection range) | [108] | |
| PMMA/P4VP | using 4-MBA as probe molecule | 10−3 M (LOD) | [109] | |
| Ag@TA@SiO2 | identify S. aureus | 0.46 pg·mL−1 (LOD) | [110] | |
| Gas | SiO2 | hydrogen detection | 3 ppm (LOD) | [112] |
| pH | Au/TPU | SERS pH sensor | for Hg2+ is 10−7 M (LOD) | [113] |
| Material | Application | Performance | Ref. | |
|---|---|---|---|---|
| Chemical | Au-SnO2 | detect ppb-level NO2 | 6 ppb (LOD) | [119] |
| PMMA | detect VOCs below lowest explosion limits | 100 ppm (LOD) | [120] | |
| PVA/CS | determination human serum samples | 0.09 μM (LOD) | [118] | |
| PVA | detect biogenic amines from food vapors | 10 ppm (LOD) | [121] | |
| Biomolecule | PAA | the detection of human immunoglobulin G | 15000 RU (sensitivity) | [122] |
| polystyrene | the detection of oestrogenic compounds | 100 ng/mL (sensitivity) | [123] | |
| Environment monitoring | PAM/GNR | detect humidity | 110 ms (response time) | [124] |
| Material | Application | Performance | Ref. | |
|---|---|---|---|---|
| Environmental monitoring | chitosan | detection of triphenyl phosphate | 0.008 ppb (LOD) | [129] |
| PVP | detect nitrite (NO2−) under visible light irradiation | 4.8 × 10−10 M (LOD) | [130] | |
| PVP/Ag2WO4/WO3 | Ag2WO4/WO3 photocatalyst was synthesized through electrospinning process combined with a facile solid chemical reaction. | - | [132] | |
| Biomedical | WO3@TiC/C | detection of hydrogen peroxide | 386 μA mM−1 cm−2 (sensitivity) | [17] |
| PVP/TTIP | utilized as UV and pH sensors | 5.68 ± 0.28 nS/pH (sensitivity) | [133] | |
| PVA/PAA | detect Escherichia coli | 102 CFU/mL (LOD) | [134] | |
| PVA/PAA | measures cancer cell metabolism and their response to anticancer drugs | 74 mV/pH (sensitivity) | [136] | |
| Energy conversion | PVP/SrNb2O6 | oxide nanofiber meshes as potential photoanode material for solar water splitting | - | [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Liu, X.; Xi, J.; Deng, L.; Yang, Y.; Li, X.; Sun, H. Recent Development of Polymer Nanofibers in the Field of Optical Sensing. Polymers 2023, 15, 3616. https://doi.org/10.3390/polym15173616
Li J, Liu X, Xi J, Deng L, Yang Y, Li X, Sun H. Recent Development of Polymer Nanofibers in the Field of Optical Sensing. Polymers. 2023; 15(17):3616. https://doi.org/10.3390/polym15173616
Chicago/Turabian StyleLi, Jinze, Xin Liu, Jiawei Xi, Li Deng, Yanxin Yang, Xiang Li, and Hao Sun. 2023. "Recent Development of Polymer Nanofibers in the Field of Optical Sensing" Polymers 15, no. 17: 3616. https://doi.org/10.3390/polym15173616
APA StyleLi, J., Liu, X., Xi, J., Deng, L., Yang, Y., Li, X., & Sun, H. (2023). Recent Development of Polymer Nanofibers in the Field of Optical Sensing. Polymers, 15(17), 3616. https://doi.org/10.3390/polym15173616






