Effect of Octene Block Copolymer (OBC) and High-Density Polyethylene (HDPE) on Crystalline Morphology, Structure and Mechanical Properties of Octene Random Copolymer
Abstract
:1. Introduction
2. Experimental Part
2.1. Material and Sample Preparation
2.2. Thermal Analysis
2.3. Morphology Analysis
2.4. Small-Angle X-ray Scattering Measurements
2.5. Dynamic Mechanical Analysis
2.6. Mechanical Testing
3. Results and Discussion
3.1. Non-Isothermal Crystallization of the ORC/OBC, ORC/HDPE and ORC/OBC/HDPE Blends with DSC
3.2. Morphology of Pure ORC, OBC, HDPE and the Blends
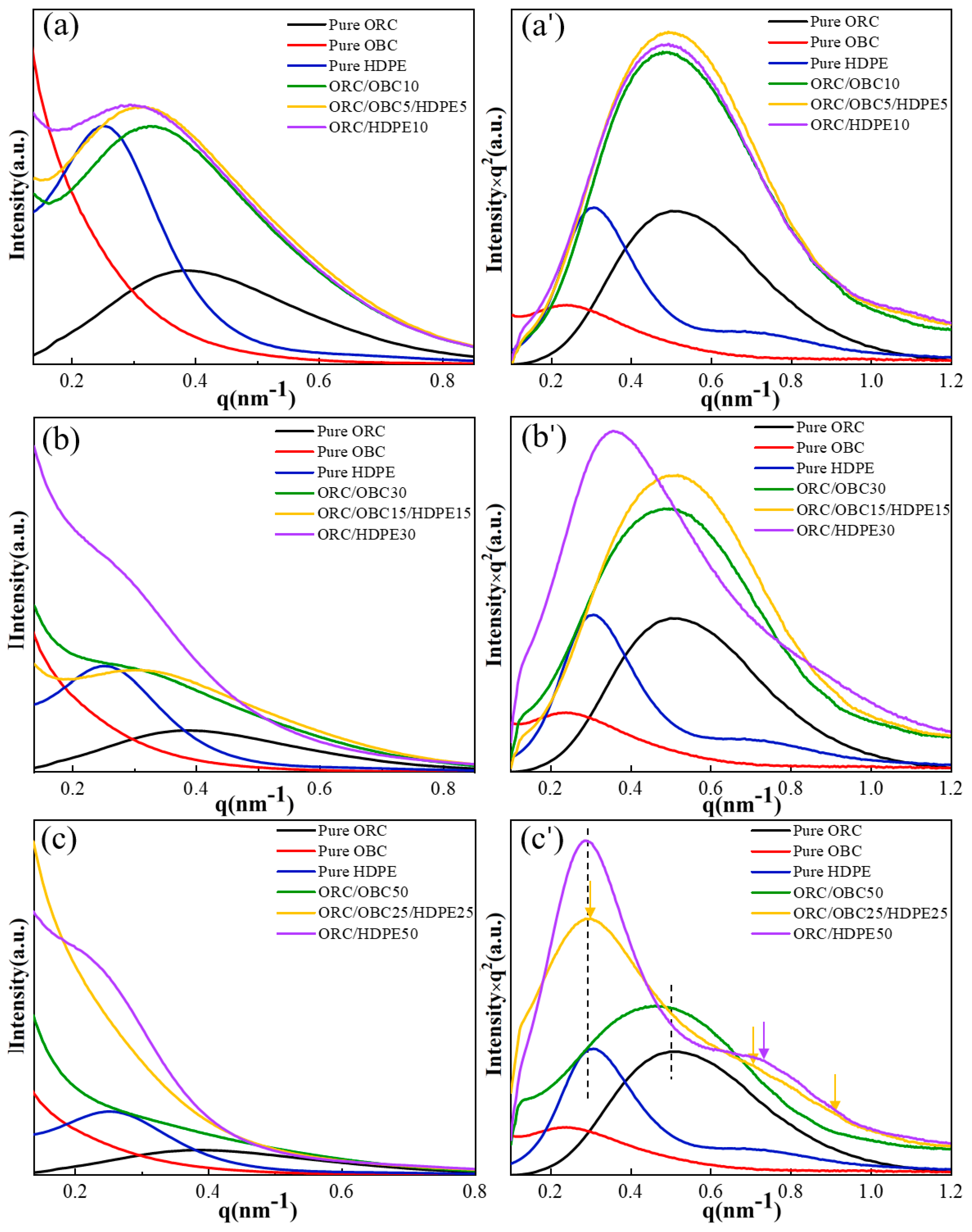
3.3. Packing Structure of the Blends with Small-Angle X-ray Scattering
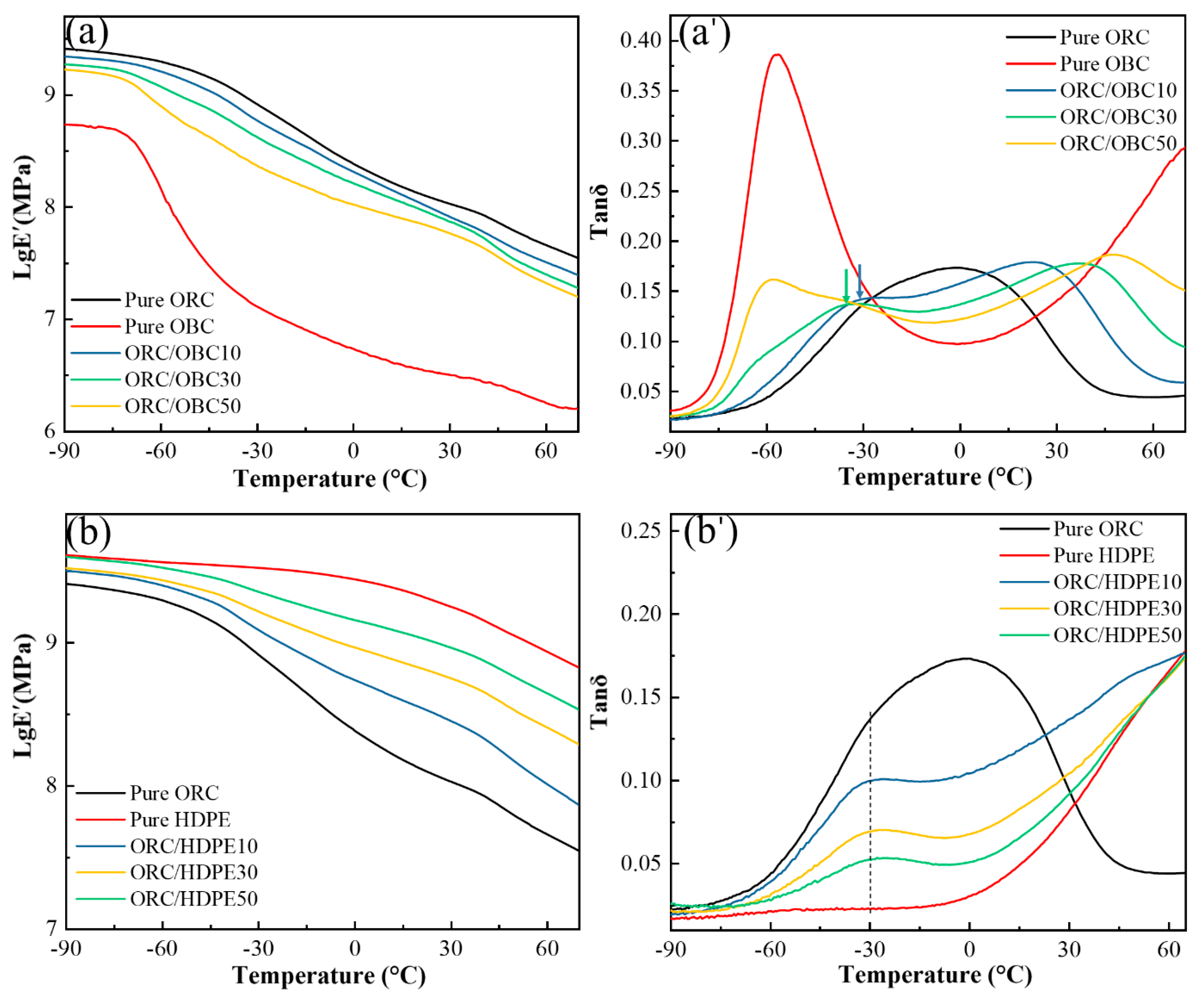
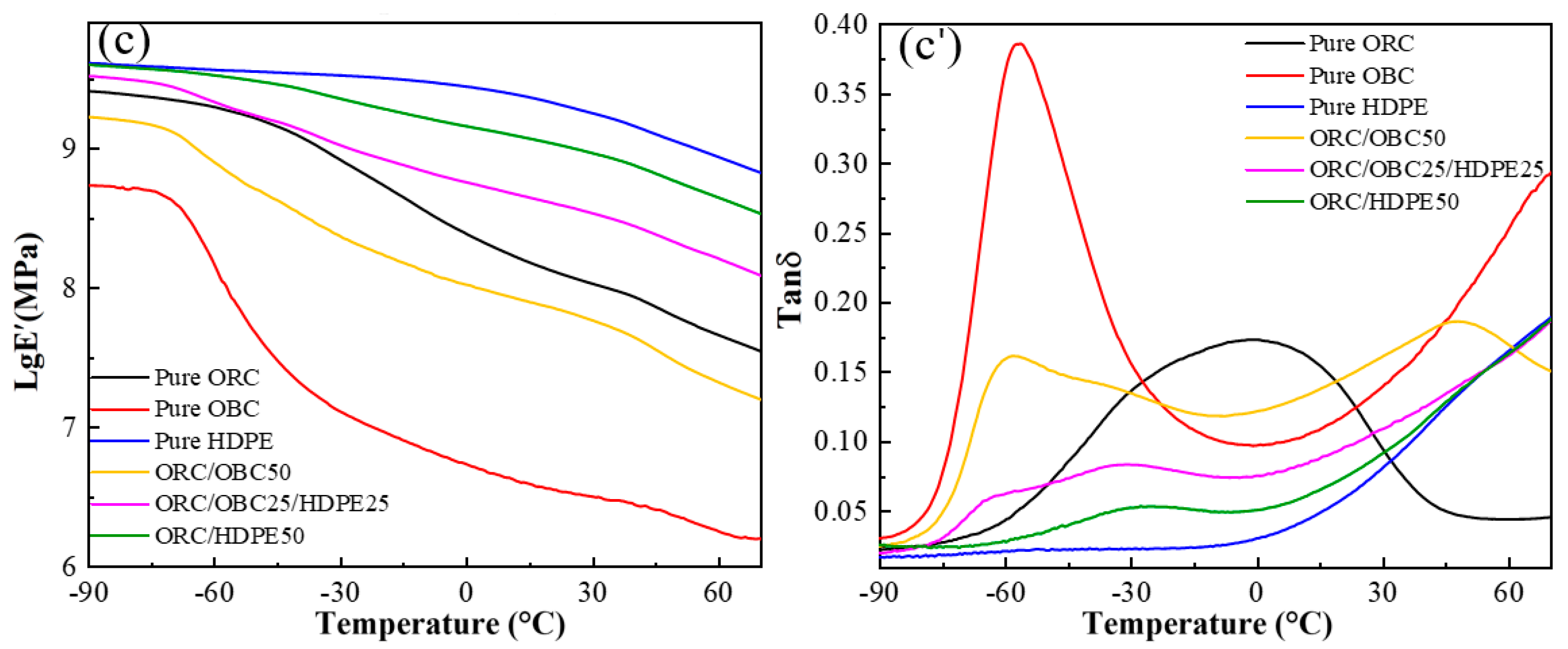
3.4. Dynamic Mechanical Behavior
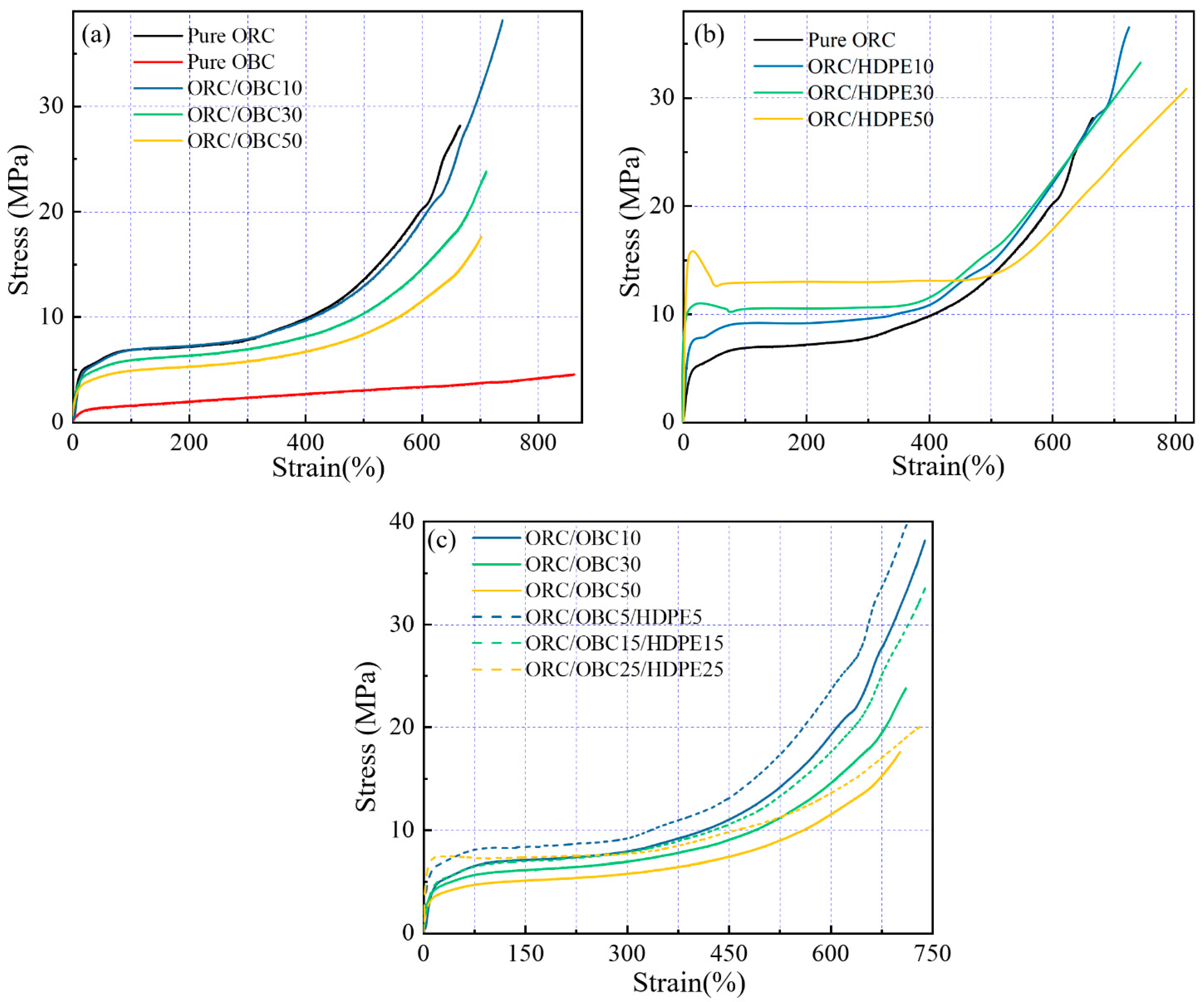
3.5. Tensile Properties of the Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Maiti, M.; Sadhu, S.; Bhowmick, A.K. Ethylene-Octene Copolymer (Engage)-Clay Nanocomposites: Preparation and Characterization. J. Appl. Polym. Sci. 2006, 101, 603–610. [Google Scholar] [CrossRef]
- Sawangpet, K.; Walong, A.; Thongnuanchan, B.; Kaesaman, A.; Sakai, T.; Lopattananon, N. Foaming and Physical Properties, Flame Retardancy, and Combustibility of Polyethylene Octene Foams Modified by Nature Rubber and Expandable Graphite. J. Vinyl Addit. Technol. 2020, 26, 423–433. [Google Scholar] [CrossRef]
- Korobko, A.; Bessonova, N.; Krasheninnikov, S.; Konyukhova, E.; Drozd, S.; Chvalun, S. Nanodiamonds as modifier of ethylene-1-octene copolymer structure and properties. Diam. Relat. Mater. 2007, 16, 2141–2144. [Google Scholar] [CrossRef]
- Tang, W.H.; Tang, J.; Yuan, H.L.; Jin, R.G. Crystallization Behavior and Mechanical Properties of Polypropylene Random Copolymer/Poly(ethylene-octene) Blends. J. Appl. Polym. Sci. 2011, 122, 461–468. [Google Scholar] [CrossRef]
- Adhikari, R.; Godehardt, R.; Lebek, W.; Frangov, S.; Michler, G.H.; Radusch, H.-J.; Calleja, F.J.B. Morphology and micromechanical properties of ethylene/1-octene copolymers and their blends with high density polyethylene. Polym. Adv. Technol. 2005, 16, 156–166. [Google Scholar] [CrossRef]
- Su, F.H.; Huang, H.X.; Zhao, Y. Microstructure and mechanical properties of polypropylene/poly (ethylene-co-octene copolymer)/clay ternary nanocomposites prepared by melt blending using supercritical carbon dioxide as a processing aid. Compos. Part B Eng. 2011, 42, 421–428. [Google Scholar] [CrossRef]
- Park, K.; Sadeghi, K.; Panda, P.K.; Seo, J.; Seo, J. Ethylene vinyl acetate/low-density polyethylene/oyster shell powder composite films: Preparation, characterization, and antimicrobial properties for biomedical applications. J. Taiwan. Inst. Chem. E 2022, 134, 104301. [Google Scholar] [CrossRef]
- HÖlzer, S.; Menzel, M.; Zia, Q.; Schubert, U.S.; Beiner, M.; Weidisch, R. Blends of ethylene-octene copolymers with different chain architectures-Morphology, thermal and mechanical behavior. Polymer 2013, 54, 5207–5213. [Google Scholar] [CrossRef]
- Reddy, C.S.; Patra, P.K.; Das, C.K. Ethylene-Octene Copolymer-Nanosilica Nanocomposites: Effects of Epoxy Resin Functionalized Nanosilica on Morphology, Mechanical, Dynamic Mechanical and Thermal Properties. Macromol. Symp. 2009, 277, 119–129. [Google Scholar] [CrossRef]
- Wang, Y.X.; Shi, Y.; Wang, C.C.; Cheng, J.H.; Wang, Y.; Shao, W.J.; Liu, L.Z. Crystallization, structure, and enhanced mechanical property of ethylene-octene elastomer crosslinked with dicumyl peroxide. J. Appl. Polym. Sci. 2021, 138, 50651. [Google Scholar] [CrossRef]
- Wang, J.F.; Wang, C.L.; Zhang, X.L.; Wu, H.; Guo, S.Y. Morphological evolution and toughening mechanism of polypropylene and polypropylene/poly(ethylene-co-octene) alternating multilayered materials with enhanced low-temperature toughness. RSC Adv. 2014, 4, 20297–20307. [Google Scholar] [CrossRef]
- Uthaipan, N.; Junhasavasdikul, B.; Vennemann, N.; Nakason, C.; Thitithammawong, A. Investigation of surface properties and elastomeric behaviors of EPDM/EOC/PP thermoplastic vulcanizates with different octene contents. J. Appl. Polym. Sci. 2017, 134, 44857. [Google Scholar] [CrossRef]
- Chum, P.S.; Swogger, K.W. Olefin polymer technologies-History and recent progress at The Dow Chemical Company. Prog. Polym. Sci. 2008, 33, 797–819. [Google Scholar] [CrossRef]
- Kamdar, A.R.; Wang, H.P.; Khariwala, D.U.; Taha, A.; Baer, E. Effect of Chain Blockiness on the Phase Behavior of Ethylene-Octene Copolymer Blends. J. Polym. Sci. Part B Polym. Phys. 2010, 47, 1554–1572. [Google Scholar] [CrossRef]
- Natta, G.; Crespi, G.; Valvassori, A.; Sartort, G. POLYOLEFIN ELSTOMERS. Rubber. Chem. Technol. 1963, 36, 1583–1668. [Google Scholar] [CrossRef]
- Zhao, Y.; Yao, C.; Chang, T.; Zhu, Y. The Influence of DMDBS on Crystallization Behavior and Crystalline Morphology of Weakly-Phase-Separated Olefin Block Copolymer. Polymers 2019, 11, 552. [Google Scholar] [CrossRef]
- Arriola, D.J.; Carnahan, E.M.; Hustad, P.D.; Kuhlman, R.L.; Wenzel, T.T. Catalytic Production of Olefin Block Copolymers via Chain Shuttling Polymerization. Science 2006, 312, 714–719. [Google Scholar] [CrossRef]
- Wang, H.P.; Khariwala, D.U.; Cheung, W.; Chum, S.P.; Hiltner, A.; Baer, E. Characterization of Some New Olefinic Block Copolymers. Macromolecules 2007, 40, 2852–2862. [Google Scholar] [CrossRef]
- Khariwala, D.; Taha, A.; Chum, S.; Hiltner, A.; Baer, E. Crystallization kinetics of some new olefinic block copolymers. Polymer 2008, 49, 1365–1375. [Google Scholar] [CrossRef]
- Wang, H.P.; Chum, S.P.; Hiltner, A.; Baer, E. Comparing Elastomeric Behavior of Block and Random Ethylene-Octene Copolymers. J. Appl. Polym. Sci. 2009, 113, 3236–3244. [Google Scholar] [CrossRef]
- Lin, Y.; Yakovleva, V.; Chen, H.; Hiltner, A.; Baer, E. Comparison of Olefin Copolymers as Compatibilizers for Polypropylene and High-Density Polyethylene. J. Appl. Polym. Sci. 2010, 113, 1945–1952. [Google Scholar] [CrossRef]
- Jin, J.; Du, J.; Xia, Q.; Liang, Y.; Han, C.C. Effect of Mesophase Separation on the Crystallization Behavior of Olefin Block Copolymers. Macromolecules 2010, 43, 10554–10559. [Google Scholar] [CrossRef]
- Zuo, F.; Burger, C.; Chen, X.M.; Mao, Y.M.; Hsiao, B.S. An in Situ X-ray Structural Study of Olefin Block and Random Copolymers under Uniaxial Deformation. Macromolecules 2010, 43, 1922–1929. [Google Scholar] [CrossRef]
- Guimarães, M.J.O.C.; Coutinho, F.M.B.; Rocha, M.C.G.; Farah, M.; Bretas, R.E.S. Effect of molecular weight and long chain branching of metallocene elastomers on the properties of high density polyethylene blends. Polym. Test. 2003, 22, 843–847. [Google Scholar] [CrossRef]
- Li, M.; Yu, L.Y.; Li, P.F.; Bin, Y.H.; Zhang, H.J. Effect of EPDM-g-MAH on properties of HDPE/OBC blends. Mater. Sci. Eng. 2017, 191, 012002. [Google Scholar] [CrossRef]
- Tabtiang, A.; Parchana, B.; Venables, R.A.; Inoue, T. Melt-Flow-Induced Phase Morphologies of a High-Density Polyethylene/Poly(ethylene-co-1-octene) Blend. J. Polym. Sci. Part B Polym. Phys. 2001, 39, 380–389. [Google Scholar] [CrossRef]
- Lee, S.H. Phase Behavior of Binary and Ternary Mixtures of Poly(ethylene-co-octene)-Hydrocarbons. J. Appl. Polym. Sci. 2005, 95, 161–165. [Google Scholar] [CrossRef]
- Wen, T.; Zhou, Y.; Liu, G.M.; Wang, F.S.; Zhang, X.Q.; Wang, D.J.; Chen, H.Y.; Walton, K.; Marchand, G.; Loos, J. Epitaxial crystallization of olefin block copolymers (OBCs) on uniaxially oriented isotactic polypropylene and high-density polyethylene films. Polymer 2012, 53, 529–535. [Google Scholar] [CrossRef]
- Kamdar, A.R.; Ayyer, R.K.; Poon, B.C.; Marchand, G.R.; Hiltner, A.; Baer, E. Effect of tie-layer thickness on the adhesion of ethylene-octene copolymers to polypropylene. Polymer 2009, 50, 3319–3328. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Fan, J.S.; Feng, J.C.; Lu, X.Y. Regulation of crystalline morphologies and mechanical properties of olefin multiblock copolymers by blending polymer with similar architecture of constituent blocks. Polymer 2015, 73, 139–148. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, G.M.; Wen, T.; Zhang, X.Q.; Dong, X.; Wang, D.J. High-density polyethylene blends regulate the tensile properties of olefin block copolymers. Acta. Polym. Sin. 2013, 5, 679–686. [Google Scholar]
- Chen, Y.H.; Ye, L. Structure and properties of PP/ORC/HDPE blends. J. Appl. Polym. Sci. 2011, 121, 1013–1022. [Google Scholar] [CrossRef]
- Auriemma, F.; De Rosa, C.; Scoti, M.; Di Girolamo, R.; Malafronte, A.; D’alterio, M.C.; Boggioni, L.; Losio, S.; Boccia, A.C.; Tritto, I. Structure and Mechanical Properties of Ethylene/1-Octene Multiblock Copolymers from Chain Shuttling Technology. Macromolecules 2019, 52, 2669–2680. [Google Scholar] [CrossRef]
- Liu, L.Z.; Hsiao, B.S.; Fu, B.X.; Ran, S.F.; Toki, S.; Chu, B.; Tsou, A.H.; Agarwal, P.K. Structure Changes during Uniaxial Deformation of Ethylene-Based Semicrystalline Ethylene-Propylene Copolymer. 1. SAXS Study. Macromolecules 2003, 36, 1920–1929. [Google Scholar] [CrossRef]
- Sinnott, K.M. Mechanical Relaxations in Single Crystals of Polyethylene. J. Polym. Sci. Part C Polym. Symp. 1966, 14, 141–172. [Google Scholar] [CrossRef]
- Simanke, A.G.; Galland, G.B.; Freitas, L.; da Jornada, J.A.H.; Quijada, R.; Mauler, R.S. Influence of the comonomer content on the thermal and dynamic mechanical properties of metallocene ethylene/1-octene copolymers. Polymer 1999, 40, 5489–5495. [Google Scholar] [CrossRef]
- Wang, C.C.; Wang, Y.X.; Shi, Y.; Liu, L.Z.; Bai, N.; Song, L.F. Effects of Dynamic Crosslinking on Crystallization, Structure and Mechanical Property of Ethylene-Octene Elastomer/EPDM Blends. Polymers 2021, 14, 139. [Google Scholar] [CrossRef]
- Popli, R.; Glotin, M.; Mandelkern, L. Dynamic Mechanical Studies of α and β Relaxations of Polyethylenes. J. Polym. Sci. Polym. Phys. Edit 1984, 22, 407–448. [Google Scholar] [CrossRef]
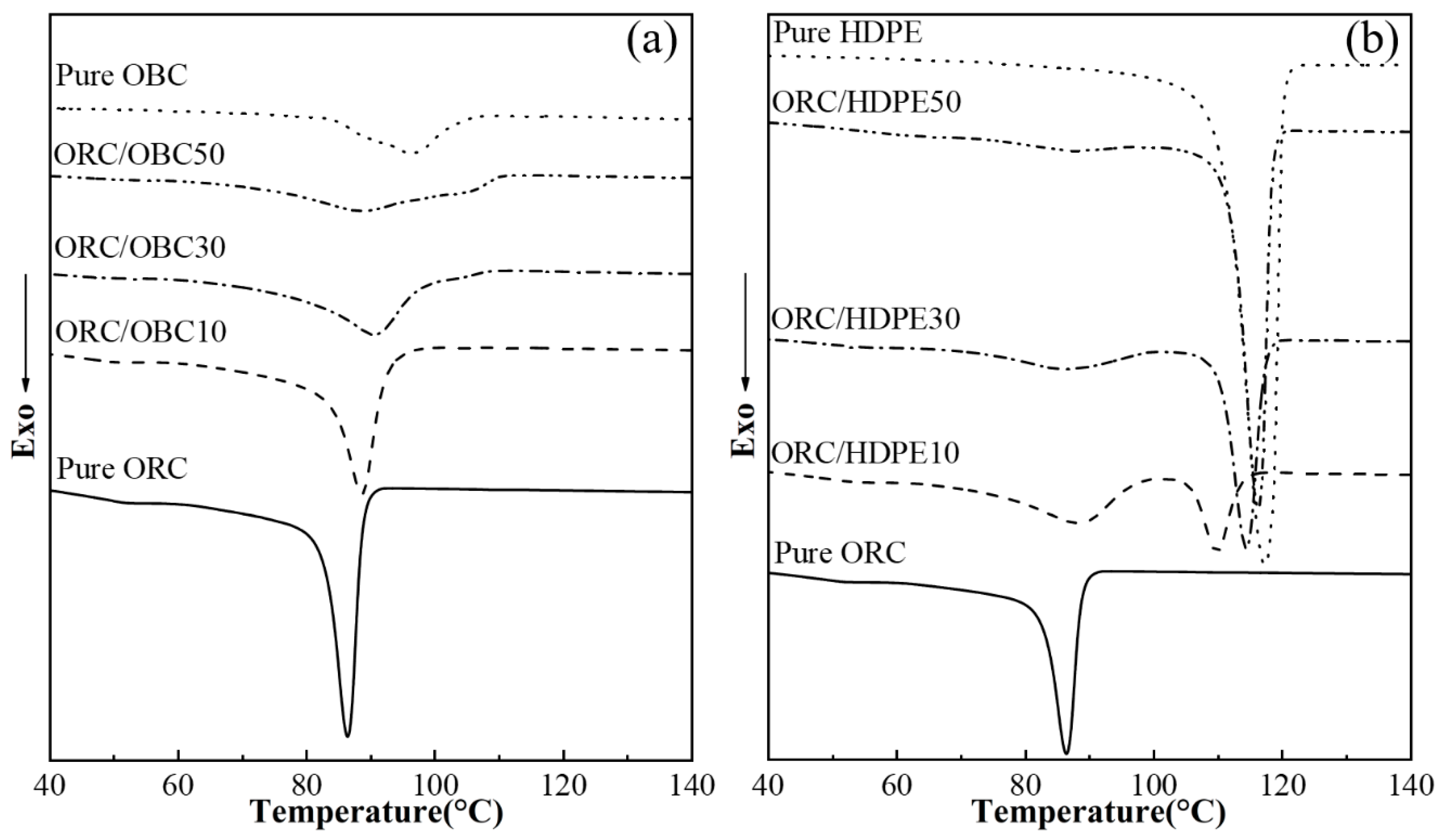
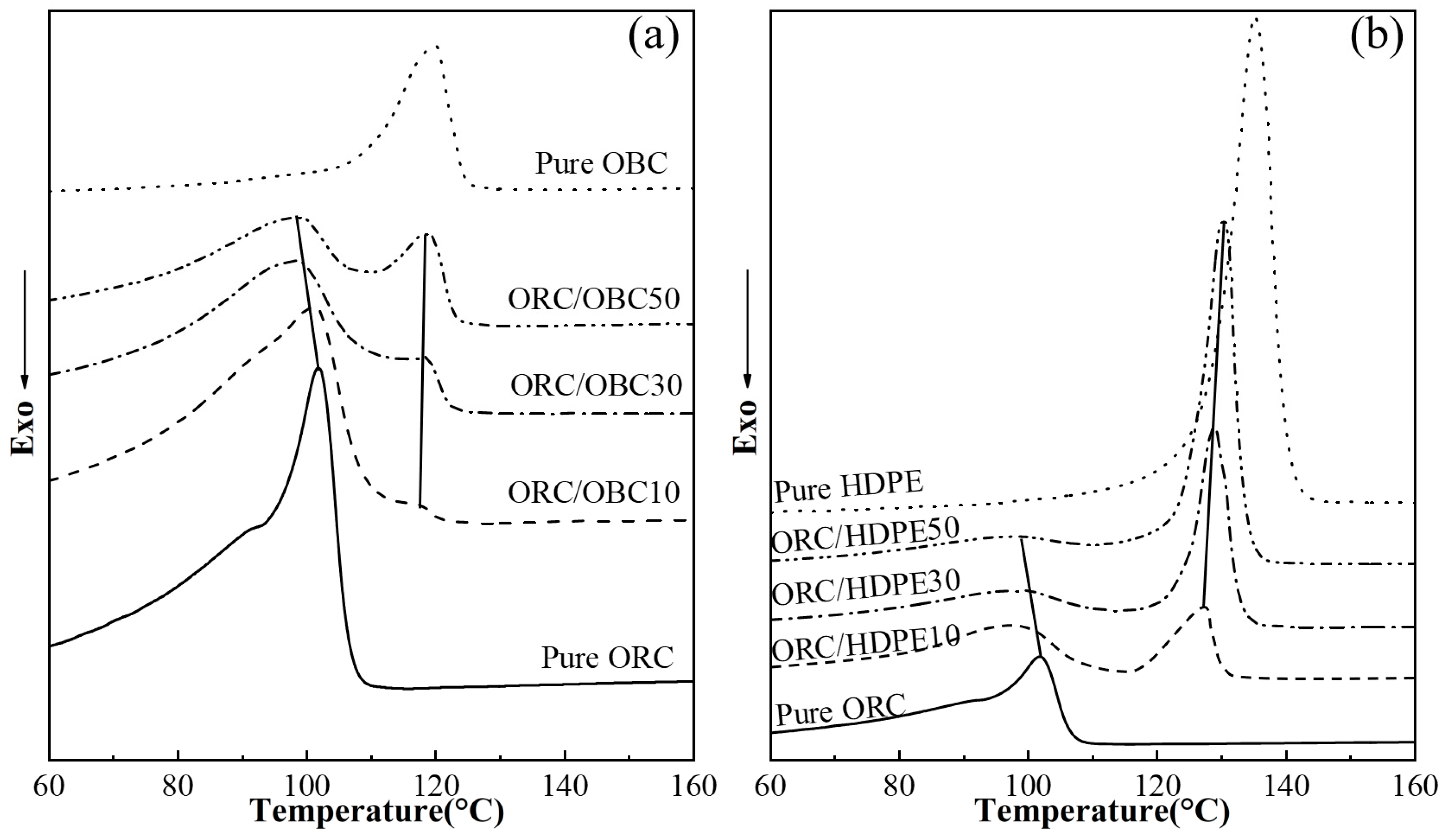
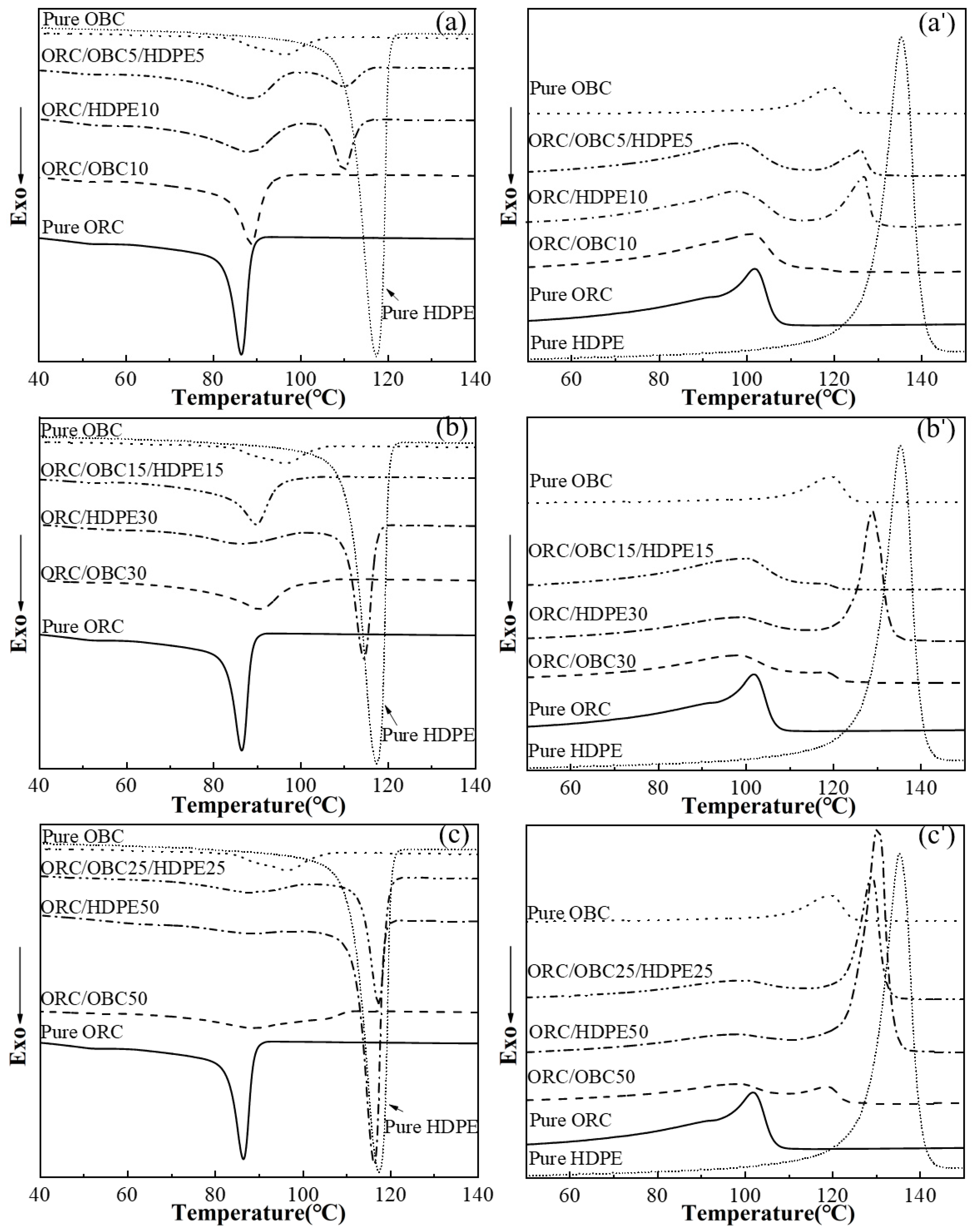

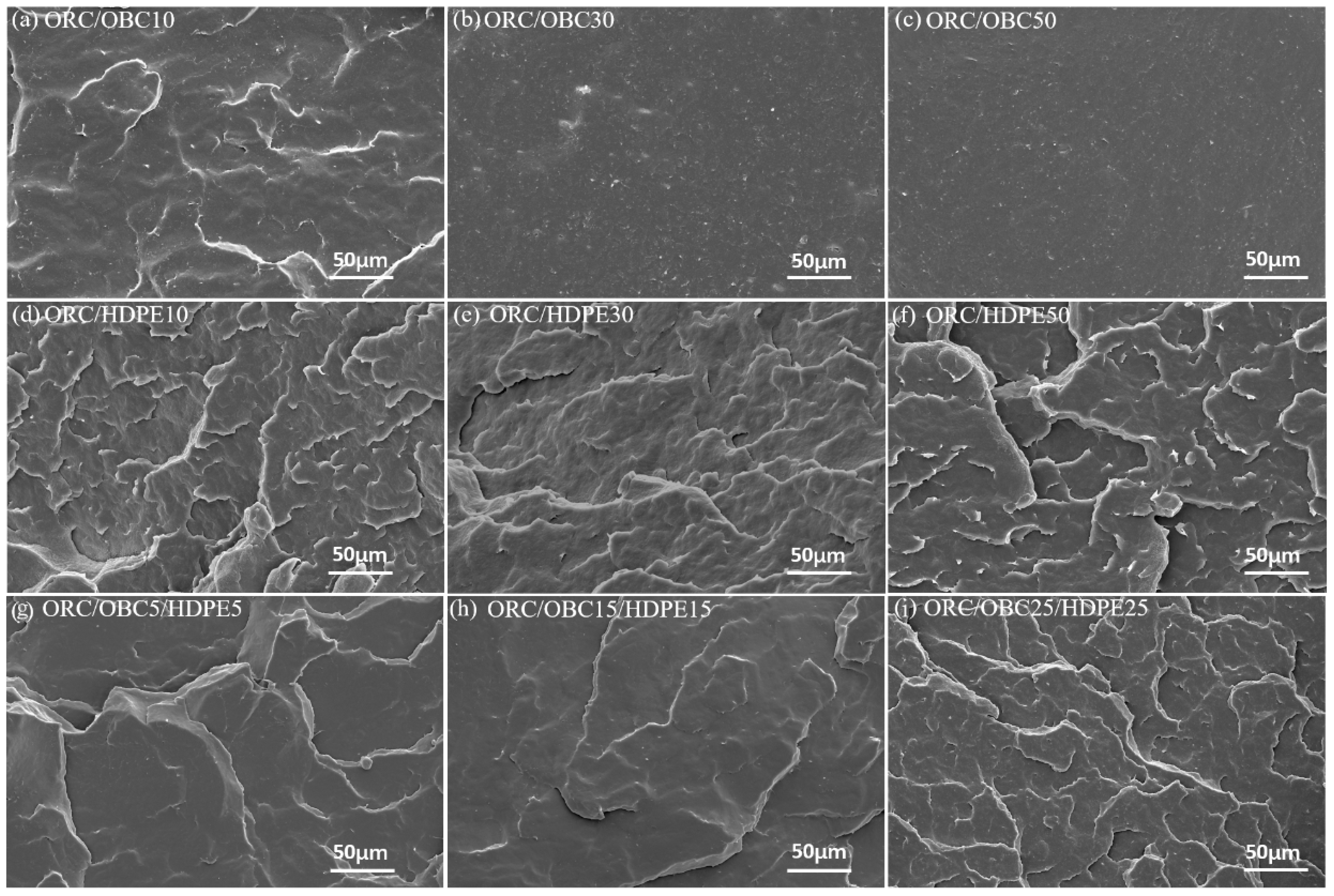


| Samples | Relative Amount by Weight (wt%) | ||
|---|---|---|---|
| ORC (ENGAGE 8480) | OBC (INFUSE 9107) | HDPE (52055L) | |
| ORC/OBC10 | 90 | 10 | 0 |
| ORC/OBC30 | 70 | 30 | 0 |
| ORC/OBC50 | 50 | 50 | 0 |
| ORC/HDPE10 | 90 | 0 | 10 |
| ORC/HDPE30 | 70 | 0 | 30 |
| ORC/HDPE50 | 50 | 0 | 50 |
| ORC/OBC5/HDPE5 | 90 | 5 | 5 |
| ORC/OBC15/HDPE15 | 70 | 15 | 15 |
| ORC/OBC25/HDPE25 | 50 | 25 | 25 |
| Sample | TC | ∆Hf (J/g) | Tm | Crystallinity (wt%) | ||
|---|---|---|---|---|---|---|
| Tc1 (°C) | Tc2 (°C) | Tm1 (°C) | Tm2 (°C) | |||
| ORC/OBC10 | 89 | - | 86.2 | 101 | 118 | 29.4 |
| ORC/OBC30 | 91 | 106 | 76.7 | 98 | 118 | 26.2 |
| ORC/OBC50 | 87 | 106 | 59.7 | 99 | 119 | 20.4 |
| Sample | TC | ∆Hf (J/g) | Tm | Crystallinity (wt%) | ||
|---|---|---|---|---|---|---|
| Tc1 (°C) | Tc2 (°C) | Tm1 (°C) | Tm2 (°C) | |||
| ORC/HDPE10 | 89 | 110 | 98.5 | 100 | 123 | 33.6 |
| ORC/HDPE30 | 86 | 115 | 124.8 | 100 | 129 | 42.6 |
| ORC/HDPE50 | 86 | 117 | 152.3 | 101 | 130 | 52.0 |
| Sample | Tensile Strength (MPa) | Elongation at Break (%) | Tensile Stress (MPa) | Permanent Set of Elongation at Break (%) | |
|---|---|---|---|---|---|
| 100% | 300% | ||||
| Pure ORC | 29.1 ± 1.2 | 668 ± 20 | 6.8 ± 0.1 | 7.8 ± 0.1 | 460 ± 13 |
| Pure OBC | 4.5 ± 0.8 | 863 ± 32 | 1.5 ± 0.0 | 2.3 ± 0.1 | 330 ± 18 |
| Pure HDPE | 30.5 ± 0.7 | 1293 ± 43 | 15.2 ± 0.2 | 14.8 ± 0.0 | 1100 ± 22 |
| ORC/OBC10 | 38.2 ± 1.1 | 740 ± 21 | 6.8 ± 0.1 | 7.8 ± 0.0 | 414 ± 11 |
| ORC/OBC30 | 23.8 ± 0.6 | 712 ± 19 | 5.3 ± 0.0 | 6.2 ± 0.1 | 391 ± 9 |
| ORC/OBC50 | 17.5 ± 0.5 | 702 ± 17 | 4.9 ± 0.0 | 5.8 ± 0.0 | 336 ± 12 |
| ORC/HDPE10 | 36.3 ± 1.0 | 701 ± 20 | 6.8 ± 0.1 | 7.9 ± 0.0 | 474 ± 15 |
| ORC/HDPE30 | 33.2 ± 0.4 | 746 ± 22 | 10.4 ± 0.1 | 10.7 ± 0.0 | 538 ± 13 |
| ORC/HDPE50 | 30.7 ± 0.8 | 821 ± 34 | 12.8 ± 0.0 | 13.0 ± 0.2 | 575 ± 15 |
| ORC/OBC5/HDPE5 | 40.1 ± 0.7 | 716 ± 22 | 8.2 ± 0.1 | 9.2 ± 0.0 | 455 ± 13 |
| ORC/OBC15/HDPE15 | 33.6 ± 0.4 | 743 ± 19 | 6.7 ± 0.0 | 7.8 ± 0.0 | 405 ± 8 |
| ORC/OBC25/HDPE25 | 20.2 ± 1.0 | 731 ± 24 | 7.3 ± 0.1 | 7.7 ± 0.0 | 538 ± 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.-X.; Zou, C.-Y.; Bai, N.; Su, Q.-F.; Song, L.-X.; Li, X.-L. Effect of Octene Block Copolymer (OBC) and High-Density Polyethylene (HDPE) on Crystalline Morphology, Structure and Mechanical Properties of Octene Random Copolymer. Polymers 2023, 15, 3655. https://doi.org/10.3390/polym15183655
Wang Y-X, Zou C-Y, Bai N, Su Q-F, Song L-X, Li X-L. Effect of Octene Block Copolymer (OBC) and High-Density Polyethylene (HDPE) on Crystalline Morphology, Structure and Mechanical Properties of Octene Random Copolymer. Polymers. 2023; 15(18):3655. https://doi.org/10.3390/polym15183655
Chicago/Turabian StyleWang, Yuan-Xia, Cun-Ying Zou, Nan Bai, Qun-Feng Su, Li-Xin Song, and Xian-Liang Li. 2023. "Effect of Octene Block Copolymer (OBC) and High-Density Polyethylene (HDPE) on Crystalline Morphology, Structure and Mechanical Properties of Octene Random Copolymer" Polymers 15, no. 18: 3655. https://doi.org/10.3390/polym15183655
APA StyleWang, Y.-X., Zou, C.-Y., Bai, N., Su, Q.-F., Song, L.-X., & Li, X.-L. (2023). Effect of Octene Block Copolymer (OBC) and High-Density Polyethylene (HDPE) on Crystalline Morphology, Structure and Mechanical Properties of Octene Random Copolymer. Polymers, 15(18), 3655. https://doi.org/10.3390/polym15183655






