Embedded Living HER2+ Cells in a 3D Gelatin–Alginate Hydrogel as an In Vitro Model for Immunotherapy Delivery for Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biopolymer Syntesis and Characterization
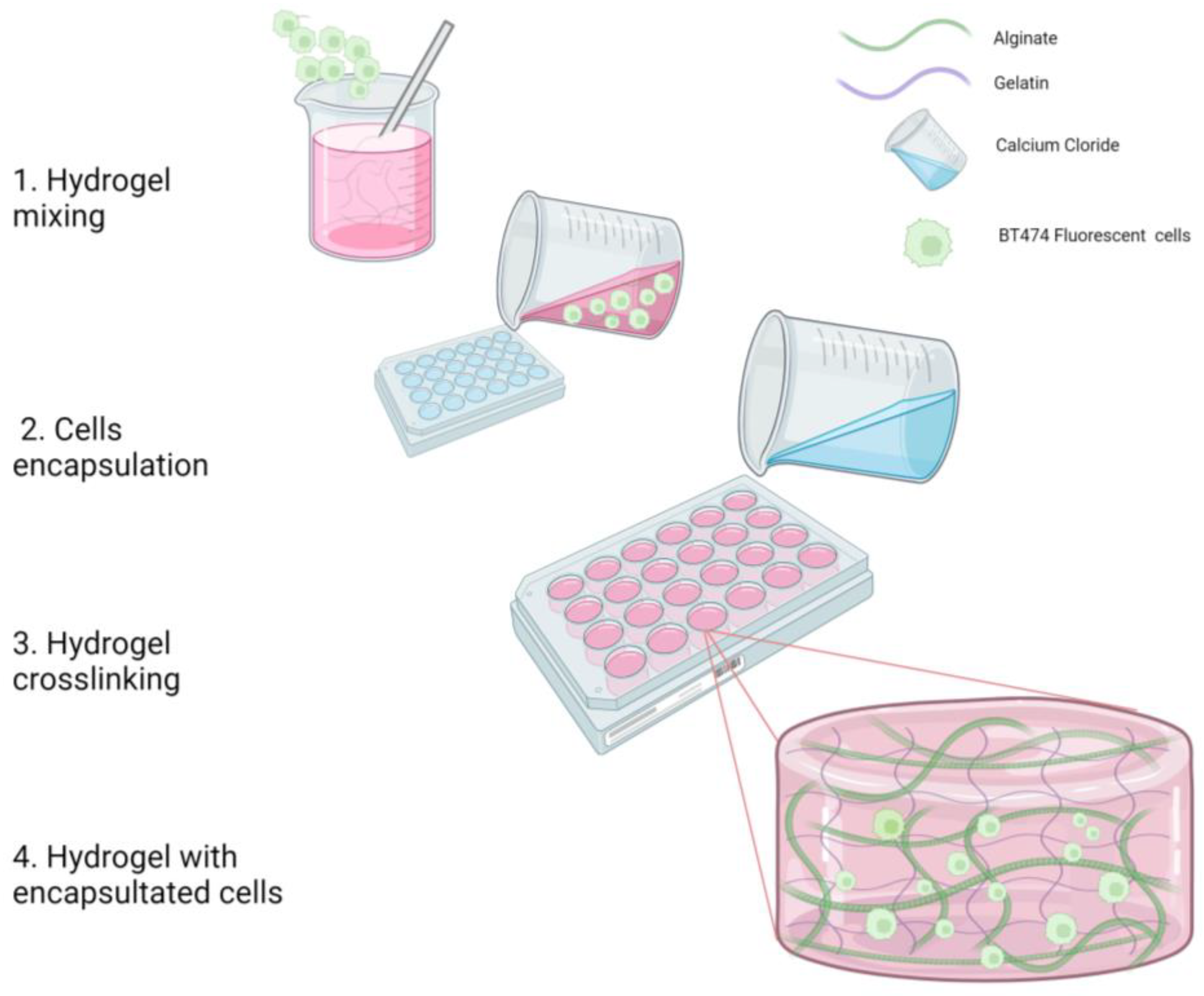
2.1.1. Hydrogel Fabrication (Biopolymer Mix) without Cells
2.1.2. Cells Encapsulation in the Biopolymer Mix
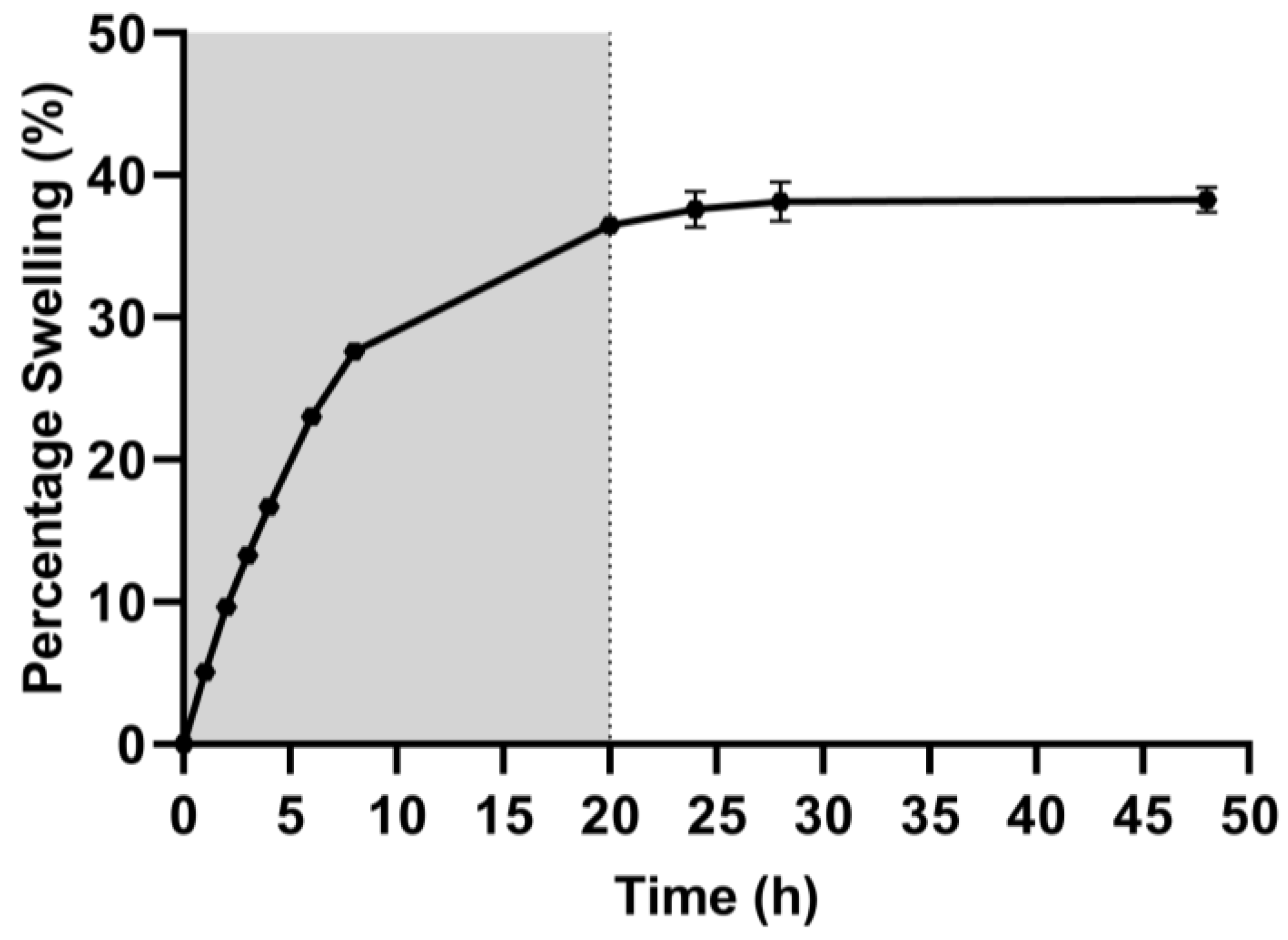
2.2. Swelling Capacity
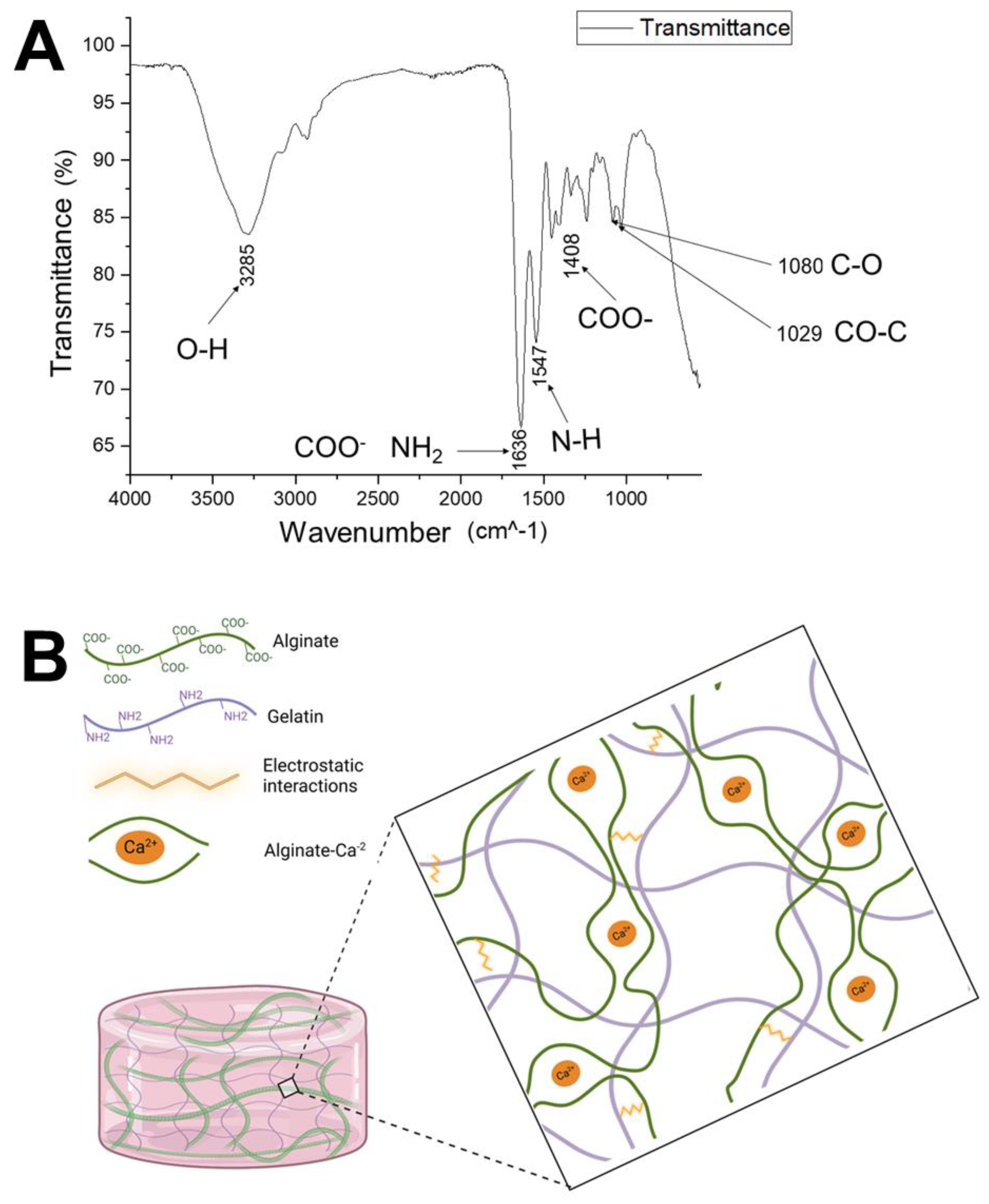
2.3. Fourier-Transform Infrared Spectroscopy (FTIR)
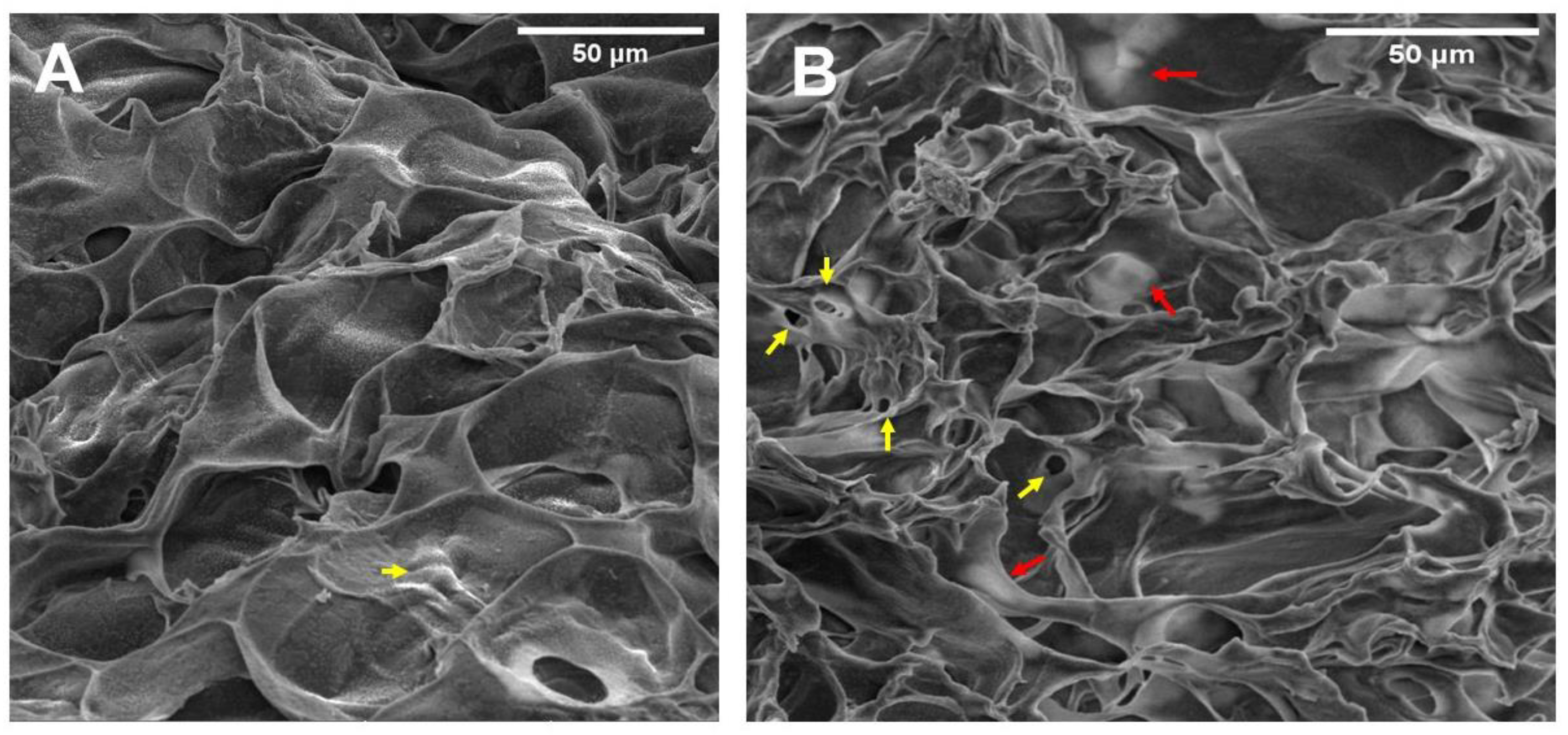
2.4. Hydrogel Microstructure and Pore Size
2.5. Hydrogel Biocompatibility and Metabolic Activity
2.6. Morphology and Distribution of Embedded Cells within Hydrogel
2.7. Evaluation of Genes Related to Hypoxia, Apoptosis, and HER2 Receptor
2.7.1. Total RNA Extraction
2.7.2. Retrotranscription of Total RNA from Embedded Cells
2.8. Trastuzumab-Fluorescein Conjugate Penetration in the Hydrogel with Embedded Cells
2.9. Statistical Analysis
3. Results and Discussion
3.1. Hydrogel Fabrication
3.2. Hydrogel Swelling Capacity
3.3. Fourier-Transform Infrared Spectroscopy (FTIR)
3.4. Hydrogel Microstructure and Pore Size
3.5. Hydrogel Biocompatibility
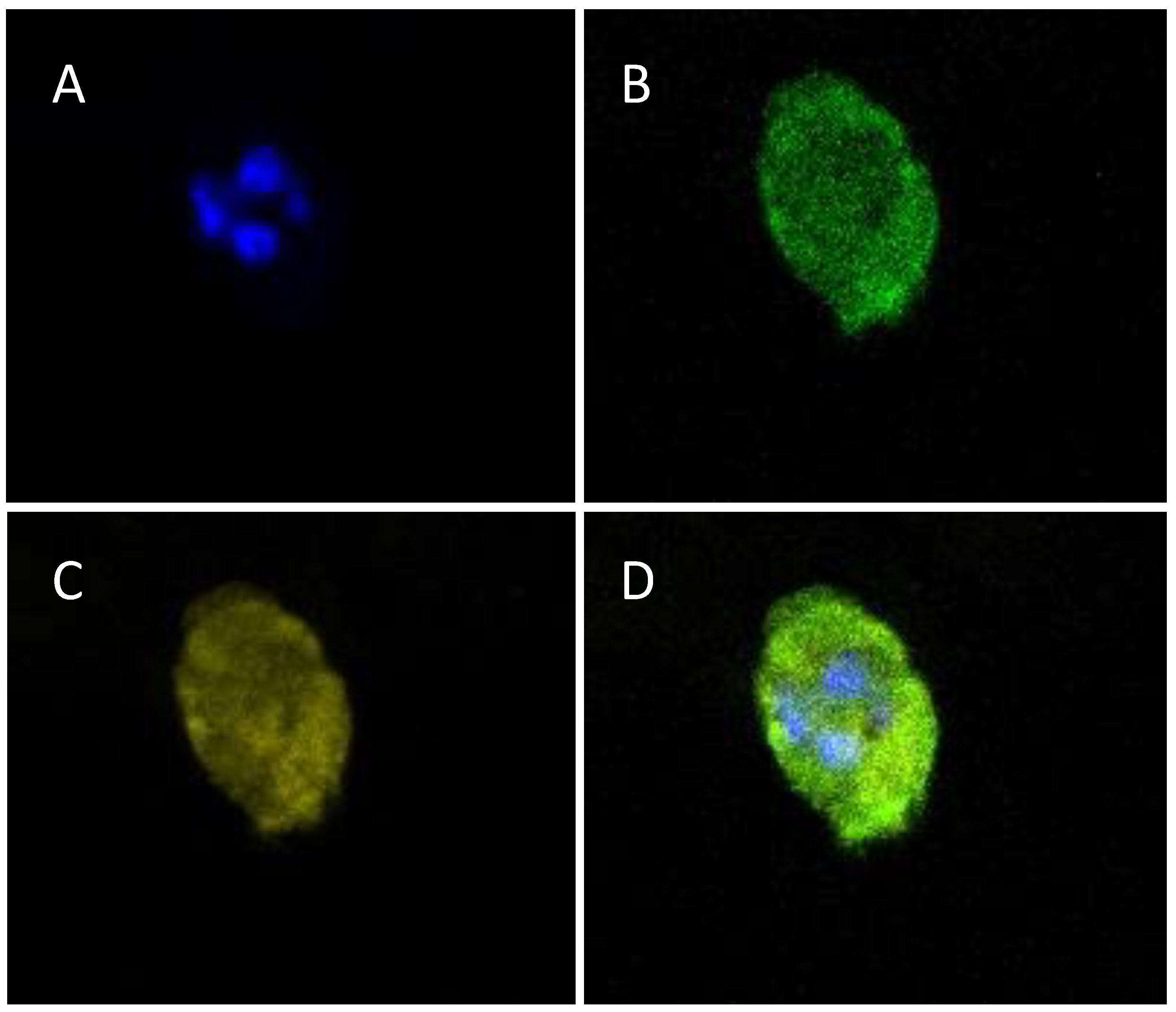
3.6. Morphology and Distribution of Living Embedded Cells within Hydrogel
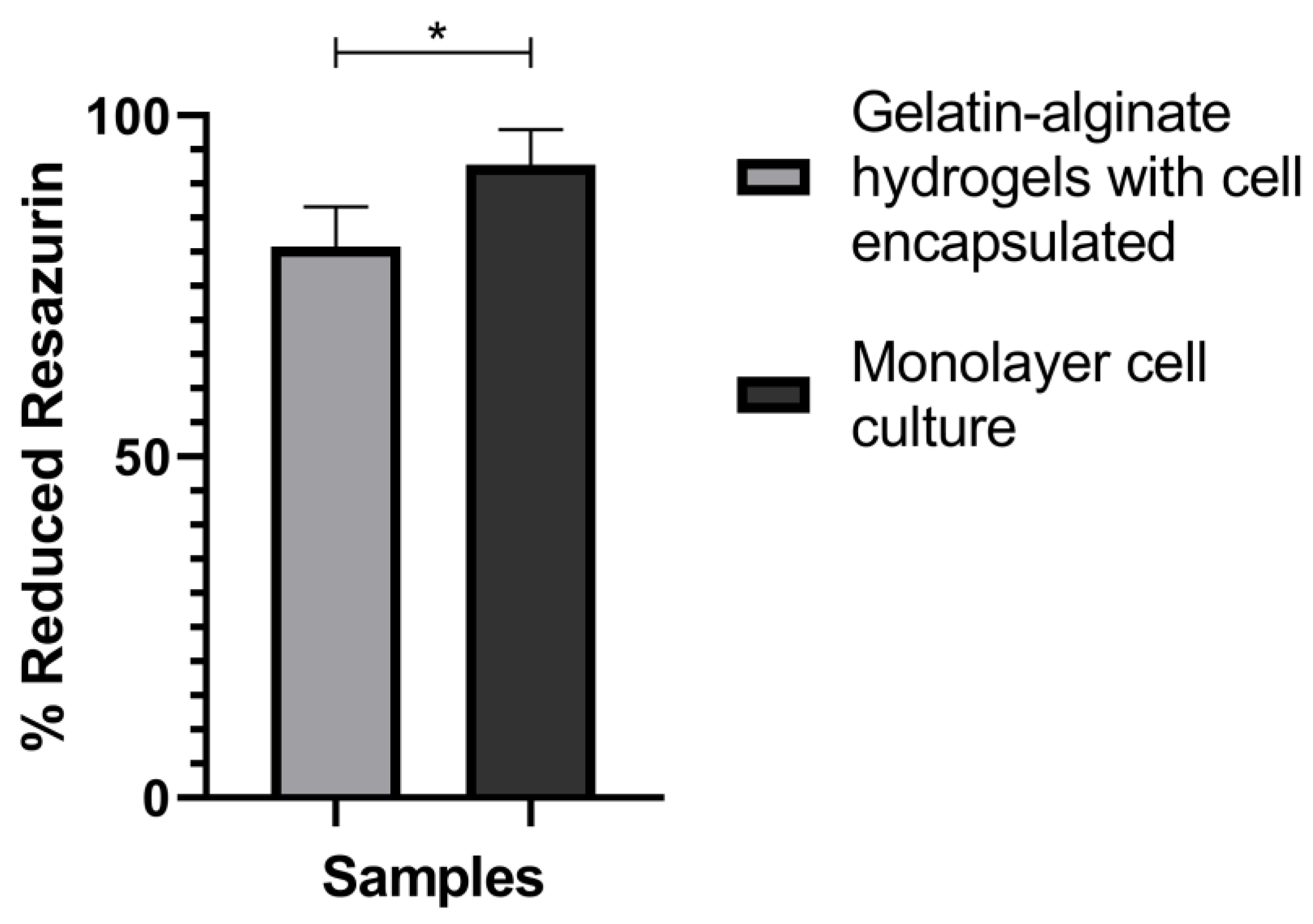
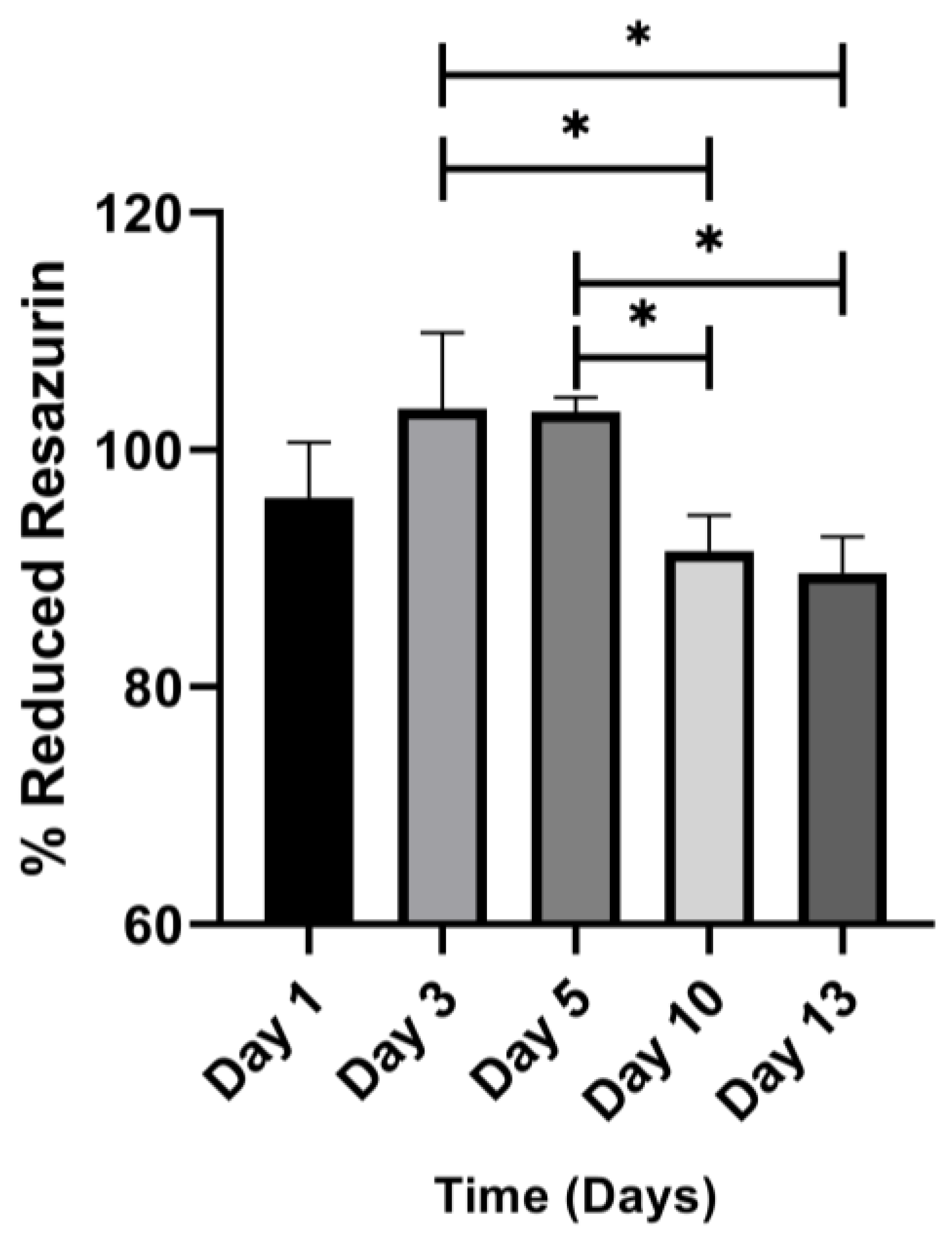
3.7. Metabolic Activity of Embedded Cell
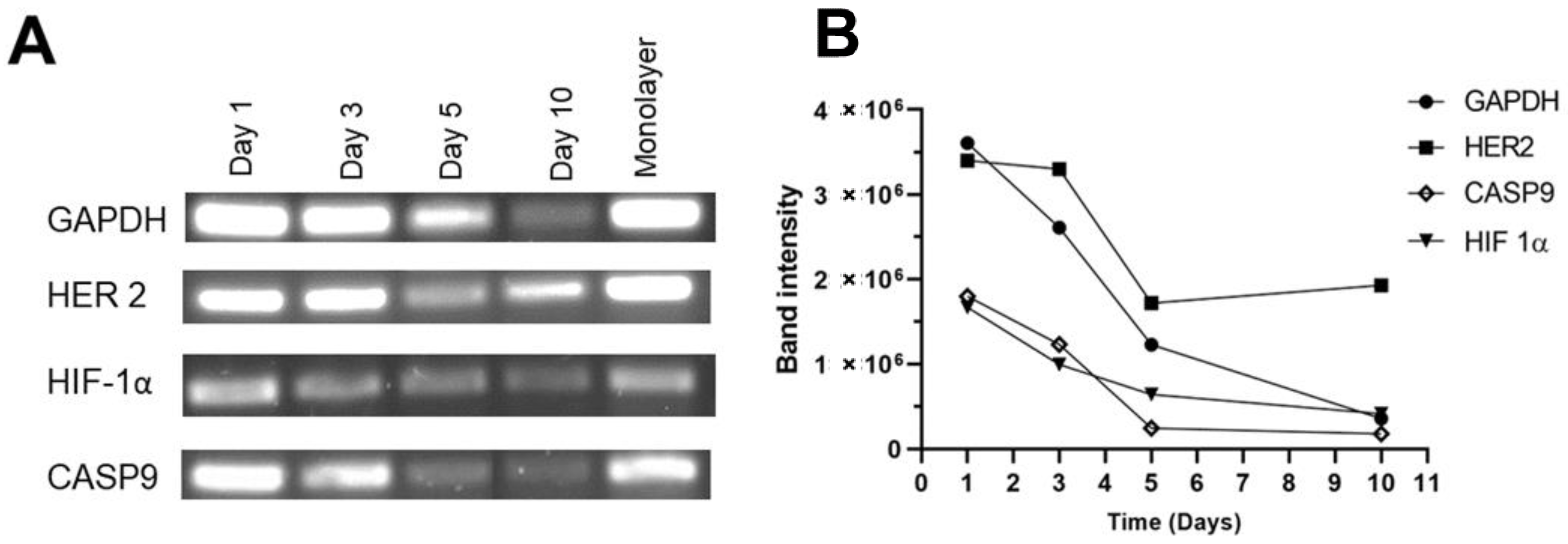
3.8. Evaluation of Genes Related to Hypoxia, Apoptosis, and HER2 Receptor
3.9. Trastuzumab-Fluorescein Conjugate Penetration into the Hydrogel
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NIEHS. National Institute of Environmental Health Sciences. Available online: www.niehs.nih.gov (accessed on 15 April 2022).
- Feng, Y.; Spezia, M.; Huang, S.; Yuan, C.; Zeng, Z.; Zhang, L.; Ji, X.; Liu, W.; Huang, B.; Luo, W.; et al. Breast Cancer Development and Progression: Risk Factors, Cancer Stem Cells, Signaling Pathways, Genomics, and Molecular Pathogenesis. Genes Dis. 2018, 5, 77–106. [Google Scholar] [CrossRef] [PubMed]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Piñeros, M.; Znaor, A.; Soerjomataram, I.; Bray, F. Global Cancer Observatory: Cancer Today. Available online: https://gco.iarc.fr/ (accessed on 22 May 2022).
- Rossi, L.; Mazzara, C.; Pagani, O. Diagnosis and Treatment of Breast Cancer in Young Women. Curr. Treat. Options Oncol. 2019, 20, 86. [Google Scholar] [CrossRef] [PubMed]
- Meisel, J.L.; Venur, V.A.; Gnant, M.; Carey, L. Evolu on of Targeted Therapy in Breast Cancer: Where precision medicine began. Am. Soc. Clin. Oncol. Educ. Book 2018, 38, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Tai, W.; Mahato, R.; Cheng, K. The Role of HER2 in Cancer Therapy and Targeted Drug Delivery. J. Control. Release 2010, 146, 264–275. [Google Scholar] [CrossRef]
- Oh, D.Y.; Bang, Y.J. HER2-Targeted Therapies—A Role beyond Breast Cancer. Nat. Rev. Clin. Oncol. 2019, 17, 33–48. [Google Scholar] [CrossRef]
- Meric-Bernstam, F.; Johnson, A.M.; Dumbrava, E.E.I.; Raghav, K.; Balaji, K.; Bhatt, M.; Murthy, R.K.; Rodon, J.; Piha-Paul, S.A. Advances in HER2-Targeted Therapy: Novel Agents and Opportunities Beyond Breast and Gastric Cancer. Clin. Cancer Res. 2019, 25, 2033–2041. [Google Scholar] [CrossRef]
- Pupa, S.M.; Ligorio, F.; Cancila, V.; Franceschini, A.; Tripodo, C.; Vernieri, C.; Castagnoli, L. HER2 Signaling and Breast Cancer Stem Cells: The Bridge behind HER2-Positive Breast Cancer Aggressiveness and Therapy Refractoriness. Cancers 2021, 13, 4778. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Nath, S.; Devi, G.R. Three-Dimensional Culture Systems in Cancer Research: Focus on Tumor Spheroid Model. Pharmacol. Ther. 2016, 163, 94–108. [Google Scholar] [CrossRef]
- Yang, J.; Ju, J.; Guo, L.; Ji, B.; Shi, S.; Yang, Z.; Gao, S.; Yuan, X.; Tian, G.; Liang, Y.; et al. Prediction of HER2-Positive Breast Cancer Recurrence and Metastasis Risk from Histopathological Images and Clinical Information via Multimodal Deep Learning. Comput. Struct. Biotechnol. J. 2022, 20, 333–342. [Google Scholar] [CrossRef]
- Cao, H.; Duan, L.; Zhang, Y.; Cao, J.; Zhang, K. Current Hydrogel Advances in Physicochemical and Biological Response-Driven Biomedical Application Diversity. Signal Transduct. Target. Ther. 2021, 6, 426. [Google Scholar] [CrossRef]
- Nosrati, H.; Pourmotabed, S.; Sharifi, E. A Review on Some Natural Biopolymers and Their Applications in Angiogenesis and Tissue Engineering. J. Appl. Biotechnol. Rep. 2018, 5, 81–91. [Google Scholar] [CrossRef]
- Afewerki, S.; Sheikhi, A.; Kannan, S.; Ahadian, S.; Khademhosseini, A. Gelatin-Polysaccharide Composite Scaffolds for 3D Cell Culture and Tissue Engineering: Towards Natural Therapeutics. Bioeng. Transl. Med. 2019, 4, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Feng, W.; Jin, C. Protocol Efficiently Measuring the Swelling Rate of Hydrogels. MethodsX 2020, 7, 100779. [Google Scholar] [CrossRef] [PubMed]
- Raghuwanshi, V.S.; Garnier, G. Characterisation of Hydrogels: Linking the Nano to the Microscale. Adv. Colloid Interface Sci. 2019, 274, 102044. [Google Scholar] [CrossRef]
- Fan, L.; Yang, H.; Yang, J.; Peng, M.; Hu, J. Preparation and Characterization of Chitosan/Gelatin/PVA Hydrogel for Wound Dressings. Carbohydr. Polym. 2016, 146, 427–434. [Google Scholar] [CrossRef]
- Wang, Q.-Q.; Liu, Y.; Zhang, C.-J.; Zhang, C.; Zhu, P. Alginate/Gelatin Blended Hydrogel Fibers Cross-linked by Ca2+ and Oxidized Starch: Preparation and Properties. Mater. Sci. Eng. C 2019, 99, 1469–1476. [Google Scholar] [CrossRef]
- Ye, J.; Yang, G.; Zhang, J.; Xiao, Z.; He, L.; Zhang, H.; Liu, Q. Preparation and Characterization of Gelatin-Polysaccharide Composite Hydrogels for Tissue Engineering. PeerJ 2021, 9, e11022. [Google Scholar] [CrossRef]
- Derkach, S.R.; Voron’ko, N.G.; Sokolan, N.I.; Kolotova, D.S.; Kuchina, Y.A. Interactions between Gelatin and Sodium Alginate: UV and FTIR Studies. J. Dispers. Sci. Technol. 2020, 41, 690–698. [Google Scholar] [CrossRef]
- Lopes, S.; Bueno, L.; de Aguiar, F., Jr.; Finkler, C. Preparation and Characterization of Alginate and Gelatin Microcapsules Containing Lactobacillus Rhamnosus. An. Acad. Bras. Cienc. 2017, 89, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Kariduraganavar, M.Y.; Kittur, A.A.; Kamble, R.R. Polymer Synthesis and Processing. In Natural and Synthetic Biomedical Polymers; Elsevier: Amsterdam, The Netherlands, 2014; pp. 1–31. [Google Scholar] [CrossRef]
- Karvinen, J.; Ihalainen, T.O.; Calejo, M.T.; Jönkkäri, I.; Kellomäki, M. Characterization of the Microstructure of Hydrazone Cross-linked Polysaccharide-Based Hydrogels through Rheological and Diffusion Studies. Mater. Sci. Eng. C 2019, 94, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Xu, L.; Zhou, Y.; Zhang, X.; Huang, X.; Wang, M.; Han, Y.; Zhai, M.; Wei, S.; Li, J. A Green Fabrication Approach of Gelatin/CM-Chitosan Hybrid Hydrogel for Wound Healing. Carbohydr. Polym. 2010, 82, 1297–1305. [Google Scholar] [CrossRef]
- Wang, J.; Fan, X.; Liu, H.; Tang, K. Self-Assembly and Metal Ions-Assisted One Step Fabrication of Recoverable Gelatin Hydrogel with High Mechanical Strength. Polym. Technol. Mater. 2020, 59, 1899–1909. [Google Scholar] [CrossRef]
- Coluccino, L.; Stagnaro, P.; Vassalli, M.; Scaglione, S. Bioactive TGF-Β1/HA Alginate-Based Scaffolds for Osteochondral Tissue Repair: Design, Realization and Multilevel Characterization. J. Appl. Biomater. Funct. Mater. 2016, 14, 42–52. [Google Scholar] [CrossRef]
- Espona-Noguera, A.; Ciriza, J.; Cañibano-Hernández, A.; Fernandez, L.; Ochoa, I.; Saenz del Burgo, L.; Pedraz, J.L. Tunable Injectable Alginate-Based Hydrogel for Cell Therapy in Type 1 Diabetes Mellitus. Int. J. Biol. Macromol. 2018, 107, 1261–1269. [Google Scholar] [CrossRef]
- Akther, F.; Little, P.; Li, Z.; Nguyen, N.-T.; Ta, H.T. Hydrogels as Artificial Matrices for Cell Seeding in Microfluidic Devices. RSC Adv. 2020, 10, 43682–43703. [Google Scholar] [CrossRef]
- Thorpe, A.; Creasey, S.; Sammon, C.; Le Maitre, C. Hydroxyapatite Nanoparticle Injectable Hydrogel Scaffold to Support Osteogenic Differentiation of Human Mesenchymal Stem Cells. Eur. Cells Mater. 2016, 32, 1–23. [Google Scholar] [CrossRef]
- Yannas, I.V.; Tzeranis, D.S.; Harley, B.A.; So, P.T.C. Biologically Active Collagen-Based Scaffolds: Advances in Processing and Characterization. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 2123–2139. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Zhang, Y.; Xu, W.; Xiao, J.; Suo, A. Growth of MCF-7 Breast Cancer Cells and Efficacy of Anti-Angiogenic Agents in a Hydroxyethyl Chitosan/Glycidyl Methacrylate Hydrogel. Cancer Cell Int. 2017, 17, 55. [Google Scholar] [CrossRef]
- Farino, C.J.; Pradhan, S.; Slater, J.H. The Influence of Matrix-Induced Dormancy on Metastatic Breast Cancer Chemoresistance. ACS Appl. Bio Mater. 2020, 3, 5832–5844. [Google Scholar] [CrossRef]
- Pradhan, S.; Slater, J.H. Tunable Hydrogels for Controlling Phenotypic Cancer Cell States to Model Breast Cancer Dormancy and Reactivation. Biomaterials 2019, 215, 119177. [Google Scholar] [CrossRef]
- Barber, R.D.; Harmer, D.W.; Coleman, R.A.; Clark, B.J. GAPDH as a Housekeeping Gene: Analysis of GAPDH MRNA Expression in a Panel of 72 Human Tissues. Physiol. Genom. 2005, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Carcereri de Prati, A.; Butturini, E.; Rigo, A.; Oppici, E.; Rossin, M.; Boriero, D.; Mariotto, S. Metastatic Breast Cancer Cells Enter Into Dormant State and Express Cancer Stem Cells Phenotype Under Chronic Hypoxia. J. Cell. Biochem. 2017, 118, 3237–3248. [Google Scholar] [CrossRef] [PubMed]
- Pranzini, E.; Raugei, G.; Taddei, M.L. Metabolic Features of Tumor Dormancy: Possible Therapeutic Strategies. Cancers 2022, 14, 547. [Google Scholar] [CrossRef]
- Semenza, G.L. Regulation of Gene Transcription by Hypoxia-Inducible Factor 1. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 67–71. [Google Scholar] [CrossRef]
- Muz, B.; de la Puente, P.; Azab, F.; Azab, A.K. The Role of Hypoxia in Cancer Progression, Angiogenesis, Metastasis, and Resistance to Therapy. Hypoxia 2015, 3, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Wang, M.; Schmid, T.; Xin, Z.; Kozhuharova, L.; Yu, W.-K.; Huang, Y.; Cai, F.; Biskup, E. Hypoxia in Breast Cancer—Scientific Translation to Therapeutic and Diagnostic Clinical Applications. Front. Oncol. 2021, 11, 652266. [Google Scholar] [CrossRef]
- Fluegen, G.; Avivar-Valderas, A.; Wang, Y.; Padgen, M.R.; Williams, J.K.; Nobre, A.R.; Calvo, V.; Cheung, J.F.; Bravo-Cordero, J.J.; Entenberg, D.; et al. Phenotypic Heterogeneity of Disseminated Tumour Cells Is Preset by Primary Tumour Hypoxic Microenvironments. Nat. Cell Biol. 2017, 19, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Brentnall, M.; Rodriguez-Menocal, L.; De Guevara, R.L.; Cepero, E.; Boise, L.H. Caspase-9, Caspase-3 and Caspase-7 Have Distinct Roles during Intrinsic Apoptosis. BMC Cell Biol. 2013, 14, 32. [Google Scholar] [CrossRef]
- Molnár, T.; Pallagi, P.; Tél, B.; Király, R.; Csoma, E.; Jenei, V.; Varga, Z.; Gogolák, P.; Odile Hueber, A.; Máté, Z.; et al. Caspase-9 Acts as a Regulator of Necroptotic Cell Death. FEBS J. 2021, 288, 6476–6491. [Google Scholar] [CrossRef]
- Azimi, T.; Loizidou, M.; Dwek, M.V. Cancer Cells Grown in 3D under Fluid Flow Exhibit an Aggressive Phenotype and Reduced Responsiveness to the Anti-Cancer Treatment Doxorubicin. Sci. Rep. 2020, 10, 12020. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Bilgin, C.C.; Fontenay, G.; Chang, H.; Henderson, M.; Han, J.; Parvin, B. Stiffness of the Microenvironment Upregulates ERBB2 Expression in 3D Cultures of MCF10A within the Range of Mammographic Density. Sci. Rep. 2016, 6, 28987. [Google Scholar] [CrossRef] [PubMed]



| Gene | Oligonucleotide Sequence 5′-3′ | Tm (°C) |
|---|---|---|
| HER 2 | FW: GGAAGTACACGATGCGGAGACT | 66.8 |
| RV: ACCTTCCTCAGCTCCGTCTCTT | 66.9 | |
| HIF-1α | FW: TATGAGCCAGAAGAACTTTTAGGC | 63.8 |
| RV: CACCTCTTTTGGCAAGCATCCTG | 70.9 | |
| GADPH | FW: GCACAGTCAAGGCCGAGAAT | 65.7 |
| RV: GCCTTCTCCATGGTGGTGAA | 65.3 | |
| CASP 9 | FW: GTTTGAGGACCTTCGACCAGCT | 68 |
| RV: CAACGTACCAGGAGCCACTCTT | 66.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Dios-Figueroa, G.T.; Aguilera-Márquez, J.d.R.; García-Uriostegui, L.; Hernández-Gutiérrez, R.; Camacho-Villegas, T.A.; Lugo-Fabres, P.H. Embedded Living HER2+ Cells in a 3D Gelatin–Alginate Hydrogel as an In Vitro Model for Immunotherapy Delivery for Breast Cancer. Polymers 2023, 15, 3726. https://doi.org/10.3390/polym15183726
De Dios-Figueroa GT, Aguilera-Márquez JdR, García-Uriostegui L, Hernández-Gutiérrez R, Camacho-Villegas TA, Lugo-Fabres PH. Embedded Living HER2+ Cells in a 3D Gelatin–Alginate Hydrogel as an In Vitro Model for Immunotherapy Delivery for Breast Cancer. Polymers. 2023; 15(18):3726. https://doi.org/10.3390/polym15183726
Chicago/Turabian StyleDe Dios-Figueroa, G. Tonantzin, Janette del Rocío Aguilera-Márquez, Lorena García-Uriostegui, Rodolfo Hernández-Gutiérrez, Tanya A. Camacho-Villegas, and Pavel H. Lugo-Fabres. 2023. "Embedded Living HER2+ Cells in a 3D Gelatin–Alginate Hydrogel as an In Vitro Model for Immunotherapy Delivery for Breast Cancer" Polymers 15, no. 18: 3726. https://doi.org/10.3390/polym15183726
APA StyleDe Dios-Figueroa, G. T., Aguilera-Márquez, J. d. R., García-Uriostegui, L., Hernández-Gutiérrez, R., Camacho-Villegas, T. A., & Lugo-Fabres, P. H. (2023). Embedded Living HER2+ Cells in a 3D Gelatin–Alginate Hydrogel as an In Vitro Model for Immunotherapy Delivery for Breast Cancer. Polymers, 15(18), 3726. https://doi.org/10.3390/polym15183726







