In Situ Synthesis of Gold Nanoparticles from Chitin Nanogels and Their Drug Release Response to Stimulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
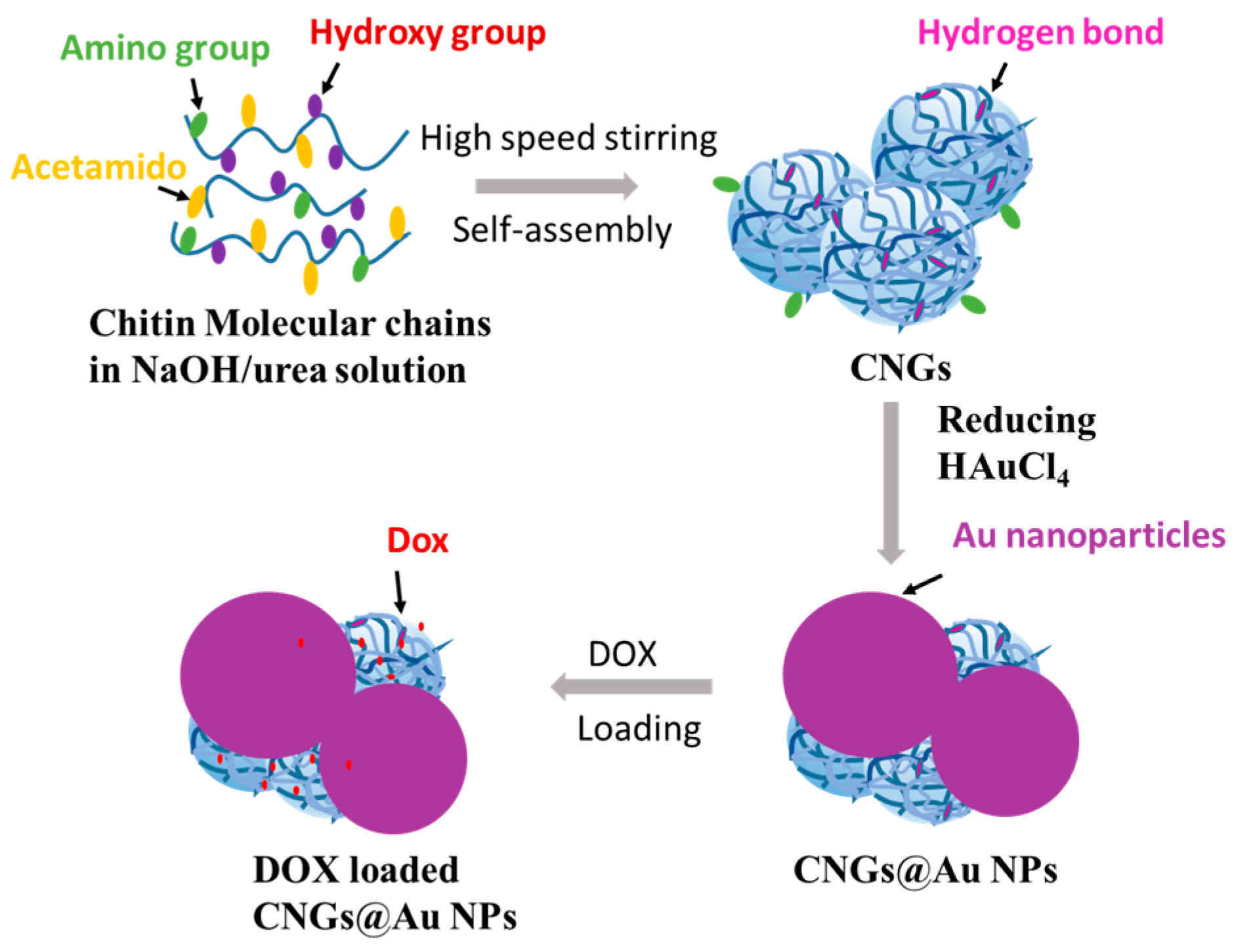
2.2. Preparation of CNGs@AuNPs
2.3. Conformal Characterization of CNGs@AuNPs
2.4. Absorption Spectroscopic Determination of CNGs@AuNPs
2.5. Experiments on the Photothermal Properties of CNGs@AuNPs
2.6. UV Standard Curve Plotting for DOX
2.7. CNGs@AuNP DOX Loading and DOX-Controlled Release Experiments
2.7.1. DOX Adsorption Experiment
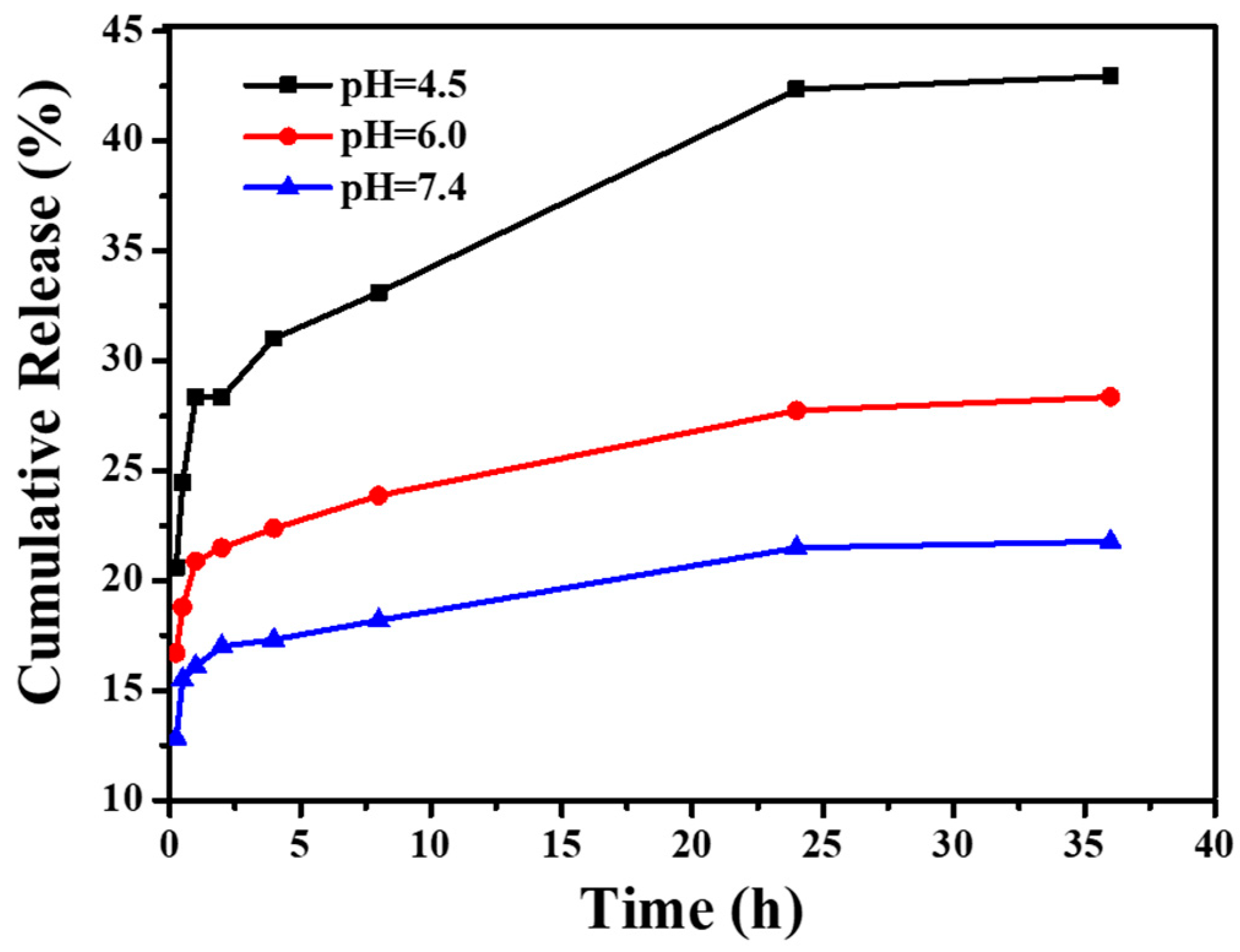
2.7.2. In Vitro Drug Release Experiments under Different pH Conditions
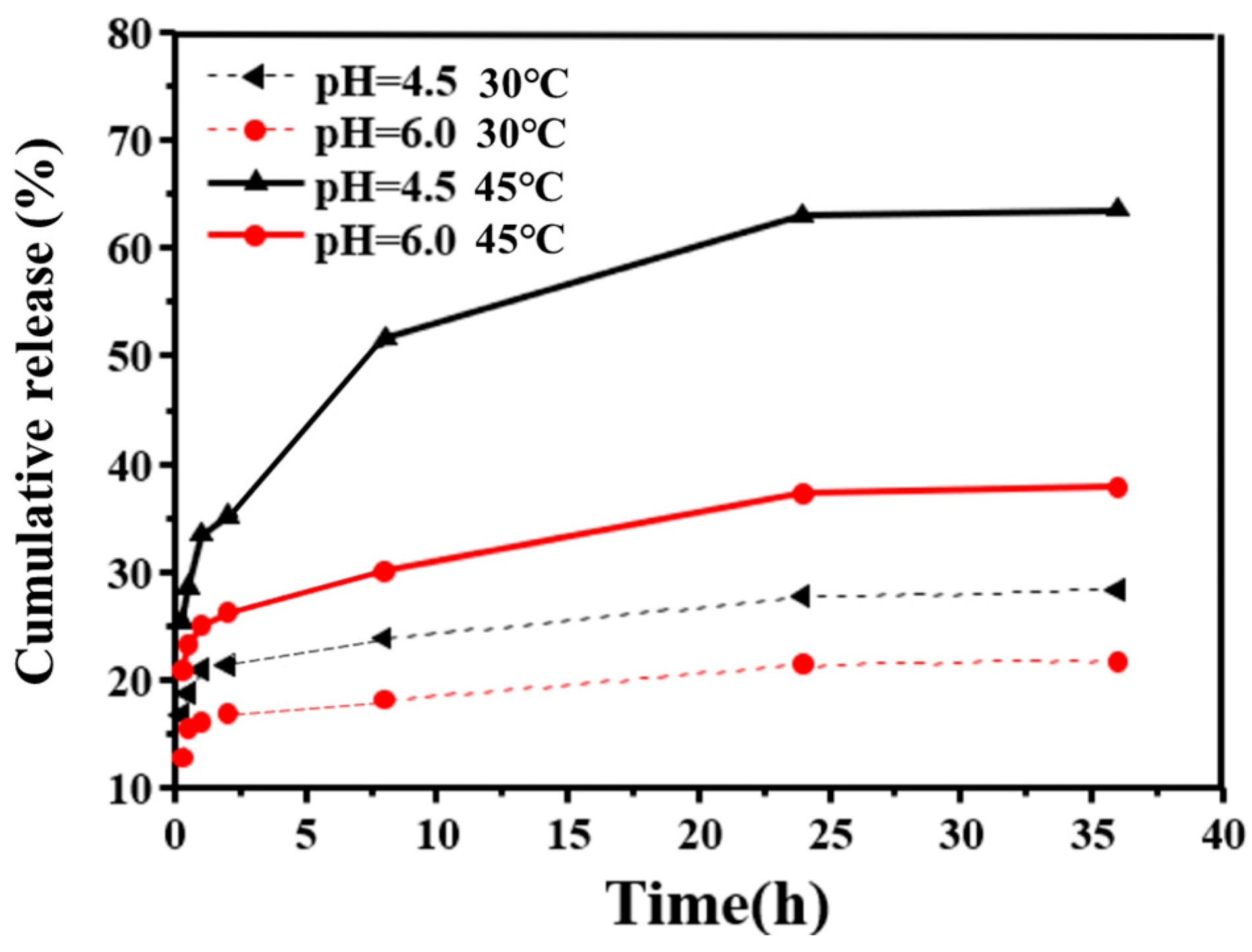
2.7.3. In Vitro Drug Release Experiments at Different Temperatures
2.7.4. Experiment under NIR Laser Stimulation
2.8. In Vitro Photothermal Therapy and Photothermal Therapy/Chemotherapy Cell Experiments
3. Results and Discussion
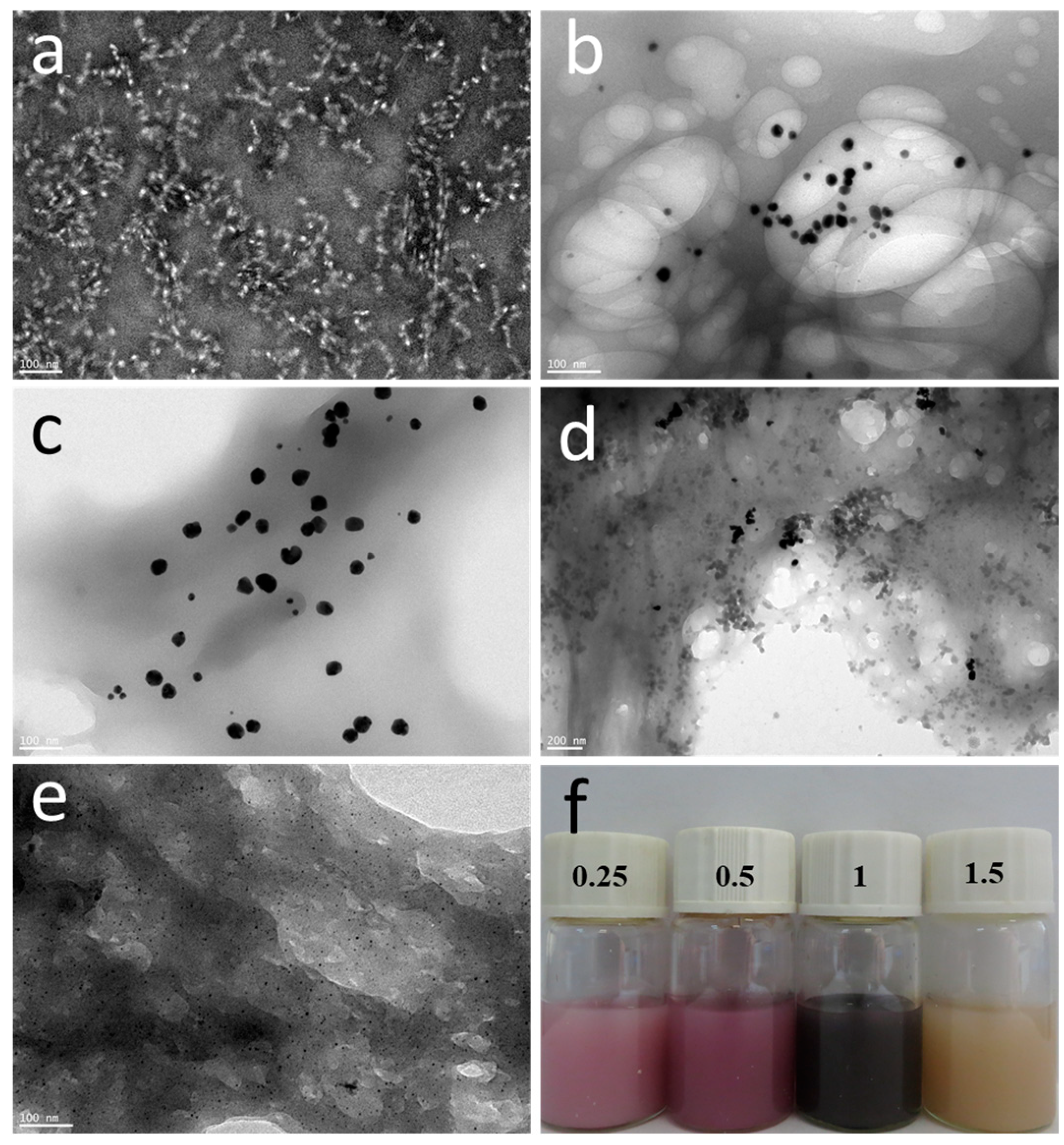
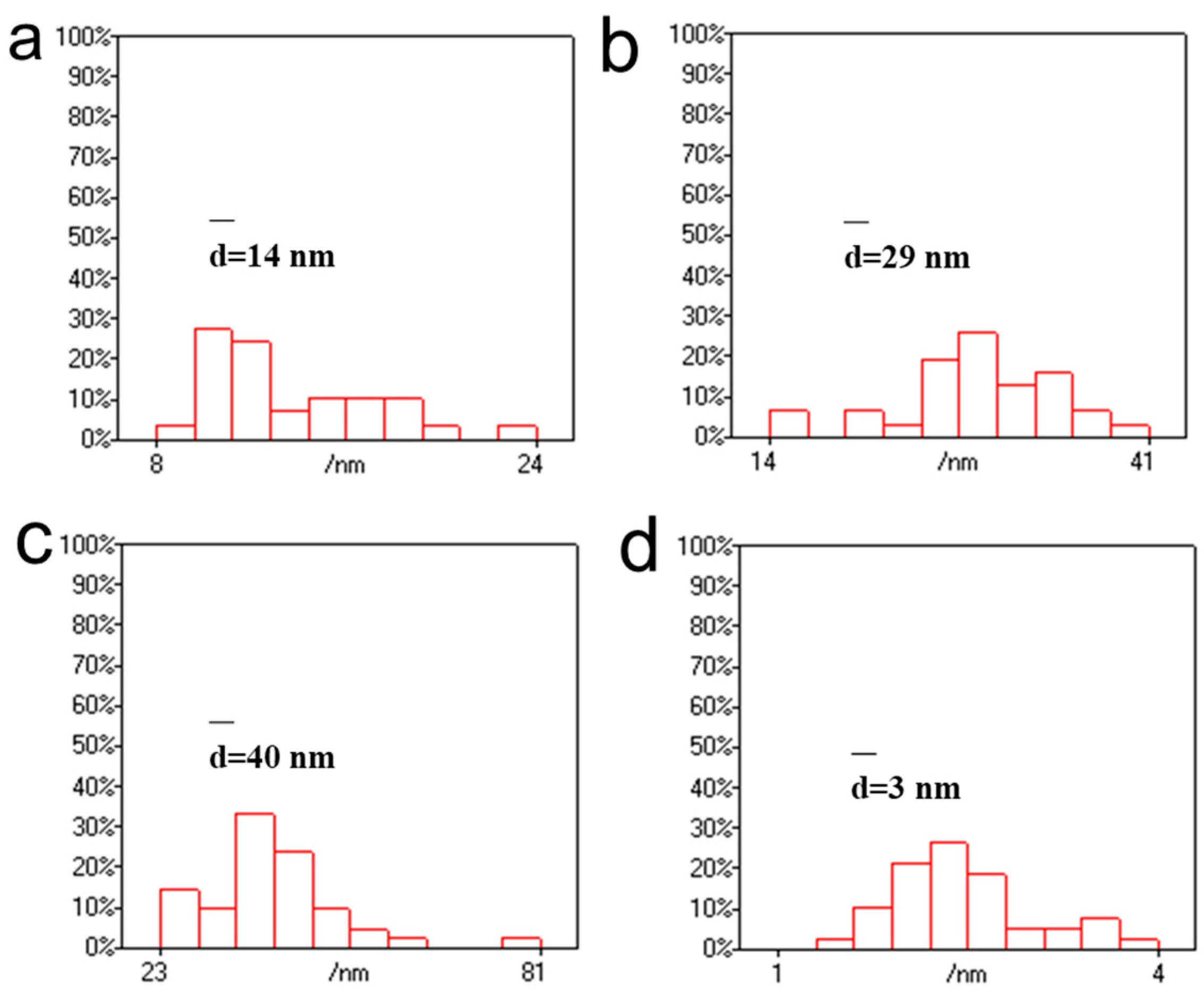
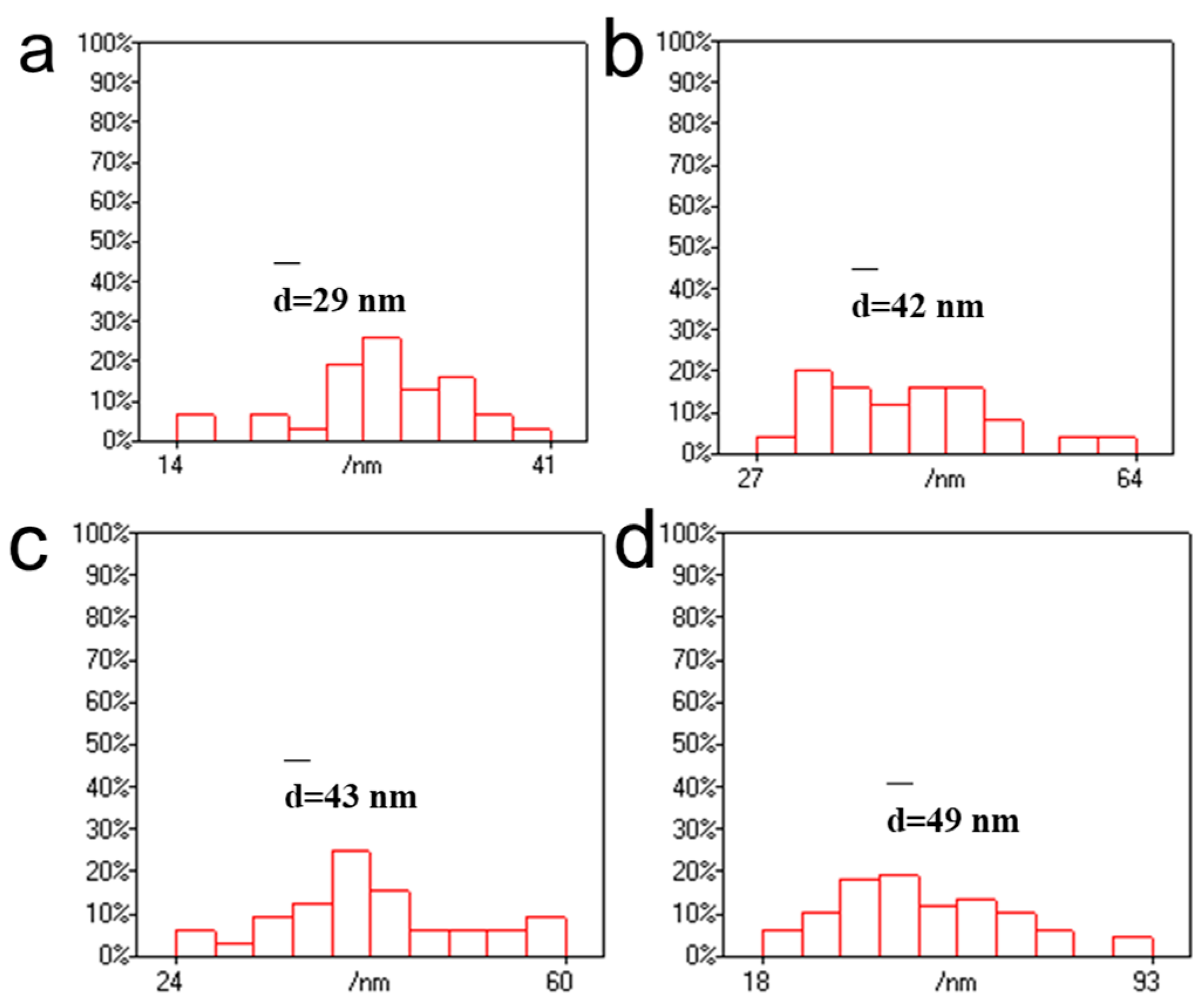
3.1. Effect of Chloroauric Acid Concentration on the Morphology of CNGs@AuNPs
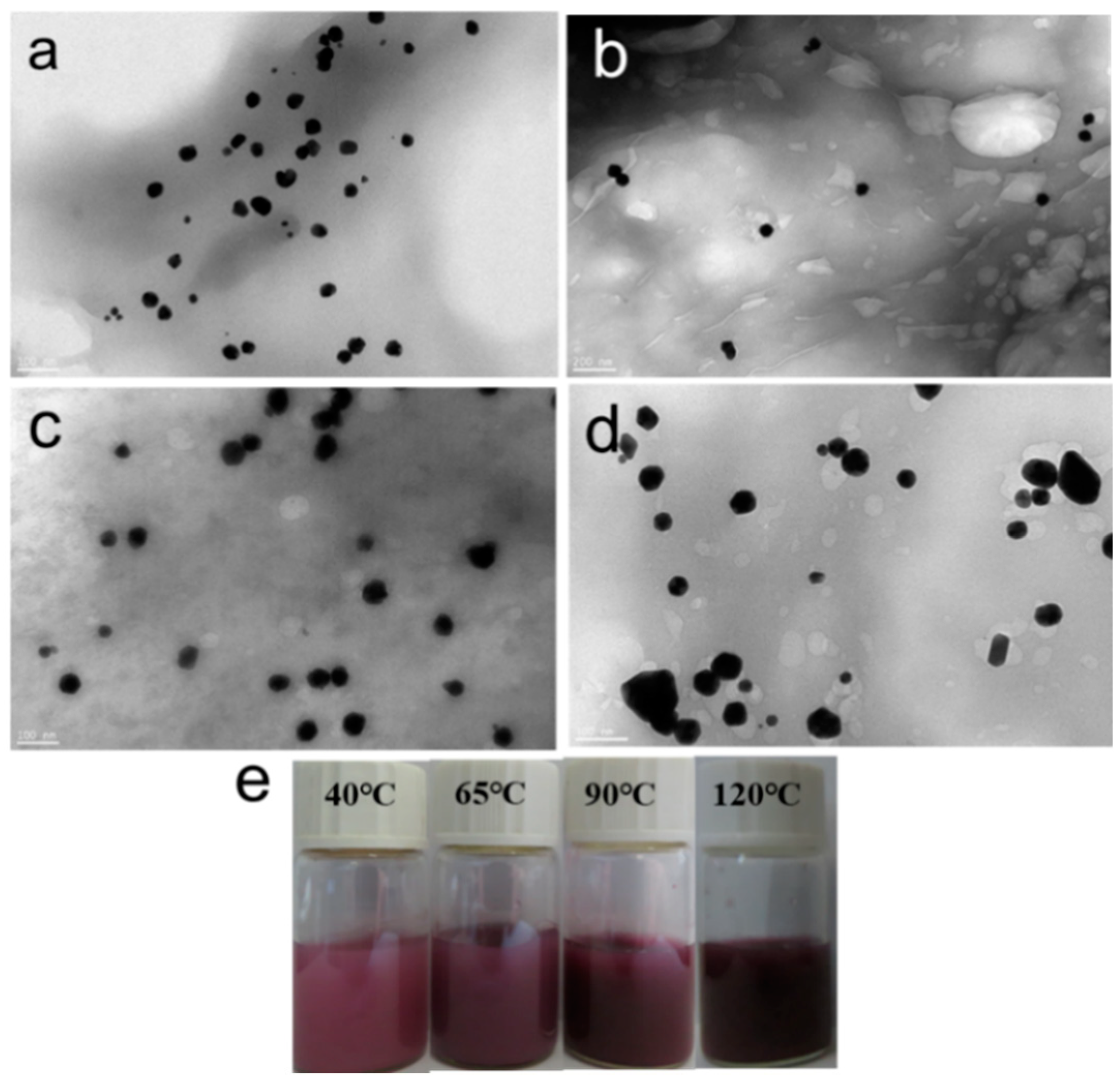
3.2. Effect of Reaction Temperature on the Morphology of CNGs@AuNPs
3.3. Absorption Spectral Analysis of CNGs@AuNPs
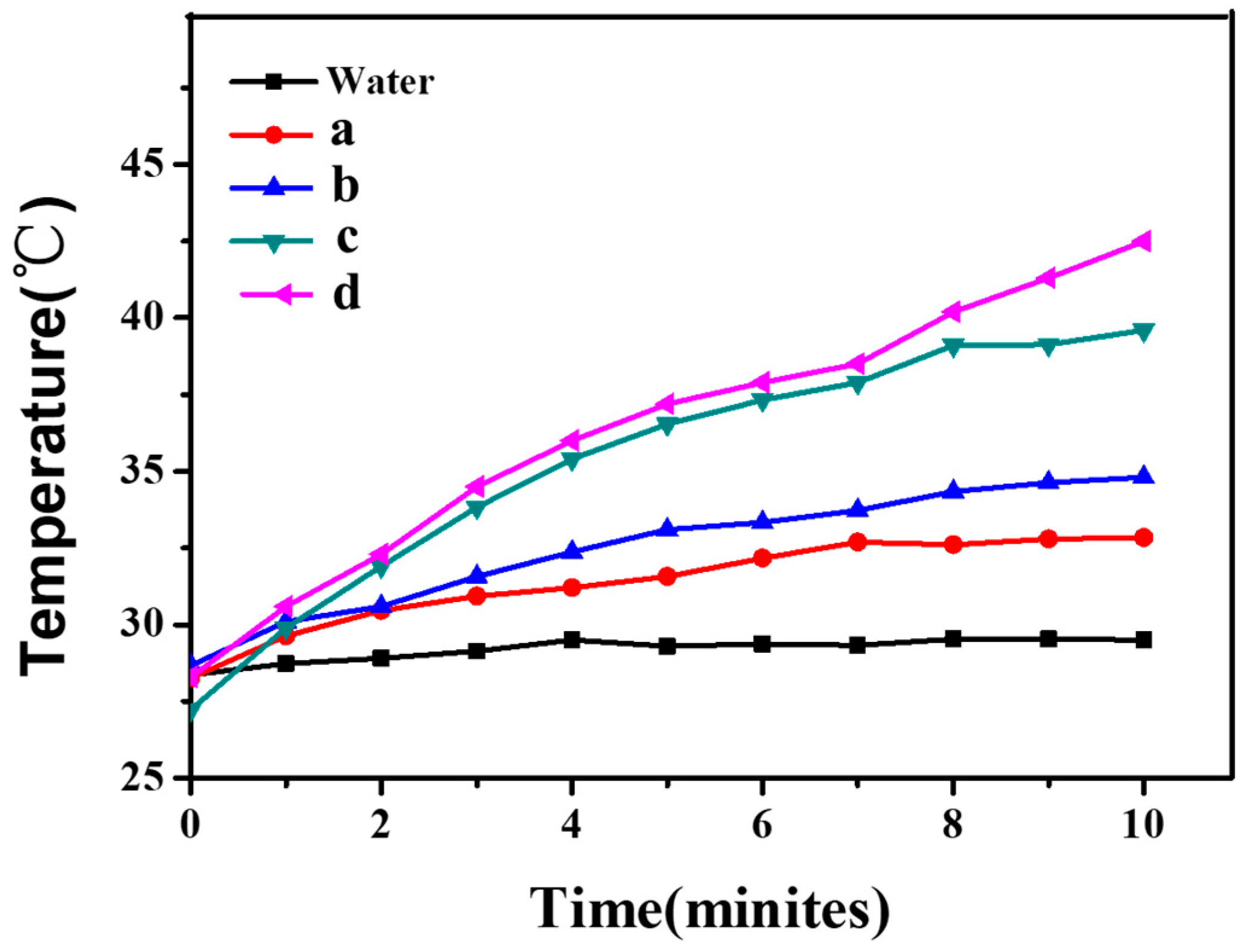
3.4. Analysis of Photothermal Properties of CNGs@AuNPs
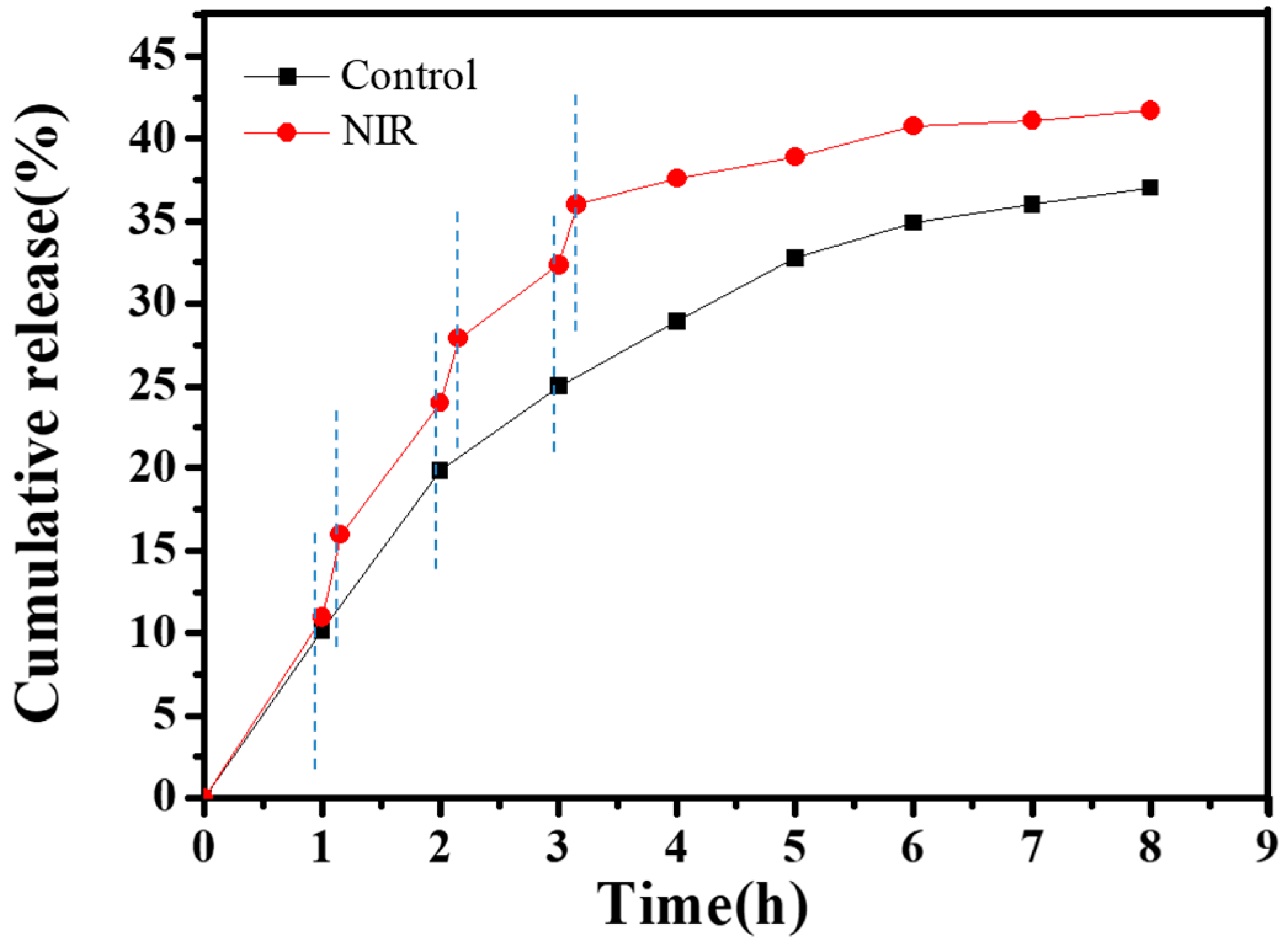
3.5. CNGs@AuNPs Drug Loading and Stimulated Release
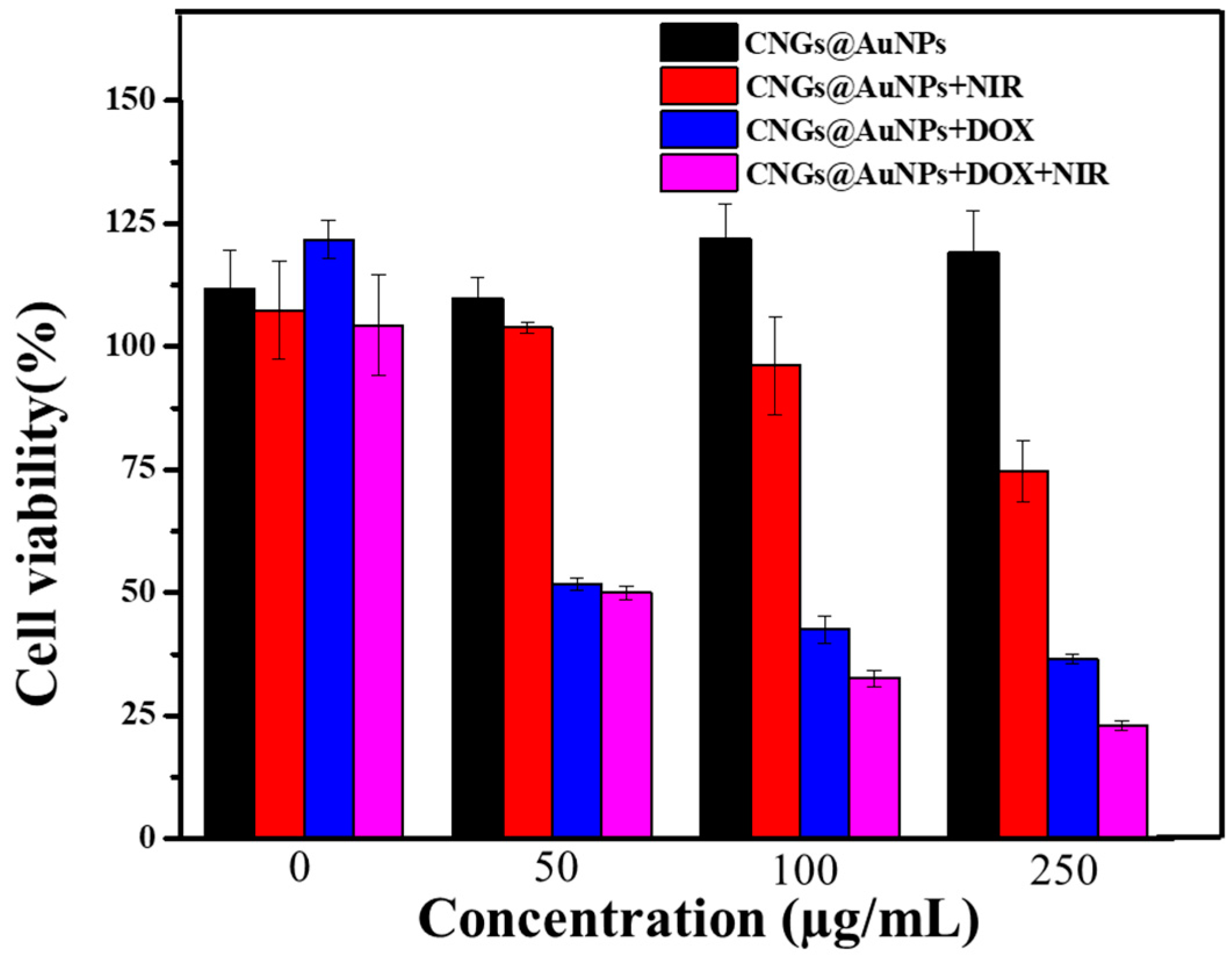
3.6. In Vitro Photothermal Therapy and Photothermal Therapy/Chemotherapy Cell Experiments
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yana, S.; Budkowski, A.; Raczkowska, J.; Donchak, V.; Melnyk, Y.; Vasiichuk, V.; Stetsyshyn, Y. Switching it up: The Promise of Stimuli-Responsive Polymer Systems in Biomedical Science. Chem. Rec. 2023, e202300217. [Google Scholar] [CrossRef]
- Liu, G.X.; Wen, Z.F.; Liu, F.Y.; Xu, Y.Q.; Li, H.J.; Sun, S.G. Multisubcellular organelle-targeting nanoparticle for synergistic chemotherapy and photodynamic/photothermal tumor therapy. Nanomedicine 2023, 18, 613–631. [Google Scholar] [CrossRef]
- Liu, S.; Shen, C.; Jiang, D.; Qian, C.; Yang, Z.; Wang, J.; Ye, W. Cascade tumor therapy platform for sensitized chemotherapy and penetration enhanced photothermal therapy. Macromol. Biosci. 2021, 22, 2100429. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Cai, L.; Dai, Y.; Liu, Y.; Zuo, F.; Ni, C.; Shi, M.; Li, J. Polydopamine-based nanoparticles for photothermal therapy/chemotherapy and their synergistic therapy with autophagy inhibitor to promote antitumor treatment. Chem. Rec. 2021, 21, 781–796. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Ye, H.; Ge, S.; Yao, Y.; Ashok, B.; Hariram, N.; Liu, H.; Tian, H.; He, Y.; Guo, G.; et al. Fabrication and properties of antimicrobial flexible nanocomposite polyurethane foams with in situ generated copper nanoparticles. J. Mater. Res. Technol. 2022, 19, 3603–3615. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; He, D.; Hai, L.; Tang, J.; Li, H.; He, X.; Wang, K. A metal–organic framework based nanocomposite with co-encapsulation of Pd@Au nanoparticles and doxorubicin for ph- and nir-triggered synergistic chemo-photothermal treatment of cancer cells. J. Mater. Chem. B 2017, 5, 4648–4659. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Tan, Y.; Wang, K.; Cai, Y.; Li, J.; Sonne, C.; Li, C. Reviewing wood-based solar-driven interfacial evaporators for desalination. Water Res. 2022, 223, 119011. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X. Gold nanoparticles for skin drug delivery. Int. J. Pharm. 2022, 625, 122122. [Google Scholar] [CrossRef]
- Ou, X.; Liu, Y.; Zhang, M.; Hua, L.; Zhan, S. Plasmonic gold nanostructures for biosensing and bioimaging. Microchim. Acta 2021, 188, 304. [Google Scholar] [CrossRef]
- Özkan, M.; Hadi, S.E.; Tunç, İ.; Midilli, Y.; Ortaç, B.; Tuncel, D. Cucurbit[7]uril-capped hybrid conjugated oligomer-gold nanoparticles for combined photodynamic-photothermal therapy and cellular imaging. ACS Appl. Polym. Mater. 2020, 2, 3840–3849. [Google Scholar] [CrossRef]
- Ziai, Y.; Rinoldi, C.; Nakielski, P.; De Sio, L.; Pierini, F. Smart plasmonic hydrogels based on gold and silver nanoparticles for biosensing application. Curr. Opin. Biomed. Eng. 2022, 24, 100413. [Google Scholar] [CrossRef]
- Bastús, N.G.; Merkoçi, F.; Piella, J.; Puntes, V. Synthesis of highly monodisperse citrate-stabilized silver nanoparticles of up to 200 nm: Kinetic control and catalytic properties. Chem. Mater. 2014, 26, 2836–2846. [Google Scholar] [CrossRef]
- Hainfeld, J.F.; O’Connor, M.J.; Lin, P.; Qian, L.P.; Slatkin, D.N.; Smilowitz, H.M. Infrared-transparent gold nanoparticles converted by tumors to infrared absorbers cure tumors in mice by photothermal therapy. PLoS ONE 2014, 9, e88414. [Google Scholar] [CrossRef] [PubMed]
- Vijayaraghavan, P.; Liu, C.H.; Vankayala, R.; Chiang, C.S.; Hwang, K.C. Designing multi-branched gold nanoechinus for nir light activated dual modal photodynamic and photothermal therapy in the second biological window. Adv. Mater. 2014, 26, 6689–6695. [Google Scholar] [CrossRef]
- Wang, R.; Du, N.; Jin, L.; Chen, W.; Ma, Z.; Zhang, T.; Xu, J.; Zhang, W.; Wang, X.; Li, M. Hyaluronic acid modified Au@SiO2@Au nanoparticles for photothermal therapy of genitourinary tumors. Polymers 2022, 14, 4772. [Google Scholar] [CrossRef] [PubMed]
- Han, H.H.; Kim, S.-J.; Kim, J.; Park, W.; Kim, C.; Kim, H.; Hahn, S.K. Bimetallic hyaluronate-modified Au@Pt nanoparticles for noninvasive photoacoustic imaging and photothermal therapy of skin cancer. ACS Appl. Mater. Interfaces 2023, 15, 11609–11620. [Google Scholar] [CrossRef]
- Lee, G.; Lee, Y.J.; Kim, Y.-J.; Park, Y. Synthesis of au-ag bimetallic nanoparticles using korean red ginseng (Panax ginseng meyer) root extract for chemo-photothermal anticancer therapy. Arch. Pharmacal Res. 2023, 46, 659–678. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, Z.; Li, J.; Shi, L.; Zhao, W.; Wang, H.; Sun, T.; Jia, T.; Sun, Y. Fabrication of the biomimetic dox/au@pt nanoparticles hybrid nanostructures for the combinational chemo/photothermal cancer therapy. J. Alloys Compd. 2021, 881, 160592. [Google Scholar] [CrossRef]
- Hirsch, L.R.; Stafford, R.J.; Bankson, J.A.; Sershen, S.R.; Rivera, B.; Price, R.E.; Hazle, J.D.; Halas, N.J.; West, J.L. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc. Natl. Acad. Sci. USA 2003, 100, 13549–13554. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Liang, J.J.; Chen, H.; Geng, D.D.; Jiao, L.; Yang, J.Y.; Qian, H.; Zhang, C.; Ding, Y. Performance of doxorubicin-conjugated gold nanoparticles: Regulation of drug location. ACS Appl. Mater. Interfaces 2017, 9, 8569–8580. [Google Scholar] [CrossRef]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape effects of gold nanoparticles in photothermal cancer therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Hiranpattanakul, P.; Jongjitpissamai, T.; Aungwerojanawit, S.; Tachaboonyakiat, W. Fabrication of a chitin/chitosan hydrocolloid wound dressing and evaluation of its bioactive properties. Res. Chem. Intermed. 2018, 44, 4913–4928. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Rangasamy, J. Multifunctional chitin nanogels for simultaneous drug delivery, bioimaging, and biosensing. ACS Appl. Mater. Interfaces 2011, 3, 3654–3665. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Nair, A.; Sabitha, M.; Chennazhi, K.P.; Tamura, H.; Nair, S.V.; Jayakumar, R. Synthesis, characterization and in vitro cytocompatibility studies of chitin nanogels for biomedical applications. Carbohydr. Polym. 2012, 87, 943–949. [Google Scholar] [CrossRef]
- Li, Z.; Li, M.; Wang, X.; Fu, G.T.; Tang, Y.W. The Use of Amino-Based Functional Molecules for the Controllable Synthesis of Noble-Metal Nanocrystals: A Minireview. Nanoscale Adv. 2021, 3, 1813–1829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, P.; Zhao, Y.; Xue, S.; Zhu, X.; Tong, J.; Zheng, S.; Chen, Y.; Shi, X.; Deng, H. A simple mechanical agitation method to fabricate chitin nanogels directly from chitin solution and subsequent surface modification. J. Mater. Chem. B 2019, 7, 2226–2232. [Google Scholar] [CrossRef]
- Hui, Y.; Zhang, S.; Wang, W. Recent progress in catalytic oxidative transformations of alcohols by supported gold nanoparticles. Adv. Synth. Catal. 2019, 361, 2215–2235. [Google Scholar] [CrossRef]
- Jiang, P.; Wang, Y.; Zhao, L.; Ji, C.; Chen, D.; Nie, L. Applications of gold nanoparticles in non-optical biosensors. Nanomaterials 2018, 8, 977. [Google Scholar] [CrossRef]
- Xuan, J.; Lirong, C.; Wei, Z. A New Linear Potentiometric Titration Method for the Determination of Deacetylation Degree of Chitosan. Carbohydr. Polym. 2003, 54, 457–463. [Google Scholar] [CrossRef]
- Guo, J.; Wang, C.; Li, C.; Liu, Y. Effect of acetylation on the physical and mechanical performances of mechanical densified spruce wood. Forests 2022, 13, 1620. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Zhao, Z.; Yang, F.; Zou, Y.; Wang, Y.; Tang, Y.; Zhou, Q.; Li, C. Low-cost and high-strength soybean meal adhesives modified by tannin–phenol–formaldehyde resin. Forests 2023, 14, 1947. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, J.A.; Wang, K.; Li, C.; Cai, Y.; Li, J.; Lam, S.S.; Sonne, C. High-performance and scalable wood-based solar-driven interfacial evaporator with corrugated structure for continuous desalination. J. Clean. Prod. 2023, 418, 138024. [Google Scholar] [CrossRef]
- Li, S.; Shi, X.; Wang, H.; Xiao, L. A Multifunctional Dual-Shell Magnetic Nanocomposite with near-Infrared Light Response for Synergistic Chemo-Thermal Tumor Therapy. J. Biomed. Mater. Res. Part B Appl. Biomater. 2020, 109, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, X.; Tian, J.; Lu, Y.; Shi, X.; Zhan, Y.; Du, Y.; Liu, H.; Deng, H. Antimicrobial activity and cytotoxicity of nanofibrous mats immobilized with polysaccharides-rectorite based nanogels. Colloids Surf. B Biointerfaces 2015, 133, 370–377. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Zhang, Q.; Chen, F.; Yue, Z.; Zhang, T.; Deng, H.; Huselstein, C.; Anderson, D.P.; Chang, P.R.; et al. Accelerated skin wound healing by soy protein isolate–modified hydroxypropyl chitosan composite films. Int. J. Biol. Macromol. 2018, 118, 1293–1302. [Google Scholar] [CrossRef]
- Huang, Y.; Fang, Y.; Chen, L.; Lu, A.; Zhang, L. One-step synthesis of size-tunable gold nanoparticles immobilized on chitin nanofibrils via green pathway and their potential applications. Chem. Eng. J. 2017, 315, 573–582. [Google Scholar] [CrossRef]
- Assan, N.; Uetake, Y.; Sakurai, H. Size-selective preparation of gold nanoparticles stabilized on chitosan using the matrix-transfer method. J. Nanopart. Res. 2023, 25, 50. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, J.; Zhan, S.; Hu, J.; Guo, Y.; Ding, L.; Huang, X.; Xiong, Y. Dynamic light scattering immunosensor based on metal-organic framework mediated gold growth strategy for the ultra-sensitive detection of alpha-fetoprotein. Sens. Actuators B Chem. 2021, 341, 130030. [Google Scholar] [CrossRef]
- Romanovskaia, E.; Slovenský, P.; Kalantarian, S.M.; Laundry-Mottiar, L.; Romanovski, V.; Halama, M.; Auinger, M.; Hedberg, Y.S. Electrochemical estimations of the gold nanoparticle size effect on cysteine-gold oxidation. J. Electrochem. Soc. 2022, 169, 021501. [Google Scholar] [CrossRef]
- Thanh, T.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Luan, C.; Zhou, Q.; Wang, Y.; Xiao, Y.; Dai, X.; Huang, X.; Zhang, X. A General Strategy Assisted with Dual Reductants and Dual Protecting Agents for Preparing Pt-Based Alloys with High-Index Facets and Excellent Electrocatalytic Performance. Small 2017, 13, 46. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.V.; Giangregorio, M.M.; Losurdo, M.; Capezzuto, P.; Bruno, G. Supported faceted gold nanoparticles with tunable surface plasmon resonance for nir-sers. Adv. Funct. Mater. 2012, 22, 5081–5088. [Google Scholar] [CrossRef]
- Yu, Y.Y.; Chang, S.S.; Lee, C.L.; Wang, C.R.C. Gold nanorods: Electrochemical synthesis and optical properties. J. Phys. Chem. B 1997, 101, 6661–6664. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, Y.C.; Wang, Q. Surface plasma resonance emission of au colloidal nanoparticles. Spectrosc. Spectr. Anal. 2003, 23, 958–961. [Google Scholar]
- Link, S.; El-Sayed, M.A. Shape and Size Dependence of Radiative, Non-Radiative and Photothermal Properties of Gold Nanocrystals. Int. Rev. Phys. Chem. 2000, 19, 409–453. [Google Scholar] [CrossRef]
- Amoozgar, Z.; Park, J.; Lin, Q.; Yeo, Y. Low Molecular-Weight Chitosan as a Ph-Sensitive Stealth Coating for Tumor-Specific Drug Delivery. Mol. Pharm. 2012, 9, 1262–1270. [Google Scholar] [CrossRef]
- He, G.; Zhu, C.; Ye, S.; Cai, W.; Yin, Y.; Zheng, H.; Yi, Y. Preparation and Properties of Novel Hydrogel Based on Chitosan Modified by Poly(Amidoamine) Dendrimer. Int. J. Biol. Macromol. 2016, 91, 828–837. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Zhu, W.; Liang, J.; Li, L.; Zheng, L.; Shi, X.; Wang, C.; Dong, Y.; Li, C.; Zhu, X. In Situ Synthesis of Gold Nanoparticles from Chitin Nanogels and Their Drug Release Response to Stimulation. Polymers 2024, 16, 390. https://doi.org/10.3390/polym16030390
Zhang J, Zhu W, Liang J, Li L, Zheng L, Shi X, Wang C, Dong Y, Li C, Zhu X. In Situ Synthesis of Gold Nanoparticles from Chitin Nanogels and Their Drug Release Response to Stimulation. Polymers. 2024; 16(3):390. https://doi.org/10.3390/polym16030390
Chicago/Turabian StyleZhang, Jianwei, Wenjin Zhu, Jingyi Liang, Limei Li, Longhui Zheng, Xiaowen Shi, Chao Wang, Youming Dong, Cheng Li, and Xiuhong Zhu. 2024. "In Situ Synthesis of Gold Nanoparticles from Chitin Nanogels and Their Drug Release Response to Stimulation" Polymers 16, no. 3: 390. https://doi.org/10.3390/polym16030390
APA StyleZhang, J., Zhu, W., Liang, J., Li, L., Zheng, L., Shi, X., Wang, C., Dong, Y., Li, C., & Zhu, X. (2024). In Situ Synthesis of Gold Nanoparticles from Chitin Nanogels and Their Drug Release Response to Stimulation. Polymers, 16(3), 390. https://doi.org/10.3390/polym16030390






