Synthesis, Characterization and Application of Biobased Unsaturated Polyester Resin Reinforced with Unmodified/Modified Biosilica Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basic Materials and Syntheses of Composites
2.1.1. Preparation and Modification of the Silica Particles
2.1.2. The Synthesis of b-UPR from Biobased Reactants
2.1.3. The Preparation of a Nanocomposite Based on b-UPR Reinforced with Unmodified and Modified Biosilica
2.1.4. The Life Extension of the Composite
2.2. Characterization Methods
3. Results
3.1. Characterization of Raw Materials, Resins, and Composites
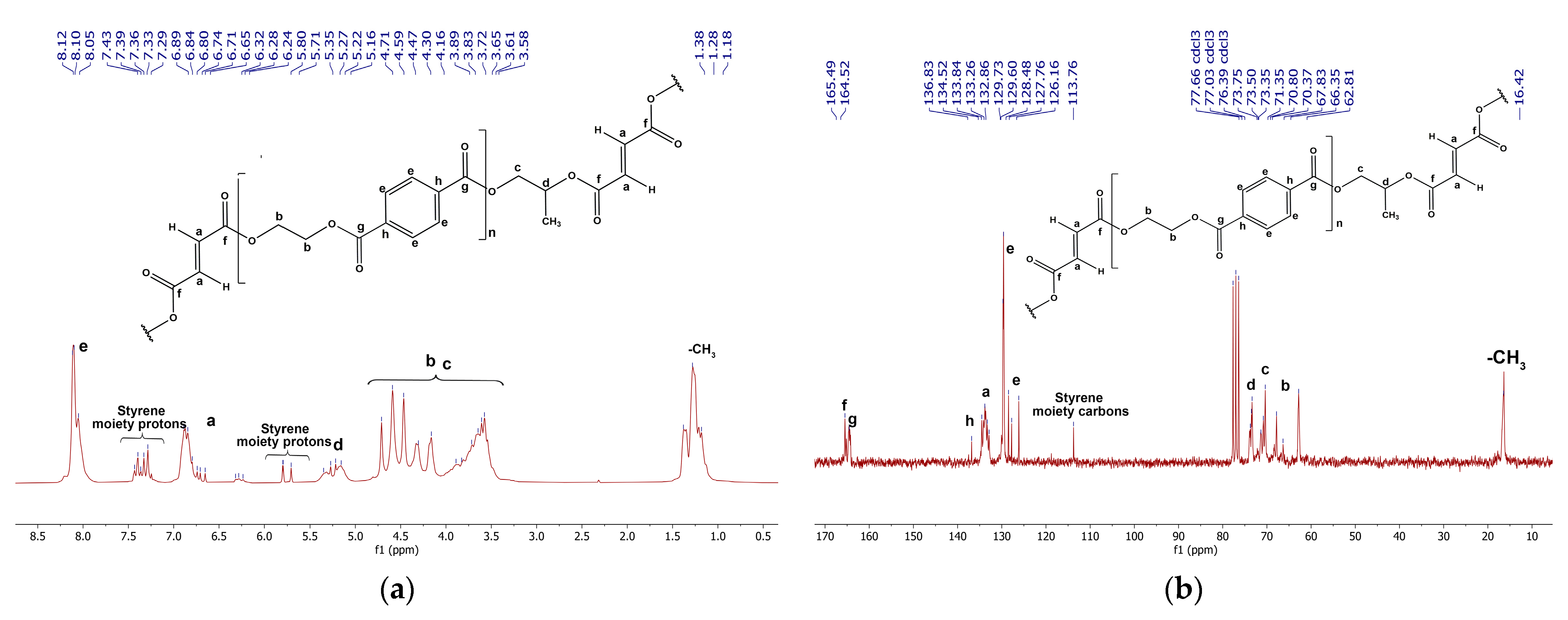
3.1.1. Analysis of Raw Materials
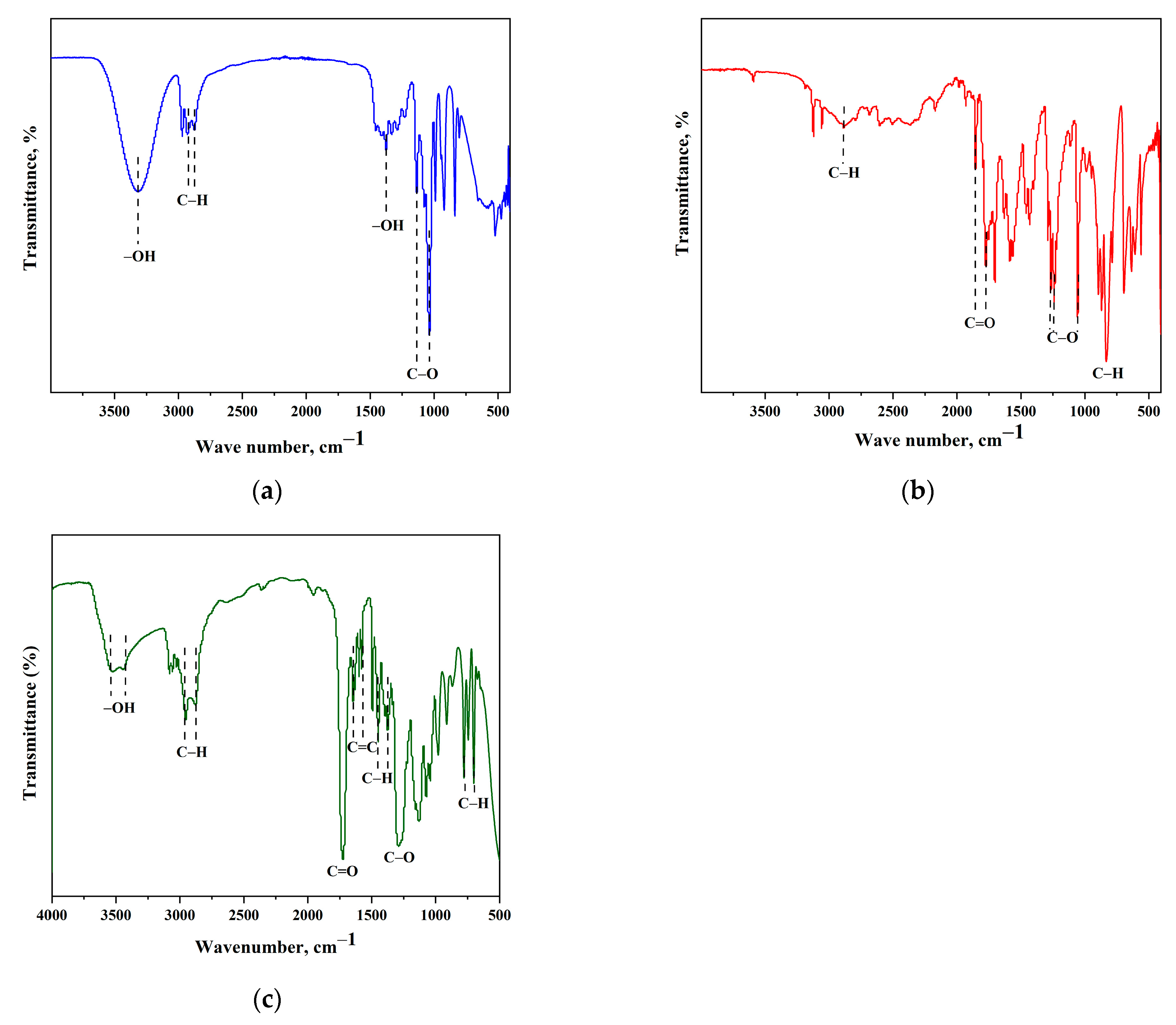
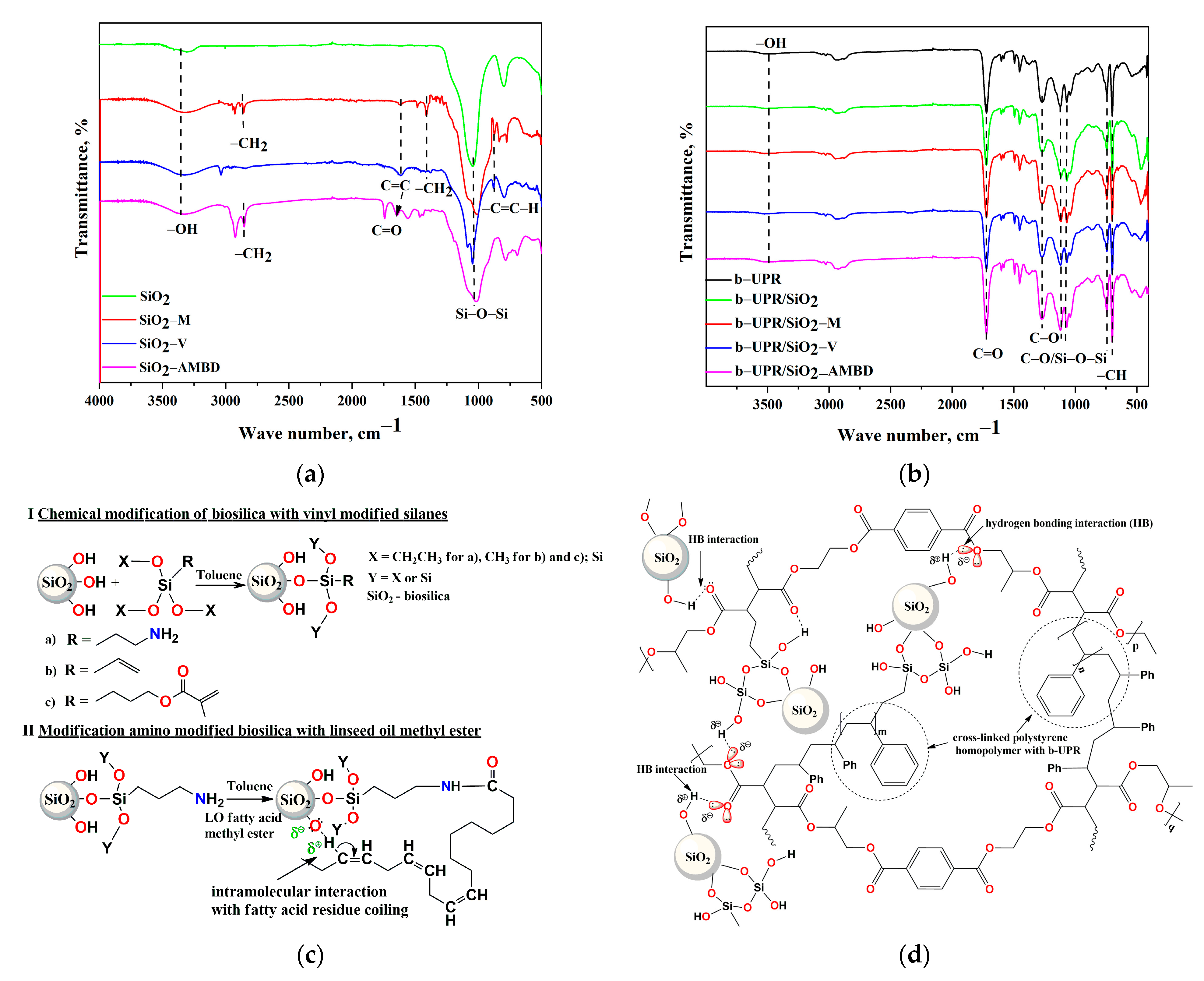
3.1.2. FTIR Analysis of Biosilica Particles and b-UPR Composites
3.1.3. Thermogravimetric Analysis
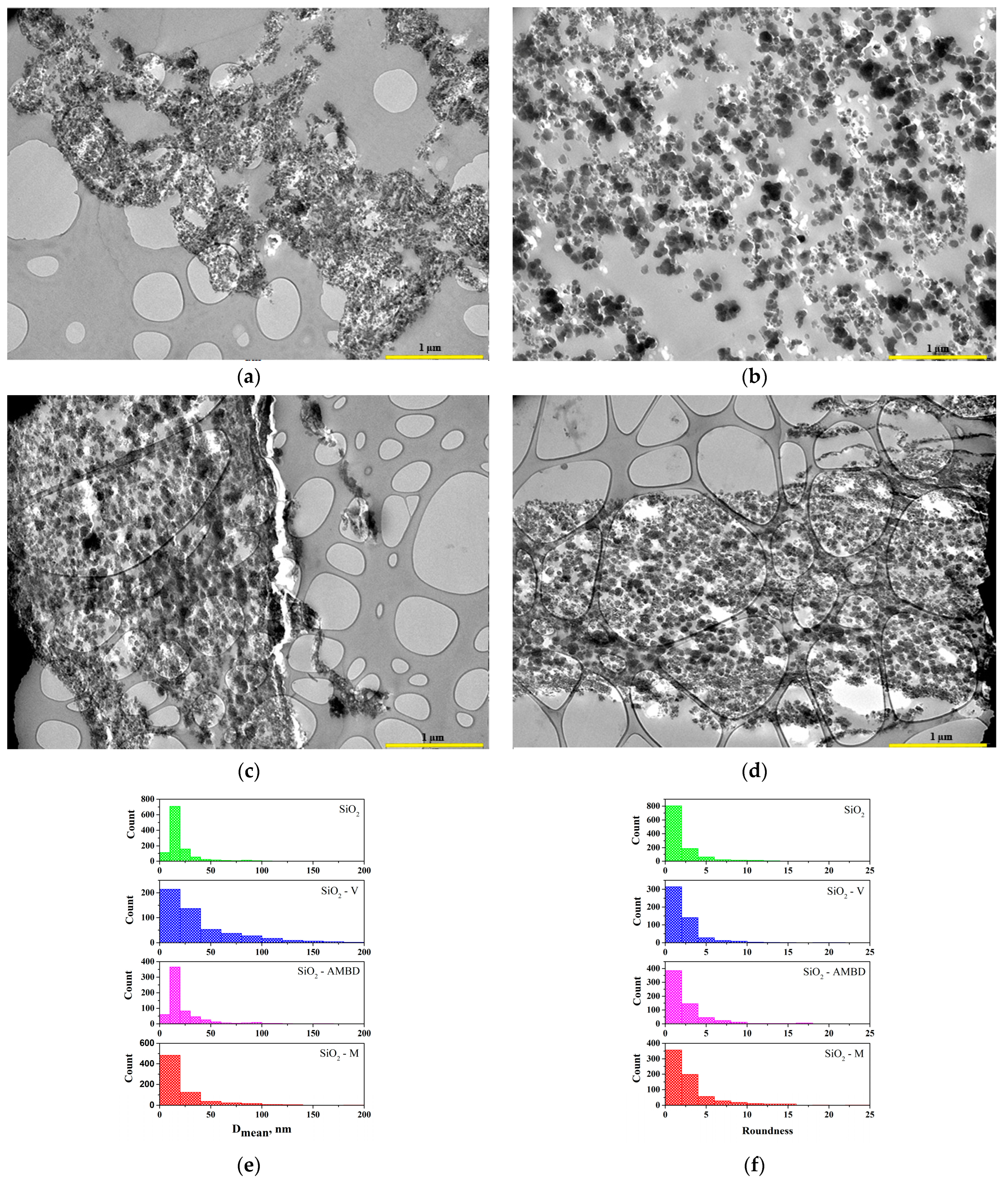
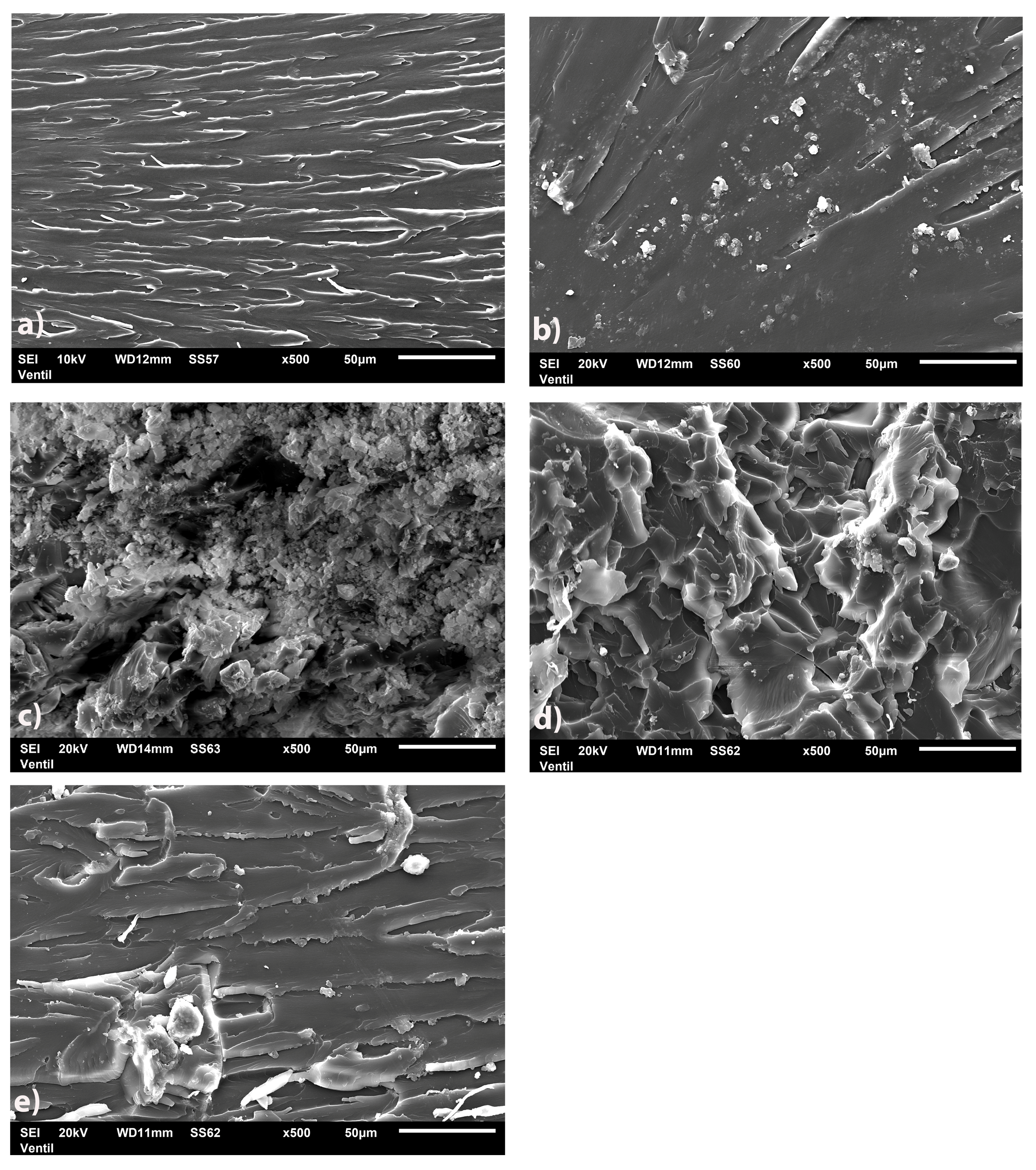
3.1.4. SEM/TEM Analysis of Biosilica Fillers, b-UPR Nanocomposite and Mechanisms
3.2. Mechanical Properties
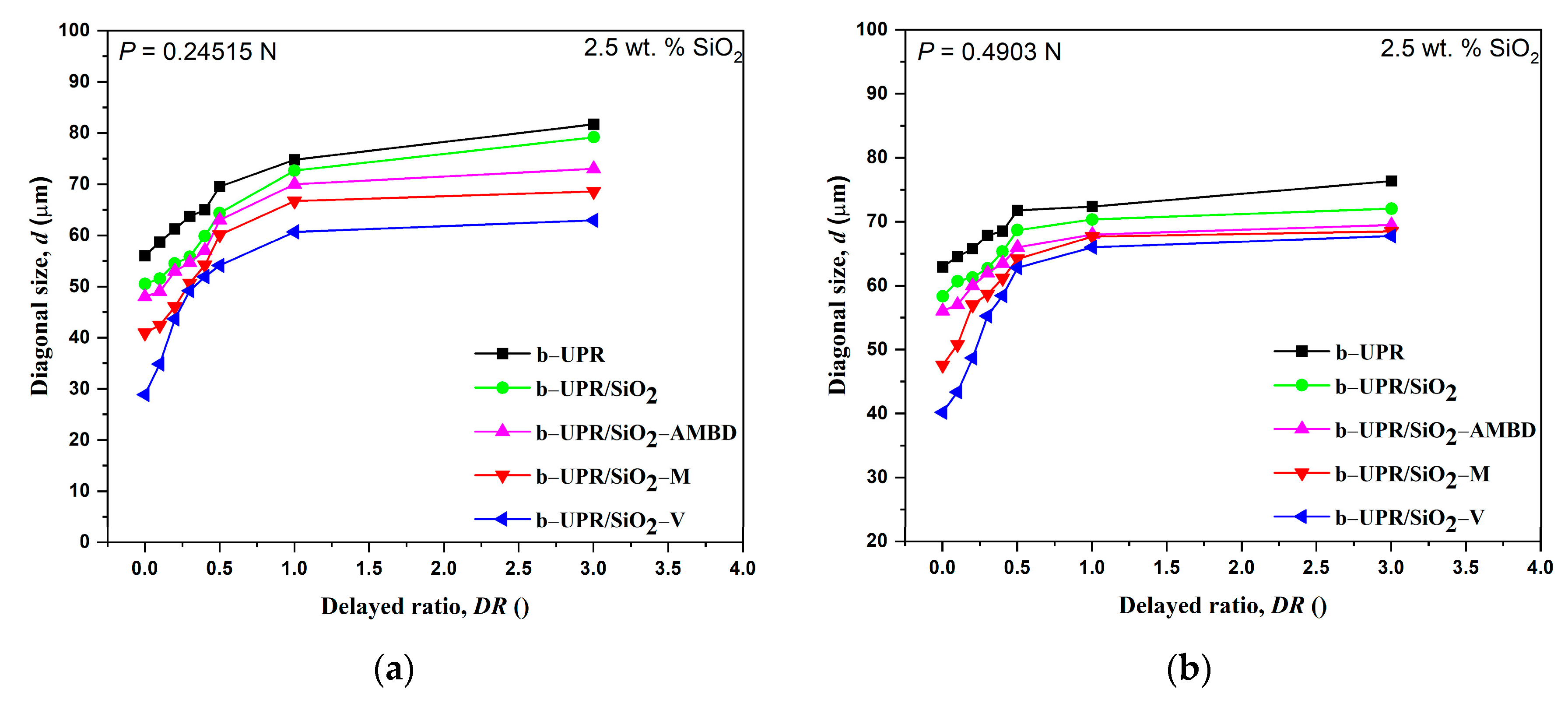
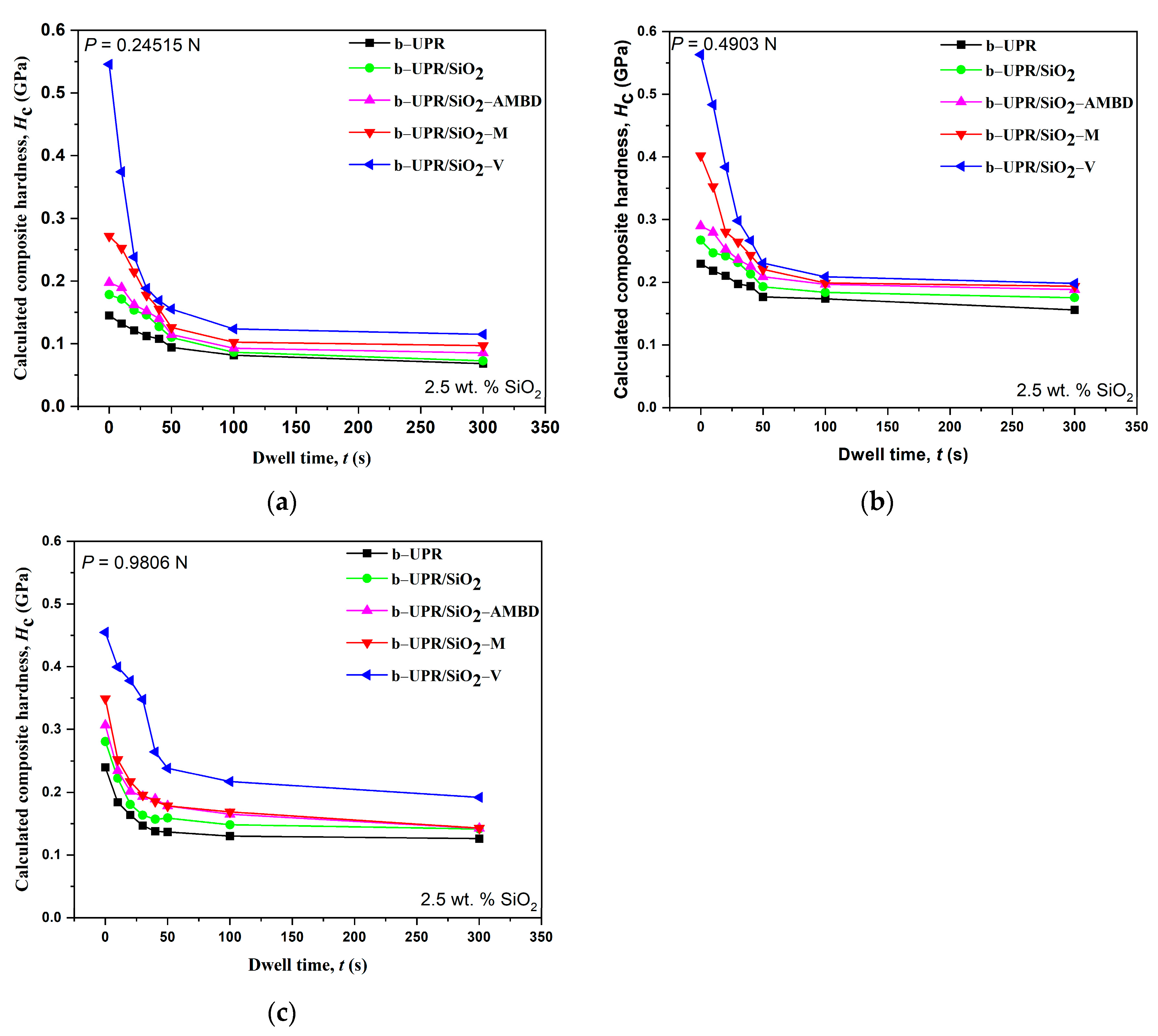
3.2.1. Microhardness
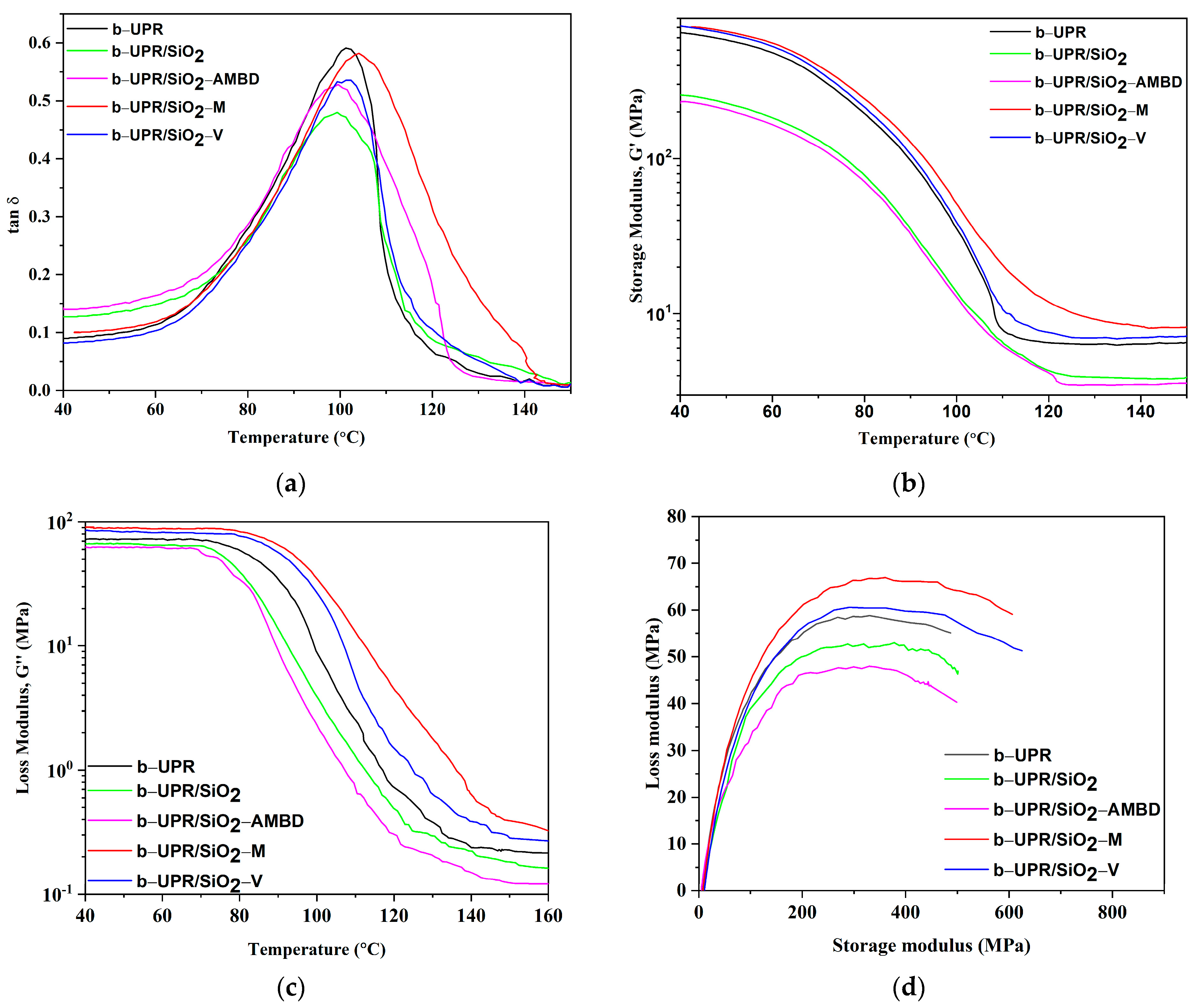
3.2.2. Dynamic Mechanical Analysis
3.2.3. Tensile Strength
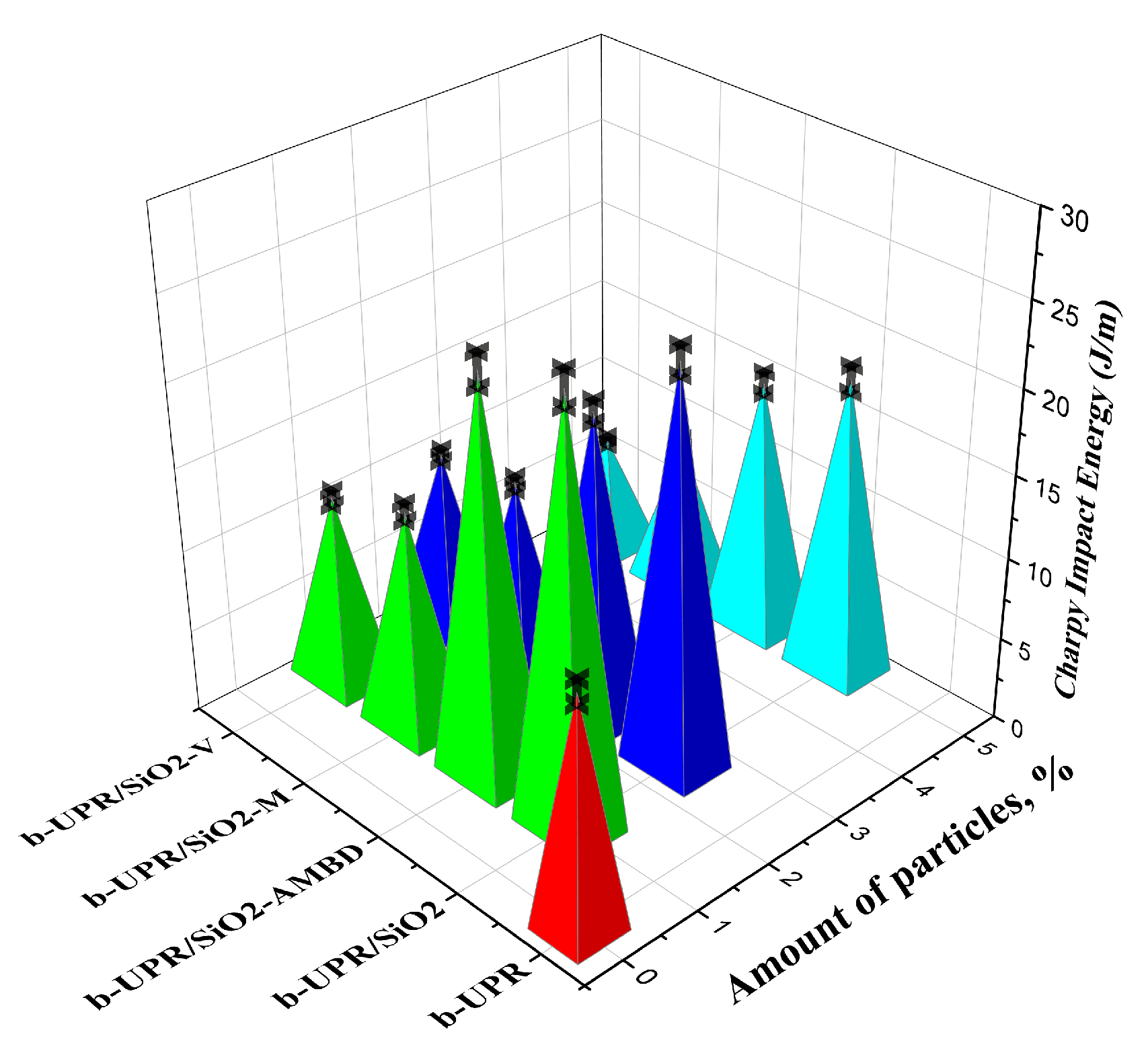
3.2.4. Charpy Testing
3.3. The Extension of the Composite’s Life Cycle
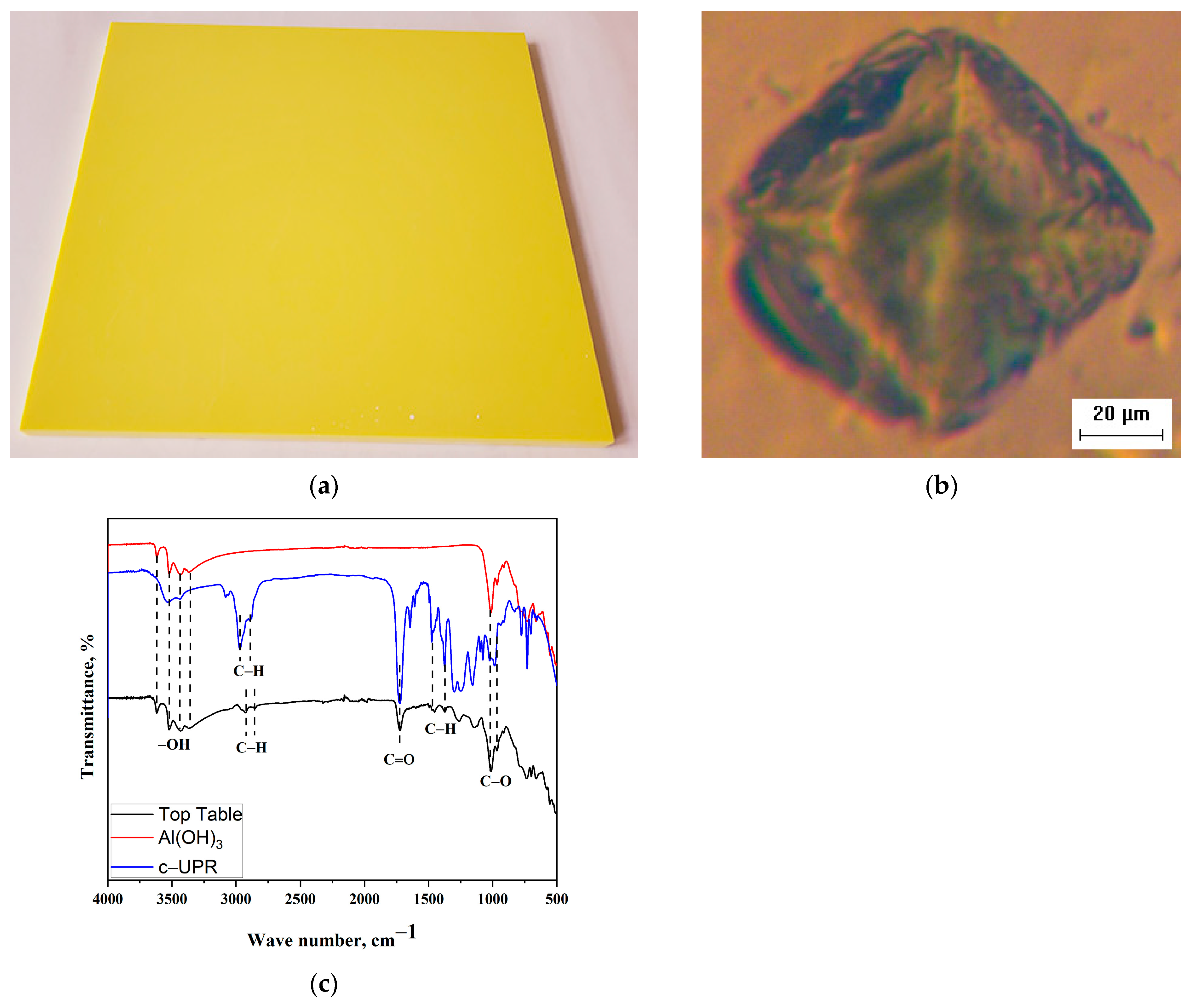
3.3.1. Properties and Characterization of Table Top Based on c-UPR Resin
3.3.2. The Results of the Flammability Test
4. Conclusions
- A method for producing b-UPR from waste PET glycolyzate and biobased PG and MAnh was presented.
- Rice husk (RH) silica particles were nanostructured and then modified with various vinyl silanes before being employed as reinforcement in b-UPR-based composites.
- The structural, morphological and mechanical properties of b-UPR and the obtained composites were investigated.
- Silica, incorporated into b-UPR resin, formed chain-like aggregates and covalent bonds in the course of cross-linking with the b-UPR matrix.
- The highest σm of 88% was obtained for 2.5 wt.% of SiO2-V addition in b-UPR/SiO2-V. Furthermore, microhardness increased by more than 200%, from 0.0668 GPa, found for UPR, to 0.205 GPa for UPR/SiO2-V (5 wt.%)
- Different modifications of SiO2 nanoparticles showed a certain influence on the dynamic-mechanical properties of the obtained nanocomposite. Tg was not significantly affected by the presence of SiO2, showing a neglectable increase for b-UPR/SiO2-M and b-UPR/SiO2-V composites, 104 °C vs. 102 °C, compared to 101 °C obtained for neat b-UPR.
- Grounded b-UPR/SiO2-V composite was used as filler for the production of c-UPR-based table top and floor materials.
- The test results of the table top material showed outstanding mechanical properties: microhardness of 0.361 GPa (42 wt.% Al(OH)3) and 0.373 GPa (55 wt.% Al(OH)3), as well as fire-resistant properties rated in category V- 0 according to the UL-94 method.
- The principles of green chemistry are implemented through minimization of waste generation, use of renewable raw materials, greener product design and minimization of solvent use.
- The continuation of the research will be devoted to the synthesis of fully biodegradable UPR, using vinyl reactive lignin and tannic acid, in order to improve fireproofing properties without addition of commercial flame-retardant additives.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Hossain, M.; Shi, H.H.; Tariq, A.; Ramakrishna, S.; Umer, R. Additive Manufacturing of Sustainable Biomaterials for Biomedical Applications. Asian J. Pharm. Sci. 2023, 18, 100812. [Google Scholar] [CrossRef] [PubMed]
- Siracusa, V.; Blanco, I. Bio-Polyethylene (Bio-PE), Bio-Polypropylene (Bio-PP) and Bio-Poly(Ethylene Terephthalate) (Bio-PET): Recent Developments in Bio-Based Polymers Analogous to Petroleum-Derived Ones for Packaging and Engineering Applications. Polymers 2020, 12, 1641. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.L. Food Packaging. In Encyclopedia of Agriculture and Food Systems; Elsevier: Amsterdam, The Netherlands, 2014; pp. 232–249. [Google Scholar]
- Nayak, S.; Khuntia, S.K. Development and Study of Properties of Moringa Oleifera Fruit Fibers/Polyethylene Terephthalate Composites for Packaging Applications. Compos. Commun. 2019, 15, 113–119. [Google Scholar] [CrossRef]
- Emamian, S.; Narakathu, B.B.; Chlaihawi, A.A.; Bazuin, B.J.; Atashbar, M.Z. Screen Printing of Flexible Piezoelectric Based Device on Polyethylene Terephthalate (PET) and Paper for Touch and Force Sensing Applications. Sens. Actuators A Phys. 2017, 263, 639–647. [Google Scholar] [CrossRef]
- Branfoot, C.; Folkvord, H.; Keith, M.; Leeke, G.A. Recovery of Chemical Recyclates from Fibre-Reinforced Composites: A Review of Progress. Polym. Degrad. Stab. 2023, 215, 110447. [Google Scholar] [CrossRef]
- Faust, K.; Denifl, P.; Hapke, M. Recent Advances in Catalytic Chemical Recycling of Polyolefins. ChemCatChem 2023, 15, e202300310. [Google Scholar] [CrossRef]
- Khalid, M.Y.; Arif, Z.U.; Ahmed, W.; Arshad, H. Recent Trends in Recycling and Reusing Techniques of Different Plastic Polymers and Their Composite Materials. Sustain. Mater. Technol. 2022, 31, e00382. [Google Scholar] [CrossRef]
- Zahedi, A.R.; Rafizadeh, M.; Taromi, F.A. Recycling of Off-Grade Pet via Partial Alcoholysis to Synthesize Functionalized Pet Oligomer Nanocomposites. Polym. Compos. 2012, 33, 1832–1839. [Google Scholar] [CrossRef]
- Padhan, R.K.; Sreeram, A. Chemical Depolymerization of PET Bottles via Combined Chemolysis Methods. In Recycling of Polyethylene Terephthalate Bottles; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–147. [Google Scholar]
- Sinha, V.; Patel, M.R.; Patel, J.V. Pet Waste Management by Chemical Recycling: A Review. J. Polym. Environ. 2010, 18, 8–25. [Google Scholar] [CrossRef]
- Patel, M.R.; Patel, J.V.; Sinha, V.K. Polymeric Precursors from PET Waste and Their Application in Polyurethane Coatings. Polym. Degrad. Stab. 2005, 90, 111–115. [Google Scholar] [CrossRef]
- Viante, M.F.; Zanela, T.M.P.; Stoski, A.; Muniz, E.C.; Almeida, C.A.P. Magnetic Microspheres Composite from Poly(Ethylene Terephthalate) (PET) Waste: Synthesis and Characterization. J. Clean. Prod. 2018, 198, 979–986. [Google Scholar] [CrossRef]
- Sinha, Y.K.; Sridhar, B.T.N.; Santhosh, M. Thermal Decomposition Study of HTPB Solid Fuel in the Presence of Activated Charcoal and Paraffin. J. Therm. Anal. Calorim. 2015, 119, 557–565. [Google Scholar] [CrossRef]
- Wang, Z.; Qiang, H.; Wang, T.; Wang, G. Tensile Behaviors of Thermal Aged HTPB Propellant at Low Temperatures under Dynamic Loading. Mech. Time-Depend. Mater. 2020, 24, 141–159. [Google Scholar] [CrossRef]
- Dîrloman, F.M.; Toader, G.; Rotariu, T.; Țigănescu, T.V.; Ginghină, R.E.; Petre, R.; Alexe, F.; Ungureanu, M.I.; Rusen, E.; Diacon, A.; et al. Novel Polyurethanes Based on Recycled Polyethylene Terephthalate: Synthesis, Characterization, and Formulation of Binders for Environmentally Responsible Rocket Propellants. Polymers 2021, 13, 3828. [Google Scholar] [CrossRef]
- Rusmirovic, J.D.; Trifkovic, K.T.; Bugarski, B.; Pavlovic, V.B.; Dzunuzovic, J.; Tomic, M.; Marinkovic, A.D. High Performances Unsaturated Polyester Based Nanocomposites: Effect of Vinyl Modified Nanosilica on Mechanical Properties. Express Polym. Lett. 2016, 10, 139–159. [Google Scholar] [CrossRef]
- Rusmirović, J.D.; Rančić, M.P.; Pavlović, V.B.; Rakić, V.M.; Stevanović, S.; Djonlagić, J.; Marinković, A.D. Cross-Linkable Modified Nanocellulose/Polyester Resin-Based Composites: Effect of Unsaturated Fatty Acid Nanocellulose Modification on Material Performances. Macromol. Mater. Eng. 2018, 303, 1700648. [Google Scholar] [CrossRef]
- Jia, W.; Si, Z.; Feng, Y.; Zhang, X.; Zhao, X.; Sun, Y.; Tang, X.; Zeng, X.; Lin, L. Oxidation of 5-[(Formyloxy)Methyl]Furfural to Maleic Anhydride with Atmospheric Oxygen Using α-MnO2/Cu(NO3)2 as Catalysts. ACS Sustain. Chem. Eng. 2020, 8, 7901–7908. [Google Scholar] [CrossRef]
- Hirunsit, P.; Luadthong, C.; Faungnawakij, K. Effect of Alumina Hydroxylation on Glycerol Hydrogenolysis to 1,2-Propanediol over Cu/Al2O3: Combined Experiment and DFT Investigation. RSC Adv. 2015, 5, 11188–11197. [Google Scholar] [CrossRef]
- Goyat, M.S.; Hooda, A.; Gupta, T.K.; Kumar, K.; Halder, S.; Ghosh, P.K.; Dehiya, B.S. Role of Non-Functionalized Oxide Nanoparticles on Mechanical Properties and Toughening Mechanisms of Epoxy Nanocomposites. Ceram. Int. 2021, 47, 22316–22344. [Google Scholar] [CrossRef]
- Shamlooh, M.; Hamza, A.; Hussein, I.A.; Nasser, M.S.; Magzoub, M.; Salehi, S. Investigation of the Rheological Properties of Nanosilica-Reinforced Polyacrylamide/Polyethyleneimine Gels for Wellbore Strengthening at High Reservoir Temperatures. Energy Fuels 2019, 33, 6829–6836. [Google Scholar] [CrossRef]
- Zou, H.; Wu, S.; Shen, J. Polymer/Silica Nanocomposites: Preparation, Characterization, Properties, and Applications. Chem. Rev. 2008, 108, 3893–3957. [Google Scholar] [CrossRef] [PubMed]
- Heng, C.; Liu, M.; Wang, K.; Deng, F.; Huang, H.; Wan, Q.; Hui, J.; Zhang, X.; Wei, Y. Biomimic Preparation of Highly Dispersible Silica Nanoparticles Based Polymer Nanocomposites. Ceram. Int. 2015, 41, 15075–15082. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface Modification of Inorganic Nanoparticles for Development of Organic–Inorganic Nanocomposites—A Review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Hayichelaeh, C.; Reuvekamp, L.A.E.M.; Dierkes, W.K.; Blume, A.; Noordermeer, J.W.M.; Sahakaro, K. Enhancing the Silanization Reaction of the Silica-Silane System by Different Amines in Model and Practical Silica-Filled Natural Rubber Compounds. Polymers 2018, 10, 584. [Google Scholar] [CrossRef]
- Siot, A.; Léger, R.; Longuet, C.; Otazaghine, B.; Caro-Bretelle, A.S.; Azéma, N. Dispersion Control of Raw and Modified Silica Particles in PMMA. Impact on Mechanical Properties, from Experiments to Modelling. Compos. Part B Eng. 2019, 157, 163–172. [Google Scholar] [CrossRef]
- Venkatesan, R.; Rajeswari, N. Nanosilica-Reinforced Poly(Butylene Adipate-Co-Terephthalate) Nanocomposites: Preparation, Characterization and Properties. Polym. Bull. 2019, 76, 4785–4801. [Google Scholar] [CrossRef]
- Picu, C.R.; Krawczyk, K.K.; Wang, Z.; Pishvazadeh-Moghaddam, H.; Sieberer, M.; Lassnig, A.; Kern, W.; Hadar, A.; Constantinescu, D.M. Toughening in Nanosilica-Reinforced Epoxy with Tunable Filler-Matrix Interface Properties. Compos. Sci. Technol. 2019, 183, 107799. [Google Scholar] [CrossRef]
- Hao, T.; Wang, Y.; Liu, Z.; Li, J.; Shan, L.; Wang, W.; Liu, J.; Tang, J. Emerging Applications of Silica Nanoparticles as Multifunctional Modifiers for High Performance Polyester Composites. Nanomaterials 2021, 11, 2810. [Google Scholar] [CrossRef]
- Bageru, A.B.; Srivastava, V.C. Preparation and Characterisation of Biosilica from Teff (Eragrostis Tef) Straw by Thermal Method. Mater. Lett. 2017, 206, 13–17. [Google Scholar] [CrossRef]
- Alves, R.H.; da Reis, T.V.S.; Rovani, S.; Fungaro, D.A. Green Synthesis and Characterization of Biosilica Produced from Sugarcane Waste Ash. J. Chem. 2017, 2017, 6129035. [Google Scholar] [CrossRef]
- Nguyen, T.K.M.; Ki, M.R.; Son, R.G.; Kim, K.H.; Hong, J.; Pack, S.P. Synthesis of Sub-50 Nm Bio-Inspired Silica Particles Using a C-Terminal-Modified Ferritin Template with a Silica-Forming Peptide. J. Ind. Eng. Chem. 2021, 101, 262–269. [Google Scholar] [CrossRef]
- Azat, S.; Korobeinyk, A.V.; Moustakas, K.; Inglezakis, V.J. Sustainable Production of Pure Silica from Rice Husk Waste in Kazakhstan. J. Clean. Prod. 2019, 217, 352–359. [Google Scholar] [CrossRef]
- Vuksanovic, M.; Mladenovic, I.; Tomic, N.; Petrovic, M.; Radojevic, V.; Marinkovic, A.; Jancic-Heinemann, R. Mechanical Properties of Biomass-Derived Silica Nanoparticles Reinforced PMMA Composite Material. Sci. Sinter. 2022, 54, 211–221. [Google Scholar] [CrossRef]
- Fernandes, I.J.; Calheiro, D.; Sánchez, F.A.L.; Camacho, A.L.D.; de Rocha, T.L.A.C.; Moraes, C.A.M.; de Sousa, V.C. Characterization of Silica Produced from Rice Husk Ash: Comparison of Purification and Processing Methods. Mater. Res. 2017, 20, 512–518. [Google Scholar] [CrossRef]
- Neopolean, P.; Karuppasamy, K. Characterization of Silane Treated Opuntia Short Fibre and Bagasse Biosilica Toughened Epoxy Resin Composite. Silicon 2022, 14, 9331–9340. [Google Scholar] [CrossRef]
- Balaji, N.; Kumar, J.V.S.P.; Ramesh, G.; Dhinakaran, V.; Gobu, N.; Maridurai, T. Investigation on DMA, Fatigue and Creep Behaviour of Rice Husk Ash Biosilica-Prickly Pear Short Fibre-Reinforced Epoxy Resin Composite. Silicon 2022, 14, 12773–12779. [Google Scholar] [CrossRef]
- Alshahrani, H.; Arun Prakash, V.R. Thermal, Mechanical and Barrier Properties of Rice Husk Ash Biosilica Toughened Epoxy Biocomposite Coating for Structural Application. Prog. Org. Coat. 2022, 172, 107080. [Google Scholar] [CrossRef]
- Crawford, R.J. Microhardness Testing of Plastics. Polym. Test. 1982, 3, 37–54. [Google Scholar] [CrossRef]
- Tomić, N.Z.; Marinković, A.D.; Balanč, B.; Obradović, V.; Pavlović, V.; Manojlović, V.; Vuksanović, M.M. High-Performance Laminate Material Based on Polyurethane and Epoxide Reinforced by Silica Particles from Rice Husk Used for Intelligent Pedestrian Crossings. Iran. Polym. J. 2021, 30, 319–330. [Google Scholar] [CrossRef]
- Rusmirović, J.D.; Radoman, T.; Džunuzović, E.S.; Džunuzović, J.V.; Markovski, J.; Spasojević, P.; Marinković, A.D. Effect of the Modified Silica Nanofiller on the Mechanical Properties of Unsaturated Polyester Resins Based on Recycled Polyethylene Terephthalate. Polym. Compos. 2017, 38, 538–554. [Google Scholar] [CrossRef]
- Rusmirovic, J. Dynamic-Mechanical and Thermal Properties of Composites Based on Unsaturated Polyester Resins and Modified Silicon-Dioxide and Cellulose Nanoparticles. Ph.D. Thesis, Unversity of Belgrade, Belgrade, Serbia, 2016. [Google Scholar]
- Nussbaumer, R.J.; Halter, M.; Tervoort, T.; Caseri, W.R.; Smith, P. A Simple Method for the Determination of Refractive Indices of (Rough) Transparent Solids. J. Mater. Sci. 2005, 40, 575–582. [Google Scholar] [CrossRef]
- Vuković, G.D.; Marinković, A.D.; Škapin, S.D.; Ristić, M.T.; Aleksić, R.; Perić-Grujić, A.A.; Uskoković, P.S. Removal of Lead from Water by Amino Modified Multi-Walled Carbon Nanotubes. Chem. Eng. J. 2011, 173, 855–865. [Google Scholar] [CrossRef]
- ASTM E384-16; Standard Test Method for Microindentation Hardness of Materials. ASTM International West Conshohocken: West Conshohocken, PA, USA, 2016. [CrossRef]
- Wu, H.; Dave, F.; Mokhtari, M.; Ali, M.M.; Sherlock, R.; McIlhagger, A.; Tormey, D.; McFadden, S. On the Application of Vickers Micro Hardness Testing to Isotactic Polypropylene. Polymers 2022, 14, 1804. [Google Scholar] [CrossRef] [PubMed]
- Marinković, A.D.; Vuksanović, M.M.; Karić, N.; Đokić, V.; Popović, M.; Jančić Heinemann, R.; Tomić, N.Z. The Effect of Natural Modifiers for Starch Hydrophobization on Performance of Composite Based on Ethylene Acrylic Acid Copolymer. Polym. Compos. 2021, 42, 1325–1337. [Google Scholar] [CrossRef]
- Rusmirović, J.; Galović, J.; Kluz, M.; Perković, S.; Brzić, S.; Bogosavljević, M.; Milojković, A.; Kovačević, T. Using Potential of Filament-Wound Carbon/Glass Polymeric Composites as Rocket Motor Thermal Insulation. Polym. Polym. Compos. 2021, 29, S1541–S1554. [Google Scholar] [CrossRef]
- Lazouzi, G.A.; Vuksanović, M.M.; Tomić, N.; Petrović, M.; Spasojević, P.; Radojević, V.; Jančić Heinemann, R. Dimethyl Itaconate Modified PMMA—Alumina Fillers Composites With Improved Mechanical Properties. Polym. Compos. 2019, 40, 1691–1701. [Google Scholar] [CrossRef]
- Song, Y.; Deng, J.; Xu, Z.; Nie, Y.; Lan, Z. Effect of Thermal Aging on Mechanical Properties and Color Difference of Glass Fiber/Polyetherimide (GF/PEI) Composites. Polymers 2021, 14, 67. [Google Scholar] [CrossRef]
- UL 94V; Standard for Tests for Flammability of Plastic Materials for Parts in Devices and Appliances. Underwriters Laboratories Inc.: Northbrook, IL, USA, 2001; ISBN 0-7629-0082-2.
- Tran, P.H.; Thi Hang, A.-H. Deep Eutectic Solvent-Catalyzed Arylation of Benzoxazoles with Aromatic Aldehydes. RSC Adv. 2018, 8, 11127–11133. [Google Scholar] [CrossRef]
- Watanabe, R.; Sugahara, A.; Hagihara, H.; Mizukado, J.; Shinzawa, H. Insight into Interfacial Compatibilization of Glass-Fiber-Reinforced Polypropylene (PP) Using Maleic-Anhydride Modified PP Employing Infrared Spectroscopic Imaging. Compos. Sci. Technol. 2020, 199, 108379. [Google Scholar] [CrossRef]
- Murillo, E.A.; López, B.L. Effect of the Maleic Anhydride Content on the Structural, Thermal, Rheological and Film Properties of the n-Butyl Methacrylate–Maleic Anhydride Copolymers. Prog. Org. Coat. 2015, 78, 96–102. [Google Scholar] [CrossRef]
- Yang, X.; Yang, D.; Zhu, X.; Nie, J.; Ma, G. Electrospun and Photocrosslinked Gelatin/Dextran–Maleic Anhydride Composite Fibers for Tissue Engineering. Eur. Polym. J. 2019, 113, 142–147. [Google Scholar] [CrossRef]
- Knežević, N.; Milanović, J.; Veličković, Z.; Milošević, M.; Vuksanović, M.M.; Onjia, A.; Marinković, A. A Closed Cycle of Sustainable Development: Effective Removal and Desorption of Lead and Dyes Using an Oxidized Cellulose Membrane. J. Ind. Eng. Chem. 2023, 126, 520–536. [Google Scholar] [CrossRef]
- Radovic, I.; Stajcic, A.; Radisavljevic, A.; Veljkovic, F.; Cebela, M.; Mitic, V.V.; Radojevic, V. Solvent Effects on Structural Changes in Self-Healing Epoxy Composites. Mater. Chem. Phys. 2020, 256, 123761. [Google Scholar] [CrossRef]
- Lee, H.; Abarghani, A.; Liu, B.; Shokouhimehr, M.; Ostadhassan, M. Molecular Weight Variations of Kerogen during Maturation with MALDI-TOF-MS. Fuel 2020, 269, 117452. [Google Scholar] [CrossRef]
- Janković, B.; Manić, N.; Radović, I.; Janković, M.; Rajačić, M. Model-Free and Model-Based Kinetics of the Combustion Process of Low Rank Coals with High Ash Contents Using TGA-DTG-DTA-MS and FTIR Techniques. Thermochim. Acta 2019, 679, 178337. [Google Scholar] [CrossRef]
- Spasojević, P.M.; Panić, V.V.; Džunuzović, J.V.; Marinković, A.D.; Woortman, A.J.J.; Loos, K.; Popović, I.G. High Performance Alkyd Resins Synthesized from Postconsumer PET Bottles. RSC Adv. 2015, 5, 62273–62283. [Google Scholar] [CrossRef]
- Kirshanov, K.; Toms, R.; Melnikov, P.; Gervald, A. Unsaturated Polyester Resin Nanocomposites Based on Post-Consumer Polyethylene Terephthalate. Polymers 2022, 14, 1602. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Zhou, Q.; Meng, Y.; Zhong, Y.; Xu, J.; Xiao, C.; Zhang, G.; Zhang, Y. Effects of Vinyl Functionalized Silica Particles on Thermal and Mechanical Properties of Liquid Silicone Rubber Nanocomposites. Polymers 2023, 15, 1224. [Google Scholar] [CrossRef]
- Rusmirović, J.D.; Ivanović, J.Z.; Pavlović, V.B.; Rakić, V.M.; Rančić, M.P.; Djokić, V.; Marinković, A.D. Novel Modified Nanocellulose Applicable as Reinforcement in High-Performance Nanocomposites. Carbohydr. Polym. 2017, 164, 64–74. [Google Scholar] [CrossRef]
- Hincapié Rojas, D.F.; Pineda Gómez, P.; Rosales Rivera, A. Production and Characterization of Silica Nanoparticles from Rice Husk. Adv. Mater. Lett. 2019, 10, 67–73. [Google Scholar] [CrossRef]
- da Maradini, G.S.; Oliveira, M.P.; da Guanaes, G.M.S.; Passamani, G.Z.; Carreira, L.G.; Boschetti, W.T.N.; Monteiro, S.N.; Pereira, A.C.; de Oliveira, B.F. Characterization of Polyester Nanocomposites Reinforced with Conifer Fiber Cellulose Nanocrystals. Polymers 2020, 12, 2838. [Google Scholar] [CrossRef]
- Mallakpour, S.; Naghdi, M. Polymer/SiO2 Nanocomposites: Production and Applications. Prog. Mater. Sci. 2018, 97, 409–447. [Google Scholar] [CrossRef]
- Hirayama, S.; Iwai, H.; Tanimoto, Y. Mechanical Evaluation of Five Flowable Resin Composites by the Dynamic Micro-Indentation Method. J. Dent. Biomech. 2014, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Giannakopoulos, A.E.; Larsson, P.-L.; Vestergaard, R. Analysis of Vickers Indentation. Int. J. Solids Struct. 1994, 31, 2679–2708. [Google Scholar] [CrossRef]
- Voyiadjis, G.Z.; Malekmotiei, L.; Samadi-Dooki, A. Indentation Size Effect in Amorphous Polymers Based on Shear Transformation Mediated Plasticity. Polymer 2018, 137, 72–81. [Google Scholar] [CrossRef]
- Han, C.-S. Influence of the Molecular Structure on Indentation Size Effect in Polymers. Mater. Sci. Eng. A 2010, 527, 619–624. [Google Scholar] [CrossRef]
- Wythers, M.C. (Ed.) Advances in Materials Science Research; Nova Science Publishers: Hauppauge, NY, USA, 2021; Volume 48, ISBN 9781685073718. [Google Scholar]
- Jawaid, M.; Abdul Khalil, H.P.S.; Hassan, A.; Dungani, R.; Hadiyane, A. Effect of Jute Fibre Loading on Tensile and Dynamic Mechanical Properties of Oil Palm Epoxy Composites. Compos. Part B Eng. 2013, 45, 619–624. [Google Scholar] [CrossRef]
- Devi, L.U.; Bhagawan, S.S.; Thomas, S. Dynamic Mechanical Analysis of Pineapple Leaf/Glass Hybrid Fiber Reinforced Polyester Composites. Polym. Compos. 2010, 31, 956–965. [Google Scholar] [CrossRef]
- Singh, T.; Gangil, B.; Ranakoti, L.; Joshi, A. Effect of Silica Nanoparticles on Physical, Mechanical, and Wear Properties of Natural Fiber Reinforced Polymer Composites. Polym. Compos. 2021, 42, 2396–2407. [Google Scholar] [CrossRef]
- Katebi Koushali, S.; Hamadanian, M.; Ghasemi, A.R.; Ashrafi, M. Investigation of Mechanical Properties of Polyester/Polyethylene Glycol/TiO2 Nanocomposites. J. Nanostruct. 2021, 11, 38–47. [Google Scholar] [CrossRef]
- Drah, A.; Kovačević, T.; Rusmirović, J.; Tomić, N.; Brzić, S.; Bogosavljavić, M.; Marinković, A. Effect of Surface Activation of Alumina Particles on the Performances of Thermosetting-Based Composite Materials. J. Compos. Mater. 2019, 53, 2727–2742. [Google Scholar] [CrossRef]
- Fidanovski, B.Z.; Popovic, I.G.; Radojevic, V.J.; Radisavljevic, I.Z.; Perisic, S.D.; Spasojevic, P.M. Composite Materials from Fully Bio-Based Thermosetting Resins and Recycled Waste Poly(Ethylene Terephthalate). Compos. Part B Eng. 2018, 153, 117–123. [Google Scholar] [CrossRef]
- Li, Z.; Muiruri, J.K.; Thitsartarn, W.; Zhang, X.; Tan, B.H.; He, C. Biodegradable Silica Rubber Core-Shell Nanoparticles and Their Stereocomplex for Efficient PLA Toughening. Compos. Sci. Technol. 2018, 159, 11–17. [Google Scholar] [CrossRef]
- Peng, Z.; Kong, L.X.; Li, S.-D.; Spiridonov, P. Poly(Vinyl Alcohol)/Silica Nanocomposites: Morphology and Thermal Degradation Kinetics. J. Nanosci. Nanotechnol. 2006, 6, 3934–3938. [Google Scholar] [CrossRef]
- Siraj, S.; Al-Marzouqi, A.H.; Iqbal, M.Z.; Ahmed, W. Impact of Micro Silica Filler Particle Size on Mechanical Properties of Polymeric Based Composite Material. Polymers 2022, 14, 4830. [Google Scholar] [CrossRef]
- Watanabe, R.; Sugahara, A.; Hagihara, H.; Sato, H.; Mizukado, J.; Shinzawa, H. Study of Matrix-Filler Interaction of Polypropylene/Silica Composite by Combined Infrared (IR) Spectroscopic Imaging and Disrelation Mapping. Compos. Part A Appl. Sci. Manuf. 2020, 128, 105658. [Google Scholar] [CrossRef]
- Adeosun, S.O.; Akpan, E.I.; Akanegbu, H.A. Thermo-Mechanical Properties of Unsaturated Polyester Reinforced with Coconut and Snail Shells. Int. J. Compos. Mater. 2015, 5, 52–64. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, B.J.; Meng, F.Z.; Hu, Q.; Pan, J.L. Influence of Aluminum Hydroxide Particles on the Properties of Polyurethane Based on Unsaturated Polyester. Adv. Mater. Res. 2013, 721, 210–214. [Google Scholar] [CrossRef]
- Chen, D.; Sun, X.; Li, B.; Liu, Y.; Zhu, T.; Xiao, S. On Crashworthiness and Energy-Absorbing Mechanisms of Thick CFRP Structures for Railway Vehicles. Polymers 2022, 14, 4795. [Google Scholar] [CrossRef] [PubMed]
- Arfiana; Saputra, A.H.; Murti, S.D.S.; Saputra, H. Study on the Flammability and Thermal Stability of Non Halogen UPR-Based Fire Retardant Composite through Combination of Carbon Black and Hydroxide Additives. Macromol. Symp. 2020, 391, 1900176. [Google Scholar] [CrossRef]
- Seraji, S.M.; Song, P.; Varley, R.J.; Bourbigot, S.; Voice, D.; Wang, H. Fire-Retardant Unsaturated Polyester Thermosets: The State-of-the-Art, Challenges and Opportunities. Chem. Eng. J. 2022, 430, 132785. [Google Scholar] [CrossRef]






| Parameter | Value | Unit | Method |
|---|---|---|---|
| Appearance | Transparent yellow | - | - |
| Non-volatile material content | 60 ± 2 | wt.% | ISO 3251 |
| Acid/Hydroxyl value (AV/HV) | 12/25 | mg KOH/g | ISO 2114 |
| Gel time, 20 °C: 2 wt.% MEKP-50 | 25–30 | Min | ISO 2535 |
| Temperature of exotherm peak | 110–120 | °C | ISO 2535 |
| Density | 1.37 ± 0.09 | kg/m3 | ISO 2811 |
| Iodine value | 51 | - | Wijs |
| Sample | nav | A | B | x | y | K |
|---|---|---|---|---|---|---|
| b-UPR | 2.2499 | 0.0060 | −0.1122 | 0.1111 | −0.0997 | 19.6738 |
| b-UPR/SiO2 | 2.2367 | 0.0121 | −0.2544 | 0.1406 | −0.2275 | 35.7390 |
| b-UPR/SiO2-AMBD | 2.3178 | 0.0078 | −0.2231 | 0.1371 | −0.1952 | 28.1794 |
| b-UPR/SiO2-M | 2.1038 | 0.1024 | −0.6910 | 0.0493 | −0.6569 | 21.2490 |
| b-UPR/SiO2-V | 2.6520 | 0.0214 | −0.9776 | 0.2458 | −0.7373 | 48.0749 |
| Sample | G′RP-50°C, MPa | G′GS-130°C, MPa | Tg, °C | tan δheight | ν × 10−3, mol m−3 |
|---|---|---|---|---|---|
| b-UPR | 581.86 | 6.35 | 101 | 0.59 | 103.37 |
| b-UPR/SiO2 | 227.83 | 3.91 | 100 | 0.48 | 40.53 |
| b-UPR/SiO2-AMBD | 207.12 | 3.47 | 100 | 0.53 | 36.85 |
| b-UPR/SiO2-M | 658.36 | 9.28 | 104 | 0.58 | 116.44 |
| b-UPR/SiO2-V | 640.05 | 6.98 | 102 | 0.53 | 113.53 |
| Sample | SiO2 Amount (wt.%) | σm (MPa) | E (GPa) | ε (%) |
|---|---|---|---|---|
| b-UPR | - | 25.5 ± 1.11 | 0.276 ±0.013 | 5.90 ± 0.25 |
| b-UPR/SiO2 | 1 | 28.7 ± 1.33 | 0.316 ± 0.015 | 5.58 ± 0.18 |
| b-UPR/SiO2-AMBD | 1 | 34.3 ± 1.54 | 0.345 ± 0.016 | 5.35 ± 0.24 |
| b-UPR/SiO2-M | 1 | 38.4 ± 1.77 | 0.351 ± 0.018 | 5.22 ± 0.21 |
| b-UPR/SiO2-V | 1 | 43.6 ± 1.97 | 0.412 ± 0.019 | 4.57 ± 0.16 |
| b-UPR/SiO2 | 2.5 | 32.1 ± 1.41 | 0.412 ± 0.020 | 5.41 ± 0.18 |
| b-UPR/SiO2-AMBD | 2.5 | 39.8 ± 1.69 | 0.490 ± 0.024 | 4.97 ± 0.26 |
| b-UPR/SiO2-M | 2.5 | 40.1 ±1.82 | 0.558 ± 0.027 | 4.81 ± 0.23 |
| b-UPR/SiO2-V | 2.5 | 47.9 ±2.18 | 0.607 ± 0.029 | 4.02 ± 0.21 |
| b-UPR/SiO2 | 5 | 28.2 ±1.35 | 0.285 ± 0.014 | 5.56 ± 0.12 |
| b-UPR/SiO2-AMBD | 5 | 27.0 ± 1.31 | 0.316 ± 0.015 | 5.78 ± 0.22 |
| b-UPR/SiO2-M | 5 | 38.9 ± 1.82 | 0.394 ± 0.019 | 5.06 ± 0.19 |
| b-UPR/SiO2-V | 5 | 41.7 ± 1.91 | 0.378 ± 0.018 | 4.73 ± 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Embirsh, H.S.A.; Stajčić, I.; Gržetić, J.; Mladenović, I.O.; Anđelković, B.; Marinković, A.; Vuksanović, M.M. Synthesis, Characterization and Application of Biobased Unsaturated Polyester Resin Reinforced with Unmodified/Modified Biosilica Nanoparticles. Polymers 2023, 15, 3756. https://doi.org/10.3390/polym15183756
Embirsh HSA, Stajčić I, Gržetić J, Mladenović IO, Anđelković B, Marinković A, Vuksanović MM. Synthesis, Characterization and Application of Biobased Unsaturated Polyester Resin Reinforced with Unmodified/Modified Biosilica Nanoparticles. Polymers. 2023; 15(18):3756. https://doi.org/10.3390/polym15183756
Chicago/Turabian StyleEmbirsh, Hifa Salah Adeen, Ivana Stajčić, Jelena Gržetić, Ivana O. Mladenović, Boban Anđelković, Aleksandar Marinković, and Marija M. Vuksanović. 2023. "Synthesis, Characterization and Application of Biobased Unsaturated Polyester Resin Reinforced with Unmodified/Modified Biosilica Nanoparticles" Polymers 15, no. 18: 3756. https://doi.org/10.3390/polym15183756







