Improving the Pervaporation Performance of PDMS Membranes for Trichloroethylene by Incorporating Silane-Modified ZSM-5 Zeolite
Abstract
:1. Introduction
2. Experimental
2.1. Materials
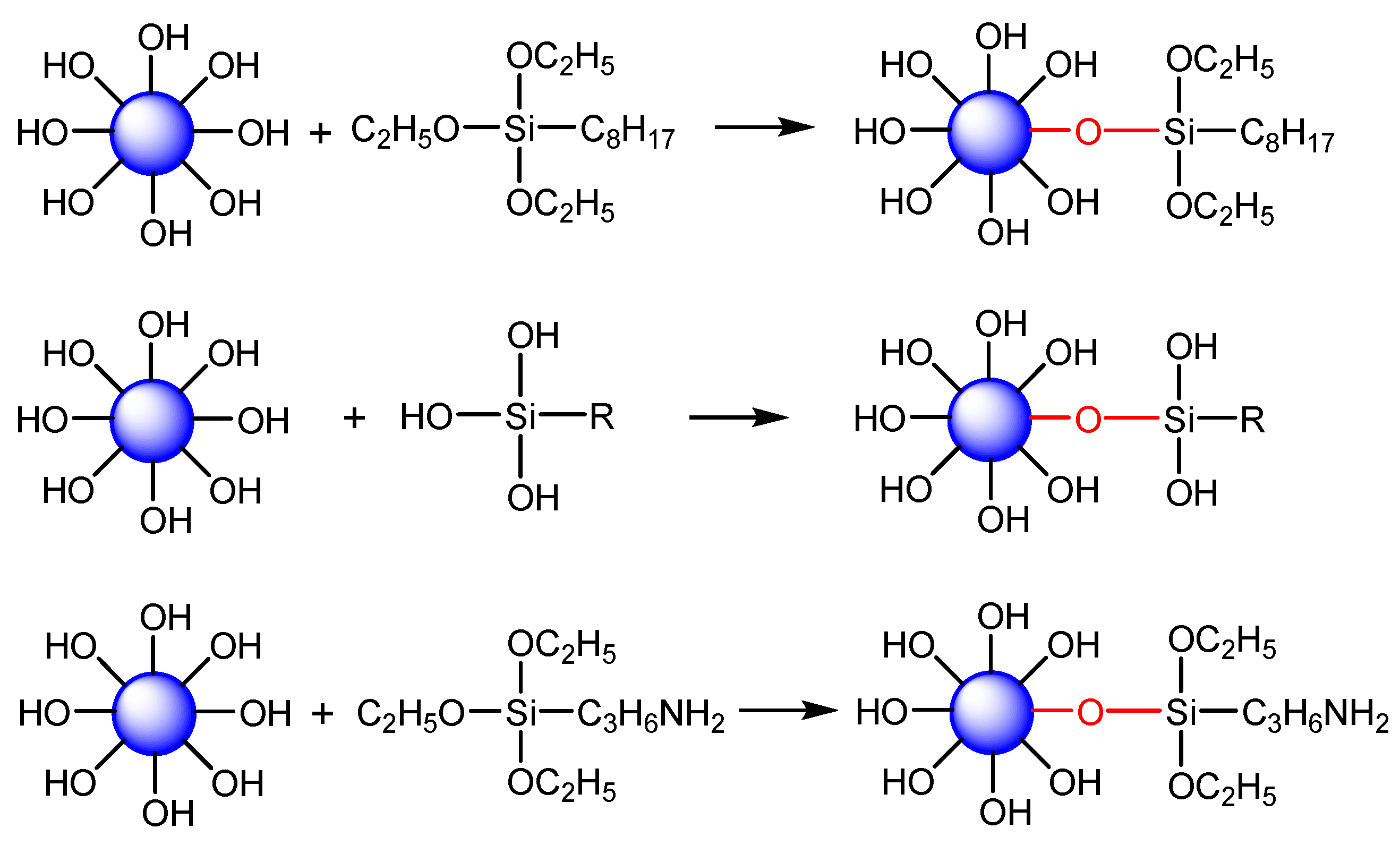
2.2. Hydrophobic Modification of ZSM-5 Zeolite
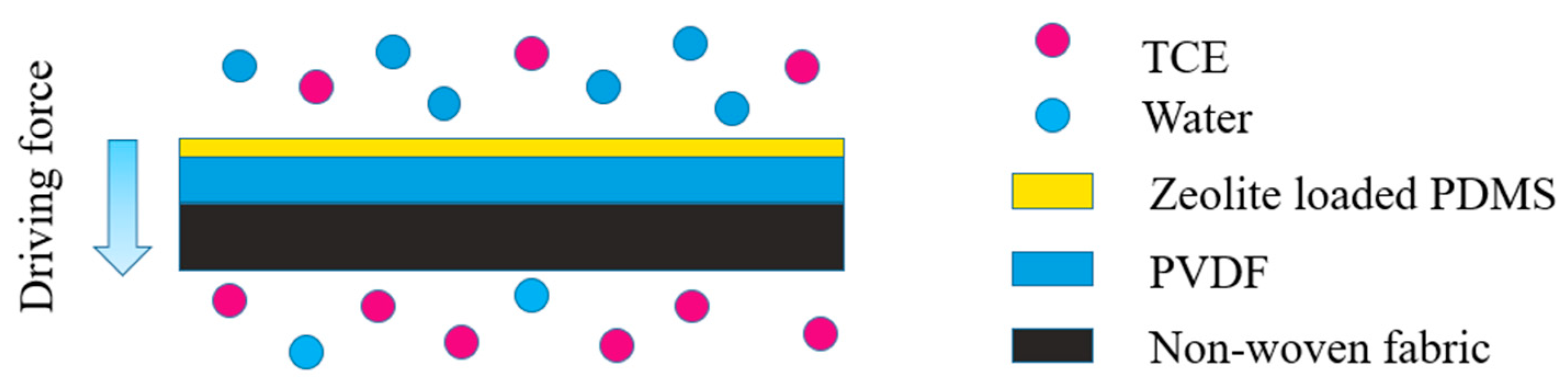
2.3. Fabrication of MMMs
2.4. Characterization
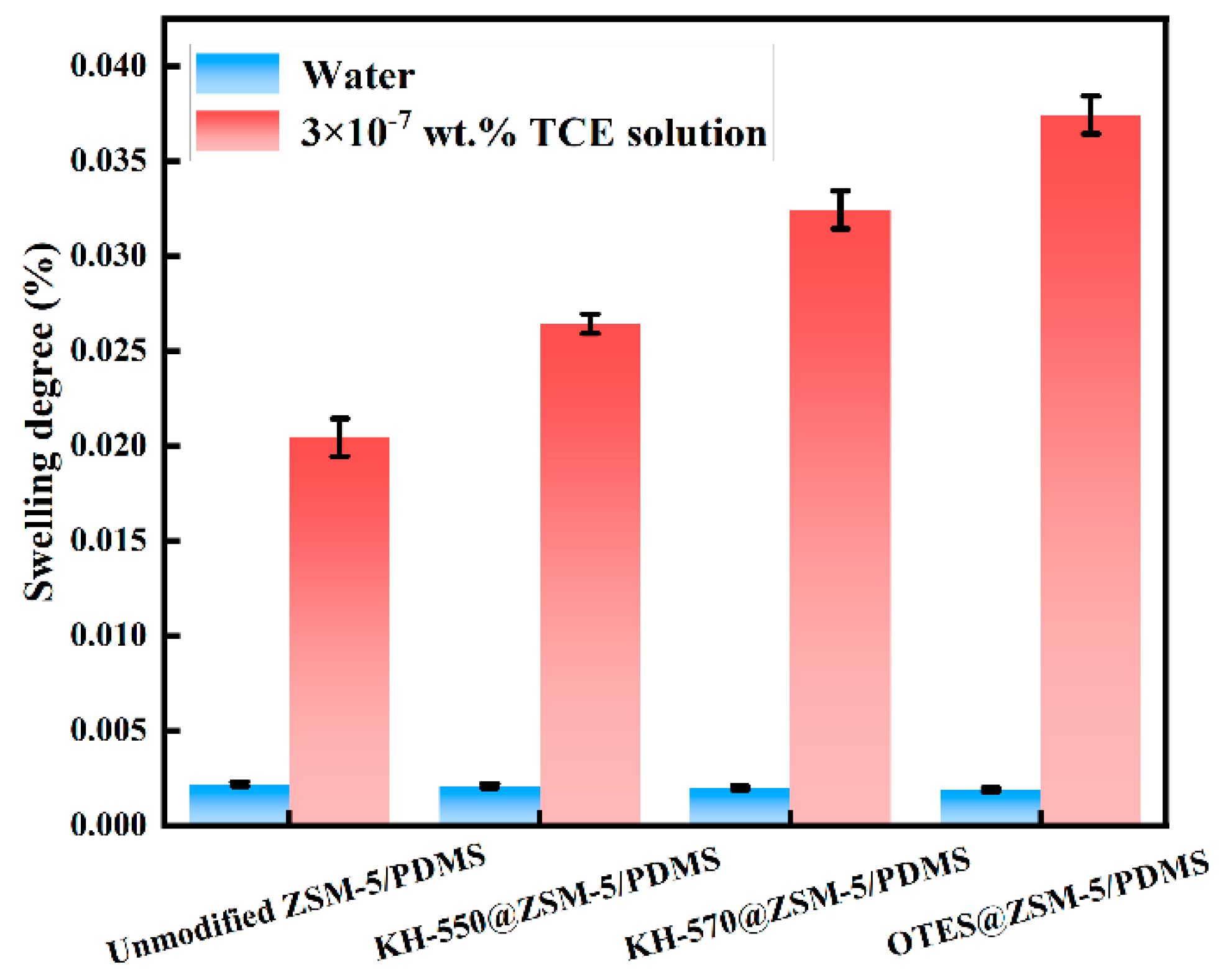
2.5. Swelling Experiment
2.6. PV Experiment
3. Results and Discussion
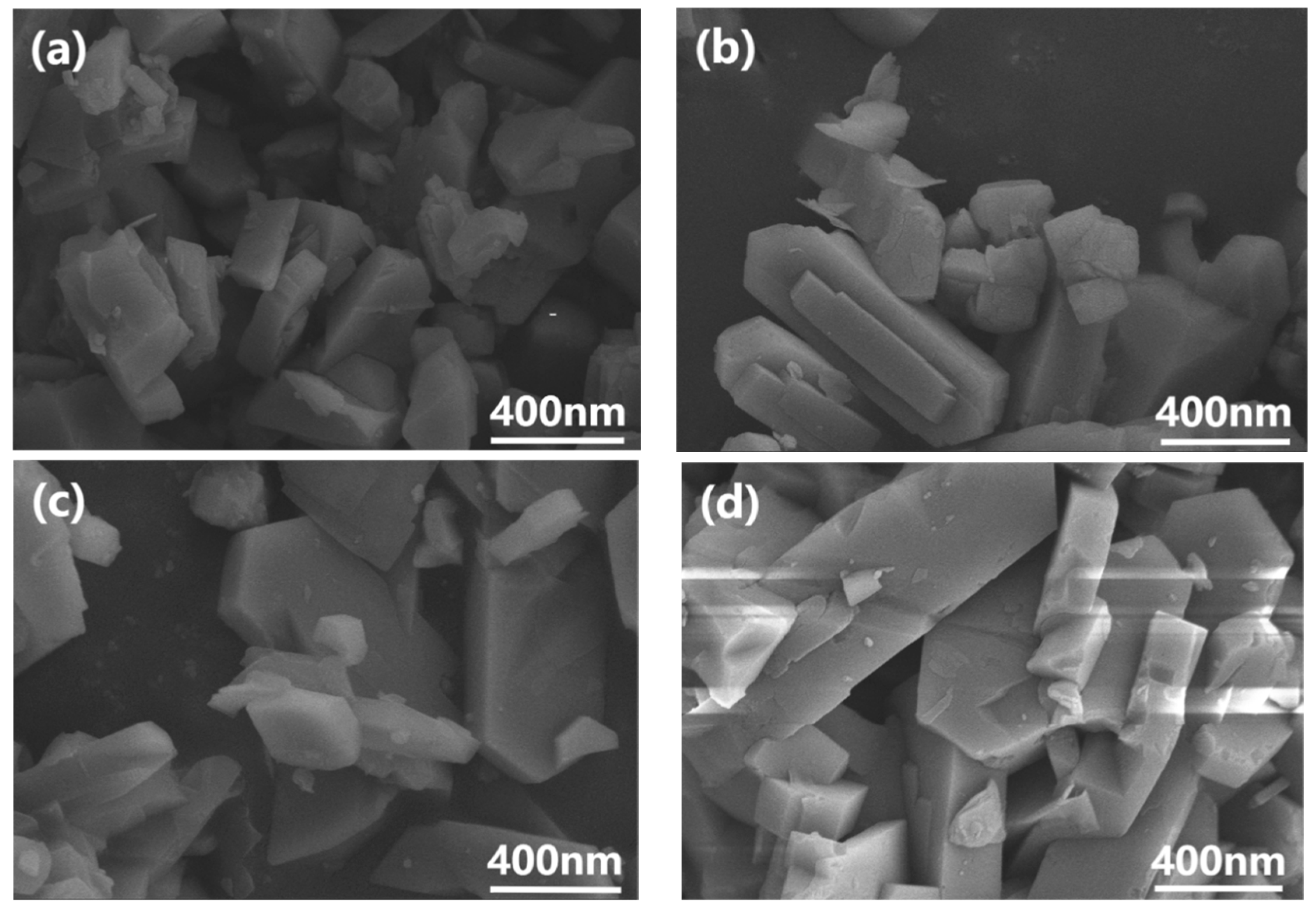
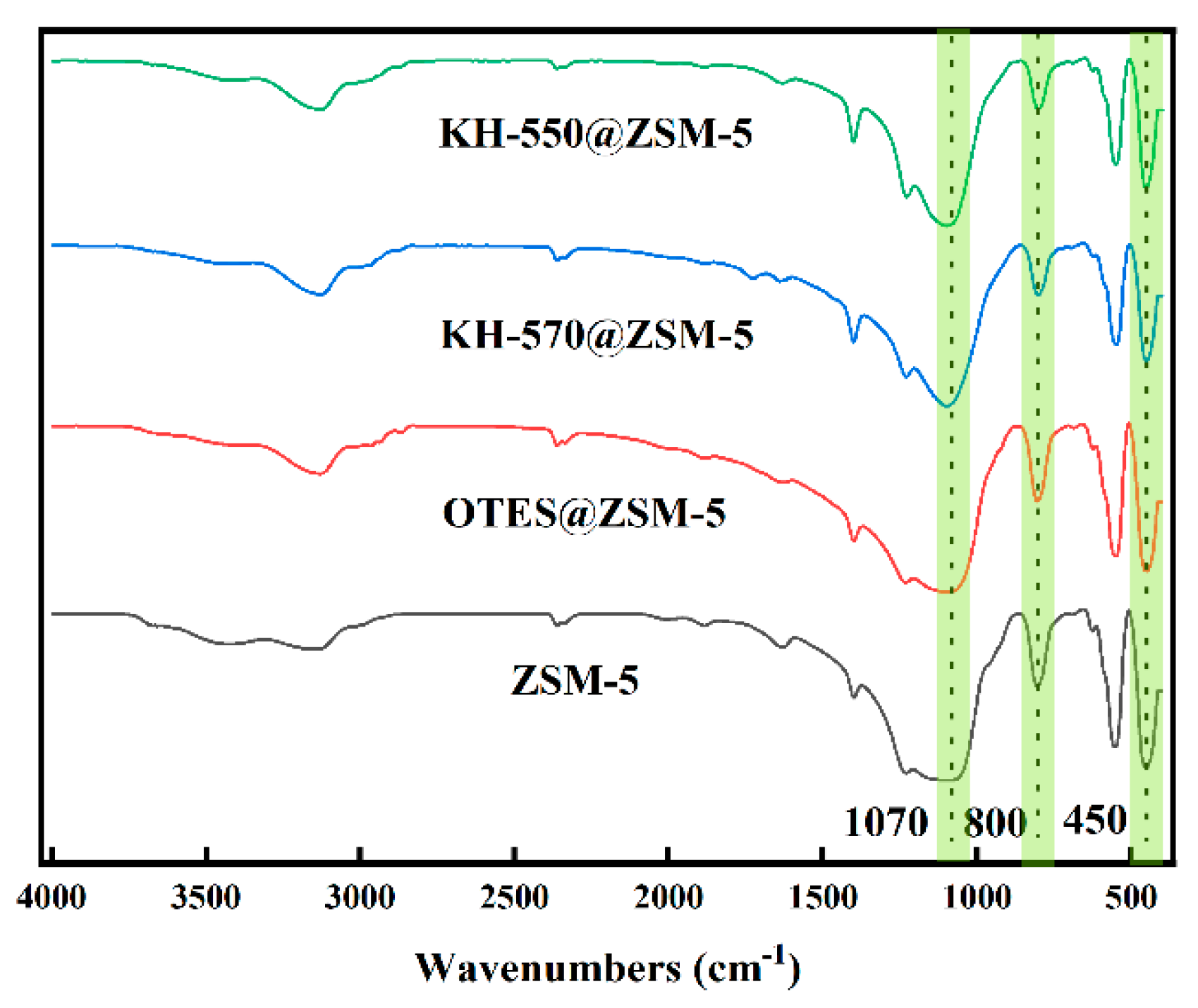
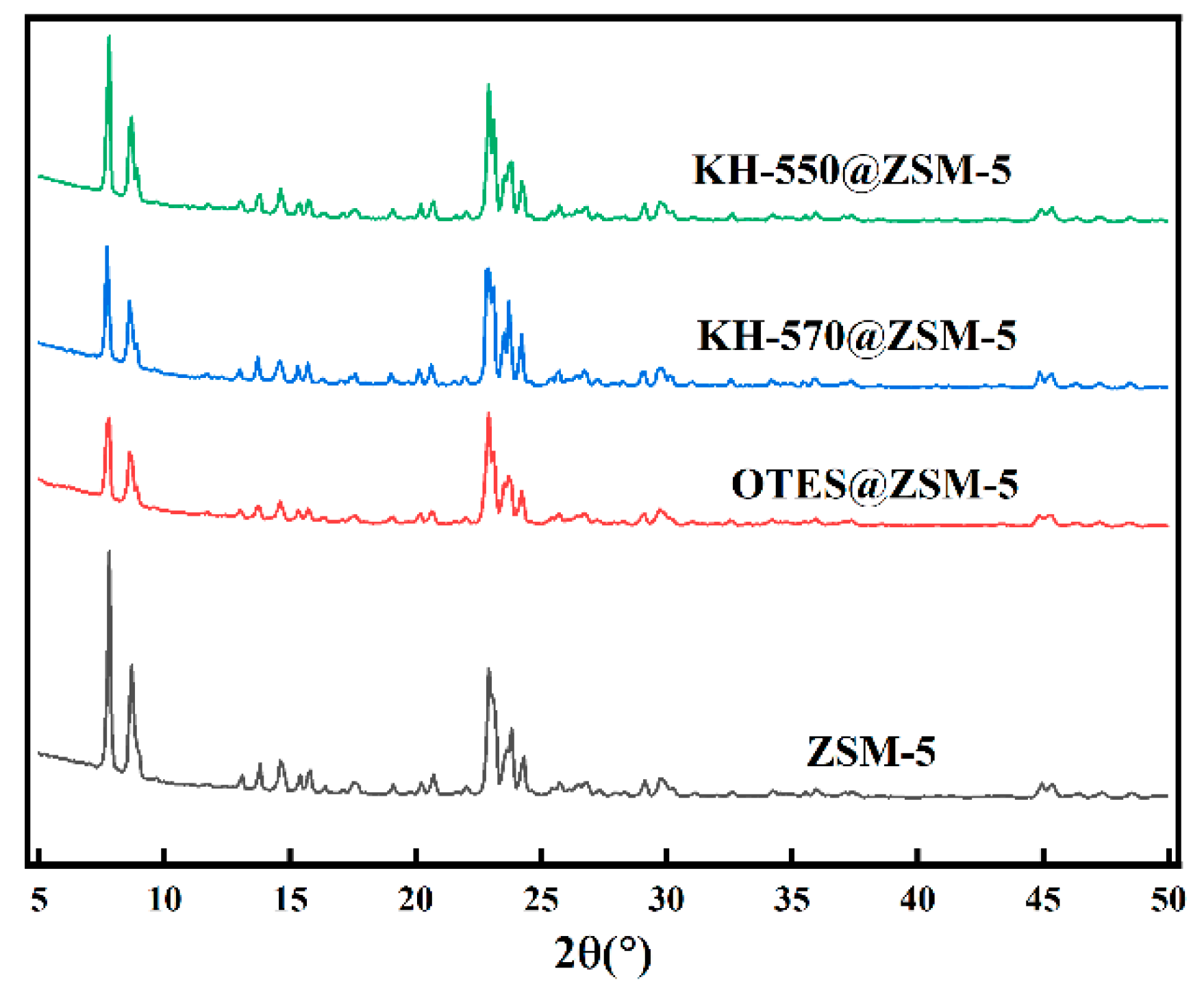
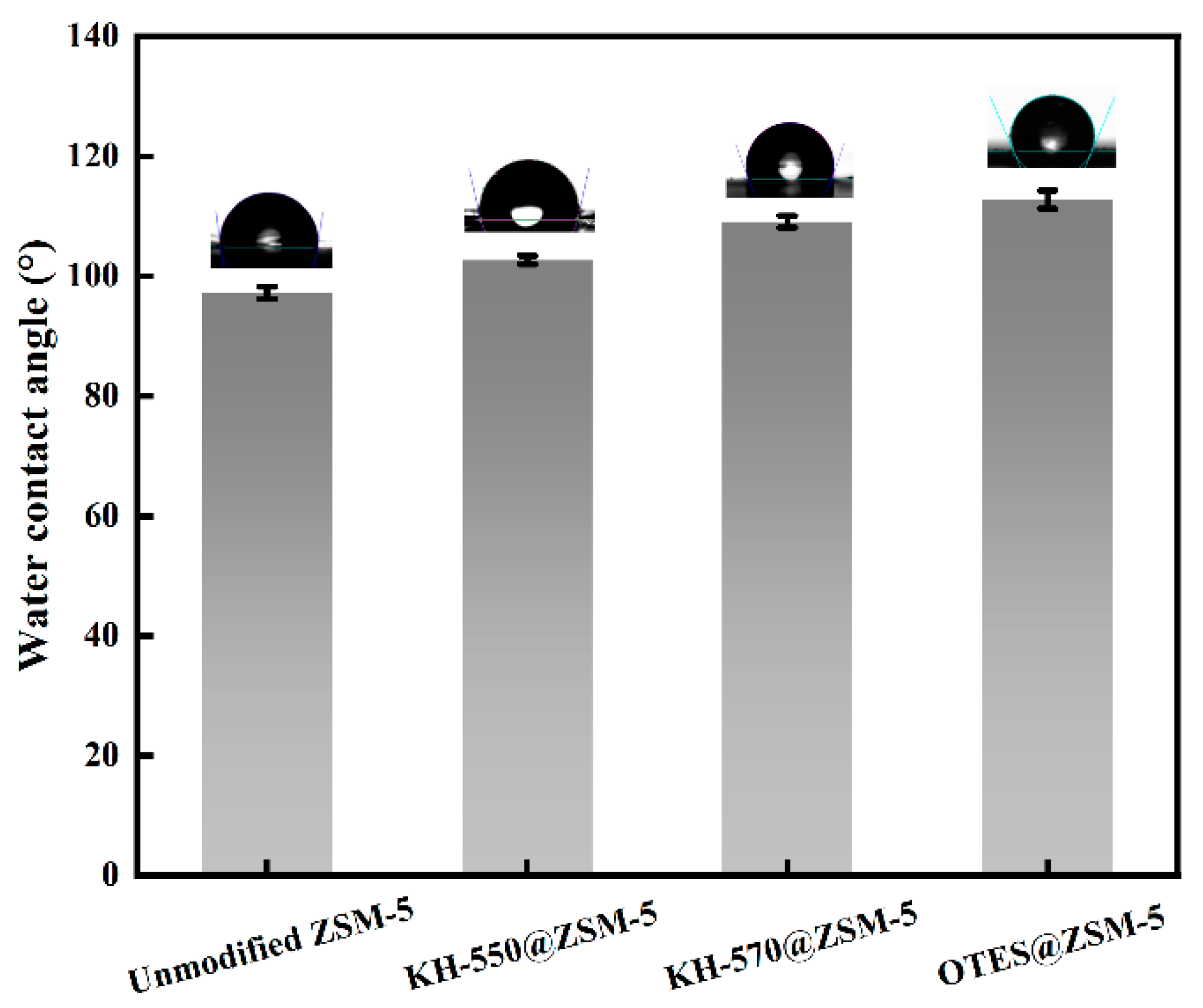
3.1. Characterization of Silane-Modified ZSM-5
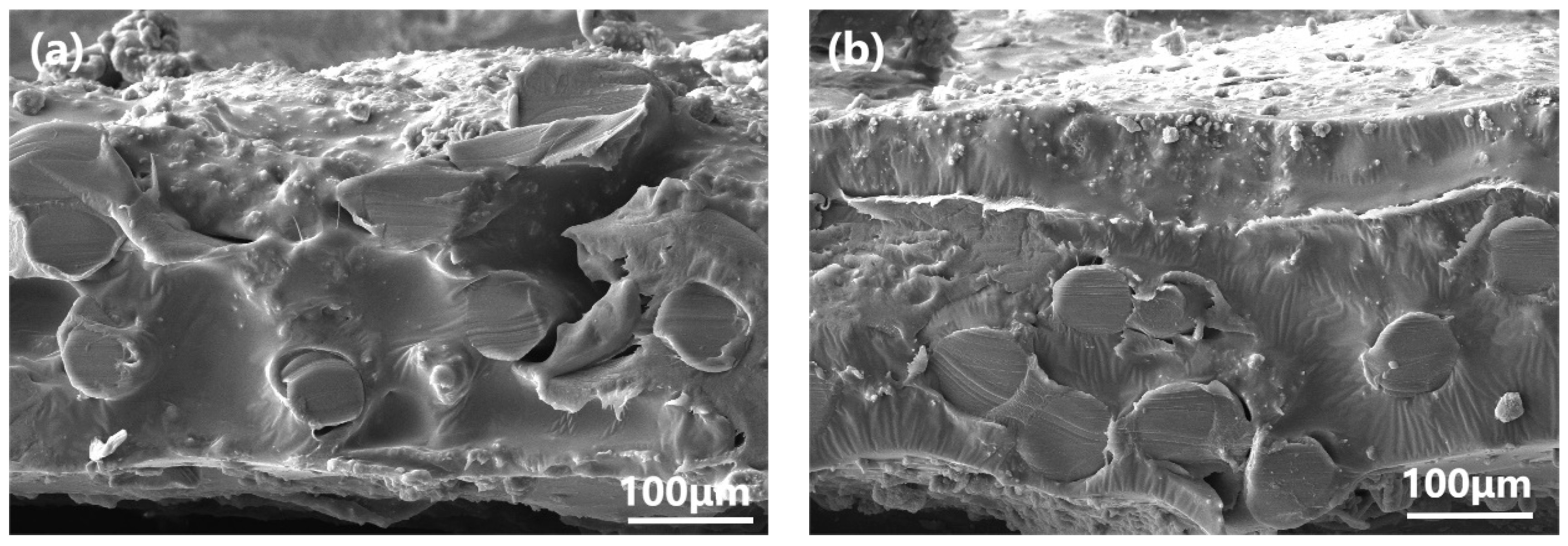
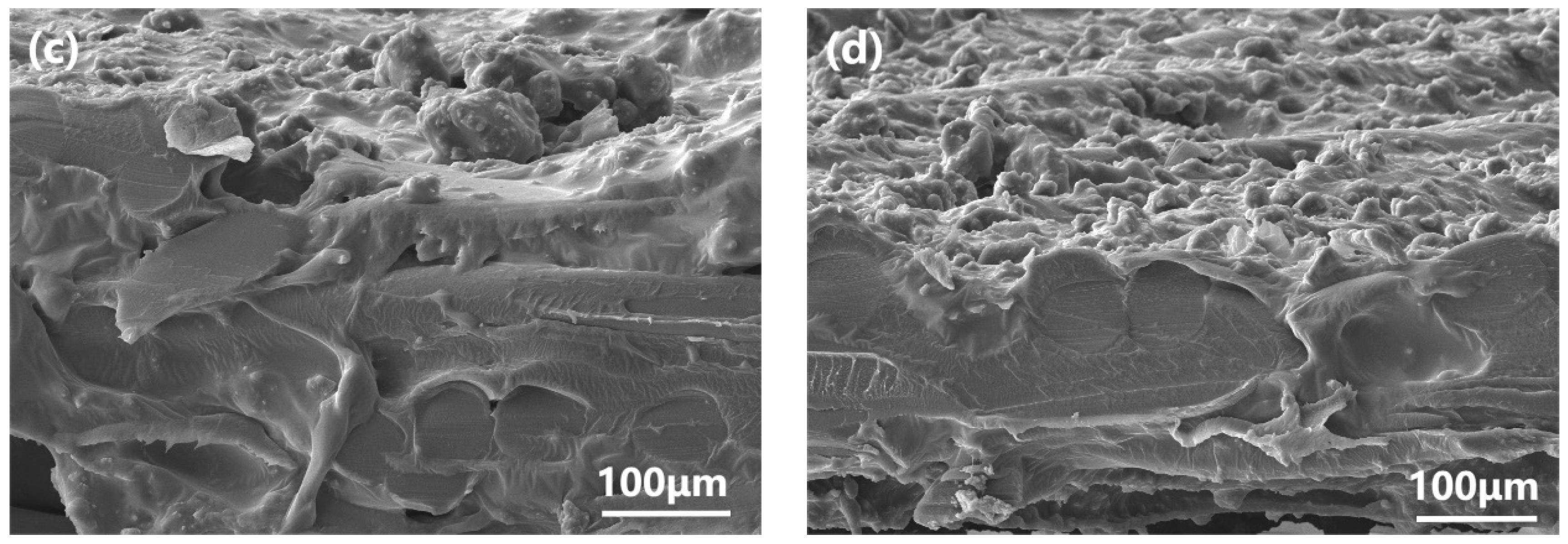
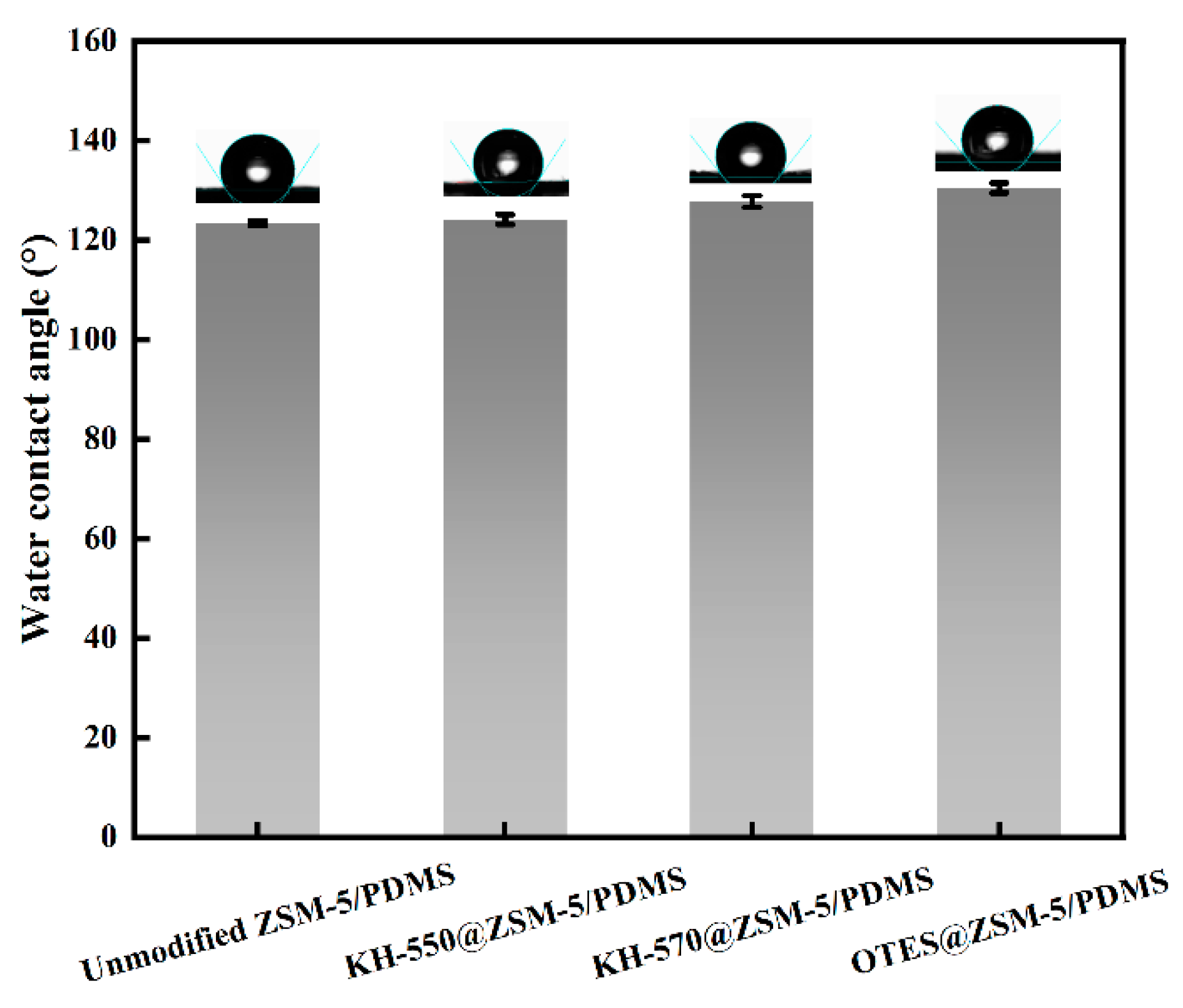
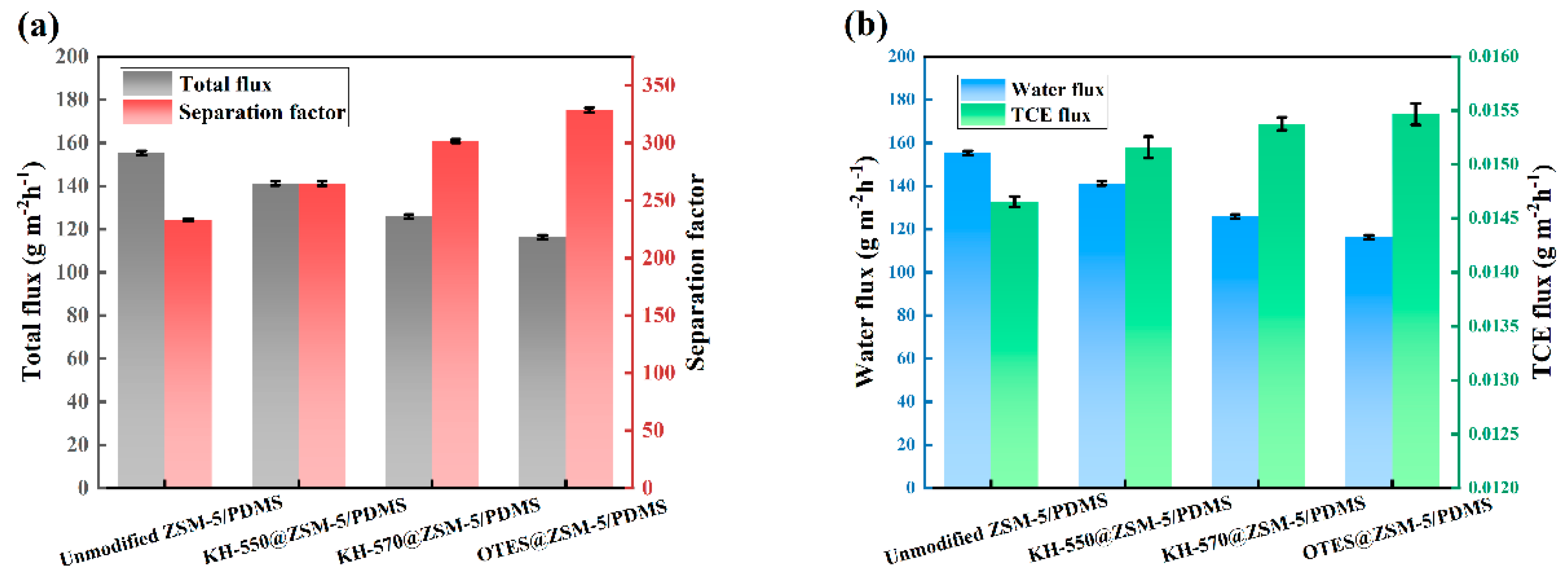
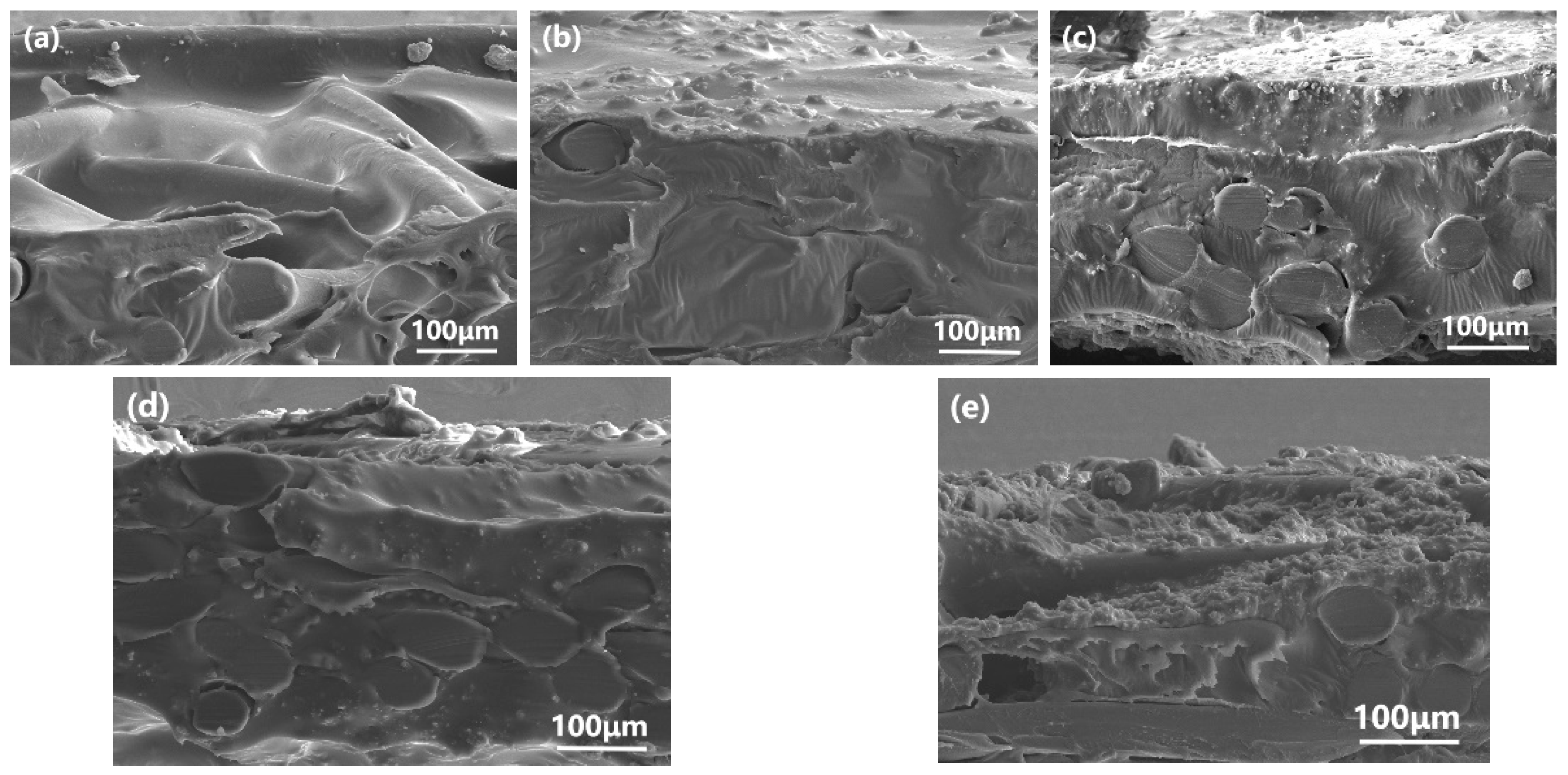
3.2. Characterization and PV Performance of the MMMs
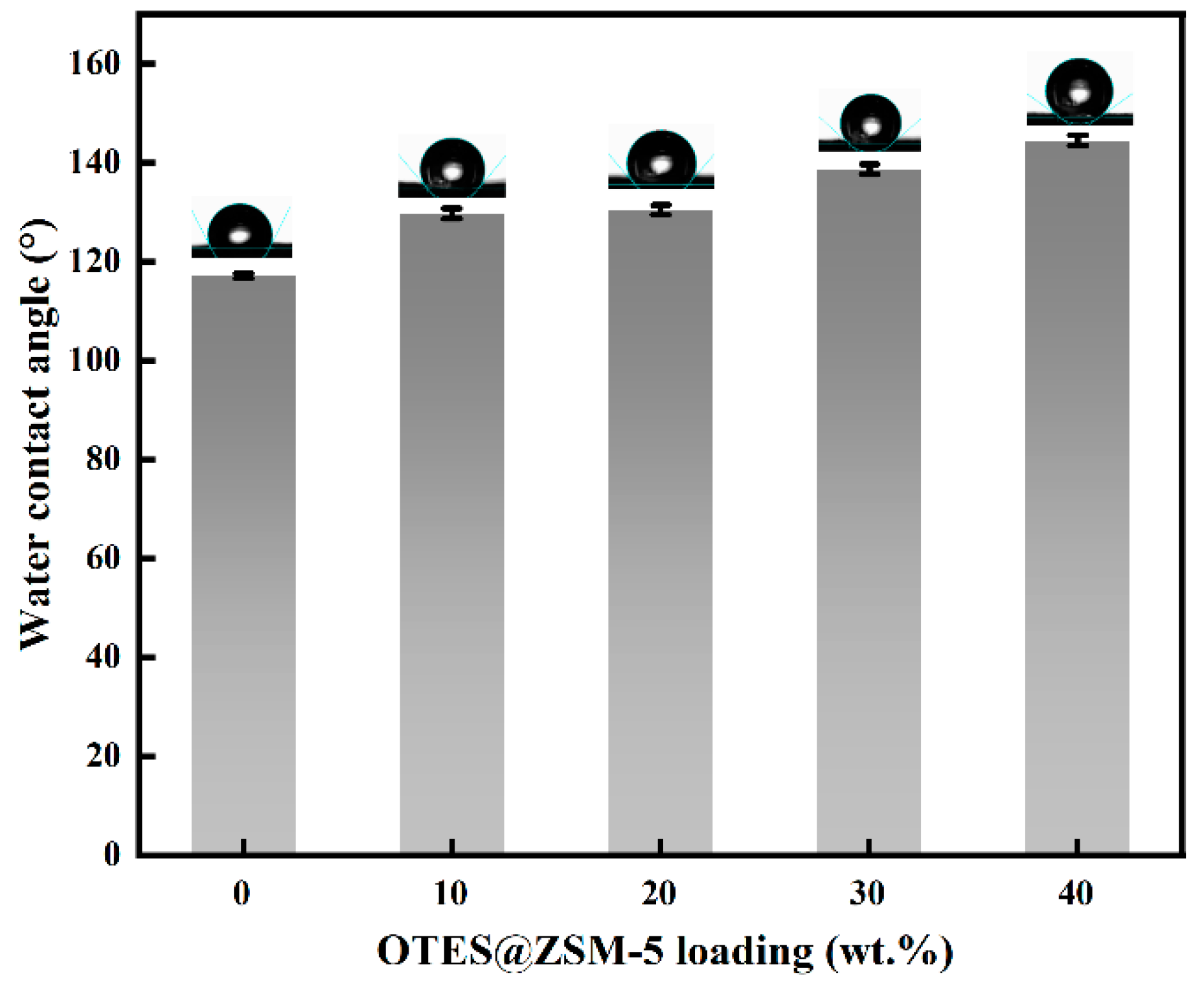
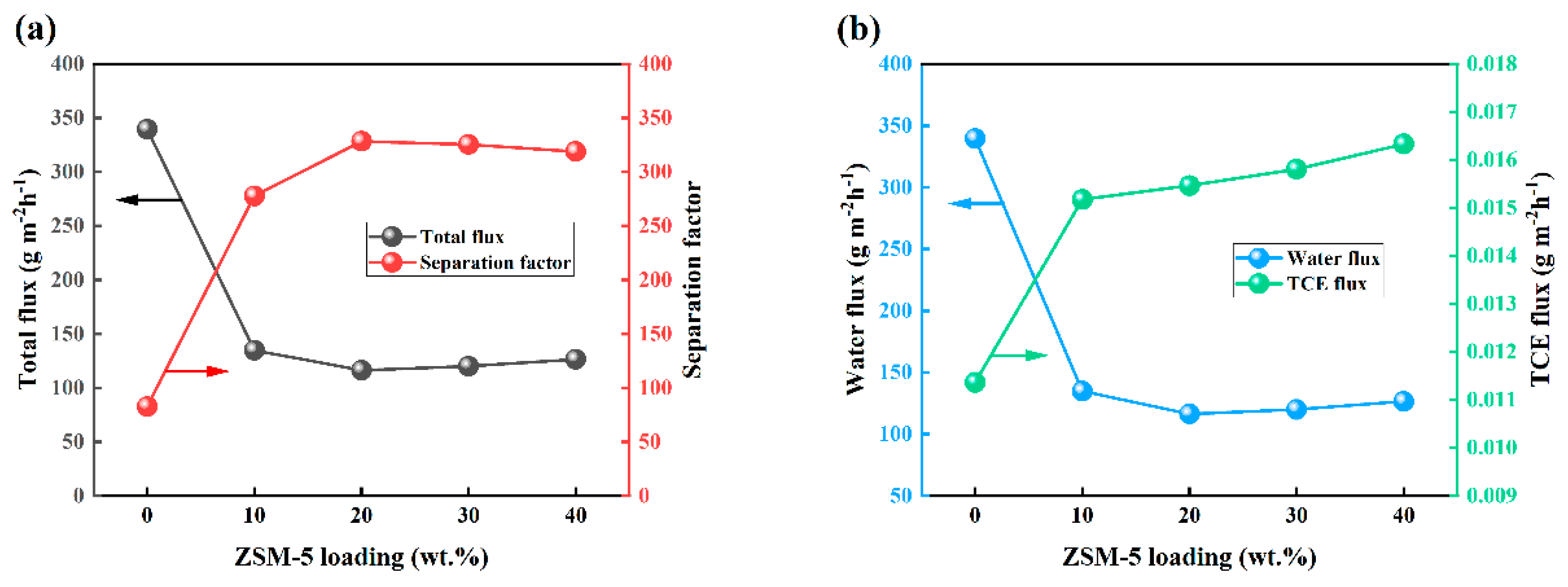
3.3. Effect of Zeolite Loading on OTES@ZSM-5/PDMS MMM
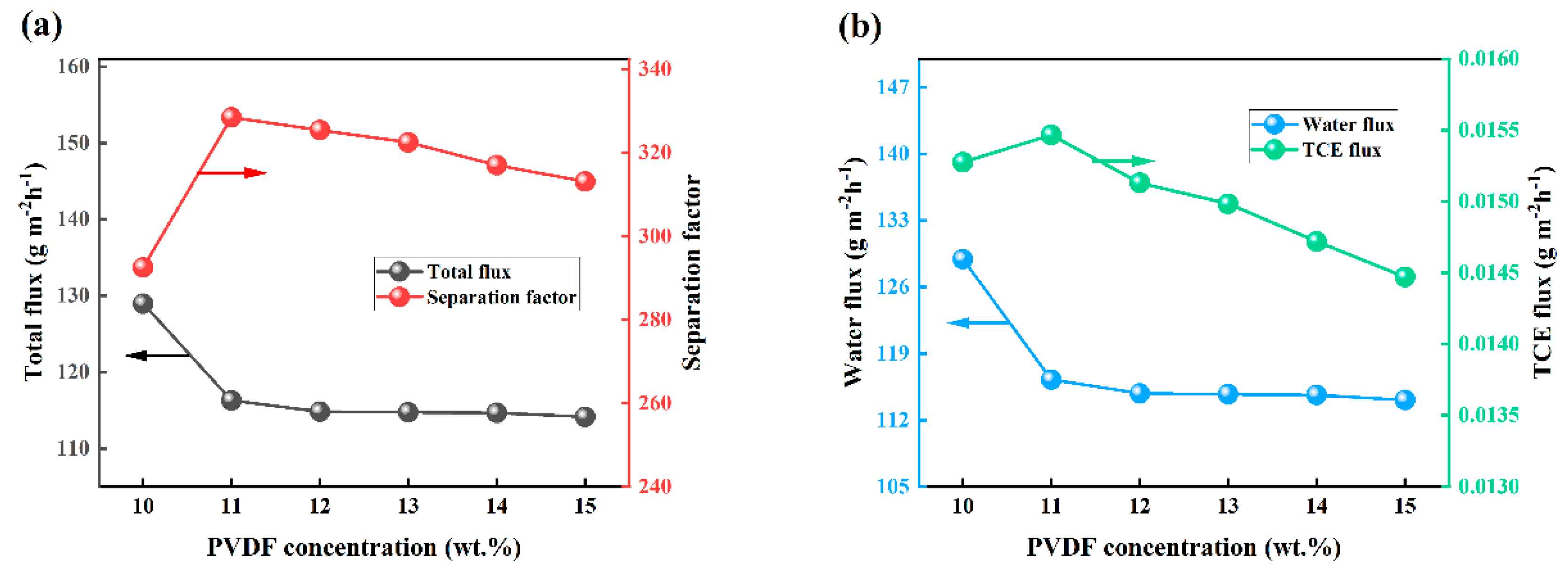
3.4. Effect of PVDF Content on OTES@ZSM-5/PDMS MMM
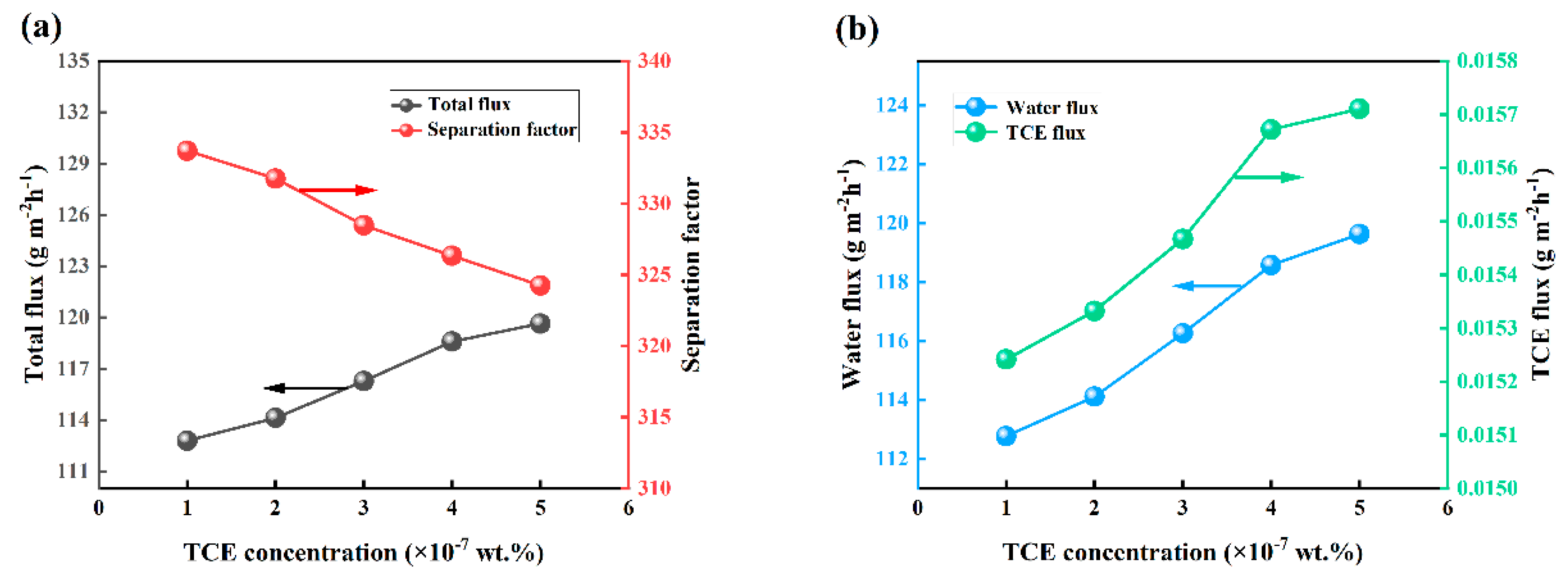
3.5. Effect of Feed Concentration on PV Performance
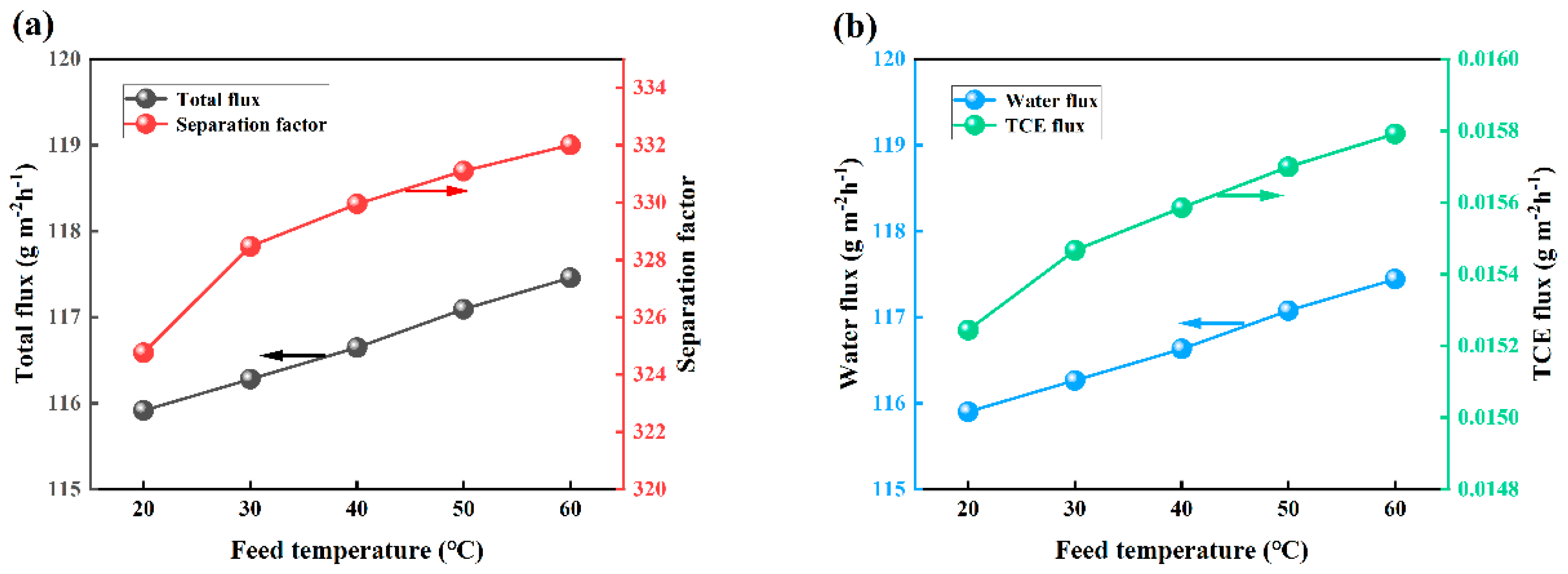
3.6. Effect of Feed Temperature on PV Performance
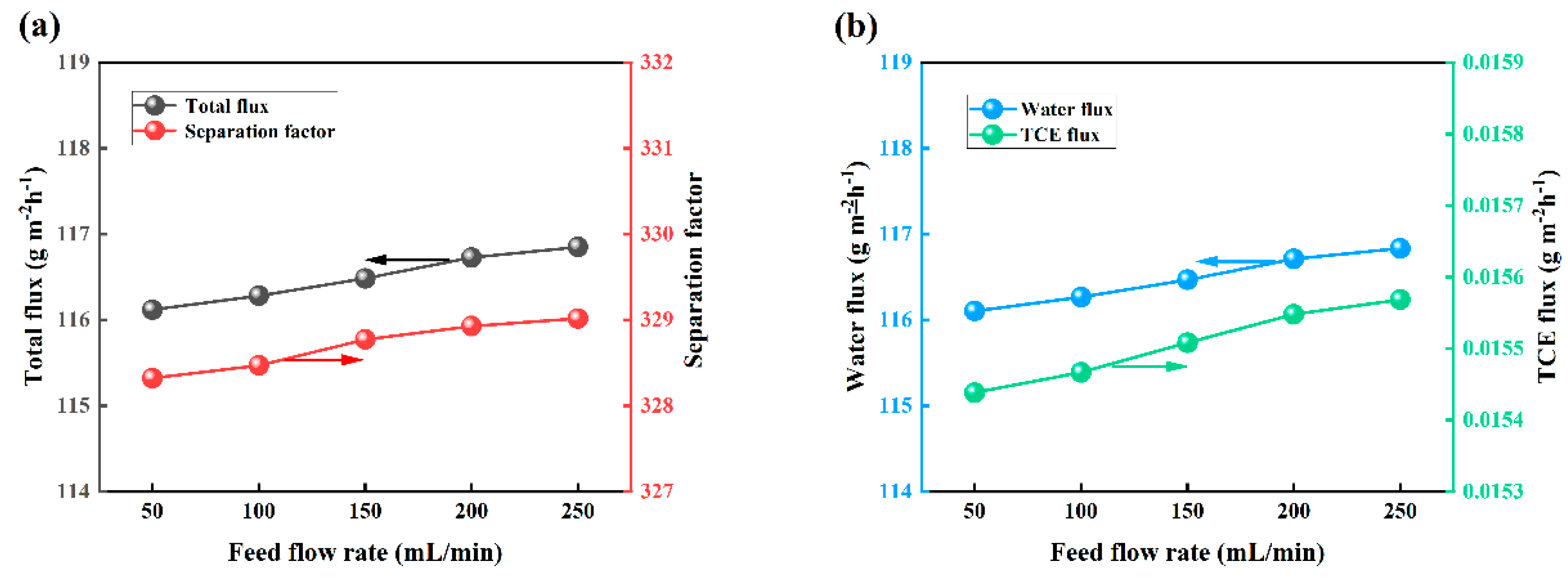
3.7. Effect of Feed Flow Rate on PV Performance
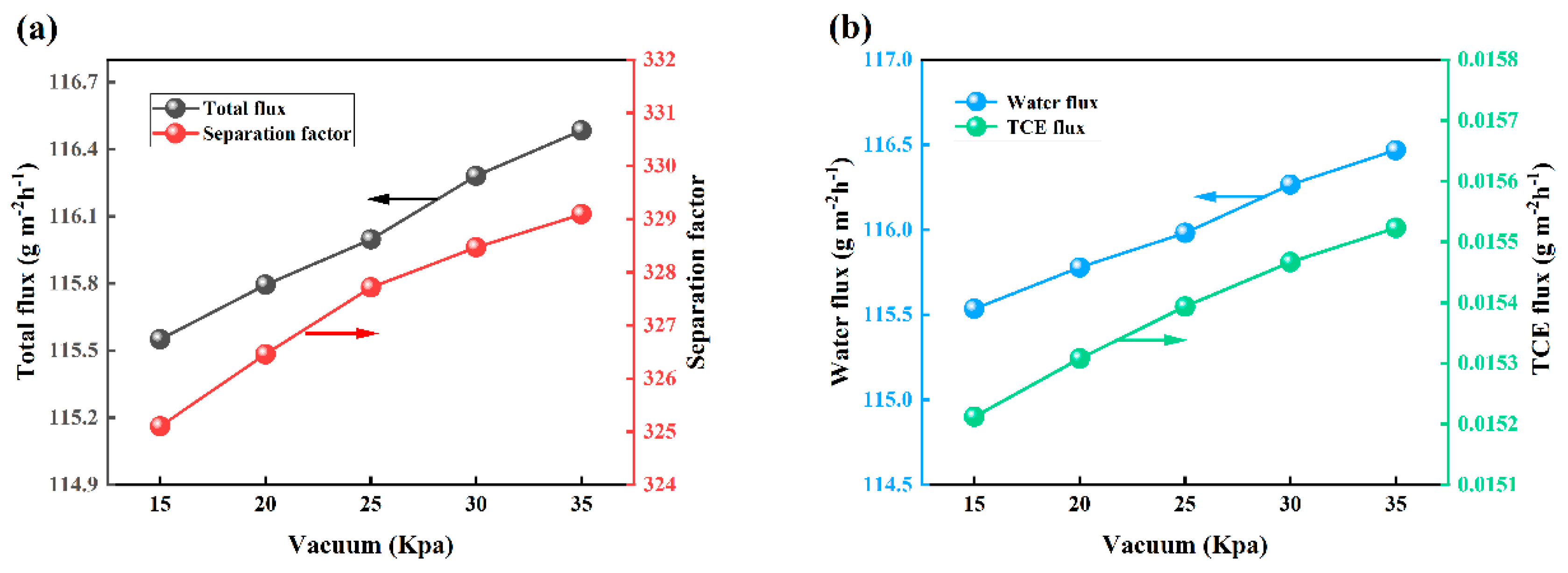
3.8. Effect of Vacuum Degree on PV Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Qin, F.; Qin, P.; Karim, M.N.; Tan, T. Preparation of PDMS membrane using water as solvent for pervaporation separation of butanol–water mixture. Green Chem. 2013, 15, 2180–2190. [Google Scholar] [CrossRef]
- Wang, T.; Ansai, T.; Lee, S.-W. Zeolite-loaded poly(dimethylsiloxane) hybrid films for highly efficient thin-film microextraction of organic volatiles in water. J. Chromatogr. B 2017, 1041–1042, 133–140. [Google Scholar] [CrossRef]
- Zhang, W.; Li, L.; Lin, K.; Xiong, B.; Li, B.; Lu, S.; Guo, M.; Cui, X. Synergetic degradation of Fe/Cu/C for groundwater polluted by trichloroethylene. Water Sci. Technol. 2012, 65, 2258–2264. [Google Scholar] [CrossRef]
- Lin, C.J.; Liou, Y.H.; Lo, S.-L. Supported Pd/Sn bimetallic nanoparticles for reductive dechlorination of aqueous trichloroethylene. Chemosphere 2009, 74, 314–319. [Google Scholar] [CrossRef]
- Chung, J.; Krajmalnik-Brown, R.; Bruce, E.R. Bioreduction of Trichloroethene Using a Hydrogen-Based Membrane Biofilm Reactor. Environ. Sci. Technol. 2008, 42, 477–483. [Google Scholar] [CrossRef] [PubMed]
- Feng, H.E.; Zhao, D. Preparation and characterization of a new class of starch-stabilized bimetallic nanoparticles for degradation of chlorinated hydrocarbons in water. Environ. Sci. Technol. 2005, 39, 3314–3320. [Google Scholar]
- Si, Z.; Liu, C.; Li, G.; Wang, Z.; Li, J.; Xue, T.; Yang, S.; Cai, D.; Li, S.; Zhao, H.; et al. Epoxide-based PDMS membranes with an ultrashort and controllable membrane-forming process for 1-butanol/water pervaporation. J. Membr. Sci. 2020, 612, 118472. [Google Scholar] [CrossRef]
- Qin, F.; Li, S.; Qin, P.; Karim, M.N.; Tan, T. A PDMS membrane with high pervaporation performance for the separation of furfural and its potential in industrial application. Green Chem. 2014, 16, 1262–1273. [Google Scholar] [CrossRef]
- Shi, G.M.; Chung, T.-S. Thin film composite membranes on ceramic for pervaporation dehydration of isopropanol. J. Membr. Sci. 2013, 448, 34–43. [Google Scholar] [CrossRef]
- Zhan, X.; Lu, J.; Tan, T.; Li, J. Mixed matrix membranes with HF acid etched ZSM-5 for ethanol/water separation: Preparation and pervaporation performance. Appl. Surf. Sci. 2012, 259, 547–556. [Google Scholar] [CrossRef]
- Kujawska, A.; Knozowska, K.; Kujawa, J.; Li, G.; Kujawski, W. Fabrication of PDMS based membranes with improved separation efficiency in hydrophobic pervaporation. Sep. Purif. Technol. 2020, 234, 116092. [Google Scholar] [CrossRef]
- Amin, K.; Mohsin., A.; Ayesha, I.; Naik, P.; Vankelecom, I.F.J.; Gilani, M.A.; Bilad, M.R.; Sajjad, Z.; Khan, A.L. ZIF-67 filled PDMS mixed matrix membranes for recovery of ethanol via pervaporation. Sep. Purif. Technol. 2018, 206, 50–58. [Google Scholar]
- Mao, H.; Li, S.-H.; Zhang, A.-S.; Xu, L.-H.; Lu, J.-J.; Zhao, Z.-P. Novel MOF-capped halloysite nanotubes/PDMS mixed matrix membranes for enhanced n-butanol permselective pervaporation. J. Membr. Sci. 2019, 595, 117543. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Ma, X.; Li, J. Modified ZSM-5/Polydimethylsiloxane Mixed Matrix Membranes for Ethanol/Water Separation via Pervaporation. Polym. Compos. 2016, 37, 1282–1291. [Google Scholar] [CrossRef]
- Pires, J.O.; Fernandes, A.C.; Duraiswami, D. Synthesis of novel hierarchical ZSM-5 monoliths and their application in trichloroethylene removal. Chin. J. Catal. 2014, 35, 1492–1496. [Google Scholar] [CrossRef]
- Chang, B.-J.; Chang, Y.-H.; Kim, D.-K.; Kim, J.-H.; Lee, S.-B. New copolyimide membranes for the pervaporation of trichloroethylene from water. J. Membr. Sci. 2005, 248, 99–107. [Google Scholar] [CrossRef]
- Cheng, C.; Liu, F.; Yang, H.K.; Xiao, K.; Xue, C.; Yang, S.-T. High-performance n-butanol recovery from aqueous solution by pervaporation with a PDMS mixed matrix membrane filled with zeolite. Ind. Eng. Chem. Res. 2020, 59, 7777–7786. [Google Scholar] [CrossRef]
- Li, D.; Yao, J.; Sun, H.; Liu, B.; van Agtmaal, S.; Feng, C. Recycling of phenol from aqueous solutions by pervaporation with ZSM-5/PDMS/PVDF hollow fiber composite membrane. Appl. Surf. Sci. 2018, 427, 288–297. [Google Scholar] [CrossRef]
- Liu, G.; Xiangli, F.; Wei, W.; Liu, S.; Jin, W. Improved performance of PDMS/ceramic composite pervaporation membranes by ZSM-5 homogeneously dispersed in PDMS via a surface graft/coating approach. Chem. Eng. J. 2011, 174, 495–503. [Google Scholar] [CrossRef]
- Xue, G.; Shi, B. Performance of various Si/Al ratios of ZSM-5-filled polydimethylsiloxane/polyethersulfone membrane in butanol recovery by pervaporation. Adv. Polym. Technol. 2018, 37, 3095–3105. [Google Scholar] [CrossRef]
- Ji, L.; Shi, B.; Wang, L. Pervaporation separation of ethanol/water mixture using modified zeolite filled PDMS membranes. J. Appl. Polym. Sci. 2015, 132, 41897. [Google Scholar] [CrossRef]
- Ramaiah, K.P.; Satyasri, D.; Sridhar, S.; Krishnaiah, A. Removal of hazardous chlorinated VOCs from aqueous solutions using novel ZSM-5 loaded PDMS/PVDF composite membrane consisting of three hydrophobic layers. J. Hazard. Mater. 2013, 261, 362–371. [Google Scholar] [CrossRef]
- Zhan, X.; Li, J.-D.; Huang, J.-Q.; Chen, C.-X.; Xia, Z.; Chen, J. Pervaporation properties of PDMS membranes cured with different cross-linking reagents for ethanol concentration from aqueous solutions. Chin. J. Polym. Sci. 2009, 27, 533–535. [Google Scholar] [CrossRef]
- Xiangli, F.; Chen, Y.; Jin, W.; Xu, N. Polydimethylsiloxane (PDMS)/Ceramic Composite Membrane with High Flux for Pervaporation of Ethanol-Water Mixtures. Ind. Eng. Chem. Res. 2007, 46, 2224–2230. [Google Scholar] [CrossRef]
- Sun, D.; Li, B.-B.; Xu, Z.-L. Pervaporation of ethanol/water mixture by organophilic nano-silica filled PDMS composite membranes. Desalination 2013, 322, 159–166. [Google Scholar] [CrossRef]
- Guan, P.; Ren, C.; Shan, H.; Cai, D.; Zhao, P.; Ma, D.; Qin, P.; Li, S.; Si, Z. Boosting the pervaporation performance of PDMS membrane for 1-butanol by MAF-6. Colloid Polym. Sci. 2021, 299, 1459–1468. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, X.; Zhang, Z.; Song, B.; Song, W. Application of polyurethane membrane with surface modified ZSM-5 for pervaporation of phenol/water mixture. J. Polym. Eng. 2017, 37, 777–784. [Google Scholar] [CrossRef]
- Vatani, M.; Raisi, A.; Pazuki, G. Pervaporation separation of ethyl acetate from aqueous solutions using ZSM-5 filled dual-layer poly(ether-block-amide)/polyethersulfone membrane. RSC Adv. 2018, 8, 4713–4725. [Google Scholar] [CrossRef]
- Li, S.; Chen, Z.; Yang, Y.; Si, Z.; Li, P.; Qin, P.; Tan, T. Improving the pervaporation performance of PDMS membranes for n-butanol by incorporating silane-modified ZIF-8 particles. Sep. Purif. Technol. 2019, 215, 163–172. [Google Scholar] [CrossRef]
- Zhan, X.; Li, J.; Fan, C.; Han, X.-L. Pervaporation separation of ethanol/water mixtures with chlorosilane modified silicalite-1/PDMS hybrid membranes. Chin. J. Polym. Sci. 2010, 28, 625–635. [Google Scholar] [CrossRef]
- Wu, Y.; Tan, H.; Li, D.; Jin, Y. Pervaporation of Aqueous Solution of Acetaldehyde Through ZSM-5 Filled PDMS Composite Membrane. Chin. J. Chem. Eng. 2012, 20, 625–632. [Google Scholar] [CrossRef]
- De, S.; Li, B.; Xu, Z.-L. Preparation and characterization of poly(dimethylsiloxane)-polytetrafluoroethylene (PDMS-PTFE) composite membrane for pervaporation of chloroform from aqueous solution. Korean J. Chem. Eng. 2013, 30, 2059–2067. [Google Scholar]
- Si, D.; Zhu, M.; Sun, X.; Xue, M.; Li, Y.; Wu, T.; Gui, T.; Kumakiri, I.; Chen, X.; Kita, H. Formation process and pervaporation of high aluminum ZSM-5 zeolite membrane with fluoride-containing and organic template-free gel. Sep. Purif. Technol. 2020, 257, 117963. [Google Scholar] [CrossRef]
- Chen, G.; Zhu, H.; Hang, Y.; Liu, Q.; Liu, G.; Jin, W. Simultaneously enhancing interfacial adhesion and pervaporation separation performance of PDMS/ceramic composite membrane via a facile substrate surface grafting approach. AIChE J. 2019, 65, e16773. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, X.; Zhao, Z.; Song, B.; Zhang, Z.; Song, W. Pervaporation performance of surface-modified zeolite/PU mixed matrix membranes for separation of phenol from water. Iran. Polym. J. 2017, 26, 193–203. [Google Scholar] [CrossRef]
- Li, Y.; Wee, L.H.; Martens, J.A.; Vankelecom, I.F.J. ZIF-71 as a potential filler to prepare pervaporation membranes for bio-alcohol recovery. J. Mater. Chem. A 2014, 2, 10034–10040. [Google Scholar] [CrossRef]
- Tian, M.; De Coninck, H.; Zhu, J.; Zhang, Y.; Yuan, S.; Van Hooreweder, B.; Van Puyvelde, P.; Van der Bruggen, B. Exploring the potential usage of 3D printed membranes combined with PVDF coating in direct contact membrane distillation. Desalination 2021, 513, 115134. [Google Scholar] [CrossRef]
- Das, A.; Abou-Nemeh; Chandra, S.; Sirkar, K.K. Membrane-moderated stripping process for removing VOCs from water in a composite hollow fiber module. J. Membr. Sci. 1998, 148, 257–271. [Google Scholar] [CrossRef]
- Zhang, K.; Li, L.; Yu, W.; Hu, M.; Zhou, Y.; Fan, X.; Liao, J.; Huang, C. Preparation of γ-methacryloxypropyl Trimethoxy Silane Cross-linked PDMS Membrane for Pervaporation Recovery of Butanol. J. Wuhan Univ. Technol. Sci. Ed. 2018, 33, 312–319. [Google Scholar] [CrossRef]
- Zong, C.; Yang, X.; Chen, D.; Chen, Y.; Zhou, H.; Jin, W. Rational tuning of the viscosity of membrane solution for the preparation of sub-micron thick PDMS composite membrane for pervaporation of ethanol-water solution. Sep. Purif. Technol. 2021, 255, 117729. [Google Scholar] [CrossRef]
- Meng, X.; Lin, C.; Zhang, Y.; Qin, H.; Cao, S.; Duan, L. Mass Transfer Behavior of Benzene in Hierarchically Structured ZSM-5. Front. Chem. 2019, 7, 502. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.; Jeong, D.; Jeong, H.-K.; Lee, Y. Pervaporation characteristics of trichlorinated organic compounds through Silicalite-1 zeolite membrane. Desalination 2009, 245, 754–762. [Google Scholar] [CrossRef]
- Li, G.; Ma, S.; Ye, F.; Zhou, L.; Wang, Y.; Lang, X.; Fan, S. Robust ZSM-5 Membranes for Efficient Bio-Oil Dehydration: Transport Mechanism and Its Implication on Structural Tuning. Ind. Eng. Chem. Res. 2021, 60, 1799–1807. [Google Scholar] [CrossRef]
- Hietaharju, J.; Kangas, J.; Tanskanen, J. Analysis of the permeation behavior of ethanol/water mixtures through a polydimethylsiloxane (PDMS) membrane in pervaporation and vapor permeation conditions. Sep. Purif. Technol. 2019, 227, 115738. [Google Scholar] [CrossRef]
- Jou, J.-D.; Yoshida, W.; Cohen, Y. A novel ceramic-supported polymer membrane for pervaporation of dilute volatile organic compounds. J. Membr. Sci. 1999, 162, 269–284. [Google Scholar] [CrossRef]
- He, X.; Wang, T.; Huang, J.; Chen, J.; Li, J. Fabrication and characterization of superhydrophobic PDMS composite membranes for efficient ethanol recovery via pervaporation. Sep. Purif. Technol. 2020, 241, 116675. [Google Scholar] [CrossRef]
- Mafi, A.; Raisi, A.; Aroujalian, A. Computational fluid dynamics modeling of mass transfer for aroma compounds recovery from aqueous solutions by hydrophobic pervaporation. J. Food Eng. 2013, 119, 46–55. [Google Scholar] [CrossRef]
- Zhang, C.; Peng, L.; Jiang, J.; Gu, X. Mass transfer model, preparation and applications of zeolite membranes for pervaporation dehydration: A review. Chin. J. Chem. Eng. 2017, 25, 1627–1638. [Google Scholar] [CrossRef]
- Wu, Y.; Xiao, Z.; Huang, W.; Zhong, Y. Mass transfer in pervaporation of active fermentation broth with a composite PDMS membrane. Sep. Purif. Technol. 2005, 42, 47–53. [Google Scholar] [CrossRef]
- Hoshi, M.; Saito, T.; Higuchi, A.; Nakagawa, T. Separation of aqueous organic solvents through crosslinked poly(acrylate-co-acrylic acid) membranes by pervaporation. Sen’i Gakkaishi 1991, 47, 644–649. [Google Scholar] [CrossRef]
- Kujawska, A.; Knozowska, K.; Kujawa, J.; Kujawski, W. Influence of downstream pressure on pervaporation properties of PDMS and POMS based membranes. Sep. Purif. Technol. 2016, 159, 68–80. [Google Scholar] [CrossRef]
- Villaluenga, J.P.G.; Cohen, Y. Numerical model of non-isothermal pervaporation in a rectangular channel. J. Membr. Sci. 2005, 260, 119–130. [Google Scholar] [CrossRef]

| Membrane | Feed Concentration (wt.%) | Feed Temperature (℃) | Particle Loading (wt.%) | Separation Factor | Total Flux (g m−2 h−1) | Reference |
|---|---|---|---|---|---|---|
| silicalite-1 | 1 × 10−4 | 30 | - | 10 | 4 | [42] |
| polyvinyl acetate | 4.57 × 10−4 | 25 | - | 110 | 310 | [45] |
| poly(acrylate-co-acrylic) | 2.01 × 10−1 | 25 | - | 108 | 638 | [50] |
| PDMS | 2.4 × 10−4 | 55 | - | 104 | 400 | [52] |
| PDMS hollow fiber | 9 × 10−4 | 20 | - | 140 | 190 | [38] |
| OTES@ZSM-5/PDMS | 3 × 10−7 | 30 | 20 | 328 | 155 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Song, X.; Zhang, Y.; Fan, J. Improving the Pervaporation Performance of PDMS Membranes for Trichloroethylene by Incorporating Silane-Modified ZSM-5 Zeolite. Polymers 2023, 15, 3777. https://doi.org/10.3390/polym15183777
Song X, Song X, Zhang Y, Fan J. Improving the Pervaporation Performance of PDMS Membranes for Trichloroethylene by Incorporating Silane-Modified ZSM-5 Zeolite. Polymers. 2023; 15(18):3777. https://doi.org/10.3390/polym15183777
Chicago/Turabian StyleSong, Xiaosan, Xichen Song, Yue Zhang, and Jishuo Fan. 2023. "Improving the Pervaporation Performance of PDMS Membranes for Trichloroethylene by Incorporating Silane-Modified ZSM-5 Zeolite" Polymers 15, no. 18: 3777. https://doi.org/10.3390/polym15183777





