Optimization of LDO-Pectin Synthesis Conditions for the Removal of Metals from Wastewater: A Comparison of Response Surface Methods and Taguchi Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Different LDH, LDO, and LDO/LDH Supported by Pectin
2.3. Experimental Design
2.3.1. RSM
2.3.2. Taguchi Method
2.4. Adsorption Experiments
2.5. Characterization
2.5.1. Morphology
2.5.2. Determination of pH at the Point of Zero Charges (pHpzc)
2.5.3. Reusability of the Modified LDO and LDH Sorbents
2.6. Kinetic and Isotherm Models
3. Results and Discussion
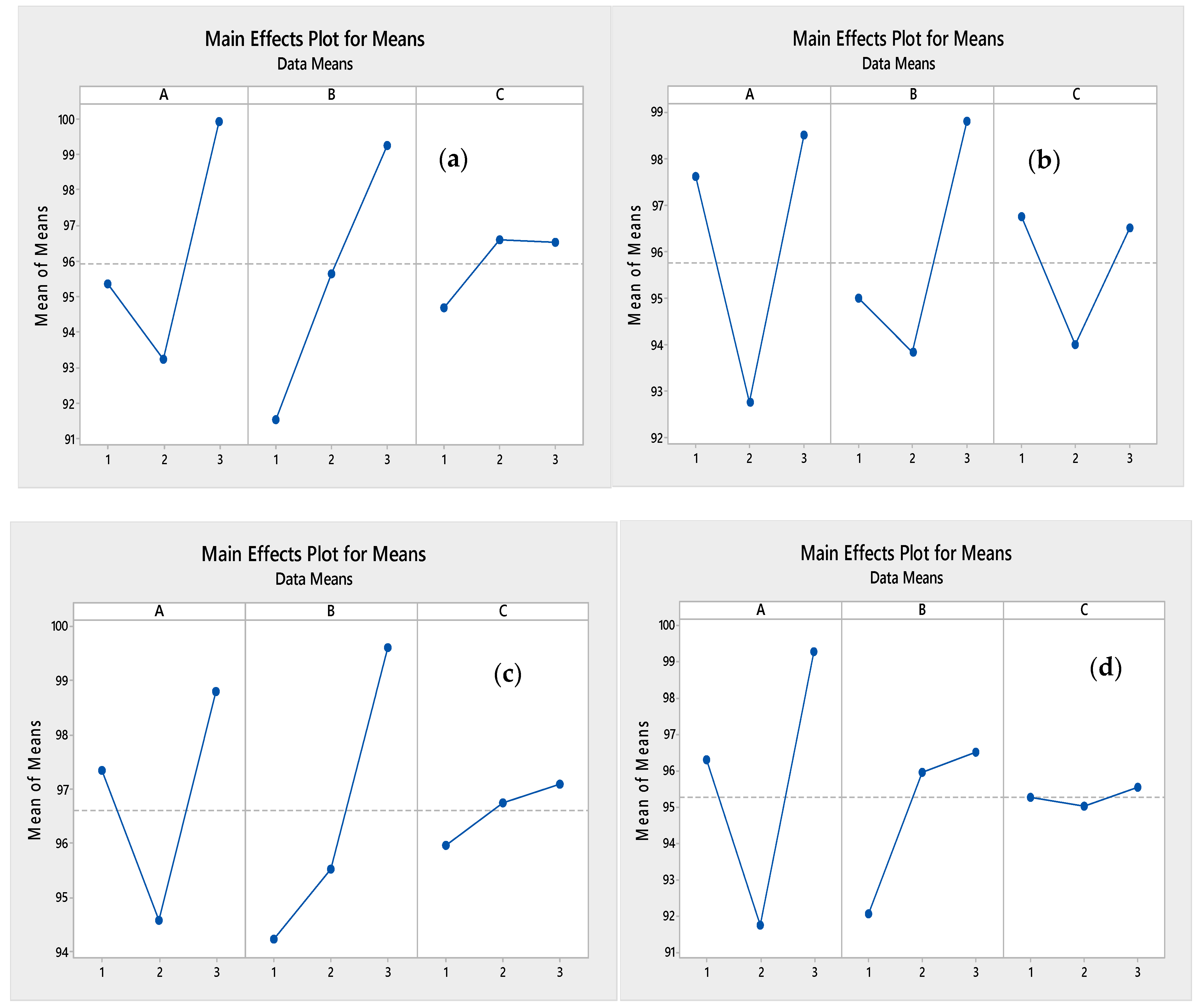
3.1. RSM and Taguchi Model Analysis
3.1.1. Analysis of Variance (ANOVA)
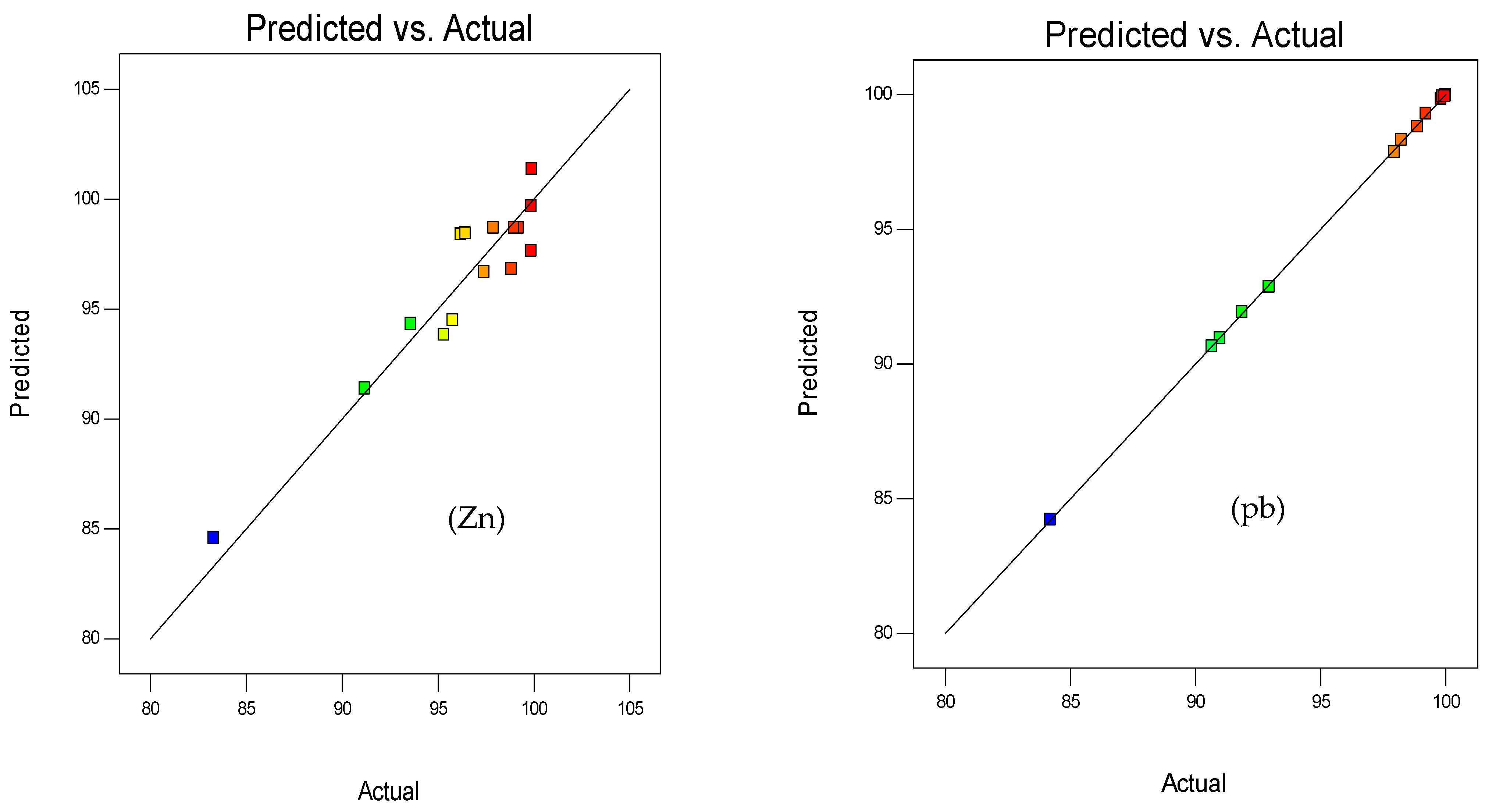
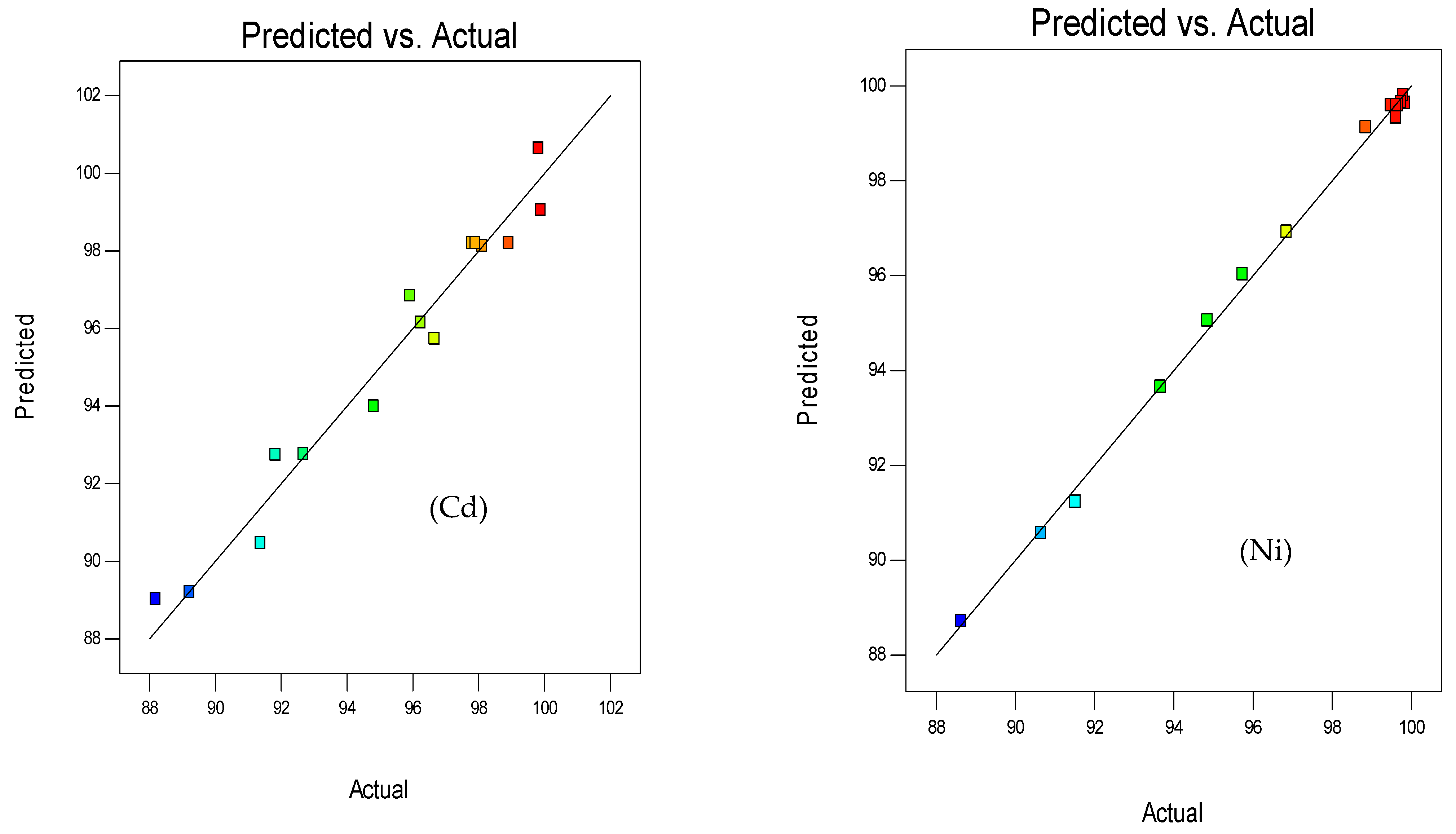
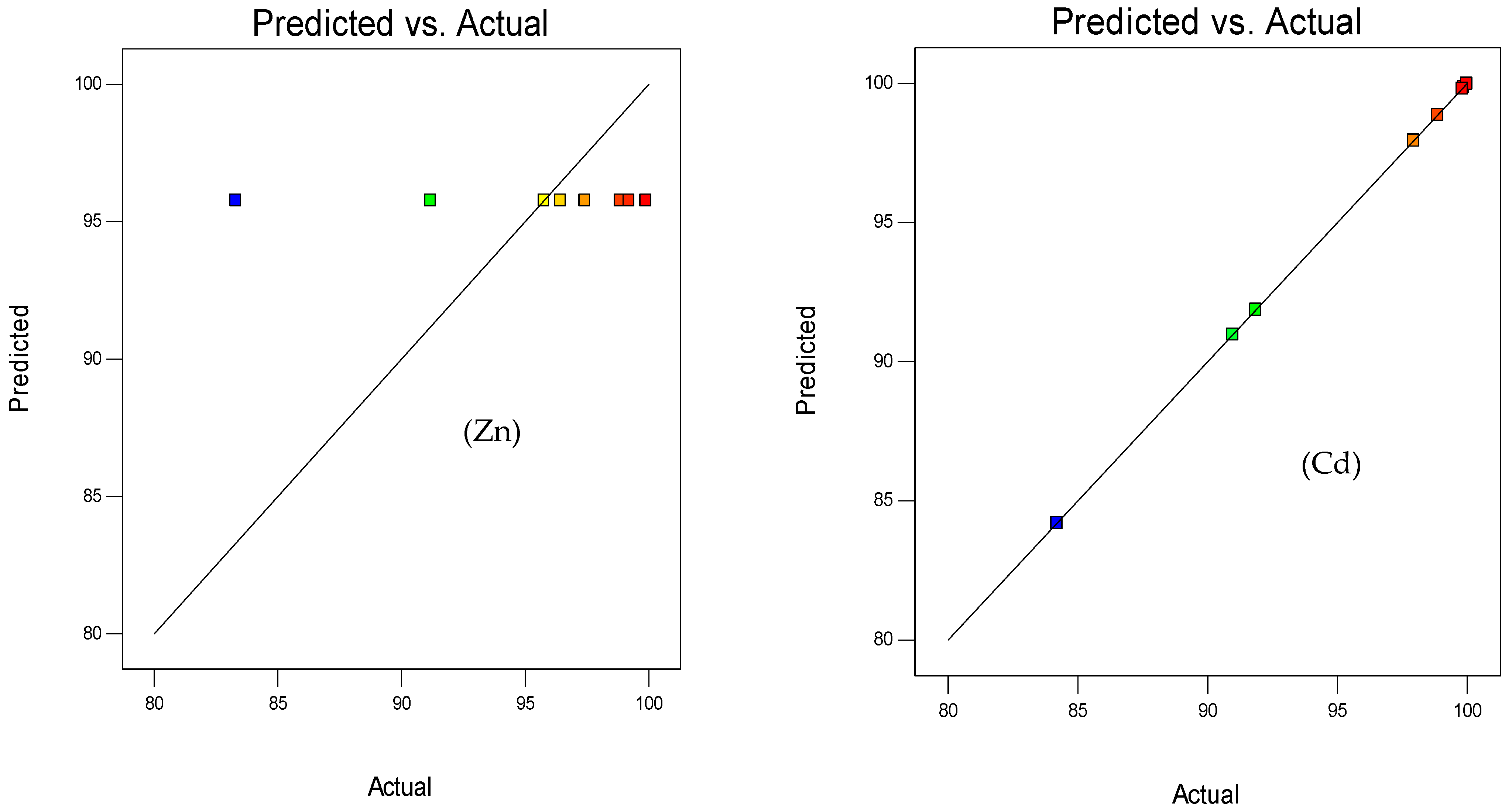
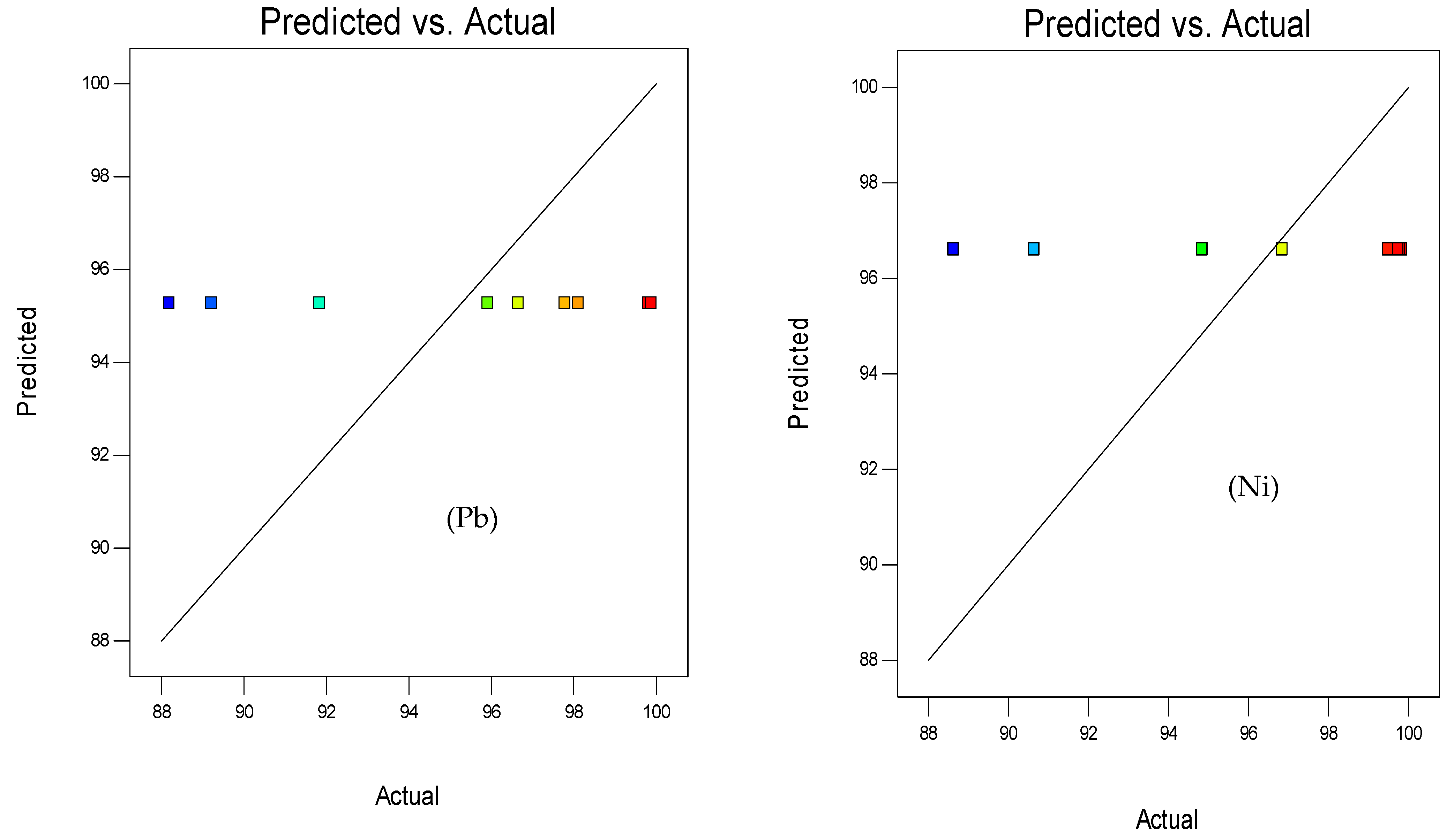
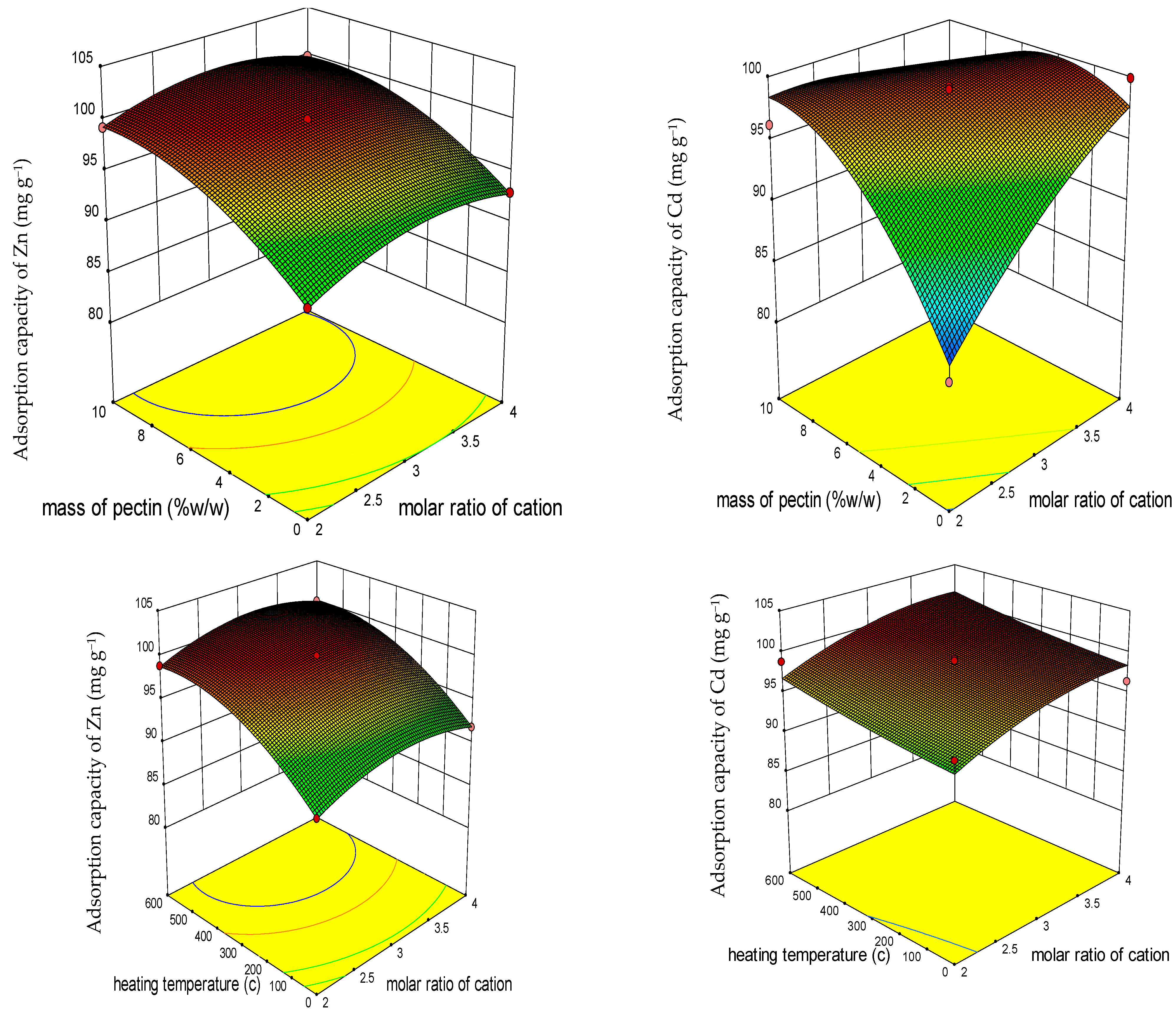
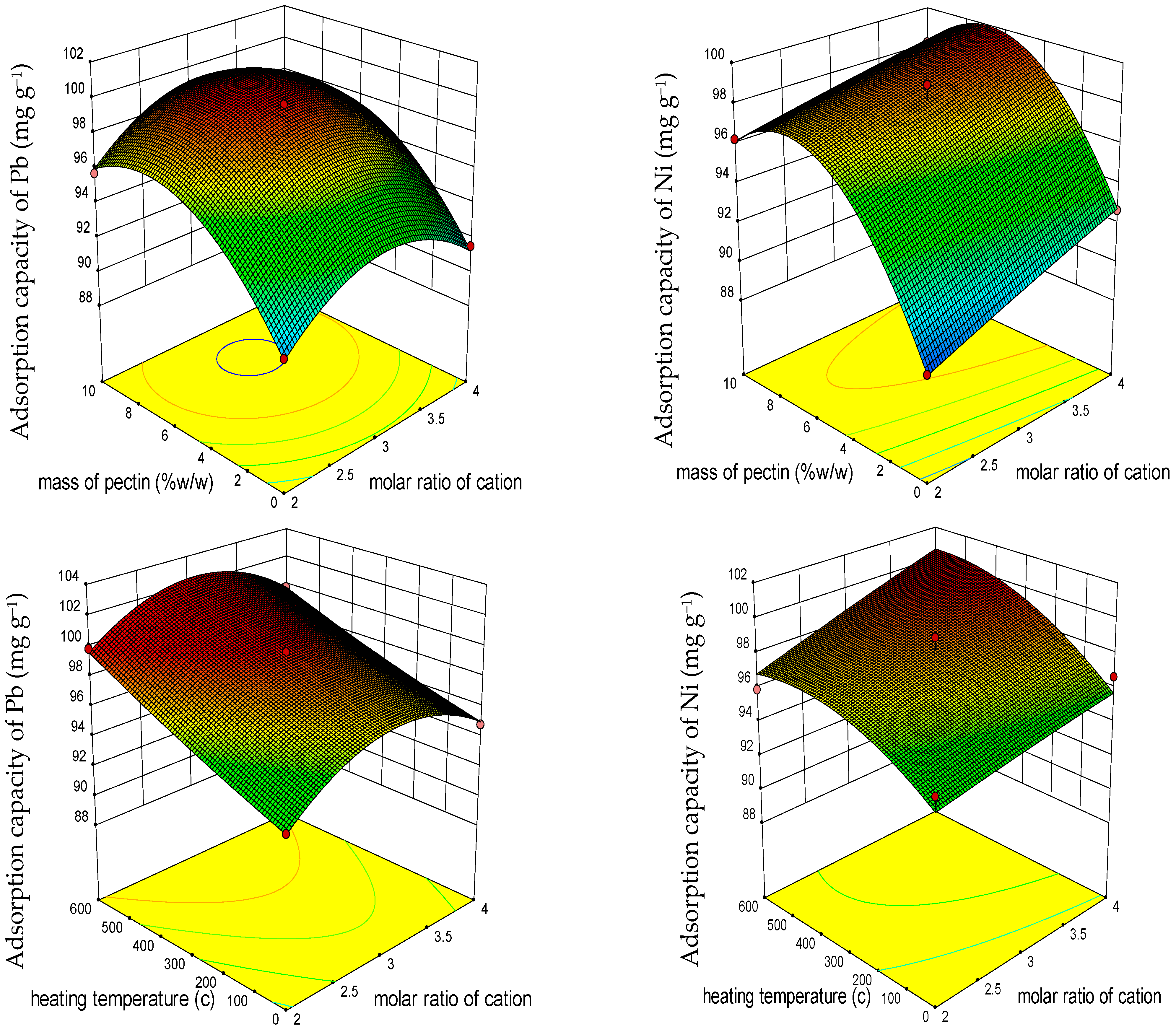
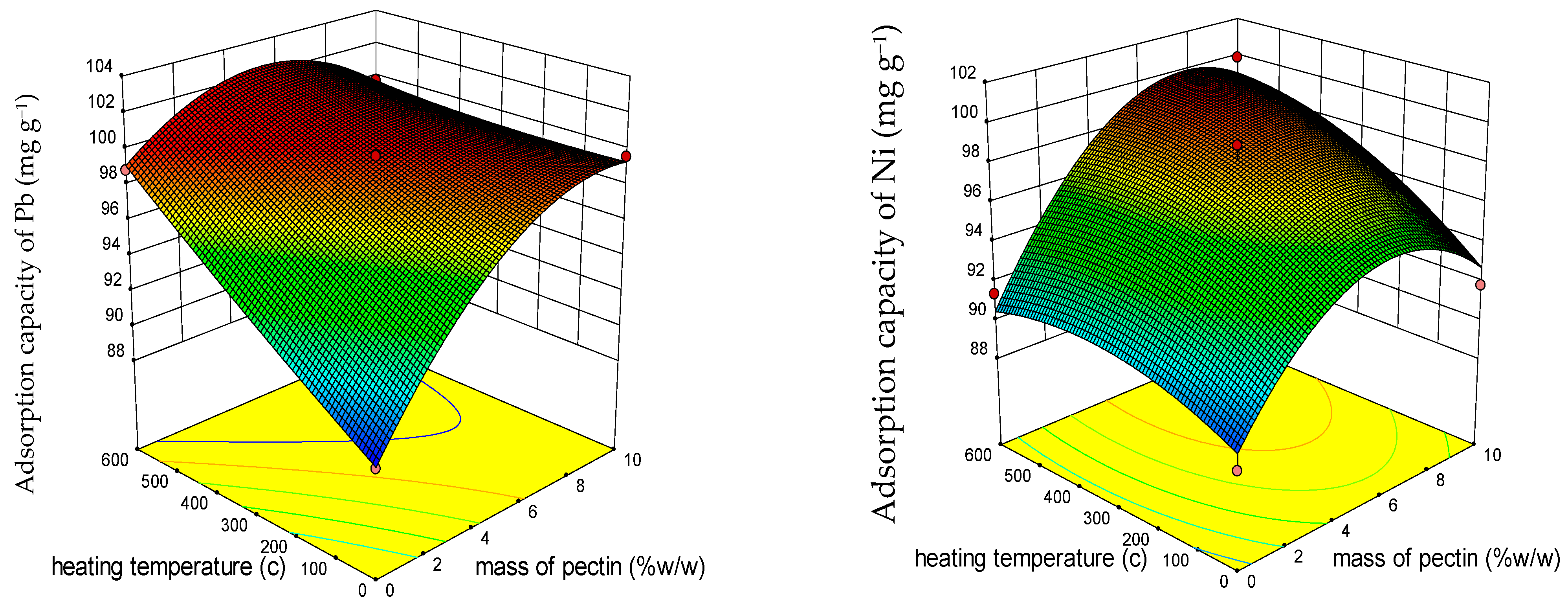
3.1.2. Response Surface 3D Plots
3.1.3. Response Surface Optimization
3.2. Characterization of the Modified LDO and LDH Sorbents
3.2.1. Influence of pHpzc
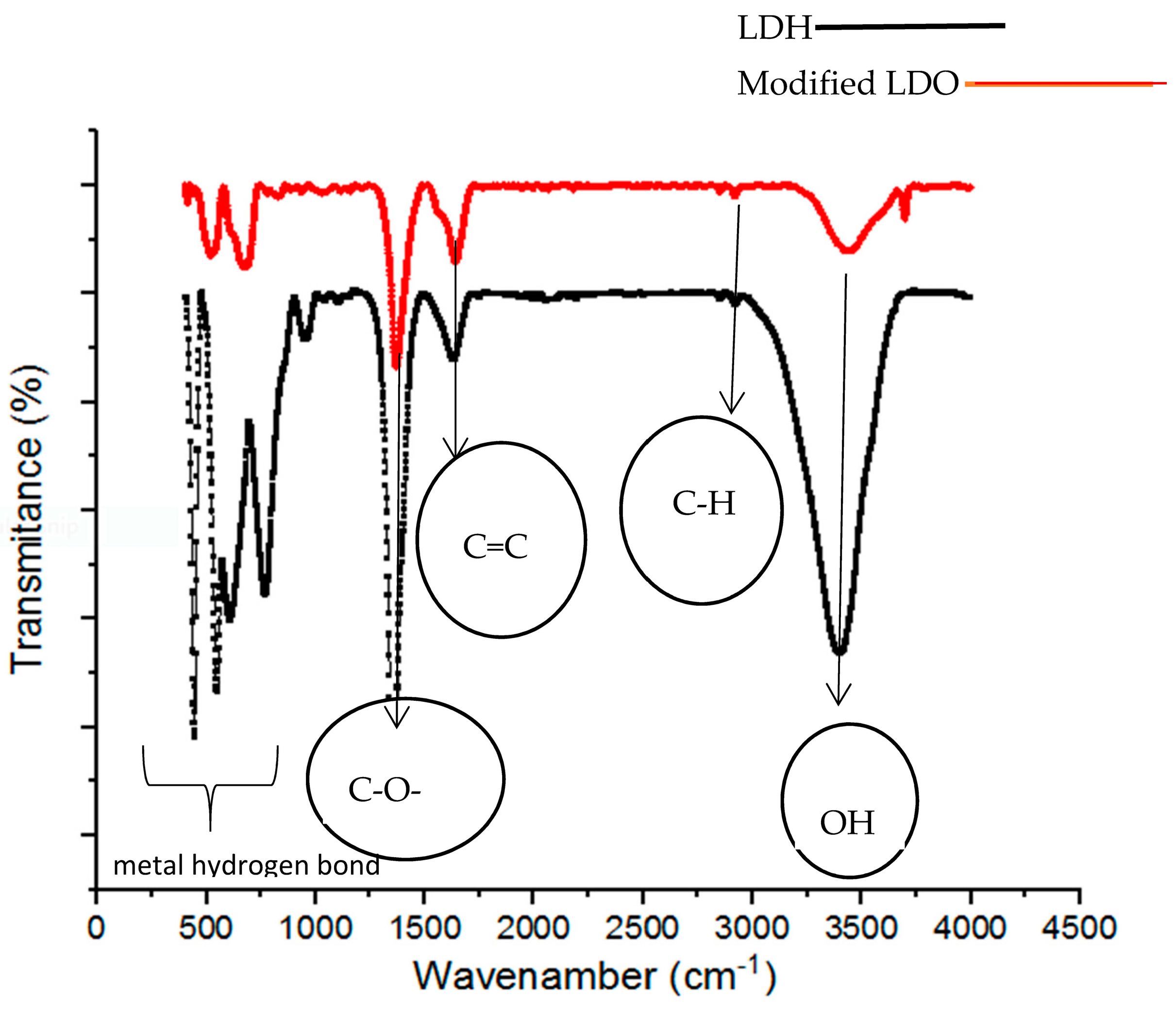
3.2.2. FTIR
3.2.3. Reusability of the Modified LDO and LDH Sorbents for HMs Adsorption
3.2.4. Morphology
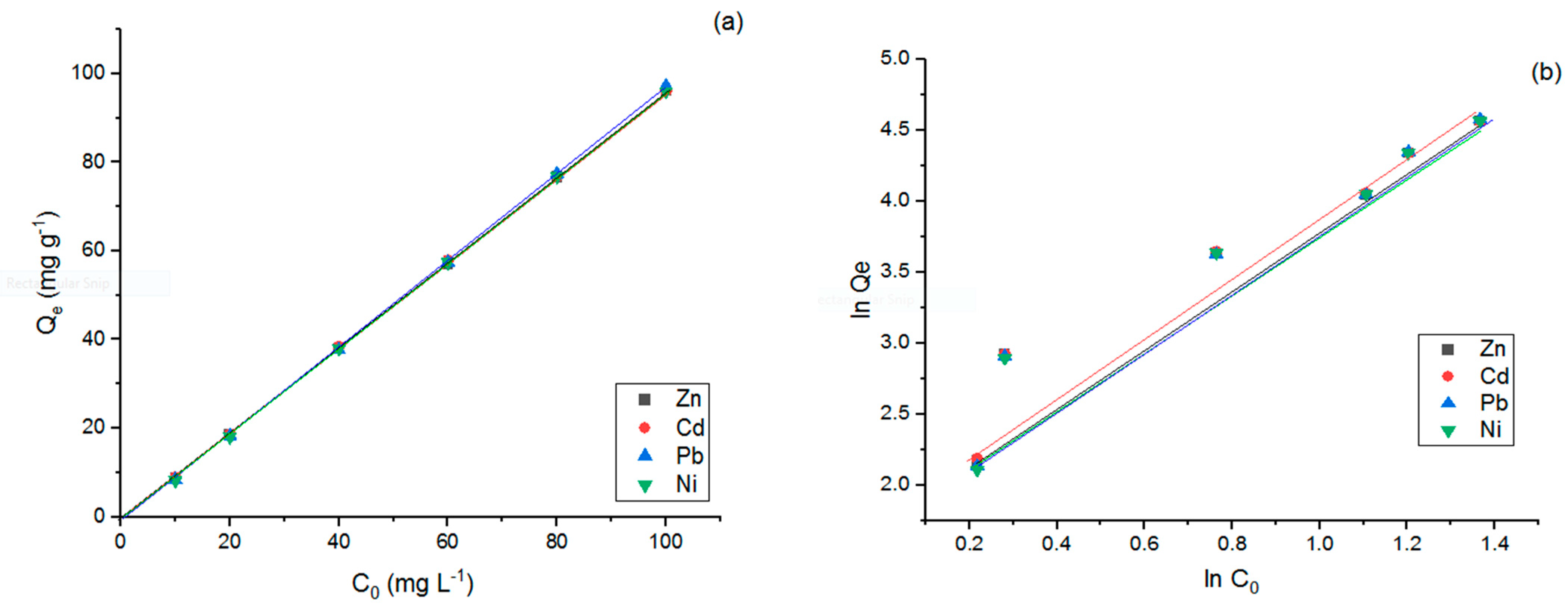
3.3. Adsorption Kinetics and Isotherms of Modified LDO Sorbent
3.4. Comparison Adsorption Capacity of Modified LDO toward Other Agricultural Sorbents for HMs Removal
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Z.; Luo, P.; Zha, X.; Xu, C.; Kang, S.; Zhou, M.; Nover, D.; Wang, Y. Overview assessment of risk evaluation and treatment technologies for heavy metal pollution of water and soil. J. Clean. Prod. 2022, 379, 134043. [Google Scholar] [CrossRef]
- Kameda, T.; Horikoshi, K.; Kikuchi, H.; Kitagawa, F.; Kumagai, S.; Saito, Y.; Kondo, M.; Jimbo, Y.; Yoshioka, T. Kinetic and equilibrium analyses of lactate adsorption by Cu-Al and Mg-Al layered double hydroxides (Cu-Al LDH and Mg-Al LDH) and Cu-Al and Mg-Al layered double oxides (Cu-Al LDO and Mg-Al LDO). Nano-Struct. Nano-Objects 2021, 25, 100656. [Google Scholar] [CrossRef]
- Azaritorbat, A.; Nasernejad, B. Optimization of the removal efficiency of o-Toluidine: Adsorption on sugarcane bagasse or biodegradation using Phanerochaete chrysosporium immobilized on bagasse? S. Afr. J. Chem. Eng. 2023, 44, 318–328. [Google Scholar] [CrossRef]
- Miao, Y.; Qi, S.; Chen, G.; Wang, X.; Zhao, W.; Wang, J.; Zhang, S.; Xin, B. Efficient removal of As, Cu and Cd and synthesis of photo-catalyst from Cu-smelting waste acid through sulfide precipitation by biogenic gaseous H2S produced by anaerobic membrane bioreactor. Chem. Eng. J. 2023, 451, 138096. [Google Scholar] [CrossRef]
- Li, J.; Chen, C.; Zhu, K.; Wang, X. Nanoscale zero-valent iron particles modified on reduced graphene oxides using a plasma technique for Cd (II) removal. J. Taiwan Inst. Chem. Eng. 2016, 59, 389–394. [Google Scholar] [CrossRef]
- Younes, M.M.; El-Sharkawy, I.I.; Kabeel, A.E.; Uddin, K.; Pal, A.; Mitra, S.; Thu, K.; Saha, B.B. Synthesis and characterization of silica gel composite with polymer binders for adsorption cooling applications. Int. J. Refrig. 2019, 98, 161–170. [Google Scholar] [CrossRef]
- Topare, N.S.; Wadgaonkar, V.S. A review on application of low-cost adsorbents for heavy metals removal from wastewater. Mater. Today Proc. 2022, 77, 8–18. [Google Scholar] [CrossRef]
- Gholami, P.; Dinpazhoh, L.; Khataee, A.; Hassani, A.; Bhatnagar, A. Facile hydrothermal synthesis of novel Fe-Cu layered double hydroxide/biochar nanocomposite with enhanced sonocatalytic activity for degradation of cefazolin sodium. J. Hazard. Mater. 2020, 381, 120742. [Google Scholar] [CrossRef]
- Amin, M.T.; Alazba, A.A.; Shafiq, M. LDH of NiZnFe and its composites with carbon nanotubes and data-palm biochar with efficient adsorption capacity for RB5 dye from aqueous solutions: Isotherm, kinetic, and thermodynamics studies. Curr. Appl. Phys. 2020, 40, 90–100. [Google Scholar] [CrossRef]
- Chen, M.; Wu, P.; Huang, Z.; Liu, J.; Li, Y.; Zhu, N.; Dang, Z.; Bi, Y. Environmental application of MgMn-layered double oxide for simultaneous efficient removal of tetracycline and Cd pollution: Performance and mechanism. J. Environ. Manag. 2019, 246, 164–173. [Google Scholar] [CrossRef]
- Hudcová, B.; Fein, J.B.; Tsang, D.C.; Komárek, M. Mg-Fe LDH-coated biochars for metal (loid) removal: Surface complexation modeling and structural change investigations. J. Chem. Eng. 2022, 432, 134360. [Google Scholar] [CrossRef]
- Nguyen, T.M.P.; Van, H.T.; Yılmaz, M.; Hoang, T.K.; Nguyen, Q.T.; Vi, T.M.H.; Nga, L.T.Q. Removal of Tetracycline from aqueous solution using composite adsorbent of ZnAl layered double hydroxide and bagasse biochar. Environ. Technol. Innov. 2022, 28, 102914. [Google Scholar] [CrossRef]
- Missau, J.; Bertuol, D.A.; Tanabe, E.H. Highly efficient adsorbent for removal of Crystal Violet Dye from Aqueous Solution by CaAl/LDH supported on Biochar. Appl. Clay Sci. 2021, 214, 106297. [Google Scholar] [CrossRef]
- Jakóbik-Kolon, A.; Bok-Badura, J.; Milewski, A.; Karoń, K. Long term and large-scale continuous studies on zinc(II) sorption and desorption on hybrid pectin-guar gum biosorbent. Polymers 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed]
- Shao, Z.; Lu, J.; Ding, J.; Fan, F.; Sun, X.; Li, P.; Fang, Y.; Hu, Q. Novel green chitosan-pectin gel beads for the removal of Cu(II), Cd(II), Hg(II) and Pb(II) from aqueous solution. Int. J. Biol. Macromol. 2021, 176, 217–225. [Google Scholar] [CrossRef]
- Esmaeili, H.; Karami, A.; Hadian, J.; Saharkhiz, M.J.; Ebrahimi, S.N. Variation in the phytochemical contents and antioxidant activity of Glycyrrhiza glabra populations collected in Iran. Ind. Crops Prod. 2019, 137, 248–259. [Google Scholar] [CrossRef]
- Mohammadi, F.; Samaei, M.R.; Azhdarpoor, A.; Teiri, H.; Badeenezhad, A.; Rostami, S. Modelling and optimizing pyrene removal from the soil by phytoremediation using response surface methodology, artificial neural networks, and genetic algorithm. Chemosphere 2019, 237, 124486. [Google Scholar] [CrossRef]
- Mohrazi, A.; Ghasemi-Fasaei, R.; Mojiri, A.; Safarzadeh, S. Investigating an electro-bio-chemical phytoremediation of multi-metal polluted soil by maize and sunflower using RSM-based optimization methodology. Environ. Exp. Bot. 2023, 211, 105352. [Google Scholar] [CrossRef]
- Razmi, B.; Ghasemi-Fasaei, R.; Ronaghi, A.; Mostowfizadeh-Ghalamfarsa, R. Application of Taguchi optimization for evaluating the capability of hydrochar, biochar, and activated carbon prepared from different wastes in multi-elements bioadsorption. J. Clean. Prod. 2022, 347, 131292. [Google Scholar] [CrossRef]
- Maran, J.P.; Sivakumar, V.; Thirugnanasambandham, K.; Sridhar, R. Optimization of microwave assisted extraction of pectin from orange peel. Carbohydr. Polym. 2013, 97, 703–709. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Y.; Sun, R.; Zhou, Y.; Tsang, D.C.; Chen, Q. Optimizing the synthesis of Fe/Al (Hydr) oxides-Biochars to maximize phosphate removal via response surface model. J. Clean. Prod. 2019, 237, 117770. [Google Scholar] [CrossRef]
- Wang, S.; Kwak, J.H.; Islam, M.S.; Naeth, M.A.; El-Din, M.G.; Chang, S.X. Biochar surface complexation and Ni(II), Cu(II), and Cd(II) adsorption in aqueous solutions depend on feedstock type. Sci. Total Environ. 2020, 712, 136538. [Google Scholar] [CrossRef]
- Wu, F.; Chen, L.; Hu, P.; Zhou, X.; Zhou, H.; Wang, D.; Lu, X.; Mi, B. Comparison of properties, adsorption performance and mechanisms to Cd(II) on lignin-derived biochars under different pyrolysis temperatures by microwave heating. Environ. Technol. Innov. 2022, 25, 102196. [Google Scholar] [CrossRef]
- Karoui, S.; Arfi, R.B.; Mougin, K.; Ghorbal, A.; Assadi, A.A.; Amrane, A. Synthesis of novel biocomposite powder for simultaneous removal of hazardous ciprofloxacin and methylene blue: Central composite design, kinetic and isotherm studies using Brouers-Sotolongo family models. J. Hazard. Mater. 2020, 387, 121675. [Google Scholar] [CrossRef]
- Liu, X.J.; Li, M.F.; Singh, S.K. Manganese-modified lignin biochar as adsorbent for removal of methylene blue. J. Mater. Res. Technol. 2021, 12, 1434–1445. [Google Scholar] [CrossRef]
- Meili, L.; Lins, P.V.; Zanta, C.L.P.S.; Soletti, J.I.; Ribeiro, L.M.O.; Dornelas, C.B.; Silva, T.L.; Vieira, M.G.A. MgAl-LDH/Biochar composites for methylene blue removal by adsorption. Appl. Clay Sci. 2019, 168, 11–20. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Chen, Y.; Li, Y. Nickel aluminum layered double oxides modified magnetic biochar from waste corncob for efficient removal of acridine orange. Bioresour. Technol. 2020, 315, 123834. [Google Scholar] [CrossRef]
- Teiri, H.; Hajizadeh, Y.; Samaei, M.R.; Pourzamani, H.; Mohammadi, F. Modelling the phytoremediation of formaldehyde from indoor air by Chamaedorea Elegans using artificial intelligence, genetic algorithm and response surface methodology. J. Environ. Chem. Eng. 2020, 8, 103985. [Google Scholar] [CrossRef]
- Razzak, S.A.; Faruque, M.O.; Alsheikh, Z.; Alsheikhmohamad, L.; Alkuroud, D.; Alfayez, A.; Hossain, S.M.Z.; Hossain, M.M. A comprehensive review on conventional and biological-driven heavy metals removal from industrial wastewater. Adv. Environ. 2022, 7, 100168. [Google Scholar] [CrossRef]
- Khan, T.A.; Chaudhry, S.A.; Ali, I. Equilibrium uptake, isotherm and kinetic studies of Cd(II) adsorption onto iron oxide activated red mud from aqueous solution. J. Mol. Liq. 2015, 202, 165–175. [Google Scholar] [CrossRef]
- Huang, J.; Yang, Z.; Wang, R.; Zhang, Z.; Feng, Z.; Xie, X. Zn–Al layered double oxides as high-performance anode materials for zinc-based secondary battery. J. Mater. Chem. 2015, 3, 7429–7436. [Google Scholar] [CrossRef]
- Debnath, B.; Majumdar, M.; Bhowmik, M.; Bhowmik, K.L.; Debnath, A.; Roy, D.N. The effective adsorption of tetracycline onto zirconia nanoparticles synthesized by novel microbial green technology. J. Environ. Manag. 2020, 261, 110235. [Google Scholar] [CrossRef] [PubMed]
- Somsesta, N.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption removal of methylene blue onto 663 activated carbon/cellulose biocomposite films: Equilibrium and kinetic studies. Mater. Chem. Phys. 2020, 240, 122221. [Google Scholar] [CrossRef]
- Yu, K.L.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.H.; Salleh, M.A.M. Biochar production 706 from microalgae cultivation through pyrolysis as a sustainable carbon sequestration and biorefinery 707 approach. Clean Technol. Environ. Policy 2018, 20, 2047–2055. [Google Scholar] [CrossRef]
- Bello, M.O.; Abdus-Salam, N.; Adekola, F.A.; Pal, U. Isotherm and kinetic studies of adsorption of methylene blue using activated carbon from ackee apple pods. Chem. Data Collect. 2021, 31, 100607. [Google Scholar] [CrossRef]
- Mpatani, F.M.; Aryee, A.A.; Kani, A.N.; Wen, K.; Dovi, E.; Qu, L.; Li, Z.; Han, R. Removal of methylene blue from aqueous medium by citrate modified bagasse: Kinetic, Equilibrium and Thermodynamic study. Bioresour. Technol. 2020, 11, 100463. [Google Scholar] [CrossRef]
- Shafiof, M.a.S.; Nezamzadeh-Ejhieh, A. A comprehensive study on the removal of Cd(II) from aqueous solution on a novel pentetic acid-clinoptilolite nanoparticles adsorbent: Experimental design, kinetic and thermodynamic aspects. Solid State Sci. 2020, 99, 106071. [Google Scholar] [CrossRef]
- Boughrara, L.; Sebba, F.Z.; Sebti, H.; Choukchou-Braham, E.; Bounaceur, B.; Kada, S.O.; Zaoui, F. Removal of Zn(II) and Ni(II) heavy metal ions by new alginic acid-ester derivatives materials. Carbohydr. Polym. 2021, 272, 118439. [Google Scholar] [CrossRef]
- Shayesteh, H.; Rahbar-Kelishami, A.; Norouzbeigi, R. Evaluation of natural and cationic surfactant modified pumice for congo red removal in batch mode: Kinetic, equilibrium, and thermodynamic studies. J. Mol. Liq. 2016, 221, 1–11. [Google Scholar] [CrossRef]
- Mouni, L.; Belkhiri, L.; Bollinger, J.-C.; Bouzaza, A.; Assadi, A.; Tirri, A.; Dahmoune, F.; Madani, K.; Remini, H. Removal of Methylene Blue from aqueous solutions by adsorption on Kaolin: Kinetic and equilibrium studies. Appl. Clay Sci. 2018, 153, 38–45. [Google Scholar] [CrossRef]
- Van, H.N.; Van, H.C.; Hoang, T.L.; Nguyen, D.K.V.; Thuc, C.N.H. The starch modified montmorillonite for the removal of Pb(II), Cd(II) and Ni(II) ions from aqueous solutions. Arab. J. Chem. 2020, 13, 7212–7223. [Google Scholar] [CrossRef]
- Lin, P.; Liu, H.; Yin, H.; Zhu, M.; Luo, H.; Dang, Z. Remediation performance and mechanisms of Cu and Cd contaminated water and soil using Mn/Al-layered double oxide-loaded biochar. J. Environ. Sci. 2023, 125, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Mohrazi, A.; Ghasemi-Fasaei, R. Removal of methylene blue dye from aqueous solution using an efficient chitosan-pectin bio-adsorbent: Kinetics and isotherm studies. Environ. Monit. Assess. 2023, 195, 339. [Google Scholar] [CrossRef] [PubMed]
- Kothavale, V.; Sharma, A.; Dhavale, R.; Chavan, V.; Shingte, S.; Selyshchev, O.; Dongale, T.; Park, H.; Zahn, D.; Salvan, G.; et al. Carboxyl and thiol-functionalized magnetic nanoadsorbents for efficient and simultaneous removal of Pb(II), Cd(II), and Ni(II) heavy metal ions from aqueous solutions: Studies of adsorption, kinetics, and isotherms. J. Phys. Chem. Solids 2023, 172, 111089. [Google Scholar] [CrossRef]
- Esfandiar, N.; Suri, R.; McKenzie, E.R. Competitive sorption of Cd, Cr, Cu, Ni, Pb and Zn from stormwater runoff by five low-cost sorbents; Effects of co-contaminants, humic acid, salinity and pH. J. Hazard. Mater. 2022, 423, 126938. [Google Scholar] [CrossRef] [PubMed]
- Pei-Ya, L.I.U.; Qin-Liang, W.E.N.; Yu-Jiao, L.I.; Chang-Xun, D.O.N.G.; Gen-Xing, P.A.N. Kinetics of specific and non-specific copper sorption on aggregates of an acidic paddy soil from the Taihu Lake region in East China. Pedosphere 2015, 25, 37–45. [Google Scholar] [CrossRef]
- Andreas, A.; Winata, Z.G.; Santoso, S.P.; Angkawijaya, A.E.; Yuliana, M.; Soetaredjo, F.E.; Ismadji, S.; Hsu, H.-Y.; Go, A.W.; Ju, Y.-H. Biocomposite hydrogel beads from glutaraldehyde-crosslinked phytochemicals in alginate for effective removal of methylene blue. J. Mol. Liq. 2021, 329, 115579. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Y.; Yang, Z.; Han, W.; Yuan, L.; Zhang, L.; Huang, X. Efficient removal of Pb(II) and Cd(II) from aqueous solutions by mango seed biosorbent. Chem. Eng. J. Adv. 2022, 11, 100295. [Google Scholar] [CrossRef]
- Moghazy, R.M.; Labena, A.; Husien, S. Eco-friendly complementary biosorption process of methylene blue using micro-sized dried biosorbents of two macro-algal species (Ulva fasciata and Sargassum dentifolium): Full factorial design, equilibrium, and kinetic studies. Int. J. Biol. Macromol. 2019, 134, 330–343. [Google Scholar] [CrossRef]
- Zhou, Y.; Lu, J.; Zhou, Y.; Liu, Y. Recent advances for dyes removal using novel adsorbents: A review. Environ. Pollut. 2019, 252, 352–365. [Google Scholar] [CrossRef]
- Makhado, E.; Pandey, S.; Modibane, K.D.; Kang, M.; Hato, M.J. Sequestration of methylene blue dye using sodium alginate poly (acrylic acid)@ ZnO hydrogel nanocomposite: Kinetic, isotherm, and thermodynamic investigations. Int. J. Biol. Macromol. 2020, 162, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Moreira, V.R.; Lebron, Y.A.R.; Lange, L.C.; Santos, L.V.S. Simultaneous biosorption of Cd(II), Ni(II) and Pb(II) onto a brown macroalgae Fucus vesiculosus: Mono-and multi-component isotherms, kinetics and thermodynamics. J. Environ. Manag. 2019, 251, 109587. [Google Scholar] [CrossRef]
- Araújo, L.D.C.B.; de Matos, H.K.; Facchi, D.P.; de Almeida, D.A.; Gonçalves, B.M.; Monteiro, J.P.; Martins, A.F.; Bonafé, E.G. Natural carbohydrate-based thermosensitive chitosan/pectin adsorbent for removal of Pb(II) from aqueous solutions. Int. J. Biol. Macromol. 2021, 193, 1813–1822. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Su, Y.; Wang, X.; Liang, C.; Tang, C.; Wei, J.; Liu, K.; Ma, J.; Yu, F.; Li, Y. Insights into the heavy metal adsorption and immobilization mechanisms of CaFe-layered double hydroxide corn straw biochar: Synthesis and application in a combined heavy metal-contaminated environment. Chemosphere 2023, 313, 137467. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wang, W.; Liu, W.; Li, P.; Cai, Y.; Chen, J.; Ding, N. Insight into different removal behaviors and mechanism of heavy metals via sulfate-loaded layered double oxide. J. Environ. Chem. Eng. 2022, 10, 107751. [Google Scholar] [CrossRef]
- Wijeyawardana, P.; Nanayakkara, N.; Law, D.; Gunasekara, C.; Karunarathna, A.; Pramanik, B.K. Performance of biochar mixed cement paste for removal of Cu, Pb and Zn from stormwater. Environ. Res. 2023, 232, 116331. [Google Scholar] [CrossRef]






| Variable | Unit | Range and Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| A: molar ratio (Mg:Al) | - | 2 | 3 | 4 |
| B: ratio of pectin to LDO | w/w% | 0 | 5 | 10 |
| C: temperature | RT | 0 | 300 | 600 |
| Run | Factor A | Factor B | Factor C |
|---|---|---|---|
| 1 | 2 | 0 | 300 |
| 2 | 4 | 0 | 300 |
| 3 | 2 | 10 | 300 |
| 4 | 4 | 10 | 300 |
| 5 | 2 | 5 | 0 |
| 6 | 4 | 5 | 0 |
| 7 | 2 | 5 | 600 |
| 8 | 4 | 5 | 600 |
| 9 | 3 | 0 | 0 |
| 10 | 3 | 10 | 0 |
| 11 | 3 | 0 | 600 |
| 12 | 3 | 10 | 600 |
| 13 | 3 | 5 | 300 |
| 14 | 3 | 5 | 300 |
| 15 | 3 | 5 | 300 |
| Factor 1 | Factor 2 | Factor 3 | |
|---|---|---|---|
| Run | A: Molar Ratio | B: Pectin/LDO | C: Temperature |
| 1 | Level 2 of A | Level 1 of B | Level 1 of C |
| 2 | Level 2 of A | Level 2 of B | Level 2 of C |
| 3 | Level 2 of A | Level 3 of B | Level 3 of C |
| 4 | Level 1 of A | Level 1 of B | Level 2 of C |
| 5 | Level 1 of A | Level 2 of B | Level 3 of C |
| 6 | Level 2 of A | Level 3 of B | Level 1 of C |
| 7 | Level 3 of A | Level 2 of B | Level 3 of C |
| 8 | Level 3 of A | Level 2 of B | Level 1 of C |
| 9 | Level 3 of A | Level 3 of B | Level 2 of C |
| Qe (mg g−1) | RE (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Run | Zn | Cd | Pb | Ni | Zn | Cd | Pb | Ni |
| 1 | 90.975 | 83.304 | 90.648 | 89.216 | 90.975 | 83.304 | 90.648 | 89.216 |
| 2 | 92.944 | 99.865 | 91.516 | 92.674 | 92.944 | 99.865 | 91.516 | 92.674 |
| 3 | 99.207 | 96.18 | 95.74 | 96.238 | 99.207 | 96.18 | 95.74 | 96.238 |
| 4 | 99.817 | 95.76 | 96.845 | 98.111 | 99.817 | 95.76 | 96.845 | 98.111 |
| 5 | 90.663 | 95.31 | 93.665 | 94.81 | 90.663 | 95.31 | 93.665 | 94.81 |
| 6 | 91.865 | 96.436 | 94.85 | 96.657 | 91.865 | 96.436 | 94.85 | 96.657 |
| 7 | 98.867 | 98.839 | 99.839 | 95.918 | 98.867 | 98.839 | 99.839 | 95.918 |
| 8 | 99.986 | 99.892 | 99.79 | 99.82 | 99.986 | 99.892 | 99.79 | 99.82 |
| 9 | 84.208 | 91.179 | 88.629 | 88.187 | 84.208 | 91.179 | 88.629 | 88.187 |
| 10 | 97.942 | 97.412 | 99.607 | 91.83 | 97.942 | 97.412 | 99.607 | 91.83 |
| 11 | 98.225 | 93.59 | 98.849 | 91.374 | 98.225 | 93.59 | 98.849 | 91.374 |
| 12 | 99.996 | 99.877 | 99.747 | 99.884 | 99.996 | 99.877 | 99.747 | 99.884 |
| 13 | 99.873 | 99.196 | 99.486 | 97.792 | 99.873 | 99.196 | 99.486 | 97.792 |
| 14 | 99.969 | 97.904 | 99.659 | 97.898 | 99.969 | 97.904 | 99.659 | 97.898 |
| 15 | 99.965 | 98.969 | 99.623 | 98.911 | 99.965 | 98.969 | 99.623 | 98.911 |
| Run | Qe of Zn | Qe of Cd | Qe of pb | Qe of Ni |
|---|---|---|---|---|
| 1 | 84.2 | 91.179 | 88.629 | 88.187 |
| 2 | 90.97 | 83.304 | 90.648 | 89.216 |
| 3 | 97.94 | 97.412 | 99.607 | 91.83 |
| 4 | 98.86 | 98.839 | 99.839 | 95.918 |
| 5 | 91.86 | 96.436 | 94.85 | 96.657 |
| 6 | 99.87 | 99.196 | 99.486 | 97.792 |
| 7 | 99.81 | 95.76 | 96.845 | 98.111 |
| 8 | 99.98 | 99.892 | 99.79 | 99.82 |
| 9 | 99.99 | 99.877 | 99.747 | 99.884 |
| Zn | A | B | C |
|---|---|---|---|
| Delta | 6.68 | 7.74 | 1.92 |
| Rank-Taguchi | 2 | 1 | 3 |
| Rank-RSM | 3 | 1 | 2 |
| Cd | |||
| Delta | 5.74 | 4.98 | 2.75 |
| Rank-Taguchi | 1 | 2 | 3 |
| Rank-RSM | 1 | 2 | 3 |
| Pb | |||
| Delta | 4.20 | 5.38 | 1.13 |
| Rank-Taguchi | 2 | 1 | 3 |
| Rank-RSM | 3 | 1 | 2 |
| Ni | |||
| Delta | 7.52 | 4.45 | 0.53 |
| Rank-Taguchi | 1 | 2 | 3 |
| Rank-RSM | 2 | 1 | 3 |
| Type of Adsorbent | Molar Ratio | The Ratio of Pectin to LDO (w/w%) | Temperature (RT) | Qe of Zn (mg g−1) | Qe of Cd (mg g−1) | Qe of Pb (mg g−1) | Qe of Ni (mg g−1) |
|---|---|---|---|---|---|---|---|
| Modified LDO (RSM) | 3.012 | 6.986 | 577.355 | 101.518 | 100.861 | 101.962 | 99.913 |
| LDH (RSM) | 2.016 | 0.000 | 0.007 | 81.357 | 83.575 | 85.390 | 87.696 |
| Modified LDO (Taguchi) | 3 | 6 | 600 | 99.990 | 95.766 | 96.605 | 95.268 |
| LDH (Taguchi) | 3 | 0 | 0 | 84.200 | 95.766 | 96.605 | 95.268 |
| W% of Element before Adsorption | W% of Element after Adsorption | |||
|---|---|---|---|---|
| Element | LDH Adsorbent | Modified LDO Adsorbent | LDH Adsorbent | Modified LDO Adsorbent |
| C | 20.53 | 21.04 | 23.81 | 17.83 |
| O | 52.25 | 46.15 | 54.26 | 48.82 |
| Mg | 17.11 | 23.38 | 10.18 | 13.7 |
| Al | 8.25 | 8.37 | 3.1 | 2.09 |
| Ni | 0.69 | 0.24 | 2.91 | 3.61 |
| Zn | 0.16 | 0.28 | 2.1 | 5.85 |
| Cd | 0.48 | 0.26 | 1.16 | 1.95 |
| Pb | 0.53 | 0.28 | 2.48 | 6.15 |
| Pseudo-Second Order | Intraparticle Diffusion | Elovich | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metal Type | Qe (exp) | K2 | Qe (cal) | R2 | SE | k3 | c | R2 | SE | R2 | SE | ||
| Zn | 99.94 | 0.0003 | 100 | 1 | 0.017 | 0.023 | 99.66 | 0.81 | 0.07 | 99.54 | 0.081 | 0.9 | 0.02 |
| Pb | 99.75 | 0.0002 | 100 | 1 | 0.005 | 0.022 | 99.47 | 0.87 | 2.2 | 99.37 | 0.078 | 0.95 | 0.43 |
| Cd | 99.52 | 0.0004 | 100 | 0.75 | 0.15 | 0.04 | 99.03 | 0.85 | 2.36 | 98.80 | 0.151 | 0.95 | 0.44 |
| Ni | 99.64 | 0.0002 | 100 | 0.74 | 0.48 | 0.024 | 99.38 | 0.84 | 2.48 | 99.25 | 0.084 | 0.93 | 0.49 |
| Metal | R2 | SE | ||
|---|---|---|---|---|
| Zn | 0.54 | 8.24 | 0.97 | 0.12 |
| Pb | 0.271 | 2.05 | 0.97 | 0.06 |
| Cd | 0.566 | 11.13 | 0.94 | 0.18 |
| Ni | 0.381 | 3.380 | 0.88 | 0.16 |
| Sorbent | Qe (mg g−1) | Concentration (mg L−1) | Metal | Contact Time (min) | Adsorbent Dose (g L−1) | References |
|---|---|---|---|---|---|---|
| Chitosan–Pectin | 97 | 100 | Pb | 60 | 0.83 | [53] |
| LDH/biochar | 24 | 50 | Cd | 300 | 4 | [54] |
| MgAl-LDO | 19.02 | 100 | Ni | 1220 | 1 | [55] |
| manure biochar | 19.8 | 10 | Cu | 1440 | 0.5 | [56] |
| MgAl-LDO-PC | 99.94 | 100 | Zn | 180 | 1 | Present research |
| MgAl-LDO-PC | 99.75 | 100 | Pb | 180 | 1 | Present research |
| MgAl-LDO-PC | 99.52 | 100 | Cd | 180 | 1 | Present research |
| MgAl-LDO-PC | 99.64 | 100 | Ni | 180 | 1 | Present research |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohrazi, A.; Ghasemi-Fasaei, R.; Mojiri, A.; Safarzadeh Shirazi, S. Optimization of LDO-Pectin Synthesis Conditions for the Removal of Metals from Wastewater: A Comparison of Response Surface Methods and Taguchi Approaches. Polymers 2023, 15, 3778. https://doi.org/10.3390/polym15183778
Mohrazi A, Ghasemi-Fasaei R, Mojiri A, Safarzadeh Shirazi S. Optimization of LDO-Pectin Synthesis Conditions for the Removal of Metals from Wastewater: A Comparison of Response Surface Methods and Taguchi Approaches. Polymers. 2023; 15(18):3778. https://doi.org/10.3390/polym15183778
Chicago/Turabian StyleMohrazi, Ava, Reza Ghasemi-Fasaei, Amin Mojiri, and Sedigheh Safarzadeh Shirazi. 2023. "Optimization of LDO-Pectin Synthesis Conditions for the Removal of Metals from Wastewater: A Comparison of Response Surface Methods and Taguchi Approaches" Polymers 15, no. 18: 3778. https://doi.org/10.3390/polym15183778
APA StyleMohrazi, A., Ghasemi-Fasaei, R., Mojiri, A., & Safarzadeh Shirazi, S. (2023). Optimization of LDO-Pectin Synthesis Conditions for the Removal of Metals from Wastewater: A Comparison of Response Surface Methods and Taguchi Approaches. Polymers, 15(18), 3778. https://doi.org/10.3390/polym15183778







