The Effect of Chitosan on Physicochemical Properties of Whey Protein Isolate Scaffolds for Tissue Engineering Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Samples Preparation
2.2. Characterization
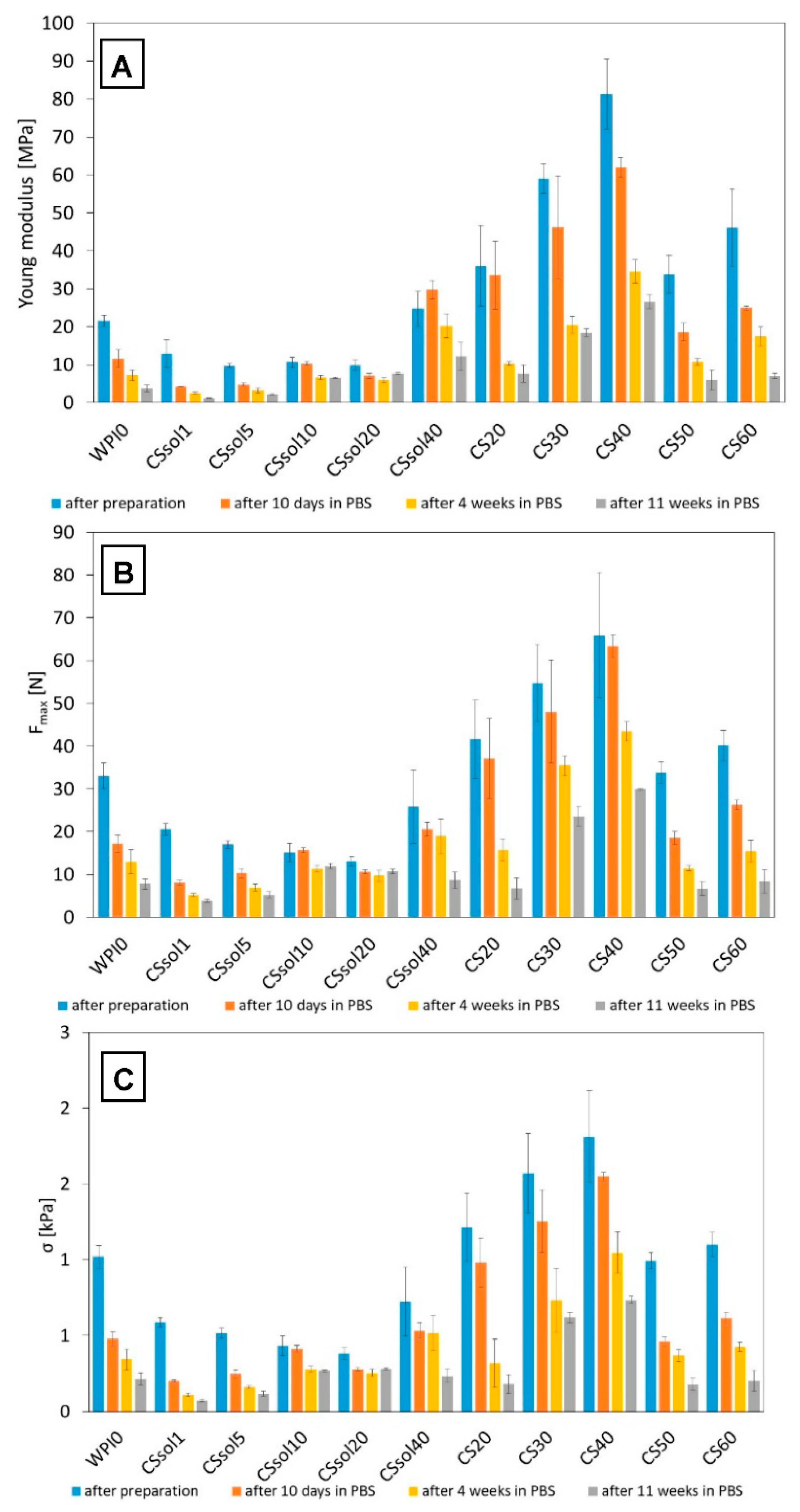
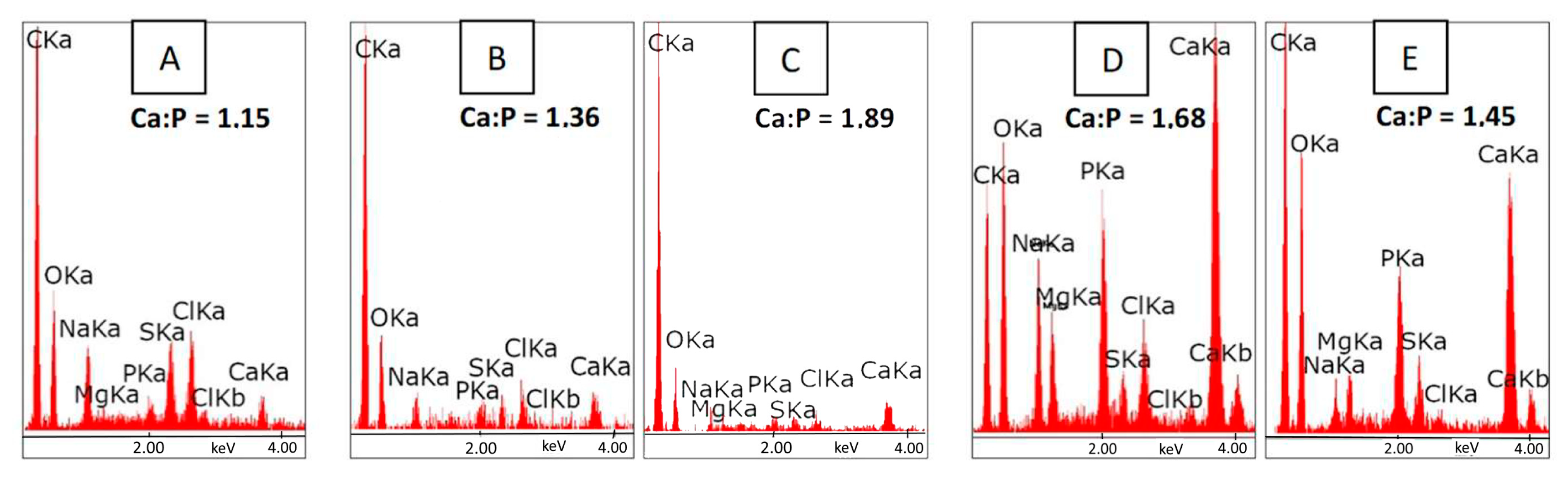
3. Results and Discussion
3.1. Bioactivity Assay
3.2. Cytotoxicity Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Olson, J.L.; Atala, A.; Yoo, J.J. Tissue Engineering: Current Strategies and Future Directions. Chonnam Med. J. 2011, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Aitken, G.; Hill, G. Benefits and associated risks of using allograft, autograft and synthetic bone fusion material for patients and service providers —A Systematic Review. JBI Libr. Syst. Rev. 2010, 8, 1–13. [Google Scholar] [CrossRef]
- Ramos, T.; Moroni, L. Tissue Engineering and Regenerative Medicine 2019: The Role of Biofabrication—A Year in Review. Tissue Eng. Part C Methods 2020, 26, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Horst, B.T.; Moiemen, N.S.; Grover, L.M. Natural polymers. In Biomaterials for Skin Repair and Regeneration; Elsevier: Amsterdam, The Netherlands, 2019; pp. 151–192. [Google Scholar]
- Deng, C.; Chang, J.; Wu, C. Bioactive scaffolds for osteochondral regeneration. J. Orthop. Transl. 2019, 17, 15–25. [Google Scholar] [CrossRef]
- Nukavarapu, S.P.; Dorcemus, D.L. Osteochondral tissue engineering: Current strategies and challenges. Biotechnol. Adv. 2013, 31, 706–721. [Google Scholar] [CrossRef]
- Duan, P.; Pan, Z.; Cao, L.; He, Y.; Wang, H.; Qu, Z.; Dong, J.; Ding, J. The effects of pore size in bilayered poly(lactide- co -glycolide) scaffolds on restoring osteochondral defects in rabbits. J. Biomed. Mater. Res. Part A 2014, 102, 180–192. [Google Scholar] [CrossRef]
- Haaparanta, A.M. Highly Porous Freeze-Dried Composite Scaffolds for Cartilage and Osteochondral Tissue Engineering; Tampere University of Technology: Tampere, Finland, 2015. [Google Scholar]
- Ansari, S.; Khorshidi, S.; Karkhaneh, A. Engineering of gradient osteochondral tissue: From nature to lab. Acta Biomater. 2018, 87, 41–54. [Google Scholar] [CrossRef]
- Ferrari, M.; Cirisano, F.; Morán, M.C. Mammalian Cell Behavior on Hydrophobic Substrates: Influence of Surface Properties. Colloids Interfaces 2019, 3, 48. [Google Scholar] [CrossRef]
- Jabbari, E. Challenges for natural hydrogels in tissue engineering. Gels 2019, 5, 30. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Téllez, D.; Téllez-Jurado, L.; Rodríguez-Lorenzo, L.M. Hydrogels for Cartilage Regeneration, from Polysaccharides to Hybrids. Polymers 2017, 9, 671. [Google Scholar] [CrossRef]
- Sharma, R.; Sharma, P.K.; Malviya, R. Polysaccharide-based Scaffolds for Bone Marrow Regeneration: Recent Work and Commercial Utility (Patent). Curr. Smart Mater. 2019, 4, 29–35. [Google Scholar] [CrossRef]
- Nooeaid, P.; Salih, V.; Beier, J.P.; Boccaccini, A.R. Osteochondral tissue engineering: Scaffolds, stem cells and applications. J. Cell. Mol. Med. 2012, 16, 2247–2270. [Google Scholar] [CrossRef]
- Lin, C.S.K.; Pfaltzgraff, L.A.; Herrero-Davila, L.; Mubofu, E.B.; Abderrahim, S.; Clark, J.H.; Koutinas, A.A.; Kopsahelis, N.; Stamatelatou, K.; Dickson, F.; et al. Food waste as a valuable resource for the production of chemicals, materials and fuels. Current situation and global perspective. Energy Environ. Sci. 2013, 6, 426–464. [Google Scholar] [CrossRef]
- Betz, A.; Buchli, J.; Göbel, C.; Müller, C. Food waste in the Swiss food service industry—Magnitude and potential for reduction. Waste Manag. 2015, 35, 218–226. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Exploitation of Food Industry Waste for High-Value Products. Trends Biotechnol. 2016, 34, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.B.; Álvarez, L.M.M.; Urtasun, N.; Baieli, M.F.; Lázaro-Martínez, J.M.; Glisoni, R.J.; María, V.; Miranda, M.V.; Cascone, O.; Wolman, F.J. Lactoferrin purification and whey protein isolate recovery from cheese whey using chitosan mini-spheres. Int. Dairy J. 2020, 109, 104764. [Google Scholar] [CrossRef]
- de Castro, R.J.S.; Domingues MA, F.; Ohara, A.; Okuro, P.K.; dos Santos, J.G.; Brexó, R.P.; Sato, H.H. Whey protein as a key component in food systems: Physicochemical properties, production technologies and applications. Food Struct. 2017, 14, 17–29. [Google Scholar] [CrossRef]
- Zajac, I.; Herreen, D.; Bastiaans, K.; Dhillon, V.; Fenech, M. The Effect of Whey and Soy Protein Isolates on Cognitive Function in Older Australians with Low Vitamin B12: A Randomised Controlled Crossover Trial. Nutrients 2018, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Khaldi, M.; Ronse, G.; André, C.; Blanpain-Avet, P.; Bouvier, L.; Six, T.; Bornay, S.; Croguennec, R.J.; Delaplace, G. Relationship between β-lactoglobulin denaturation and fouling mass distribution in a plate heat exchanger. In Proceedings of the Heat Exchanger Fouling and Cleaning Conference, Dublin, Ireland, 7–12 June 2015. [Google Scholar]
- Nicolai, T.; Britten, M.; Schmitt, C. β-Lactoglobulin and WPI aggregates: Formation, structure and applications. Food Hydrocoll. 2011, 25, 1945–1962. [Google Scholar] [CrossRef]
- Galani, D.; Apenten, R.K.O. Heat-induced denaturation and aggregation of β-lactoglobulin: Kinetics of formation of hydrophobic and disulphide-linked aggregates. Int. J. Food Sci. Technol. 1999, 34, 467–476. [Google Scholar] [CrossRef]
- Douglas, T.E.; Vandrovcová, M.; Kročilová, N.; Keppler, J.K.; Zárubová, J.; Skirtach, A.G.; Bačáková, L. Application of whey protein isolate in bone regeneration: Effects on growth and osteogenic differentiation of bone-forming cells. J. Dairy Sci. 2018, 101, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Puyol, P.; Pérez, M.D.; Horne, D.S. Heat-induced gelation of whey protein isolates (WPI): Effect of NaCl and protein concentration. Food Hydrocoll. 2001, 15, 233–237. [Google Scholar] [CrossRef]
- Liu, K.; Kong, X.L.; Li, Q.M.; Zhang, H.L.; Zha, X.Q.; Luo, J.P. Stability and bioavailability of vitamin D3 encapsulated in composite gels of whey protein isolate and lotus root amylopectin. Carbohydr. Polym. 2020, 227, 115337. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, M.; Kudlackova, R.; Zima, A.; Slosarczyk, A.; Ziabka, M.; Jelen, P.; Shkarina, S.; Cecilia, A.; Zuber, M.; Baumbach, T.; et al. Novel multicomponent organic–inorganic WPI/gelatin/CaP hydrogel composites for bone tissue engineering. J. Biomed. Mater. Res. Part A 2019, 107, 2479–2491. [Google Scholar] [CrossRef]
- Gupta, D.; Kocot, M.; Tryba, A.M.; Serafim, A.; Stancu, I.C.; Jaegermann, Z.; Pamuła, E.; Reilly, G.C.; Douglas, T.E.L. Novel naturally derived whey protein isolate and aragonite biocomposite hydrogels have potential for bone regeneration. Mater. Des. 2020, 188, 108408. [Google Scholar] [CrossRef]
- Carson, M.; Keppler, J.K.; Brackman, G.; Dawood, D.; Vandrovcova, M.; Fawzy El-Sayed, K.; Coenye, T.; Schwarz, K.; Clarke, S.A.; Skirtach, A.G.; et al. Whey protein complexes with green tea polyphenols: Antimicrobial, osteoblast-stimulatory and 1 antioxidant activities. Cells Tissues Organs 2019, 206, 106–118. [Google Scholar] [CrossRef]
- Rouabhia, M.; Gilbert, V.; Hongxum, W.; Subirade, M. In vivo evaluation of whey protein-based biofilms as scaffolds for cutaneous cell cultures and biomedical applications. Biomed. Mater. 2007, 2, S38–S44. [Google Scholar] [CrossRef] [PubMed]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Ji, C.; Khademhosseini, A.; Dehghani, F. Enhancing cell penetration and proliferation in chitosan hydrogels for tissue engineering applications. Biomaterials 2011, 32, 9719–9729. [Google Scholar] [CrossRef]
- Dash, M.; Chiellini, F.; Ottenbrite, R.M.; Chiellini, E. Chitosan—A versatile semi-synthetic polymer in biomedical applications. Prog. Polym. Sci. 2011, 36, 981–1014. [Google Scholar] [CrossRef]
- Domalik-Pyzik, P.; Chłopek, J.; Pielichowska, K. Chitosan-Based Hydrogels: Preparation, Properties, and Applications; Springer: Cham, Switzerland, 2019; pp. 1665–1693. [Google Scholar]
- Sultankulov, B.; Berillo, D.; Sultankulova, K.; Tokay, T.; Saparov, A. Progress in the Development of Chitosan-Based Biomaterials for Tissue Engineering and Regenerative Medicine. Biomolecules 2019, 9, 470. [Google Scholar] [CrossRef]
- Kosowska, K.; Domalik-Pyzik, P.; Sekuła-Stryjewska, M.; Noga, S.; Jagiełło, J.; Baran, M.; Lipińska, L.; Zuba-Surma, E.; Chłopek, J. Gradient chitosan hydrogels modified with graphene derivatives and hydroxyapatite: Physiochemical properties and initial cytocompatibility evaluation. Int. J. Mol. Sci. 2020, 21, 4888. [Google Scholar] [CrossRef] [PubMed]
- Zavyalova, O.; Gajewska, S.; Sionkowska, A.; Medicum, C. Characteristics of Physicochemical and Rheological Properties of Chitosan Hydrogels Based on. Eng. Biomater. 2021, 36, 2015. [Google Scholar] [CrossRef]
- Sionkowska, A.; Musiał, K. Fish Collagen and Chitosan Mixtures as a Promising Biomaterial for Potential Use in Medicine and. Eng. Biomater. 2022, 164, 16–24. [Google Scholar] [CrossRef]
- Jiang, T.; Deng, M.; James, R.; Nair, L.S.; Laurencin, C.T. Micro- and nanofabrication of chitosan structures for regenerative engineering. Acta Biomater. 2014, 10, 1632–1645. [Google Scholar] [CrossRef] [PubMed]
- Shamekhi, M.A.; Rabiee, A.; Mirzadeh, H.; Mahdavi, H.; Mohebbi-kalhori, D.; Eslaminejad, B. Fabrication and characterization of hydrothermal cross-linked chitosan porous scaffolds for cartilage tissue engineering applications. Mater. Sci. Eng. C 2017, 80, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Teixeira, L.M.; Dijkstra, P.J.; Karperien, M.; Van Blitterswijk, C.A.; Zhong, Z.Y.; Feijen, J. Injectable chitosan-based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2544–2551. [Google Scholar] [CrossRef]
- Yu, P.; Bao, R.Y.; Shi, X.J.; Yang, W.; Yang, M.B. Self-assembled high-strength hydroxyapatite/graphene oxide/chitosan composite hydrogel for bone tissue engineering. Carbohydr. Polym. 2017, 155, 507–515. [Google Scholar] [CrossRef]
- Gritsch, L.; Maqbool, M.; Mouriño, V.; Ciraldo, F.E.; Cresswell, M.; Jackson, P.R.; Lovell, C.; Boccaccini, A.R. Chitosan/hydroxyapatite composite bone tissue engineering scaffolds with dual and decoupled therapeutic ion delivery: Copper and strontium. J. Mater. Chem. B 2019, 7, 6109–6124. [Google Scholar] [CrossRef]
- Demirtaş, T.T.; Irmak, G.; Gümüşderelio, M. A bioprintable form of chitosan hydrogel for bone tissue engineering. Biofabrication 2017, 9, 3. [Google Scholar] [CrossRef]
- Arakawa, C.; Ng, R.; Tan, S.; Kim, S.; Wu, B.; Lee, M. Photopolymerizable chitosan–collagen hydrogels for bone tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 164–174. [Google Scholar] [CrossRef]
- Chicatun, F.; Griffanti, G.; McKee, M.D.; Nazhat, S.N. Collagen/chitosan composite scaffolds for bone and cartilage tissue engineering. In Biomedical Composites; Elsevier: Amsterdam, The Netherlands, 2017; pp. 163–198. [Google Scholar]
- Malafaya, P.B.; Reis, R.L. Bilayered chitosan-based scaffolds for osteochondral tissue engineering: Influence of hydroxyapatite on in vitro cytotoxicity and dynamic bioactivity studies in a specific double-chamber bioreactor. Acta Biomater. 2009, 5, 644–660. [Google Scholar] [CrossRef]
- Gough, J.E.; Scotchford, C.A.; Downes, S. Cytotoxicity of glutaraldehyde crosslinked collagen/poly(vinyl alcohol) films is by the mechanism of apoptosis. J. Biomed. Mater. Res. 2002, 61, 121–130. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, F.C.; Coimbra, J.S.D.R.; de Oliveira, E.B.; Zuñiga, A.D.G.; Rojas, E.E.G. Food Protein-polysaccharide Conjugates Obtained via the Maillard Reaction: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1108–1125. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Li, C.; Bin, Z.; Huang, Q.; You, L.J.; Chen, C.; Fu, X.; Liu, R.H. Physicochemical properties and bioactivity of whey protein isolate-inulin conjugates obtained by Maillard reaction. Int. J. Biol. Macromol. 2020, 150, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Miralles, B.; Martínez-Rodríguez, A.; Santiago, A.; van de Lagemaat, J.; Heras, A. The occurrence of a Maillard-type protein-polysaccharide reaction between β-lactoglobulin and chitosan. Food Chem. 2007, 100, 1071–1075. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H. Characterization of the interactions between chitosan/whey protein at different conditions. Food Sci. Technol. 2019, 39, 163–169. [Google Scholar] [CrossRef]
- de Souza, H.K.S.; Bai, G.; Gonçalves, M.D.P.; Bastos, M. Whey protein isolate-chitosan interactions: A calorimetric and spectroscopy study. Thermochim. Acta 2009, 495, 108–114. [Google Scholar] [CrossRef]
- Zheng, Z.; Luo, Y.; Yao, L.; Lu, J.; Bu, G. Effects of Maillard reaction conditions on the functional properties of WPI chitosan oligosaccharide conjugates. J. Food Sci. Technol. 2014, 51, 3794–3802. [Google Scholar] [CrossRef]
- Yang, N.; Ashton, J.; Gorczyca, E.; Kasapis, S. In-vitro starch hydrolysis of chitosan incorporating whey protein and wheat starch composite gels. Heliyon 2017, 3, e00421. [Google Scholar] [CrossRef]
- Hu, Y.; He, C.; Jiang, C.; Liao, Y.; Xiong, H.; Zhao, Q. Complexation with whey protein fibrils and chitosan: A potential vehicle for curcumin with improved aqueous dispersion stability and enhanced antioxidant activity. Food Hydrocoll. 2020, 104, 105729. [Google Scholar] [CrossRef]
- Bohner, M.; Lemaitre, J. Can bioactivity be tested in vitro with SBF solution? Biomaterials 2009, 30, 2175–2179. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological evaluation of medical devices — Part 5: Tests for in vitro cytotoxicity. International Organization for Standardization: Geneva, Switzerland.
- Ikeda, T.; Ikeda, K.; Yamamoto, K.; Ishizaki, H.; Yoshizawa, Y.; Yanagiguchi, K.; Yamada, S.; Hayashi, Y. Fabrication and Characteristics of Chitosan Sponge as a Tissue Engineering Scaffold. BioMed Res. Int. 2014, 2014, 786892. [Google Scholar] [CrossRef]
- Stenhamre, H.; Nannmark, U.; Lindahl, A.; Gatenholm, P.; Brittberg, M. Influence of pore size on the redifferentiation potential of human articular chondrocytes in poly(urethane urea) scaffolds. J. Tissue Eng. Regen. Med. 2011, 5, 578–588. [Google Scholar] [CrossRef]
- Ajmeri, J.R.; Ajmeri, C.J. Developments in nonwoven materials for medical applications. In Advances in Technical Nonwovens; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 227–256. [Google Scholar]
- Wang, S.; Yu, P.; Li, X.; Zhao, Z.; Dong, Y.; Li, X. Design and fabrication of functional hydrogels with specific surface wettability. Colloids Interface Sci. Commun. 2023, 52, 100697. [Google Scholar] [CrossRef]
- Guo, Z.; Bo, D.; He, Y.; Luo, X.; Li, H. Degradation properties of chitosan microspheres/poly(L-lactic acid) composite in vitro and in vivo. Carbohydr. Polym. 2018, 193, 1–8. [Google Scholar] [CrossRef]
- Tampieri, A.; Sandri, M.; Landi, E.; Pressato, D.; Francioli, S.; Quarto, R.; Martin, I. Design of graded biomimetic osteochondral composite scaffolds. Biomaterials 2008, 29, 3539–3546. [Google Scholar] [CrossRef]
- Manjubala, I.; Scheler, S.; Bössert, J.; Jandt, K.D. Mineralisation of chitosan scaffolds with nano-apatite formation by double diffusion technique. Acta Biomater. 2006, 2, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Eliaz, N.; Metoki, N. Calcium phosphate bioceramics: A review of their history, structure, properties, coating technologies and biomedical applications. Materials 2017, 10, 334. [Google Scholar] [CrossRef] [PubMed]
- Gbassi, G.; Yolou, F.; Sarr, S.; Atheba, P.; Amin, C.; Ake, M. Whey proteins analysis in aqueous medium and in artificial gastric and intestinal fluids. Int. J. Biol. Chem. Sci. 2012, 6, 4. [Google Scholar] [CrossRef]
- Jain, A.; Thakur, D.; Ghoshal, G.; Katare, O.P.; Shivhare, U.S. Microencapsulation by Complex Coacervation Using Whey Protein Isolates and Gum Acacia: An Approach to Preserve the Functionality and Controlled Release of β-Carotene. Food Bioprocess Technol. 2015, 8, 1635–1644. [Google Scholar] [CrossRef]
- Mohan, C.O.; Gunasekaran, S.; Ravishankar, C.N. Chitosan-capped gold nanoparticles for indicating temperature abuse in frozen stored products. npj Sci. Food 2019, 3, 2. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, S.; Bisiriyu, I.O.; Akinremi, C.A.; Amolegbe, S.A. Synthesis, Spectroscopic, Surface and Catalytic Reactivity of Chitosan Supported Co(II) and Its Zerovalentcobalt Nanobiocomposite. J. Inorg. Organomet. Polym. Mater. 2017, 27, 114–121. [Google Scholar] [CrossRef]
- Ayad, M.M.; Amer, W.A.; Kotp, M.G.; Minisy, I.M.; Rehab, A.F.; Kopecký, D.; Fitl, P. Synthesis of silver-anchored polyaniline-chitosan magnetic nanocomposite: A smart system for catalysis. RSC Adv. 2017, 7, 18553–18560. [Google Scholar] [CrossRef]
- Nunes, A.P.L.; Peres, A.E.C.; Chaves, A.P.; Ferreira, W.R. Effect of alkyl chain length of amines on fluorapatite and aluminium phosphates floatabilities. J. Mater. Res. Technol. 2019, 8, 3623–3634. [Google Scholar] [CrossRef]
- Ramos, A.P.; Ybarra, G.O.; Pazos, L.M.; Parodi, M.B.; Rodríguez, L.; Hernández, M.L.; Ruíz, J.E.G. Manufacture of nanosized apatite coatings on titanium with different surface treatments using a supersaturated calcification solution. Quim. Nova 2016, 39, 1159–1164. [Google Scholar] [CrossRef]
- Plastun, V.O.; Prikhozhdenko, E.S.; Gusliakova, O.I.; Raikova, S.V.; Douglas, T.E.L.; Sindeeva, O.A.; Mayorova, O.A. WPI Hydrogels with a Prolonged Drug-Release Profile for Antimicrobial Therapy. Pharmaceutics 2022, 14, 1199. [Google Scholar] [CrossRef]
- Norris, K.; Kocot, M.; Tryba, A.M.; Chai, F.; Talari, A.; Ashton, L.; Parakhonskiy, B.V.; Samal, S.K.; Blanchemain, N.; Pamuła, E.; et al. Marine-Inspired Enzymatic Mineralization of Dairy-Derived Whey Protein Isolate (WPI) Hydrogels for Bone Tissue Regeneration. Mar. Drugs 2020, 18, 294. [Google Scholar] [CrossRef]
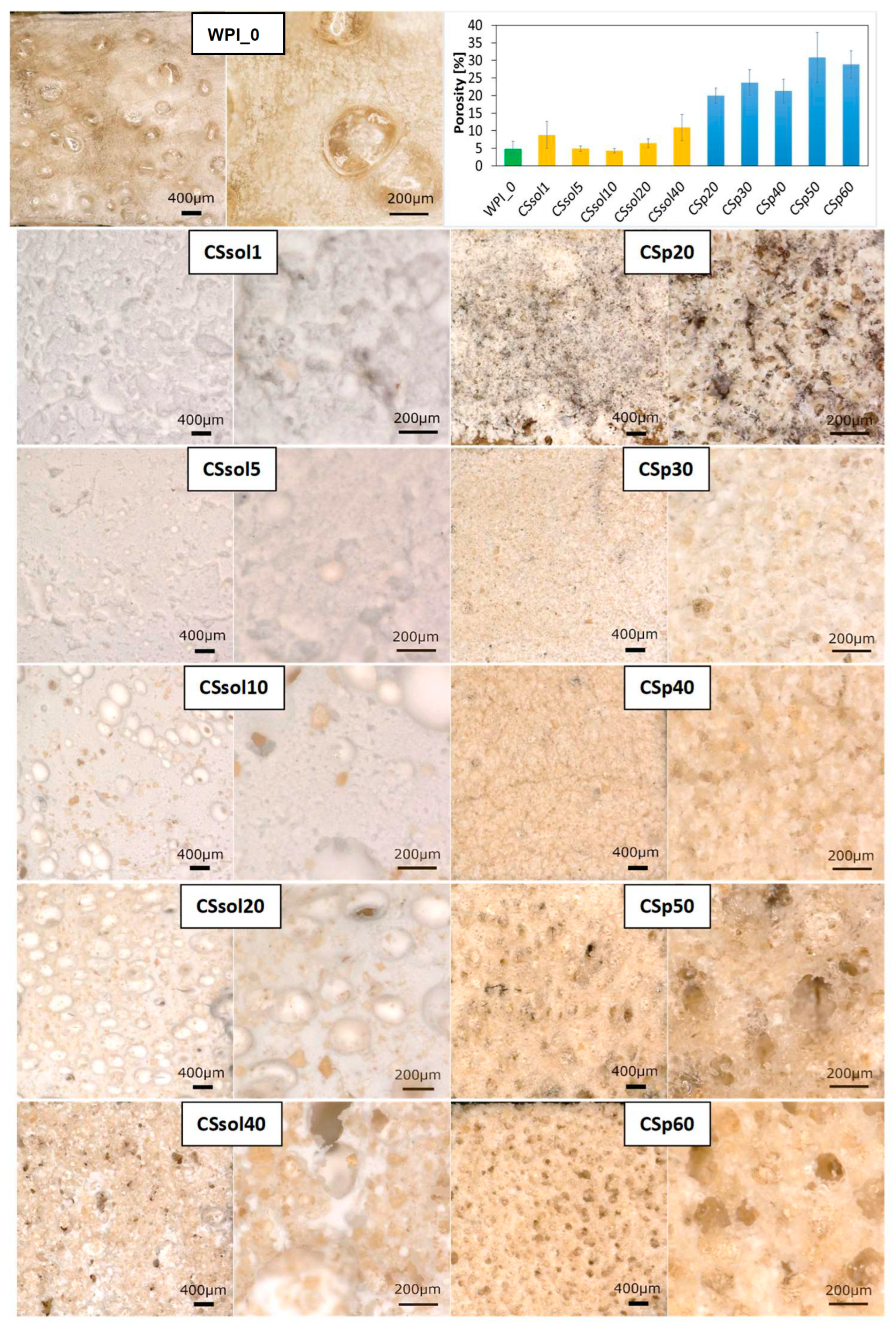
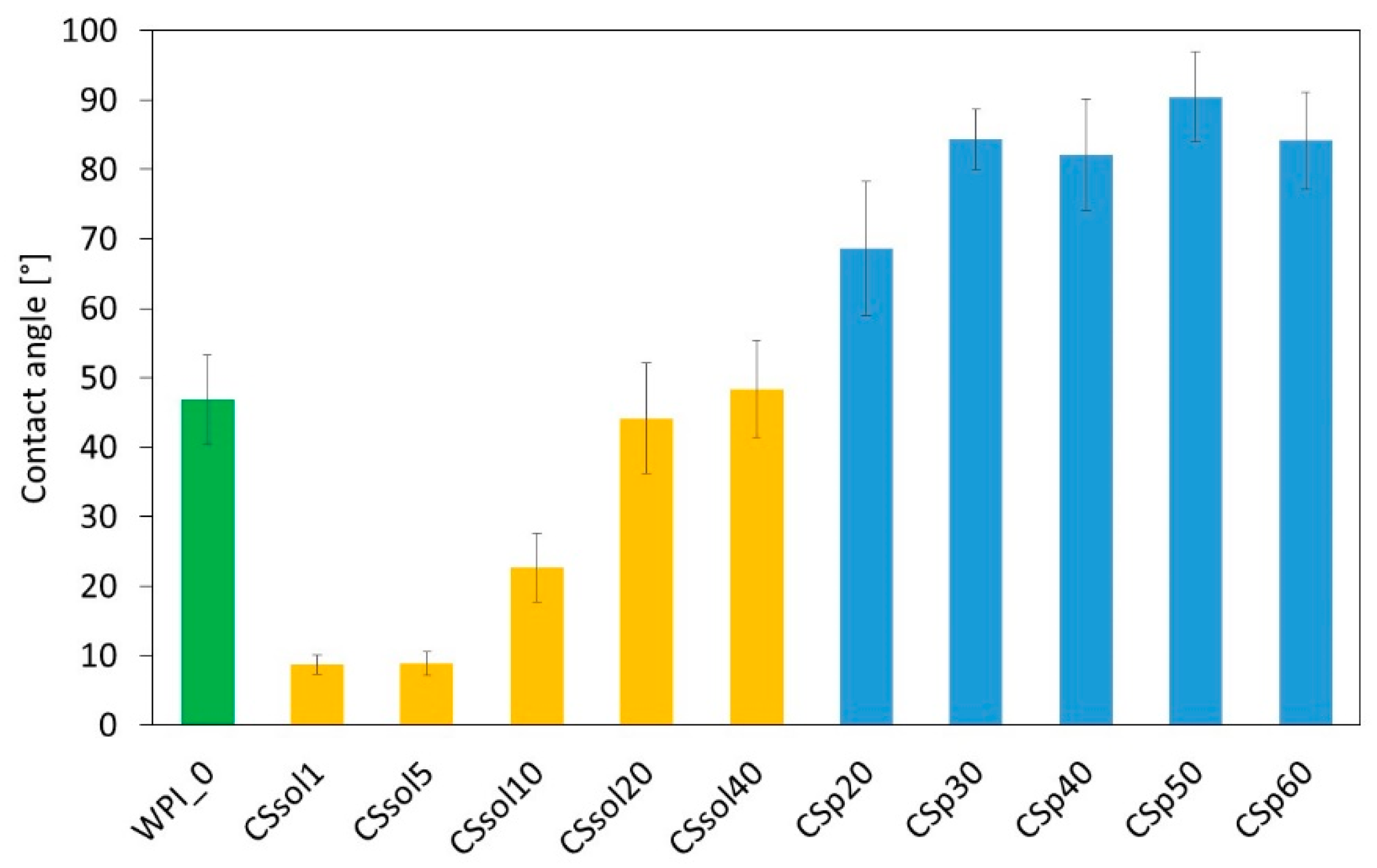
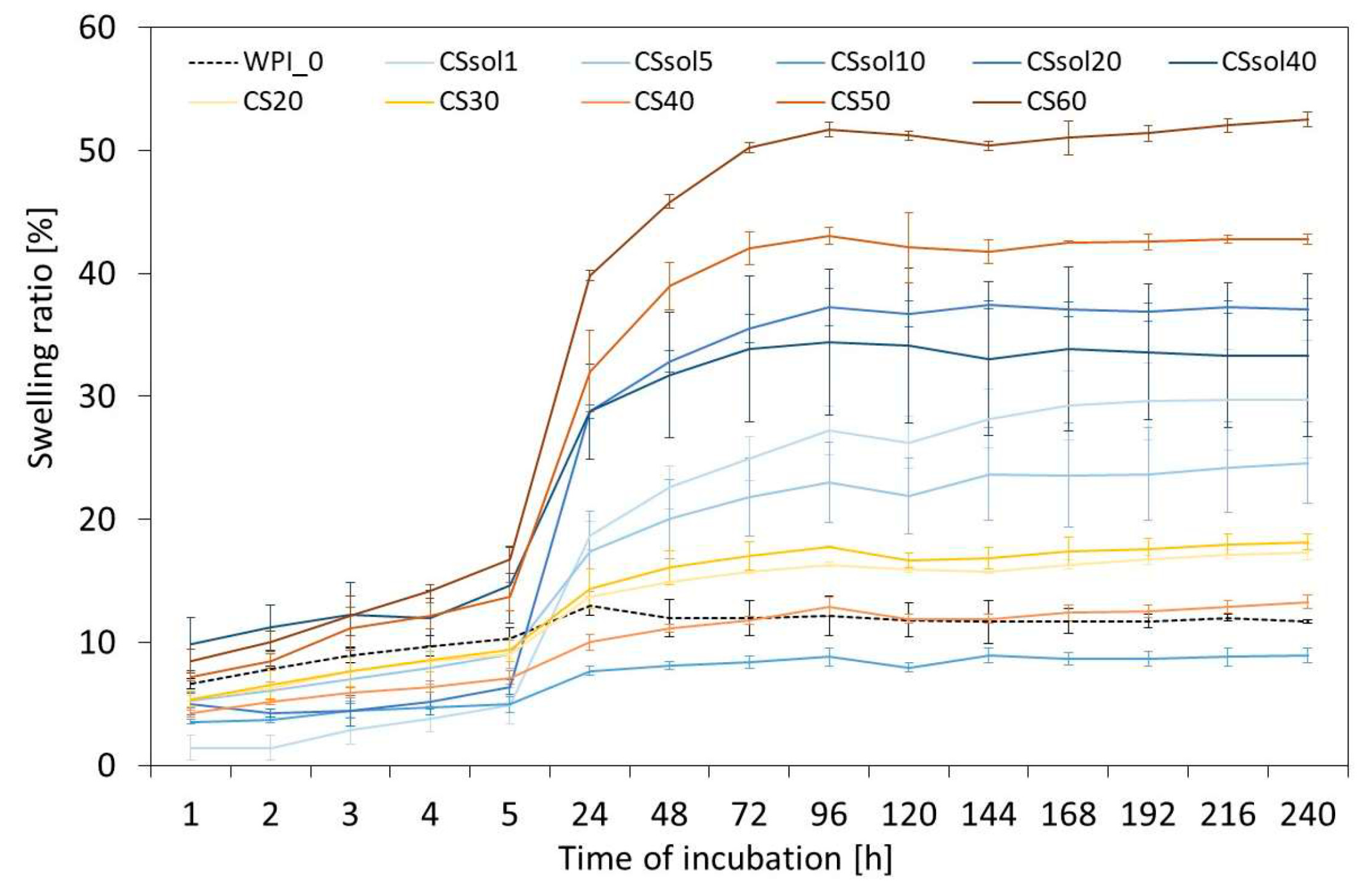
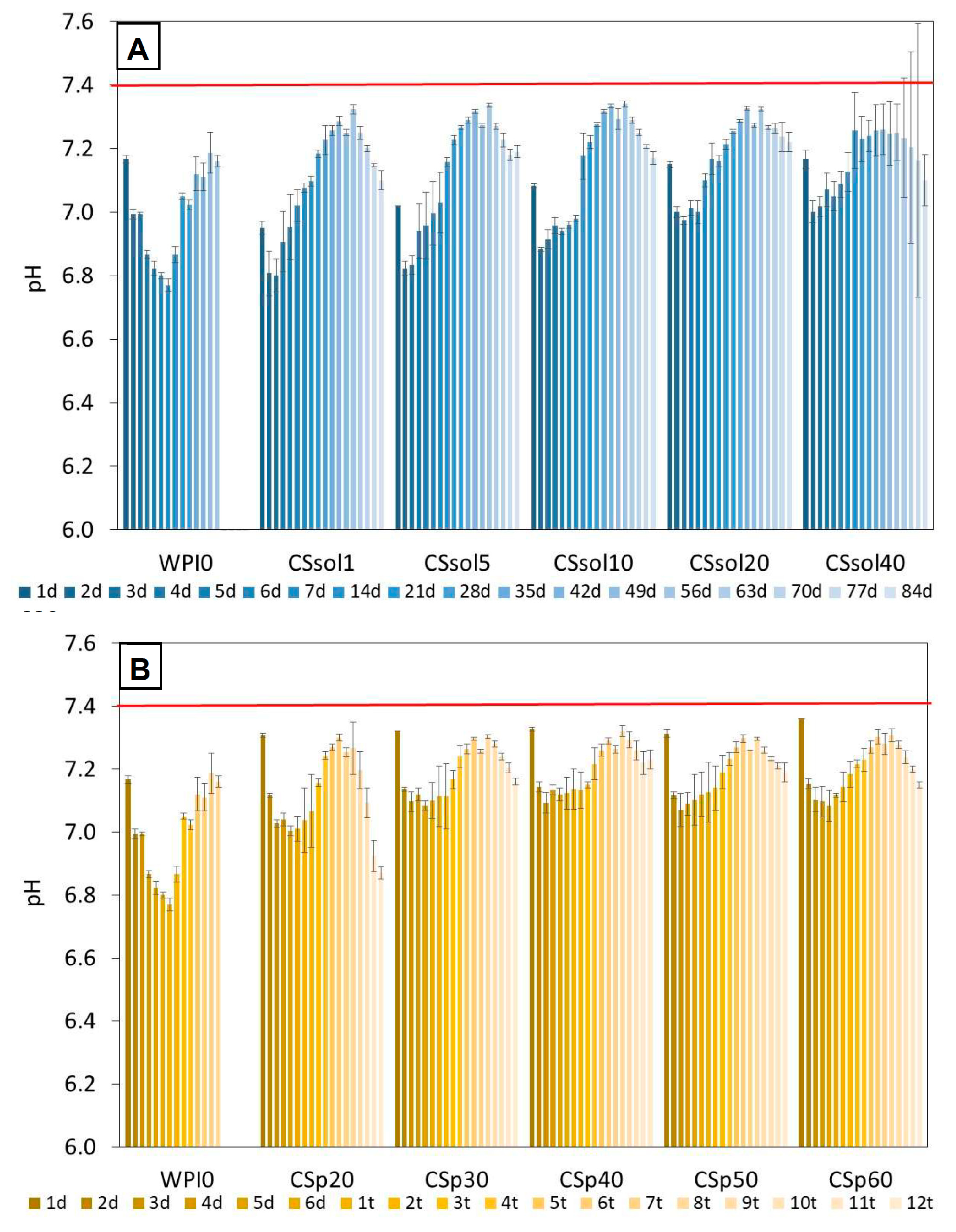





| Sample | CS Dissolved in 2% AcAc [%] | CS Powder Suspended in Water [%] | WPI [%] |
|---|---|---|---|
| CSsol1 | 1 | - | 40 |
| CSsol5 | 5 | - | 40 |
| CSsol10 | 10 | - | 40 |
| CSsol20 | 20 | - | 40 |
| CSsol40 | 40 | - | 40 |
| CSp20 | - | 20 | 40 |
| CSp30 | - | 30 | 40 |
| CSp40 | - | 40 | 40 |
| CSp50 | - | 50 | 40 |
| CSp60 | - | 60 | 40 |
| WPI | 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaweł, M.; Domalik-Pyzik, P.; Douglas, T.E.L.; Reczyńska-Kolman, K.; Pamuła, E.; Pielichowska, K. The Effect of Chitosan on Physicochemical Properties of Whey Protein Isolate Scaffolds for Tissue Engineering Applications. Polymers 2023, 15, 3867. https://doi.org/10.3390/polym15193867
Gaweł M, Domalik-Pyzik P, Douglas TEL, Reczyńska-Kolman K, Pamuła E, Pielichowska K. The Effect of Chitosan on Physicochemical Properties of Whey Protein Isolate Scaffolds for Tissue Engineering Applications. Polymers. 2023; 15(19):3867. https://doi.org/10.3390/polym15193867
Chicago/Turabian StyleGaweł, Martyna, Patrycja Domalik-Pyzik, Timothy E. L. Douglas, Katarzyna Reczyńska-Kolman, Elżbieta Pamuła, and Kinga Pielichowska. 2023. "The Effect of Chitosan on Physicochemical Properties of Whey Protein Isolate Scaffolds for Tissue Engineering Applications" Polymers 15, no. 19: 3867. https://doi.org/10.3390/polym15193867
APA StyleGaweł, M., Domalik-Pyzik, P., Douglas, T. E. L., Reczyńska-Kolman, K., Pamuła, E., & Pielichowska, K. (2023). The Effect of Chitosan on Physicochemical Properties of Whey Protein Isolate Scaffolds for Tissue Engineering Applications. Polymers, 15(19), 3867. https://doi.org/10.3390/polym15193867









