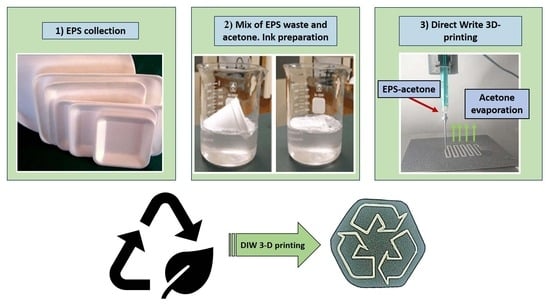
Novel and Accessible Physical Recycling for Expanded Polystyrene Waste with the Use of Acetone as a Solvent and Additive Manufacturing (Direct Ink-Write 3D Printing)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
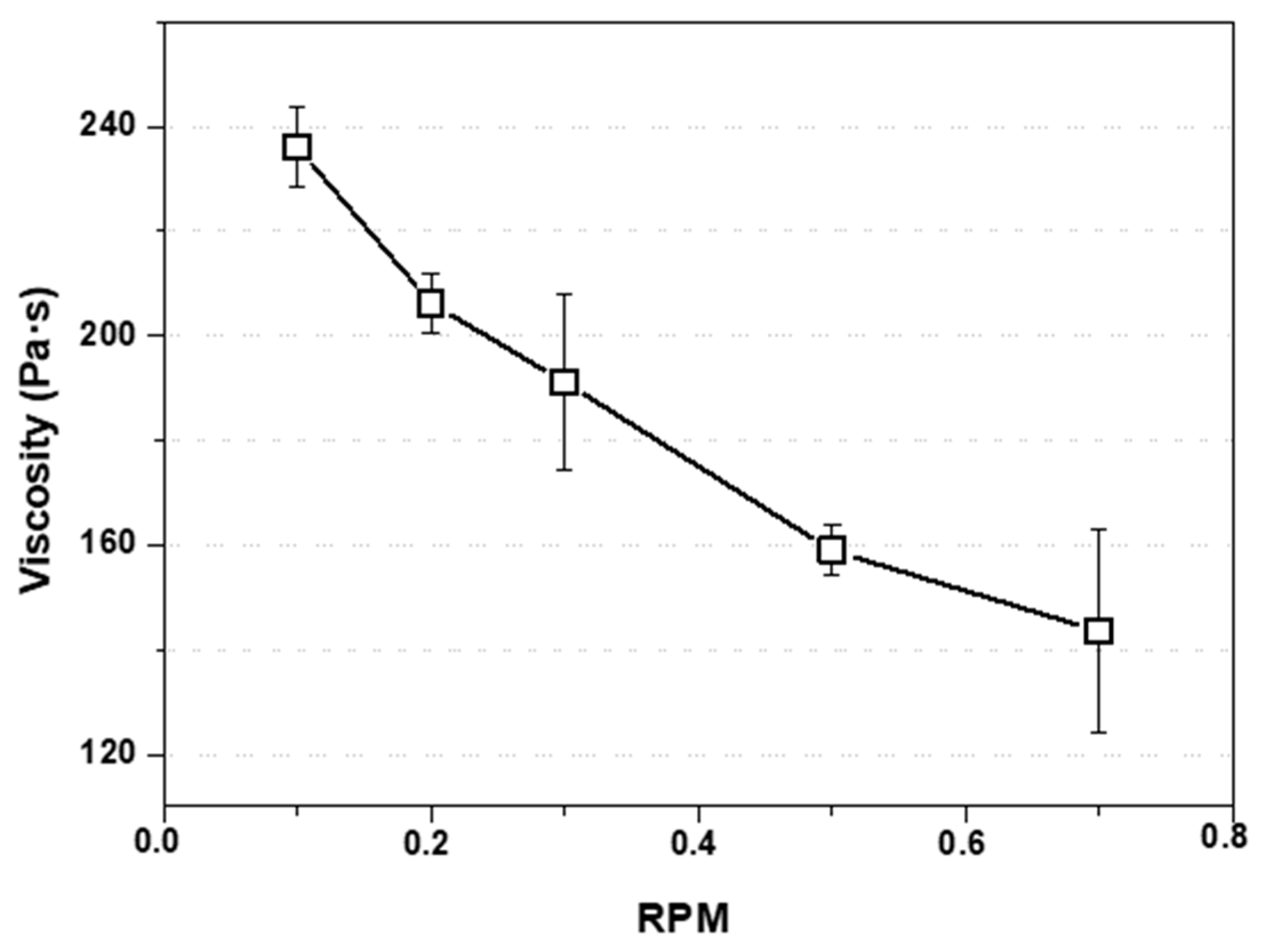
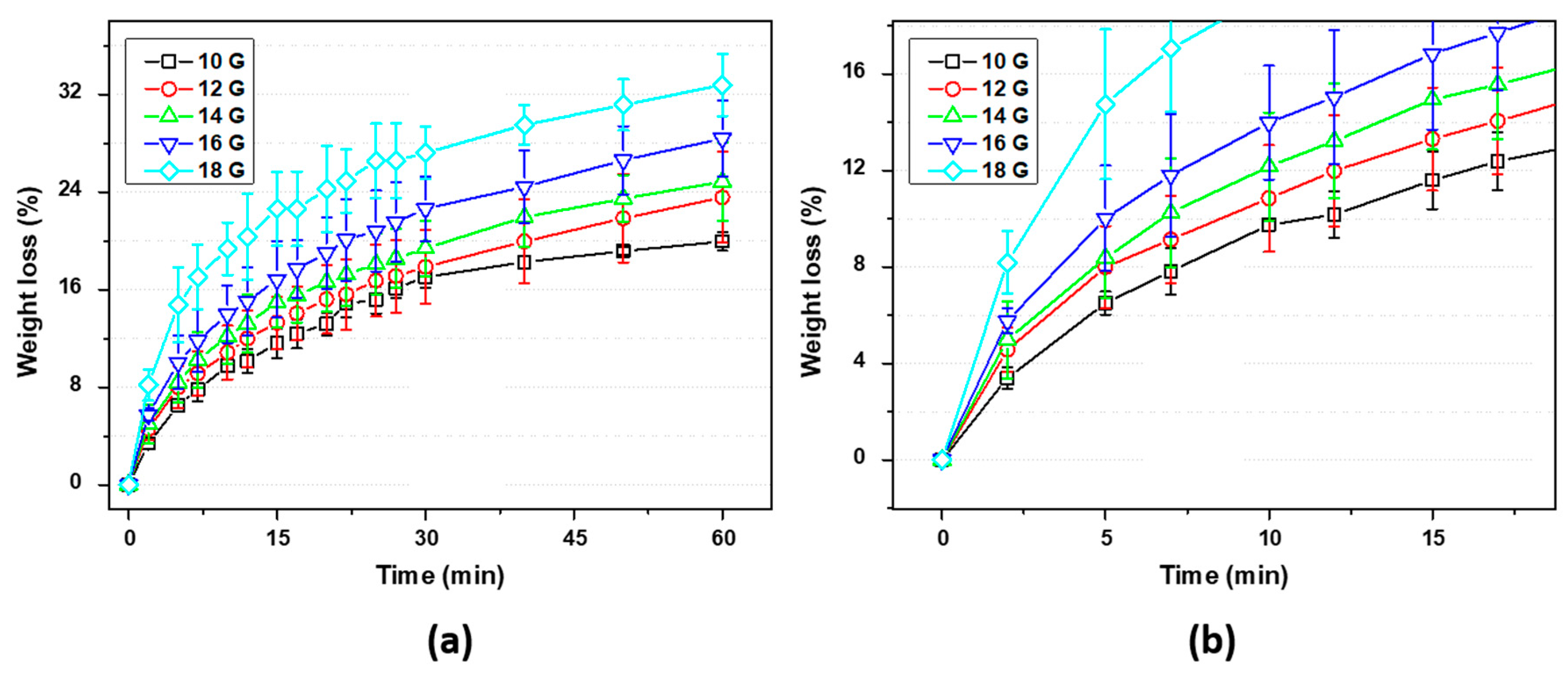
2.2. Preparation and Evaluation of the EPS-Ink
2.3. Manufacturing by 3D Printing and Optimization of the Process
2.4. Comparative Analysis of 3D-Printed Material vs. Original Material
3. Results
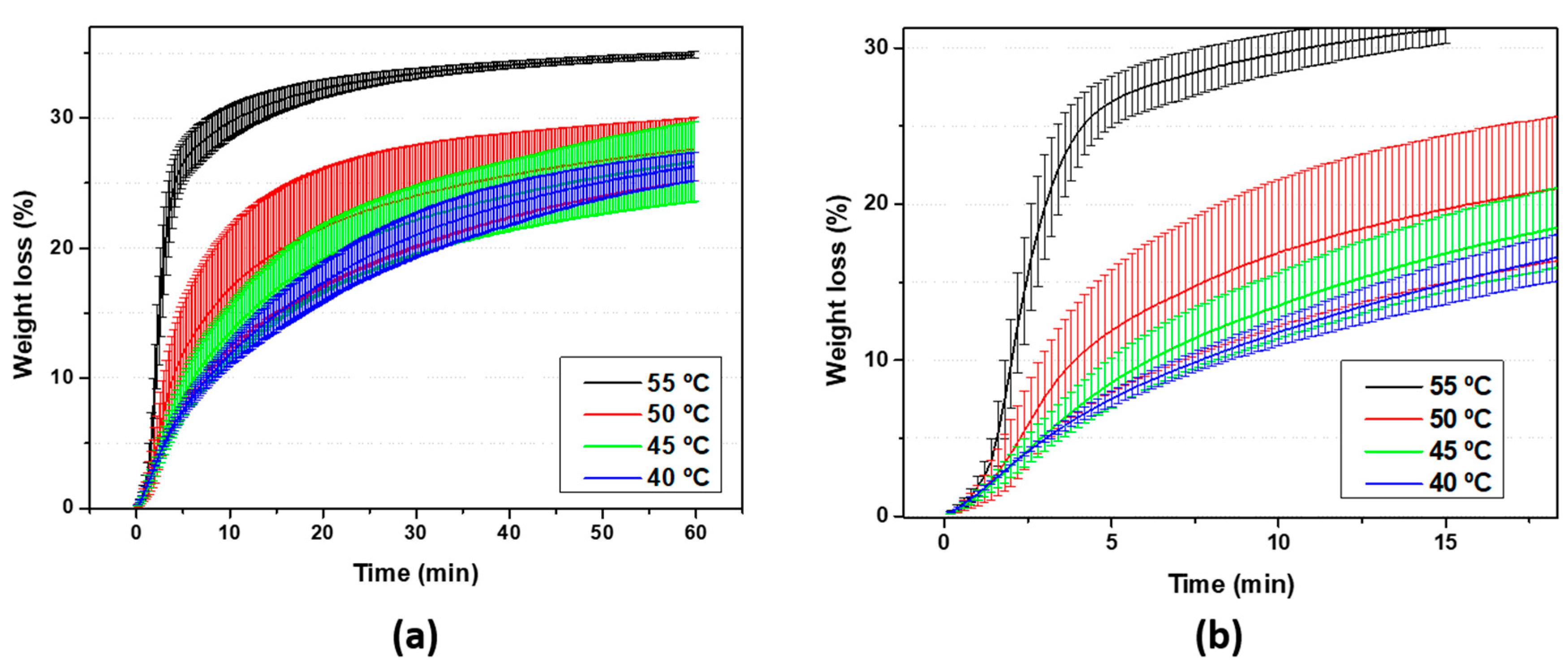
3.1. EPS as Waste Diluted in Acetone: Evaluation and Preparation of the Ink for 3D Printing
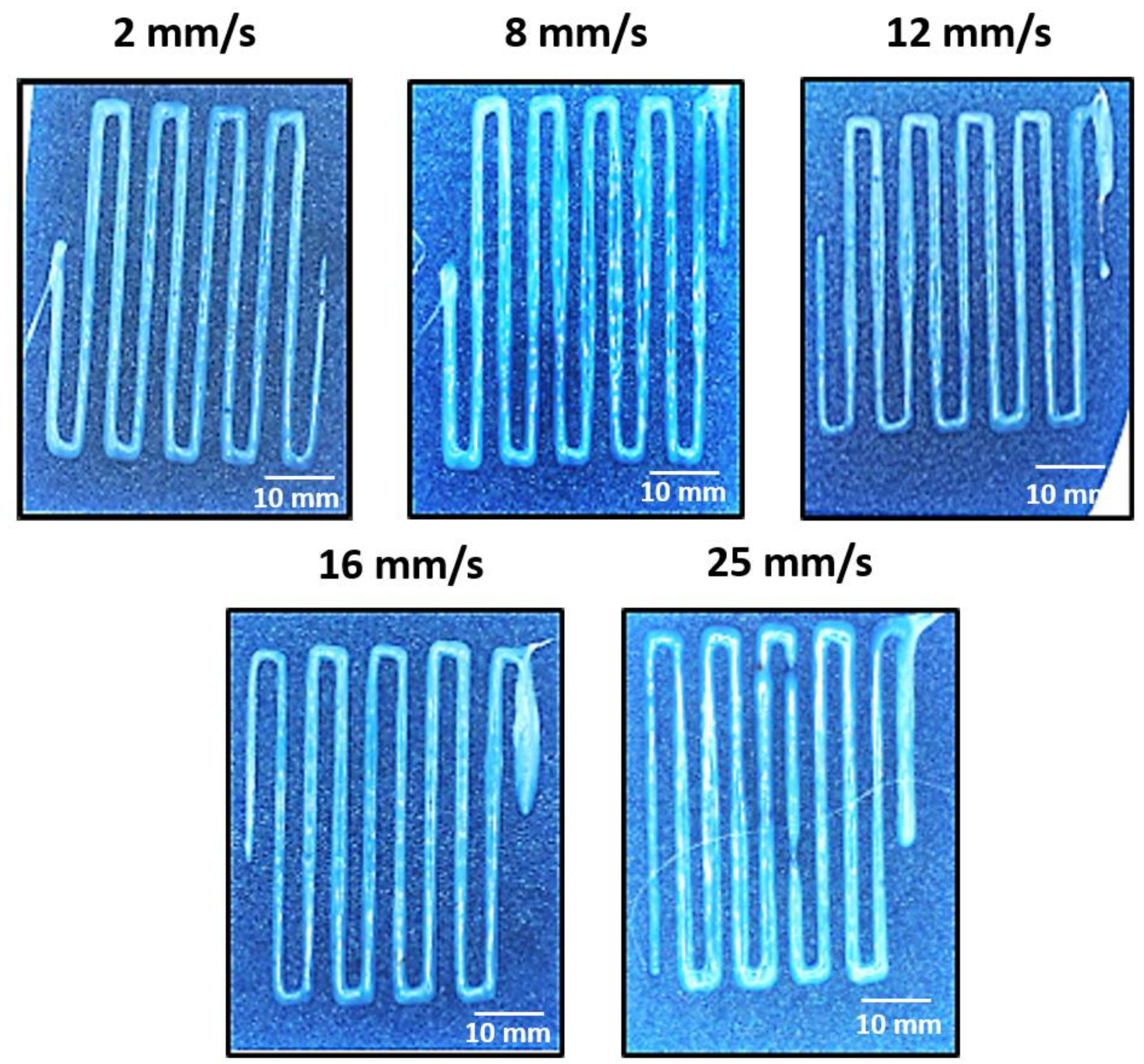
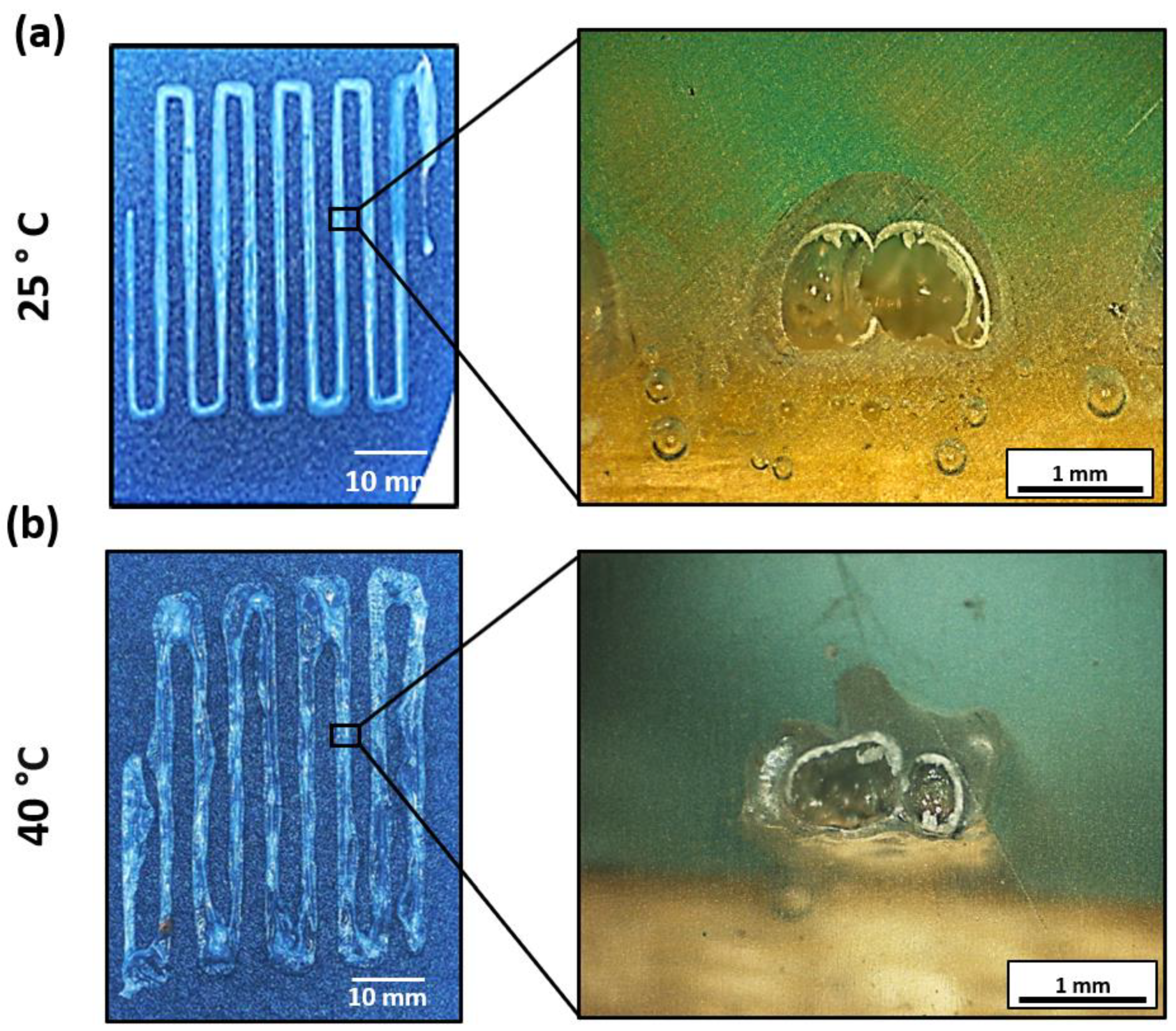
3.2. 3D Printing Parameter Optimization
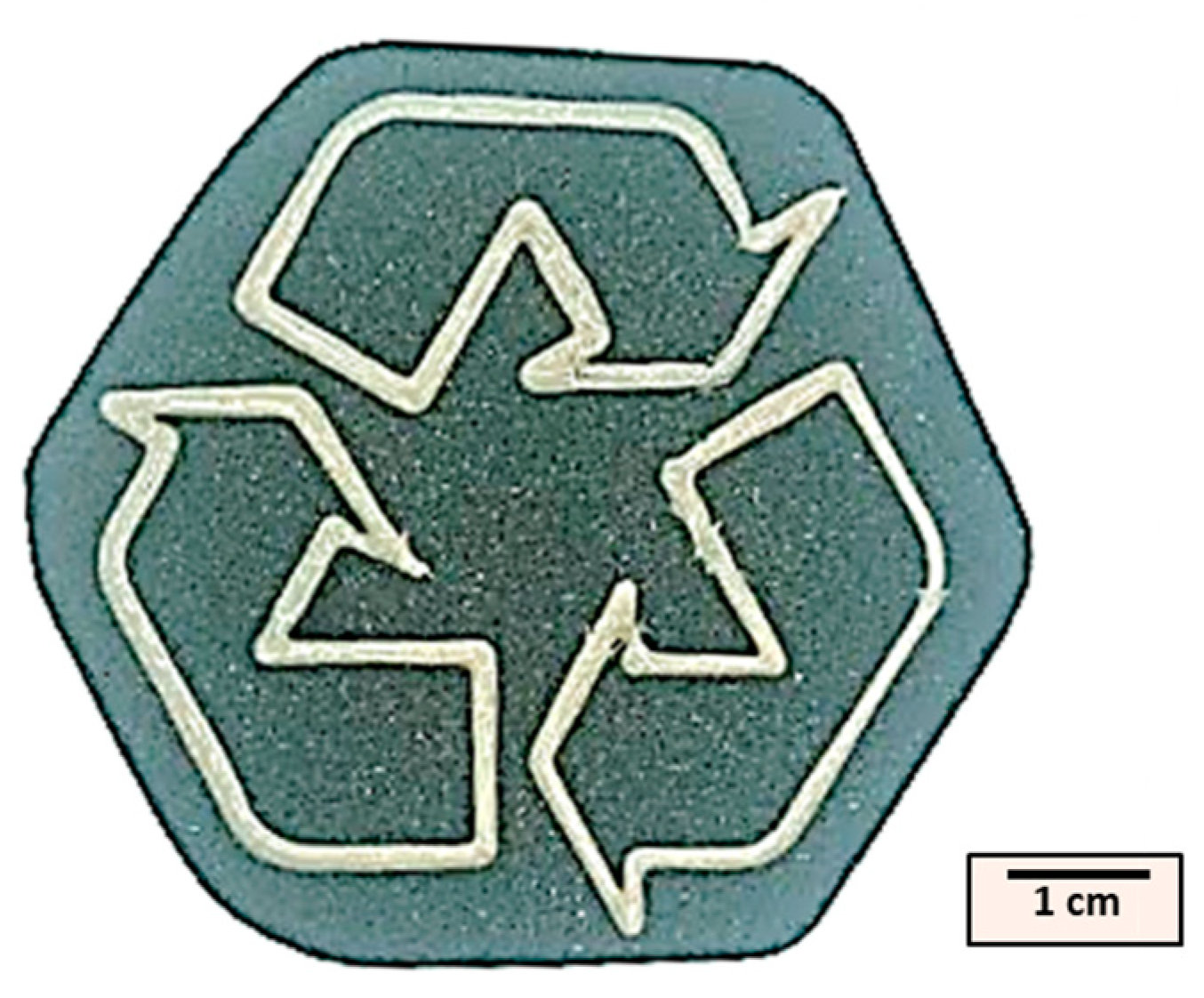
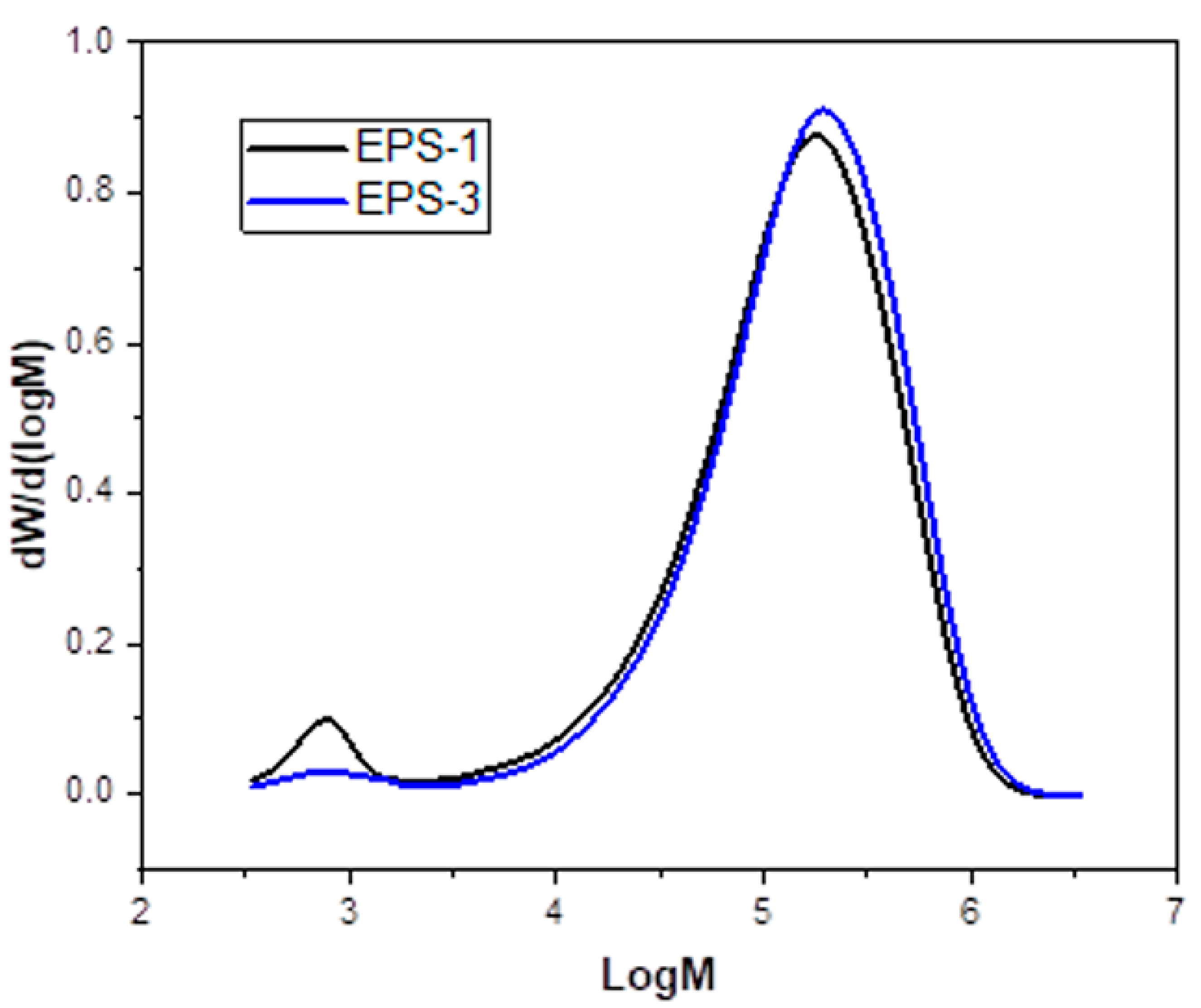
3.3. Comparative Analysis of Printed Material vs. Original Material
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Korey, M.; Li, K.; Copenhaver, K.; Tekinalp, H.; Celik, S.; Kalaitzidou, K.; Ruan, R.; Ragauskas, A.J.; Ozcan, S. Plastic Waste Upcycling toward a Circular Economy. Chem. Eng. J. 2022, 428, 131928. [Google Scholar] [CrossRef]
- Morales, I.; Muñoz, M.; Costa, C.S.; Alonso, J.M.; Silva, J.M.; Multigner, M.; Quijorna, M.; Ribeiro, M.R.; de la Presa, P. Induction Heating in Nanoparticle Impregnated Zeolite. Materials 2020, 13, 4013. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Azad, N.; Dutta, D.; Yadav, B.R.; Kumar, S. A Critical Review and Future Perspective of Plastic Waste Recycling. Sci. Total. Environ. 2023, 881, 163433. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, Migration and Toxicology of Microplastics in Soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef] [PubMed]
- Lisiecki, M.; Damgaard, A.; Ragaert, K.; Astrup, T.F. Circular Economy Initiatives Are No Guarantee for Increased Plastic Circularity: A Framework for the Systematic Comparison of Initiatives. Resour. Conserv. Recycl. 2023, 197, 107072. [Google Scholar] [CrossRef]
- European Commission. The European Green Deal. 52019DC0640; European Commission: Brussels, Belgium, 2018.
- von Vacano, B.; Mangold, H.; Vandermeulen, G.W.M.; Battagliarin, G.; Hofmann, M.; Bean, J.; Künkel, A. Sustainable Design of Structural and Functional Polymers for a Circular Economy. Angew. Chem. Int. Ed. 2023, 62, e202210823. [Google Scholar] [CrossRef]
- Shanmugam, V.; Das, O.; Neisiany, R.E.; Babu, K.; Singh, S.; Hedenqvist, M.S.; Berto, F.; Ramakrishna, S. Polymer Recycling in Additive Manufacturing: An Opportunity for the Circular Economy. Mater. Circ. Econ. 2020, 2, 11. [Google Scholar] [CrossRef]
- Anwar, M.K.; Shah, S.A.R.; Alhazmi, H. Recycling and Utilization of Polymers for Road Construction Projects: An Application of the Circular Economy Concept. Polymers 2021, 13, 1330. [Google Scholar] [CrossRef]
- Thakur, S.; Verma, A.; Sharma, B.; Chaudhary, J.; Tamulevicius, S.; Thakur, V.K. Recent Developments in Recycling of Polystyrene Based Plastics. Curr. Opin. Green Sustain. Chem. 2018, 13, 32–38. [Google Scholar] [CrossRef]
- Ho, B.T.; Roberts, T.K.; Lucas, S. An Overview on Biodegradation of Polystyrene and Modified Polystyrene: The Microbial Approach. Crit. Rev. Biotechnol. 2018, 38, 308–320. [Google Scholar] [CrossRef]
- Siyal, A.N.; Memon, S.Q.; Khuhawar, M. Recycling of Styrofoam Waste: Synthesis, Characterization and Application of Novel Phenyl Thiosemicarbazone Surface. Pol. J. Chem. Technol. 2012, 14, 11–18. [Google Scholar] [CrossRef]
- Goshayeshi, B.; Abbas-Abadi, M.S.; Kusenberg, M.; Lemonidou, A.; Van Geem, K.M. Prospects of Physical, Mechanical, and Advanced Recycling of End-of-Life Polystyrenes: Towards Full Recyclability. Curr. Opin. Green Sustain. Chem. 2023, 42, 100821. [Google Scholar] [CrossRef]
- Yang, M.; Sui, W.Y.; Qin, Y.; Nie, Y.J. Study on Recycling of Waste Styrofoam for Adhesive. Adv. Mater. Res. 2011, 181–182, 975–978. [Google Scholar] [CrossRef]
- Cao, R.; Zhang, M.-Q.; Hu, C.; Xiao, D.; Wang, M.; Ma, D. Catalytic Oxidation of Polystyrene to Aromatic Oxygenates over a Graphitic Carbon Nitride Catalyst. Nat. Commun. 2022, 13, 4809. [Google Scholar] [CrossRef]
- Dilberoglu, U.M.; Gharehpapagh, B.; Yaman, U.; Dolen, M. The Role of Additive Manufacturing in the Era of Industry 4.0. Procedia Manuf. 2017, 11, 545–554. [Google Scholar] [CrossRef]
- Haleem, A.; Javaid, M. Additive Manufacturing Applications in Industry 4.0: A Review. J. Ind. Integr. Manag. 2019, 4, 1930001. [Google Scholar] [CrossRef]
- Nguyen, K.Q.; Vuillaume, P.Y.; Hu, L.; López-Beceiro, J.; Cousin, P.; Elkoun, S.; Robert, M. Recycled, Bio-Based, and Blended Composite Materials for 3D Printing Filament: Pros and Cons—A Review. Mater. Sci. Appl. 2023, 14, 148–185. [Google Scholar] [CrossRef]
- Mikula, K.; Skrzypczak, D.; Izydorczyk, G.; Warchoł, J.; Moustakas, K.; Chojnacka, K.; Witek-Krowiak, A. 3D Printing Filament as a Second Life of Waste Plastics—A Review. Environ. Sci. Pollut. Res. 2021, 28, 12321–12333. [Google Scholar] [CrossRef]
- Dhinakaran, V.; Kumar, K.M.; Ram, P.B.; Ravichandran, M.; Vinayagamoorthy, M. A Review on Recent Advancements in Fused Deposition Modeling. Mater. Today Proc. 2020, 27, 752–756. [Google Scholar] [CrossRef]
- Yoshimura, T.; Kimura, T. Soluble Material for Three Dimensional Molding. EP 3 656 545 B1, 2 October 2015. [Google Scholar]
- Turku, I.; Kasala, S.; Kärki, T. Characterization of Polystyrene Wastes as Potential Extruded Feedstock Filament for 3D Printing. Recycling 2018, 3, 57. [Google Scholar] [CrossRef]
- Bartolomei, S.S.; da Silva, F.L.F.; de Moura, E.A.B.; Wiebeck, H. Recycling Expanded Polystyrene with a Biodegradable Solvent to Manufacture 3D Printed Prototypes and Finishing Materials for Construction. J. Polym. Environ. 2022, 30, 3701–3717. [Google Scholar] [CrossRef]
- Chu, J.S.; Koay, S.C.; Chan, M.Y.; Choo, H.L.; Ong, T.K. Recycled Plastic Filament Made from Post-consumer Expanded Polystyrene and Polypropylene for Fused Filamant Fabrication. Polym. Eng. Sci. 2022, 62, 3786–3795. [Google Scholar] [CrossRef]
- William, L.J.W.; Koay, S.C.; Chan, M.Y.; Pang, M.M.; Ong, T.K.; Tshai, K.Y. Recycling Polymer Blend made from Post-used Styrofoam and Polypropylene for Fuse Deposition Modelling. J. Physics: Conf. Ser. 2021, 2120, 012020. [Google Scholar] [CrossRef]
- Biasetto, L.; Franchin, G.; Elsayed, H.; Boschetti, G.; Huang, K.; Colombo, P. Direct Ink Writing of Cylindrical Lattice Structures: A Proof of Concept. Open Ceram. 2021, 7, 100139. [Google Scholar] [CrossRef]
- Dong, J.; Li, Y.; Lin, P.; Leeflang, M.; van Asperen, S.; Yu, K.; Tümer, N.; Norder, B.; Zadpoor, A.; Zhou, J. Solvent-Cast 3D Printing of Magnesium Scaffolds. Acta Biomater. 2020, 114, 497–514. [Google Scholar] [CrossRef]
- Tolbert, J.W.; Hammerstone, D.E.; Yuchimiuk, N.; Seppala, J.E.; Chow, L.W. Solvent-Cast 3D Printing of Biodegradable Polymer Scaffolds. Macromol. Mater. Eng. 2021, 306, 2100442. [Google Scholar] [CrossRef]
- AMETEK Brookfield Inc. More Solutions to Sticky Problems; AMETEK Brookfield Inc.: Middleborough, MA, USA, 2017; pp. 1–62. [Google Scholar]
- García-Sobrino, R.; Lago, E.; Goñi, C.; Ramos, V.; García, C.; Reinecke, H.; Elvira, C.; Rodríguez-Hernández, J.; Gallardo, A.; Martínez-Campos, E. Fabrication of 3D Cylindrical Thermosensitive Hydrogels as Supports for Cell Culture and Detachment of Tubular Cell Sheets. Biomater. Adv. 2023, 144, 213210. [Google Scholar] [CrossRef]
- Cortés, A.; Jiménez-Suárez, A.; Campo, M.; Ureña, A.; Prolongo, S.G. Assessment of Manufacturing Parameters for New 3D-Printed Heating Circuits Based on CNT-Doped Nanocomposites Processed by UV-Assisted Direct Write. Appl. Sci. 2021, 11, 7534. [Google Scholar] [CrossRef]
- Dortmund Data Bank Acetone Vapor Pressure. Available online: http://www.ddbst.com/en/EED/PCP/VAP_C4.php (accessed on 1 January 2023).
- Dortmund Data Bank Acetone Heat of Vaporization. Available online: http://www.ddbst.com/en/EED/PCP/HVP_C4.php (accessed on 1 January 2023).
- Pretsch, E.; Clerc, T.; Seibl, J.; Simon, W. Tablas Para La Elucidacion Estructural de Compuestos Organicos Por Metodos Espectroscopicos, 1st ed.; Editorial Alhambra S.A.: Madrid, Spain, 1908. [Google Scholar]
- Cella, R.F.; Mumbach, G.D.; Andrade, K.L.; Oliveira, P.; Marangoni, C.; Bolzan, A.; Bernard, S.; Machado, R.A.F. Polystyrene Recycling Processes by Dissolution in Ethyl Acetate. J. Appl. Polym. Sci. 2018, 135, 46208. [Google Scholar] [CrossRef]
- Cáceres, C.A.; Zborowski, L.; Canevarolo, S.V. Thermo-Mechanical Degradation and VOC Emission of Unstabilized and Stabilized Polypropylene Copolymer during Multiple Extrusions. Mater. Res. 2011, 14, 569–575. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Lee, Y.-J.; Park, J.-R.; Lee, D.-H.; Yoon, K.-B. Control of Molecular Weight Distribution for Polypropylene Obtained by Commercial Ziegler-Natta Catalyst: Effect of Electron Donor. Macromol. Res. 2011, 19, 622–628. [Google Scholar] [CrossRef]
- Yang, W.; Dong, Q.; Liu, S.; Xie, H.; Liu, L.; Li, J. Recycling and Disposal Methods for Polyurethane Foam Wastes. Procedia Environ. Sci. 2012, 16, 167–175. [Google Scholar] [CrossRef]
- WHO—EPA Environmental Health Criteria 207. Acetone. Available online: https://www.inchem.org/documents/ehc/ehc/ehc207.htm (accessed on 1 January 2023).
- National Center for Biotechnology Information PubChem Compound Summary for CID 180, Acetone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acetone (accessed on 1 January 2023).
- Sanchirico, R.; Di Benedetto, A.; Garcia-Agreda, A.; Russo, P. Study of the Severity of Hybrid Mixture Explosions and Comparison to Pure Dust–Air and Vapour–Air Explosions. J. Loss Prev. Process Ind. 2011, 24, 648–655. [Google Scholar] [CrossRef]
- Abdelkhalik, A.; Askar, E.; Markus, D.; Brandes, E.; El-Sayed, I.; Hassan, M.; Nour, M.; Stolz, T. Explosion Regions of Propane, Isopropanol, Acetone, and Methyl Acetate/Inert Gas/Air Mixtures. J. Loss Prev. Process Ind. 2016, 43, 669–675. [Google Scholar] [CrossRef]




| Needle Diameter | 10 G | 12 G | 14 G | 16 G | 18 G |
|---|---|---|---|---|---|
| Average width (mm) | 2.30 ± 0.26 | 1.94 ± 0.18 | 1.83 ± 0.17 | 1.84 ± 0.16 | 1.65 ± 0.35 |
| Ribbons width deviation (%) | 11.49 | 9.21 | 9.42 | 8.44 | 21.31 |
| Printing Speed (mm/s) | 2 | 8 | 12 | 16 | 25 |
|---|---|---|---|---|---|
| Average width (mm) | 1.84 ± 0.22 | 2.15 ± 0.24 | 1.93 ± 0.22 | 2.04 ± 0.37 | 3.06 ± 0.47 |
| Ribbons width deviation (%) | 11.73 | 11.06 | 11.26 | 18.38 | 15.24 |
| Bed Temperature (°C) | 25 | 40 |
|---|---|---|
| Average width (mm) | 1.93 ± 0.22 | 2.46 ± 0.63 |
| Ribbons width deviation (%) | 11.26 | 25.73 |
| Sample/Label | EPS−1 | EPS−3 |
|---|---|---|
| Mn (g/mol) × 104 | 6.55 | 7.71 |
| Mw (g/mol) × 104 | 21.32 | 23.71 |
| PDI | 3.26 | 3.07 |
| Tg (°C) | 100.0 | 104.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Sobrino, R.; Cortés, A.; Calderón-Villajos, R.; Díaz, J.G.; Muñoz, M. Novel and Accessible Physical Recycling for Expanded Polystyrene Waste with the Use of Acetone as a Solvent and Additive Manufacturing (Direct Ink-Write 3D Printing). Polymers 2023, 15, 3888. https://doi.org/10.3390/polym15193888
García-Sobrino R, Cortés A, Calderón-Villajos R, Díaz JG, Muñoz M. Novel and Accessible Physical Recycling for Expanded Polystyrene Waste with the Use of Acetone as a Solvent and Additive Manufacturing (Direct Ink-Write 3D Printing). Polymers. 2023; 15(19):3888. https://doi.org/10.3390/polym15193888
Chicago/Turabian StyleGarcía-Sobrino, Rubén, Alejandro Cortés, Rocío Calderón-Villajos, Jorge G. Díaz, and Marta Muñoz. 2023. "Novel and Accessible Physical Recycling for Expanded Polystyrene Waste with the Use of Acetone as a Solvent and Additive Manufacturing (Direct Ink-Write 3D Printing)" Polymers 15, no. 19: 3888. https://doi.org/10.3390/polym15193888
APA StyleGarcía-Sobrino, R., Cortés, A., Calderón-Villajos, R., Díaz, J. G., & Muñoz, M. (2023). Novel and Accessible Physical Recycling for Expanded Polystyrene Waste with the Use of Acetone as a Solvent and Additive Manufacturing (Direct Ink-Write 3D Printing). Polymers, 15(19), 3888. https://doi.org/10.3390/polym15193888